Abstract
Pharmaceutical compounds were emerging contaminants, and the accumulation of pharmaceutical compounds in the environment increased the risk to humans and ecosystems. In this study, electron beam irradiation was applied to degrade indomethacin (IDM) in aqueous solution. IDM degradation followed pseudo-first-order kinetics and 300 μM IDM could be completely degraded at only 2 kGy. According to the quenching experiment, the dose constant ratios of oxidative radicals (•OH) and reductive radicals (e−aq and •H) could be calculated as k•OH: ke aq and •H=4.79:1. As the concentration of H2O2 increased from 0 to 10 mM, the dose constant increased from 1.883 to 2.582 kGy−1. However, degradation effect would be restrained in the existence of NO−3, NO−2, CO2−3, HCO−3, SO2−, and humic acid due to their competition for the active species. Theoretical calculation revealed the radical attacking sites of IDM molecule and the most probable pathways were proposed with identification of intermediates. The attack of •OH mainly resulted in the cleavage of amide bond, indole ring opening, demethoxylation, and •OH addition. Dechlorination and the reduction of the carbonyl group occurred on IDM molecular through the reduction of e−aq and •H. The intