Abstract
Microplastics and associated adverse effects have been on the global agenda in recent years. Because of its importance as a model organism for studies on developmental biology, Xenopus laevis has been chosen as the study animal in in vitro teratogenesis studies. FETAX test uses early-stage embryos of X. laevis to measure the potential of substances to cause mortality, malformation, and growth inhibition in developing embryos. The aim of this study was to examine the effects of high molecular weight polyvinyl chloride (HMW-PVC) on parental X. laevis frogs and their embryos using the FETAX test. To this purpose, a HMW-PVC dose of 1% of body weight/twice each week was provided to frogs by oral gavage throughout 6 weeks. After the procedure, oocytes and sperms of HMW-PVC-exposed frogs were fertilized and FETAX was applied to selected embryos. After the completion of a 96-h incubation period, tadpoles were examined, their live/dead status were determined, their lengths were measured, and their anomalies were photographed. Besides, excised organs of the parental frogs were referred to histopathology examination. On the other hand, the mRNA expression levels of Hsp70, Myf5, Bmp4, Pax6, and Esr1 genes were determined by applying real-time quantitative PCR method to cDNA which was synthesized from the total RNA of embryos. The results showed that treatment with HMW-PVC dose of 1% of body weight/twice each week caused malformations and decreased viability. Hsp70 and Pax6 gene expression levels significantly decreased in all assay groups, as compared with controls. Lung and intestine tissues showed normal appearance in histopatological examination. Further research is required to explain the whole effects of HMW-PVC exposure on X. laevis embryos.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the 1960s, plastic pollution is a growing environmental issue in marine and freshwater systems, where it can spread to most remote aquatic habitats around the globe (Eerkes-Medrano et al. 2015; Shabbir et al. 2020; Bharath et al. 2021). Annual plastic production increased from 1.5 to 359 million metric tonnes in last 60 years (Wright et al. 2013; Weis et al. 2015; Lusher et al. 2017; Smith et al. 2018; Banaee et al. 2019; Bhagat et al. 2020; Brandts et al. 2021). As of 2050, it is estimated that the amount of plastic pollutants will exceed fish biomass in the seas (Eerkes-Medrano et al. 2015). Each passing day, plastic debris increasingly accumulate in terrestrial and aquatic habitats worldwide. As a result of this increased level of plastic pollution in aquatic ecosystems, aquatic species are inevitably exposed to and contaminated with plastic particles. A 2016 UN document reported that over 800 animal species contaminated with plastic via ingestion or entanglement, a figure which is 69% greater than the figure reported in a 1977 review, which estimated only 247 contaminated species (Smith et al. 2018).
Depending on size, plastic particles are generally classified as macroplastics and microplastics. The biological consequences of macroplastic (particle size: >5 mm) debris on wildlife have been well documented (Wright et al. 2013; Kaposi et al. 2014; Banaee et al. 2020). Likewise, microplastics (particle size: ≤5 mm) are also recognized as prominent pollutants in aquatic environments (Browne et al. 2007; Barnes et al. 2009; Hopewell et al. 2009; Meeker et al. 2009; Oehlmann et al. 2009; Ryan et al. 2009; Shaxson 2009; Song et al. 2009; Browne et al. 2011; Goldstein et al. 2012; Claessens et al. 2011; Jovanović et al. 2018; Malinich et al. 2018). The most prevalent types of synthetic polymers used in plastic production are polyvinylchloride (PVC), polyethylene (PE), and polypropylene (PP). The figures published in 2007 revealed that PVC, PE, and PP comprised 19%, 21%, and 24% of world’s plastics production, respectively (Browne et al. 2007).
Microplastics in aquatic environments are composed of diverse polymers and typically found as fragments fibers, films, and pellets (Wang et al. 2020). Some of those particles are heavier than seawater and are expected to sink to the ocean floor. Those particles contain polyester, PVC, polyamide, and acrylics. Other particles are lighter than seawater and found floating at the ocean or sea surface. Those polymers include polystyrene (PS), PP, and PE (Hu et al. 2016; Smith et al. 2018; De Felice et al. 2018). Bharath et al. (2021) investigated the quantity of microplastic contaminants in water and soil samples at Veeranam lake in Tamil Nadu, India. They found that the amount of total plastic particles were in the range of 92–604 items/kg with a mean value of 309 items/kg in sediment. This study found that the collected water and sediment samples deposited with polymer types of nylon (39%), PS (19%), PP (15%), PE (23%), and PVC (4%) (Bharath et al. 2021).
Microplastic ingestion could cause external or internal abrasions and ulcers; and gastrointestinal tract obstructions, which can result in satiation and subsequent starvation and body weakness (Jovanović 2017; Wibowo et al. 2019; Ahrendt et al. 2020). Microplastic ingestion can also cause reproductive failure and initiate impairment of feeding ability, diminished predator avoidance, drowning, the potential transfer of toxic substances from sea/ocean water, and ultimately death (Wibowo et al. 2019). It is also likely that smaller organisms such as invertebrates suffer from such detrimental effects of microplastics ingestion (Wright et al. 2013).
A thorough understanding of the effects of microplastic particles on an organism necessitate to examine the effects of microplastic exposure on mRNA expression profiles of some early development genes (Myf5, Esr1, Bmp4, Pax6) and a stress response gene (Hsp 70). The mRNA expression of Myf5 and Esr1 genes are important for normal development of X. laevis embryo (Segundo et al. 2013). Myf5 gene is a member of the myogenic regulatory factor (MRF) gene family and plays a main role in determining myogenic cell destiny at the onset of skeletal muscle formation in the embryo (Giordani et al. 2007). In X. laevis, XMyf5 expression occurs in the presomitic mesoderm region (dorsal–lateral marginal zone; DLMZ) during gastrulation and this region subsequently differentiates into myotomal muscle cells. Therefore, the loss of XMyf5 leads to reduced convergent extension in the paraxial mesoderm, a slight delay in segmentation and a general deterioration of the somite borders and structure, the events which occur in different stages of Xenopus embryo development (Keren et al. 2005; Maguire et al. 2012). Esr1 gene as a member of Hairy/enhancer of split (HES) gene family is a downstream effector of notch signaling pathway, which is a fundamental part of the segmentation clock that controls the regular formation of somites from the presegmental plate mesoderm during embryo development. HES genes encode proteins which are targets of the Notch signaling pathway in neural precursors, but their exact role in neurogenesis is not known. So, Esr1 gene is important for normal somitogenesis and neuronal differentiation (Lamar et al. 2005). Bone morphogenetic protein-4 (BMP-4), the growth factor which is a member of transforming growth factor-3 superfamily, is identified as a very effective ventralizing factor in Xenopus embryos. BMP-4 is expressed in the ventral marginal zone during gastrulation (Fainsod et al. 1994).
The Pax6 gene encodes a key transcription factor which plays a key role in regulating bilaterian eye and central nervous system morphogenesis (Elso et al. 2013). Pax6 function deficiency results in several phenotypes (Vasilyeva et al. 2018). Those phenotypes include eye malformations known as aniridia in humans, small eye in mice, and eyeless in Drosophila. Misexpression of Pax6 gene causes the formation of ectopic eye structures in Xenopus (Onuma et al. 2002).
Heat shock proteins (HSP) are a group of evolutionary conserved chaperons which play a key role in cellular response to heat stress/shock. One of the most important of these chaperone proteins is heat shock protein 70 (Hsp70) (Campanella et al. 2014). Hsp70 is a potent survival protein which protects cells against numerous cell death, stimuli including oxidative stress-inducing heat shock, staurosporine, tumor necrosis factor (TNF), and anti-cancer drugs (Gyrd-Hansen et al. 2004; Piri et al. 2016).
The African clawed frog X. laevis is a suitable model organism for teratogenicity studies ( Bonfanti et al. 2018; Babalola et al. 2019). The advantages that have made X. laevis a classical model organism to study early vertebrate embryonic development are as follows: (1) the ease with which female frogs can be induced to lay eggs using commercially available hCG; (2) their eggs can easily be harvested and fertilized in vitro for synchronized development; (3) their eggs and embryos have relatively large sizes; (4) frog exhibits external fertilization, so oocytes and sperms can easily be fertilized ex utero (Lee-Liu et al. 2017).
Even though the awareness regarding consequences of exposure to microplastic particles has started to rise in the early 2000s, to date, there are a limited number of studies focused on this issue. These studies investigated the effects of microplastic particles on various organisms including frogs, zebrafish, seabream, larval fish fathead minnow, and red tilapia (Hu et al. 2016; Lu et al. 2016; Baumann et al. 2016; De Felice et al. 2018; Jovanović et al. 2018; Malinich et al. 2018; Ding et al. 2020). As a consequence of sharing the same habitat, X. laevis frogs and freshwater fishes are exposed to the same harmful substances, including microplastic particles. Because the microplastics which accumulated in the body of fishes enter the human body through the food chain and because X. laevis is a model organism for all vertebrates, the results obtained from the studies which aimed to investigate the detrimental effects of microplastics on X. laevis, can also be considered relevant for human studies. In this regard, in a review published in 2018, Smith et al. suggested attenuation and elimination strategies which targeted the microplastic life cycle and recommended more research to evaluate the effects of microplastics on humans. This study concluded that there were research gaps in this field and due to the nutritional importance of seafood consumption, it is a critical priority to design studies focusing on these research gaps (Smith et al. 2018). Therefore, we aimed to examine the effects of HMW-PVC on adult male and female X. laevis frogs and their embryos using the frog embryo teratogenesis assay of Xenopus (FETAX) test. We also examined mRNA expression levels of Hsp70, Myf5, Bmp4, Pax6, and Esr1 genes which are involved in early development and general stress response.
Materials and methods
All procedures and methods used in the present study were implemented according to American Society for Testing Materials guide (ASTM guide 2004).
Test substances
FETAX solution and the DeBoers Tris (DBT) reagent and microplastic particles (HMW-PVC in powder form, catalog number 81387;2) were obtained from Sigma-Aldrich (Jovanović et al. 2018; Anbumani and Kakkar 2018). The follicle-stimulating hormone (FSH) and human chorionic gonadotropin (hCG, Pregnyl, 5000 IU) were obtained from Serono and Organon, respectively.
Test organisms
Twelve adult frogs (6 female, 6 male) were acquired from Cukurova University Medical School’s Department of Physiology (Adana, Turkey). The frogs were maintained in 95–60–44 cm3 aquaria. The aquaria were exposed to 12-h photoperiod consisting 12-h light and 12-h darkness and the temperature was set at 23 °C (±2 °C). Frogs were fed ad libitum (once per week) with chicken liver (Boga Pekmezekmek et al. 2013).
In vitro fertilization and application of FETAX procedure
In vitro fertilization was performed in four groups of adult frogs: The first group was designed as control group which includes normal female and male frogs embryos; second, third, and fourth groups were study groups which included normal females+assay males, assay females+normal males, and assay females+assay males embryos, respectively. A 480 embryos (360 assay and 120 control embryos), which developed from gametes of HMWPVC-exposed frogs, were examined by microscope (Olympus SZ-61). Experiments were repeated three times in all study and control groups. A HMW-PVC dose, which was determined according to 1% of body weight/twice each week, was provided by oral gavage to frogs in study groups throughout 6 weeks (Segundo et al. 2013; Jovanović et al. 2018). Six weeks later, in vitro fertilized embryos were produced as described in standard FETAX procedure. In order to produce embryos, in vitro fertilization of oocytes were performed following procedure described by Lindi et al. (2001). Ovulation was induced by hCG injection of 700–1000IU as a single dose. After 16 h of injection, female frogs laid their eggs on the petri dishes. Sperms were obtained from minced frog testes and then –2 mL of DBT (119nM NaCl, 1.8nM CaCl2 and 15nM Tris–HCl; pH value of 7.5) solution were added to sperm-containing petri plates for preparing sperm suspension to be used in insemination procedure. Insemination was performed by addition of sperm suspensions to petri plates which contained eggs. A 10 mL of FETAX solution (625mg/L NaCI, 96mg/mL NaHCO3, 70 mg/mL MgSO4, 60 mg/mL CaSO4-2H2O, 30mg/mL KCI, 15mg/mL CaCI2; a pH between 7.8 and 8.0) was added to each petri plate, 2 min after addition of sperm suspension. Several minutes following sperm addition, the presence of successful insemination was detected according to the upward positioning of animal pole (the dark side) within the eggs. Irregularly segmented eggs were discarded. Normal tables were used for determining the embryos between the midblastula (stage 8) and early gastrula (stage 11), and then FETAX procedures were applied to stage 8 and stage 11 embryos (Nieuwkoop and Faber 1994; Boga Pekmezekmek et al. 2013). Petri dishes containing embryos were maintained at 23 (±1)°C.
The FETAX solutions were changed at 24, 48, and 72 h of FETAX procedure. After the end of a 4-day (96 h) incubation period, embryos/tadpoles were counted and photographed according to their lengths from tail to head. Olympus SZ-61 model ocular micrometer which magnifies by a factor of 10 was used to measure the length of embryos. After the completion of FETAX procedure, the number of alive tadpoles was ascertained and then the tadpoles were fixed in formalin solution of 3.0% (pH=7.0). The notochord curvature was taken into account, when measuring the length of tadpoles showing curved posture. A dissecting microscope was utilized for determining and examining abnormally developed and dead embryos. Irritability which is described as having the ability to respond an external stimulus, structural integrity and pigmented skin were accepted as signs of embryonic death at the 24 (stages 26 and 27) and 48 h (stage 37 and 39), while the absence of visible heartbeats was determined as sign of death at the 72 (stage 42) and 96 h (stage 45-46) (Dawson and Bantle 1987). The number of embryos having malformations were also determined and their stages of development were recorded in all petri plates. The normal table of X. leavis was used in identifying normal embryos (Nieuwkoop and Faber 1994; Hurney et al. 2015). Abnormal embryos were determined according to the presence of structural anomalies including head, trunk, and tail malformations and some other anomalies (Yamaguchi and Shinagawa 1983; Kao and Elinson 1988; Boga Pekmezekmek et al. 2013).
Histopathology
The excised organs of adult X. leavis were fixed in 10% formaldehyde solution. After fixation, macroscopic tissue slices were taken from fixed organs and tissue processing procedure were started. Tissue processing was carried out by using Thermo Scientific Excelsior ES Tissue Processor which makes automatic reagent rotation and dehydrate tissues by using alcohol. After processing, 4-μm sections were cut from the paraffine-embedded tissues, stained with hematoxylin-eozin dye, and examined under light microscope.
RNA extraction, cDNA synthesis, and RT-PCR
Total RNA were isolated from whole Xenopus newborn tadpoles by using TRI-reagent (Sigma-Aldrich) following the manufacturer’s guidance. Then, total RNA quality was measured using the spectrophotometric method by the 260/280 ratio (Thermo Fisher Multiskan GO Reader). For cDNA synthesis, the Applied Biosystems High Capacity cDNA Reverse Transcription Kit (Thermo Fisher) was used. Reverse transcription of RNA was carried out in 20 μl of final volume containing 10 μl RNA (2 μg), 2 μl of random primers, 1 μl of reverse transcriptase, 0.8 μl of dNTP, and 2 μl of 10 × RT buffer. The reaction was incubated at 25 °C for 10 min, and then the temperature was raised to 37 °C for 60 min., and finally it was raised to 85 °C for 5 min. The cDNAs were preserved at −20 °C until they were used for RT-qPCR. Two microliters of the cDNAs were used for RT-PCR analysis of gene expression. The qPCR reactions were performed in 96-well plates using SYBR Green Master Mix (Applied Biosystem). Reactions were performed following the manufacturer’s guidance with the total volume of 25 μl for each sample. Each plate was contained 12.5 μl of Sybr Green Master Mix, 4 μl of cDNA template, 20 pmol primers of 2.5 μl, and 6 μl of water. All quantitative PCR reactions were performed in Applied Biosystem 7500 RT-PCR device. The quantitative PCR cycling conditions were 50 °C for 2 min., 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. Gene expression studies were conducted in the Biotechnology Research and Application Center at Cukurova University.
Gapdh was used as an internal reference gene for its stability. All primer sequences are provided in Table 1. The 2−ΔΔCt formula (Rao et al. 2013) using threshold cycle (Ct) values obtained from replicates were used for calculating the alterations in expression levels of Hsp70, Myf5, Bmp4, Pax6, and Esr1 genes.
Statistical analysis
All data were analyzed using IBM SPSS 20.0 software package (IBM 2011). One-way ANOVA test was used in inter-group comparisons. The homogeneity of variances in multiple comparisons was controlled with Tamhane test. The chi-square test was used for comparing the proportions of normal, abnormal, and dead embryos. Inter-group differences of gene expression were evaluated using one-way ANOVA with Tukey’s test and Kruskal Wallis with Man-Whitney U tests for homogeneous and inhomogeneous data, respectively. The gene expression analysis results were reported as mean ± standard error. The statistical significance level was considered as p≤0.05.
Results
The effects of HMW-PVC on embryo development and viability
Control group embryos showed normal, abnormal, and death ratios of 97.5%, 2.5%, 0%, respectively. The second group which includes embryos produced from gametes of normal female and assay male frogs showed normal, abnormal, and death ratios of 60%, 5%, 35%, respectively. The normal, abnormal, and death ratios of the third group which includes embryos produced from gametes of assay female and normal male frogs were 62.5%, 5%, 32.5%, respectively. The last (fourth) group which includes embryos developed from gametes of assay female and male frogs showed normal, abnormal, and death ratios of 57.5%, 7.5%, and 35%, respectively. Abnormal and dead embryo ratios were significantly increased in all assay groups in comparison with controls (p<0001) (Table 2, Fig. 1).
Weight and length measurements results
Adult male and female frogs which were fed by liver and provided HMW-PVC at the abovementioned dose were examined in terms of weight change. Accordingly, the frogs were weighed before and after the experiment. The average weight gain of 2.835 g (an increase of 3.8%) and the average weight loss of 7.49 g (a decrease of 23.9%) were recorded in female and male frogs, respectively. Length measurement results in all embryo groups were provided as means±SE (mm) and shown in Table 3, Fig. 2. The results were as followed. The average length in control group (normal female and male frogs embryos) were 7.95±0.0045 mm. The average lengths of second (normal females+assay males), third (assay females+normal males), and fourth (assay females and assay male frogs) assay groups were *7.30±0.0063, **6.2±0.0032, **6.09±0.0014 mm, respectively. Average length values in assay groups were found to be significantly shorter than the control value (*p<0.01; **p<0.001).
Length values of first generation Xenopus embryos after 6 weeks treatment of parental frogs with HMW-PVC (95%CI). One-way ANOVA test was used in inter-group comparisons and data were presented as mean ± SD. Significant differences between the assay groups’ average length values and the control value are shown by asteriks (*p<0.01;**p<0.001)
Gene expression analysis results
The results of mRNA expressions of Hsp70, Myf5, Bmp4, Pax6, and Esr1 are shown in Fig. 3. The mRNA expression levels of Hsp70 and Pax6 genes significantly decreased in all assay groups as compared to the control group (group 1) (p < 0.05). Bmp4 mRNA expression, however, significantly decreased only in groups 3 and 4 as compared with the control group (p < 0.05). The expression levels of Myf5 and Esr1 genes were not changed as compared to the control group (p > 0.05) (Fig. 3).
The changes of expression levels of Bmp4, Myf5, Esr1, Hsp70, and Pax6 genes of X. leavis larvae. One-way ANOVA test was used in inter-group comparisons and data were presented as mean ± SD. Significant differences between the mRNA expression levels in the study groups and the control group were shown by asteriks (*p < 0.05)
Histopathological examination results
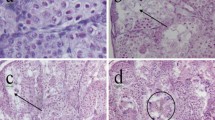
Histopatological analysis of lung and small intestine tissues of X. leavis which is exposed to HMW-PVC showed normal alveoli structure and congested blood vessels in lung (H&EX 100), and uneffected intestinal villi in small intestine (H&EX 200) (Figs. 4, 5, 6, and 7).
Discussion
Morphological results
The results of present study showed that abnormal and dead embryo ratios were significantly increased in all embryo groups which their parents were exposed to HMW-PVC substance, as compared with control group (p<0001). In a similar study, De Felice et al. (2018) investigated possible harmful effects of exposure to polystyrene microplastics (PSμPs; Ø = 3 μm) on X. laevis tadpoles. They seeded larvae at the developmental stage 36, prior to mouth opening, in petri dishes and exposed to a nominal concentration of 0.125, 1.25, and 12.5 μg mL−1 PSμPs in FETAX under semi-static conditions, and allowed larvae to develop until stage 46. They reported that exposure of X. laevis tadpoles to the abovementioned concentration of PSμPs did not significantly affect tadpoles’survival (De Felice et al. 2018). Although the study of De Felice et al. (2018) and our study were conducted with tadpoles of same species (X. laevis), the difference in experimental setups, particularly, directy providing HMW-PVC substance to parental X. laevis frogs at the extremely high concentration (1% of body weight/twice each week) in our study, could be considered the main cause of the difference between the results of these two studies. In another study, Assas et al. (2020) reported that 3 weeks’ exposure of Japanese medaka (freshwaterfish) to polystyrene microplastics (107 beads/L) did not cause significant effects on survival and reproduction (Assas et al. 2020). When compared with the study of Assas et al., a longer exposure period and the higher concentration applied in our study were considered as important factors which could have affected the difference in our results. In a similar study, Malinich et al. 2018 investigated the effects of exposure to increasing densities of polyethylene microspheres on the foraging and growth responses of Pimephales promelas larvae by using Artemia nauplii as prey. In this study, P. promelas larvae were exposed to differential plastic particle treatments (control, medium [250 particles/L], and high [500 particles/L]) for 15 days and 30 days, and found that polyethylene microspheres had no or statistically non-significant effect on larval survivorship. Unlike our study, this study used an experimental setup which includes exposure of P. promelas larvae to polyethylene microspheres in tanks containing water and polyethylene microspheres at the abovementioned densities, mimicking the natural exposure conditions (Malinich et al. 2018). Thus, when compared to our study, P. promelas larvae ability to select food, the use of different method and shorter exposure durations could be considered as factors which caused difference in results in the study of Malinich et al. In general, conflicting results obtained from different studies might be attributed to variations in exposure concentration, duration, and the procedure applied.
Our results showed that body weights of female and male frogs, which were provided HMW-PVC by oral gavage (twice per week), differentiated at the end of a 6-week period. After 6 weeks, male frogs lost 23.9% of their weights on average, while female frogs increased their body weight at the average rate of 3.8%. These results suggest that female frogs are less effected/or vice versa from stress caused by microplastic particle exposure than male frogs. To our knowledge, there is no similar finding in related literature and further studies are to be conducted to confirm our observations.
Length values of second, third, and fourth embryo groups were significantly decreased as compared with control group (p<0.01, p<0.001, p<0.001, respectively). The third group embryos which includes assay females and normal males showed more decreased average body length value than the second group embryos which include assay males and normal females. It was seen that the embryos produced from assay females were more harmfully effected from HMW-PVC exposure than the embryos produced from assay males, when their average body length values were compared. Interestingly, it was seen that HMW-PVC exposure caused weight loss in the embryos of assay males against length decrease in the embryos of assay females. Our results are in agreement with a previous study demonstrating that the ingestion of micro-polystyrene particles negatively affected body growth of Crepidula onyx (the slipper limpet) larvae (Lo and Chan 2018). The accurate interpretation of these results require more extensive and similarly designed studies conducted on embryos of various species.
Hu et al. (2016) reported that they exposed X. tropicalis tadpoles to polystyrene microspheres (1 and 10 μm) by filter-feeding for 48 h. They reported that microspheres were seen in digestive tract and gills of tadpoles within 1 h and in feces after 6 h. The number of tadpoles which did not ingest microspheres was significantly higher than the number of tadpoles which ingested microspheres at 12 and 24 h after exposure. They suggested that tadpoles might have the ability to ingest or egest microspheres, rapidly. They concluded that aquatic vertebrate organisms might swallow more microplastic particles if the amount of microplastic particles continue to increase and available food decrease in aquatic ecosystems (Hu et al. 2016). Lu et al. (2016) studied the uptake, tissue accumulation, and toxic effects of polystyrene microplastics in zebrafish. Their results revealed that following 7 days of exposure, 5 μm diameter microplastics accumulated in fish gut and liver and gills, while 20-μm diameter microplastics accumulated only in fish gut and gills. The results of metabolomic analysis showed that microplastic exposure stimulated metabolic profile alterations in fish liver and harmfully effected whole-body lipid and energy metabolism in zebrafishes. Their results offer new insights about the damaging effects of microplastics on zebrafish (Lu et al. 2016). These results are in agreement with our results and further confirm that different aquatic organisms in contaminated waters can ingest/accumulate microplastic particles and may suffer from their toxic effects, paving the way for further research aimed to delineate the toxic effects of microplastic accumulation on different animal species.
Malinich et al. (2018) reported that 15- and 30-day exposures to polyethylene microspheres at the densities of 250 particles/L and 500 particles/L, did not significantly change the total length of Pimephales promelas larvae. The use of different species and experimental setup in the study of Malinich et al. could have caused different results as compared to our study (Malinich et al. 2018). In another study, it was reported that polystyrene microplastics ingestion did not affect body length of X. laevis tadpoles during early-life stages (De Felice et al. 2018). Jovanović et al. (2018) examined the effects of 6 common types of virgin microplastics (polyvinyl chloride high molecular weight, polyamide, ultra-high molecular weight polyethylene, polystyrene, average molecular weight medium density polyethylene, polyvinyl chloride low molecular weight) on gilt-head seabream (Sparus aurata) after 45 day’ exposure at 0.1 g kg−1 bodyweight day−1. In their experiments, they used seven (six-study and a control) water tanks of 500 L, each of which contained water, a type of virgin microplastics mixed into the fish feed and 50 fishes. They concluded that the dietary exposure to six common types of virgin plastic particles did not change the growth rate, induce stress or cause pathology in fish (Sparus aurata), and similarly, did not lead to microplastics accumulation in the digestive tract of fish (Jovanović et al. 2018). Contrary to the findings of Jovanović et al. (2018), De Felice et al. (2018) reported that exposure of X. laevis tadpoles to 0.125, 1.25, and 12.5 μg mL−1 of PSμPs caused the presence of polystyrene microplastics in their digestive tract and they concluded that tadpoles can ingest polystyrene microplastics, but ingested particles did not change tadpole development and swimming behavior at least during early-life stages, even at unrealistic high concentrations. The results of these studies, which are similar to our study in terms of research design, were in conflict with our results. The use of different species and variations in exposure concentration and duration might be accepted as important factors which caused conflicting results.
In the present study, histopatological analysis of lung and small intestine tissues of X. leavis frogs exposed to HMW-PVC showed normal alveoli structure and congested blood vessels in lung (H&EX 100), and uneffected intestinal villi in small intestine (H&EX 200). Similarly, Jovanović et al. (2018) dissected and microscopically examined the gastrointestinal tract, liver, pancreas, spleen, and mesentery of gilt-head seabream (Sparus aurata) after exposure to 6 common types of virgin microplastics at 0.1 g kg−1 bodyweight day−1, and found that some large microplastic particles remained trapped in the liver of the fish, and 5.3% of all the livers analyzed contained at least one particle. Besides, histopathological examination results in their study revealed that there was no difference between the control and the treatments in terms of the scored histopathology features and for all organs examined. Because we used HMW-PVC in powder form and could not examine HMW-PVC content of the liver and other organs of X. laevis in our study, the question of as to whether X. laevis’ organs including the liver retain microplastic particles ingested needs to be answered in future studies.
Molecular results
In the present study, the mRNA expression levels of Myf5 and Esr1 genes in assay groups were not significantly different from the mRNA expression level in control group. However, Segundo et al. (2013) investigated the effects of 2 contaminants of environmental concern (bisphenol A [BPA] and chlorpyrifos [CPF]), 1 emerging pollutant (methylparaben [MPB]), and 2 effluent samples from wastewater treatment plants on embryo survival and the mRNA expression levels of Myf5, Esr1, Bmp4, Pax6, and Hsp70 genes during X. laevis embryo development. They treated Xenopus embryos with various doses of contaminants for different durations; 4, 24, and 96 h. They reported the following results. BPA caused statistically significant decreases in mRNA levels for Esr1 at 12 mg/L after 4 h and 24 h post-exposure and at 3 mg/L and 6 mg/L after 96 h post-exposure. However, the expression of hsp70 mRNA showed significant increases after 24h-treatments with ≥3 mg/L BPA. Animals treated with CPF displayed significant increase of mRNA levels at 0.1 mg/L after 24 h post-exposure, and significant decreases at 0.1 mg/L and 2.5 mg/L after 96 h post-exposure. CPF treatment caused significantly increased mRNA levels of hsp70 at the 3 concentrations tested after 24 h post-exposure. Exposure to MPB caused significant decreases in mRNA levels for Esr1 at 240 mg/L, and for myf5 at concentrations ≥120 mg/L as well as induction of mRNA pax6 expression at 30 mg/L and 120 mg/L after 96 h post-exposure. MPB led to statistically significant induction of hsp70 at 240 mg/L after 24 h post-exposure and dose–response induction for concentrations ≥60 mg/L after 96 h post-exposure. They concluded that the levels of Hsp70, Pax6, and Esr1 mRNAs were differentially altered at early developmental stages and that the alterations in mRNA expression levels were specific to the contaminant species and the exposure time (Lamar and Kintner 2005; Segundo et al. 2013).
In the present study, BMP-4 gene expression significantly decreased in third and fourth embryo groups. These results showed that the expression level of BMP-4 was negatively affected in embryos which developed from HMW-PVC-exposed female frogs or HMW-PVC-exposed frog eggs. Besides, tail malformations were observed in these embryo groups (Fig. 1), confirming the previous data which revealed that BMP-4 RNA plays role in tail formation. Fainsod et al. (1994) demonstrated that BMP-4 could suppress the expression of genes which are active in Spemann’s organizer, and that BMP-4 RNA can play major role in mesoderm patterning, trunk, and tail formations in embryos. They reported that BMP-4 expression begins in the ventral marjinal zone and encompasses the animal cap, respecting only the organizer region and that temporal regulation of the BMP-4 gene is important for normal embryonic development (Fainsod et al. 1994; Piccolo et al. 1996). Sadlon et al. (2004) reported that mice lacking BMP4 die in utero.
The expression of x-Pax6 gene is important for development of forebrain subdivisions at embryonic stages 32, 35, and 40 in Xenopus (Bachy et al. 2002). Accordingly, Moreno et al. have demonstrated x-Pax6 expression in the forebrain of Xenopus at early developmental stages (Moreno et al. 2008). In the present study, Pax6 mRNA expression significantly decreased in all assay groups, as compared to the controls. Given that microcephaly and anencephaly conditions were occurred in embryo groups 2 and 4 (B and D in Fig. 1), and these conditions are closely related to forebrain development, our findings suggest that the reduced level of Pax6 mRNA expression adversely effected brain development and caused microcephaly and anencephaly conditions to occur (Ackerman 1992; Chen et al. 2009). Consistently, Peng et al. (2004) temporarily exposed X. leavis embryos to alcohol and demonstrated that alcohol dose-dependently caused growth retardation and microcephaly. They showed that inserting xPax6 expression plasmid to embryos by microinjection dose-dependently rescued alcohol-induced microcephaly in X. leavis embryos (Peng et al. 2004). Confirmation of these results requires carefully designed studies.
The expression level of Hsp70 mRNA significantly decreased in all embryo (assay) groups, which produced from HMW-PVC-exposed parental frogs. The decrease in Hsp70 mRNA expression level might have led to development of embryos vulnerable to abnormalities or having deficit in response to death-inducing stimuli. Incomplete response to unfavorable conditions might have caused higher percentages of abnormal and dead embryos.
Conclusion
Six-week treatment of HMW-PVC at a dose of 1% of body weight/twice each week on parental frogs led to increased ratios of malformed and dead embryos and decreased average embryo lengths. These results infer that treatment of parental frogs with HMW-PVC showed effects on their gametes and subsequently on embryos which were produced from those gametes. Decrease in expression levels of Pax6 and Hsp70 genes during X. laevis embryo development imply that both the developmental process and the stress response mechanism of embryos were affected from parental HMW-PVC treatment. Normal histologic appearance in histopathology examination suggest that the detrimental effects of parental HMW-PVC treatment on embryos may not reached tissue level change that can be readily diagnosed by histopathological tissue analysis, particularly in terms of lung and intestine tissues. Therefore, it can be thought that exposure times exceeding 6 weeks might cause tissue level changes. Further carefully designed studies are needed to establish malformation-causing mutations and transcript levels of important developmental genes as biomarkers of sub-lethal effects in an environmental risk-assessment framework.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Ackerman S (1992) Discovering the brain (1992). Washington (DC): National Academies Press (US)
Ahrendt C, Perez-Venegas DJ, Urbina M, Gonzalez C, Echeveste P, Aldana M, Pulgar J, Galbán-Malagón CC (2020) Microplastic ingestion cause intestinal lesions in the intertidal fish Girella laevifrons. Mar Pollut Bull 2020 151:110795. https://doi.org/10.1016/j.marpolbul.2019.110795
Anbumani S, Kakkar P (2018) Ecotoxicological effects of microplastics on biota: a review. Environ Sci Pollut Res Int 25(15):14373–14396. https://doi.org/10.1007/s11356-018-1999-x
Assas M, Qiu X, Chen K, Ogawa H, Xu H, Shimasaki Y, Oshima Y (2020) Bioaccumulation and reproductive effects of fluorescent microplastics in medaka fish. Mar Pollut Bull 158:111446. https://doi.org/10.1016/j.marpolbul.2020.111446
ASTM (American Society forTesting Materials) (2004) Standart guide for conducting the frog embryo-teratogenesis assay—Xenopus, FETAX, E1439-98. In: ASTM Standards on 381 Biological Effects and Environmental Fate. vol.11.05.Philadelphia, pp. 447–457.
Babalola JO, Truter C, van Wyk HJ (2019) Mortality, teratogenicity and growth inhibition of threeglyphosate formulations using Frog Embryo TeratogenesisAssay-Xenopus. J Appl Toxicol 39:1257–1266
Bachy I, Berthon J, Rétaux S (2002) Defining pallial and subpallial divisions in the developing Xenopus forebrain. Mech Dev 117(1-2):163–172
Bharath MK, Srinivasalu S, Natesan U, Ayyamperumal R, Kalam SN, Anbalagan S, Sujatha K, Alagarasan C (2021) Microplastics as an emerging threat to the freshwater ecosystems of Veeranam lake in south India: a multidimensional approach. Chemosphere 264(Pt2):128502. https://doi.org/10.1016/j.chemosphere.2020.12850
Banaee M, Soltanian S, Sureda A, Gholamhosseini A, Haghi BN, Akhlaghi M, Derikvandy A (2019) Evaluation of single and combined effects of cadmium and micro-plastic particles on biochemical and immunological parameters of common carp (Cyprinus carpio). Chemosphere 236:124335. https://doi.org/10.1016/j.chemosphere.2019.07.066
Banaee M, Gholamhosseini A, Sureda A, Soltanian S, Fereidouni MS, Ibrahim ATA (2020) Effects of microplastic exposure on the blood biochemical parameters in the pond turtle (Emys orbicularis). Environ Sci Pollut Res 28:9221–9234. https://doi.org/10.1007/s11356-020-11419-2
Barnes DKA, Galgani F, Thompson RC, Morton B (2009) Accumulation and Fragmentation of plastic debris in global environments. Philos Trans R Soc Lond Ser B Biol Sci 364(1526):1985–1998
Baumann L, Schmidt-Posthaus H, Segner H, Wolf JC (2016) Comment on “Uptake and Accumulation of Polystyrene Microplastics in zebrafish (Danio rerio) and Toxic Effects in Liver. Environ Sci Technol 50(22): 12521-12522.
Bhagat J, Zang L, Nishimura N, Shimada Y (2020) Zebrafish: an emerging model to study microplastic and nanoplastic toxicity. Sci Total Environ 728:138707. https://doi.org/10.1016/j.scitotenv.2020.138707
Brandts I, Barría C, Martins MA, Franco-Martínez L, Barreto A, Tvarijonaviciute A, Tort L, Oliveira M, Teles M (2021) Waterborne exposure of gilthead seabream (Sparus aurata) to polymethylmethacrylate nanoplastics causes effects at cellular and molecular levels. J Hazard Mater 403:123590. https://doi.org/10.1016/j.jhazmat.2020.123590
Boga Pekmezekmek A, Binokay US, Akıllıoglu K, Sertdemir Y (2013) Evaluation of E330-induced developmental toxicity using FETAX. Turk J Biol 37:265–272
Bonfanti P, Saibene M, Bacchetta R, Mantecca P, Colombo AA (2018) A glyphosate micro-emulsion formulation displays teratogenicity in Xenopus laevis. Aquat Toxicol 195:103-113. doi: 10.1016/j.aquatox.2017.12.007.
Browne MA, Galloway T, Thompson R (2007) Microplastic-an emerging contaminant of potential concern? Integr Environ Assess Manag 3(4):559–566
Browne MA, Crump P, Nivens SJ, Teuten E, Tonkin A, Galloway T, Thompson R (2011) Accumulation of microplastics on shorelines worldwide: sources and sinks. Environ Sci Technol 45(21):9175e9179
Campanella C, Bavisotto CC, Gammazza AM, Nikolic D, Rappa F, David S, Cappello F, Bucchieri F, Fais S (2014). Exosomal heat shock proteins as new players in tumour cell-to-cell communication. JCB [Internet]. [cited 15Jun.2021];3(1).
Chen L, Melendez J, Campbell K, Kuan CY, Zheng Y (2009) Rac1 deficiency in the forebrain results in neural progenitor reduction and microcephaly. Dev Biol 325(1):162–170
Claessens M, De Meester S, Van Landuyt L, De Clerck K, Janssen CR (2011) Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar Pollut Bull 62(10):2199e2204–2199e2204
Dawson DA, Bantle JA (1987) Development of a reconstituted water medium and preliminary validation of the frog embryo teratogenesis assay-xenopus (FETAX). J Appl Toxicol 7(4):237–244
De Felice B, Bacchetta R, Santo N, Tremolada P, Parolini M (2018) Polystyrene microplastics did not affect body growth and swimming activity in Xenopus laevis tadpoles. Environ Sci Pollut Res 25:34644–34651
Ding J, Huang Y, Liu S, Zhang S, Zou H, Wang Z, Zhu W, Geng J (2020) Toxicological effects of nano- and micro-polystyrene plastics on red tilapia: are larger plastic particles more harmless? J Hazard Mater 396:122693. https://doi.org/10.1016/j.jhazmat.2020.122693
Eerkes-Medrano D, Thompson RC, Aldridge DC (2015) Microplastics in freshwater systems: a review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res 75:63–82
Elso C, Lu X, Weisner PA, Thompson HL, Skinner A, Carver E, Stubbs L (2013) A reciprocal translocation dissects roles of Pax6 alternative promoters and upstream regulatory elements in the development of pancreas, brain, and eye. Genesis 51(9):630–646. https://doi.org/10.1002/dvg.22409 Epub 2013 Jul 23
Fainsod A, Steinbeisser H, De Robertis EM (1994) On the function of BMP-4 in patterning the marginal zone of the Xenopus embryo. EMBO J 13(21):5015–5025
Giordani J, Bajard L, Demignon J, Daubas P, Buckingham M, Maire P (2007) Six proteins regulate the activation of Myf5 expression in embryonic mouse limbs. PNAS 104(27):11310–11315
Goldstein MC, Rosenberg M, Cheng L (2012) Increased oceanic microplastic debris enhances oviposition in an endemic pelagic insect. Biol Lett 8(5):817–820
Gyrd-Hansen M, Nylandsted J, Jäättelä M (2004) Heat shock protein 70 promotes cancer cell viability by safeguarding lysosomal integrity. Cell Cycle 3(12):1484–1485
Hopewell J, Dvorak R, Kosior E (2009) Plastics recycling: challenges and opportunities. Philos Trans R Soc Lond Ser B Biol Sci 364(1526):2115–2126
Hu L, Su L, Xue Y, Mu J, Zhu J, Xu J, Shi H (2016) Uptake, accumulation and elimination of polystyrene microspheres in tadpoles of Xenopus tropicalis. Chemosphere 164:611–617
Hurney CA, Babcock SK, Shook DR, Pelletier TM, Turner SD, Maturo J, Cogbill S, Snow MC, Kinch K (2015) Normal table of embryonic development in the four-toed salamander, Hemidactylium scutatum. Mech Dev 136:99–110. https://doi.org/10.1016/j.mod.2014.12.007
IBM Corp Released (2011) IBM SPSS statistics for windows, Version 20.0. Armonk NY:IBM Corp.
Jovanović B (2017) Ingestion of microplastics by fish and its potential consequences from a physical perspective. Integr Environ Assess Manag 13(3):510–515. https://doi.org/10.1002/ieam.1913
Jovanović B, Gökdağ K, Güven O, Emre Y, Whitley EM, Kideys AE (2018) Virgin microplastics are not causing imminent harm to fish after dietary exposure. Mar Pollut Bull 130:123–131
Kao KR, Elinson R (1988) The entire mesodermal mantle behaves as Spemann’s organizer in dorsoanterior enhanced Xenopus laevis embryos. Dev Biol 127(1):64–77
Kaposi KL, Mos B, Kelaher BP, Dworjanyn SA (2014) Ingestion of microplastic has limited impact on a marine larva. Environ Sci Technol 48(3):1638–1645
Keren A, Bengal E, Frank D (2005) p38 MAP kinase regulates the expression of XMyf5 and affects distinct myogenic programs during Xenopus development. Dev Biol 228:73–86
Lamar E, Kintner C (2005) The Notch targets Esr1 and Esr10 are differentially regulated in Xenopus neural precursors. Development 132:3619–3630
Lee-Liu D, Méndez-Olivos EE, Muñoz R, Juan Larraín J (2017) The African clawed frog Xenopus laevis: a model organism to study regeneration of the central nervous system. Review Neurosci Lett 652:82–93. https://doi.org/10.1016/j.neulet.2016.09.054
Lindi C, Montorfano G, Rossi F, Gornati R, Rizzo AM (2001) Effect of ethanol exposure on Xenopus embryo lipid composition. AlcoholAlcohol 36:388–392
Lo HKA, Chan KYK (2018) Negative effects of microplastic exposure on growth and development of Crepidula onyx. Environ Pollut 233:588–595
Lu Y, Zhang Y, Deng Y, Jiang W, Zhao Y, Geng J, Ding L, Ren H (2016) Uptake and accumulation of polystyrene microplastics in zebrafish (Danio Rerio) and toxic effects in liver. Environ Sci Technol 50(7):4054–4060
Lusher AL, Hollman PCH, Mendoza-Hill JJ (2017) Microplastics in fisheries and aquaculture: status of knowledge on their occurrence and implications for aquatic organisms and food safety. FAO Fisheries and Aquaculture Technical Paper No. 615. Rome Italy.
Maguire RJ, Isaacs HV, Pownall ME (2012) Early transcriptional targets of MyoDlink myogenesis and somitogenesis. Dev Biol 371:256–268
Malinich TD, Chou N, Sepulveda MS, Höök TO (2018) No Evidence of microplastic impacts on consumption or growth of larval Pimephales promelas. Environ Toxicol Chem 37(11):2912–2918
Meeker JD, Sathyanarayana S, Swan SH (2009) Phthalates and other additives in plastics: human exposure and associated health outcomes. Philos Trans R Soc B 364:2097–2113
Moreno N, Rétaux S, González A (2008) Spatio-temporal expression of Pax6 in Xenopus forebrain. Brain Res 6(1239):92–99. https://doi.org/10.1016/j.brainres.2008.08.052
Nieuwkoop PD, Faber J (1994) Normal table of Xenopus laevis (Daudin). Newyork&London: Garland Publishing INC
Oehlmann J, Schulte-Oehlmann U, Kloas W, Jagnytsch O, Lutz I, Kusk KO, Wollenberger L, Santos EM, Paull GC, Van Look KJW, Tyler CR (2009) A critical analysis of the biological impacts of plasticizers on wildlife. Philos Trans R Soc Lond Ser B Biol Sci 364(1526):2047–2062
Onuma Y, Takahashi S, Asashima M, Kurata S, Gehring WJ (2002) Conservation of Pax6 function and upstream activation by Notch signaling in eye development of frogs and flies. Proc Natl Acad Sci U S A 99(4):2020–2025
Peng Y, Yang PH, Ng SS, Wong OG, Liu J, He ML, Kung HF, Lin MC (2004) A critical role of Pax6 in alcohol-induced fetal microcephaly. Neurobiol Dis 16(2):370–376
Piccolo S, Sasa Y, Lu B, Robertis EMD (1996) Dorsoventral patterning in xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell 86(4:589–598
Piri N, Kwong JM, Gu L, Caprioli J (2016) Heat shock proteins in the retina: focus on HSP70 and alpha crystallins in ganglion cell survival. Prog Retin Eye Res 52:22–46
Rao X, Huang X, Zhou Z, Lin X (2013) An improvement of the 2ˆ(–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath 3(3):71–85
Ryan PG, Moore CJ, van Franeker JA, Moloney CL (2009) Monitoring the abundance of plastic debris in the marine environment. Philos Trans R Soc Lond Ser B Biol Sci 364(1526):1999–2012
Sadlon TJ, Lewis ID, D'Andrea RJ (2004) BMP4: its role in development of the hematopoietic system and potential as a hematopoietic growth factor. Stem Cells 22(4):457–474
Shabbir S, Faheem M, Ali N, Kerr PG, Wang LF, Kuppusamy S, Li Y (2020) Periphytic biofilm: an innovative approach for biodegradation of microplastics. Sci Total Environ 15(717):137064
Segundo LS, Martini F, Pablos MV (2013) Gene expression responses for detecting sublethal effects of xenobiotics and whole effluents on a Xenopus laevis embryo assay. Environ Toxicol Chem 32(9):2018–2025
Shaxson L (2009) Structuring policy problems for plastics, the environment and human health: reflections from the UK. Philos Trans R Soc Lond Ser B Biol Sci 364(1526):2141–2151
Smith M, Love DC, Rochman CM, Neff RA (2018) Microplastics in seafood and the implications for human health. Curr Environ Health Rep 5:375–386
Song JH, Murphy RJ, Narayan R, Davies GBH (2009) Biodegradable and compostable alternatives to conventional plastics. Philos Trans R Soc Lond Ser B Biol Sci 364(1526):2127–2139
Vasilyeva TA, Voskresenskaya AA, Pozdeyeva NA, Marakhonov AV, Zinchenko RA (2018) Pax6 gene characteristic and causative role of Pax6 mutations in inherited eye pathologies. Russ J Genet 54:995–1002
Wang C, Xing R, Sun M, Ling W, Shi W, Cui S, An L (2020) Microplastics profile in a typical urban river in Beijing. Sci Total Environ 15(743):140708. https://doi.org/10.1016/j.scitotenv.2020.140708
Weis J, Andrews CJ, Dyksen JE, et al. (2015) Human health impact of microplastics and nanoplastics. NJDEP - Science Advisory Board.
Wibowo YG, Maryani AT, Rosanti D, Rosarina D (2019) Microplastic in marine environment and its impact.Sainmatika 16:1.
Wright A, Thompson RC, Galloway TS (2013) The physical impacts of microplastics on marine organisms. A review Stephanie L Environ Pollut 178:483–492
Yamaguchi Y, Shinagawa A (1983) Marked alteration at midblastula transition in the effect of lithium on formation of the larval body pattern of Xenopus laevis. Develop Growth Differ 31(6):531–541
Funding
This study was supported by the Çukurova University Research Fund (I.U.BAP) (Project no: TSA-2019-11793).
Author information
Authors and Affiliations
Contributions
Ayper Boga Pekmezekmek: ınvestigation, writing—original draft-,supervision, project administration; Mustafa Emre: ınvestigation, Methodology, Supervision; Seyda Erdogan: histopathological analysis; Bertan Yılmaz: genetic analysis; Yaşar sertdemir: statistics; Erdal Tunc: writing—review and editing; Yılmaz Emre: supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The study entitled “Effects of high-molecular-weight polyvinyl chloride on X. laevis adultes and embryos: the mRNA expression profiles of Myf5, Esr1, Bmp4, Pax6, and Hsp70 genes during early embryonic development” was approved by Cukurova University Health Sciences Assay Application and Research Center. Decision No: 4(5) on July 8, 2019.
Additional information
Responsible Editor: Bruno Nunes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pekmezekmek, A.B., Emre, M., Erdogan, S. et al. Effects of high-molecular-weight polyvinyl chloride on Xenopus laevis adults and embryos: the mRNA expression profiles of Myf5, Esr1, Bmp4, Pax6, and Hsp70 genes during early embryonic development. Environ Sci Pollut Res 29, 14767–14779 (2022). https://doi.org/10.1007/s11356-021-16527-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16527-1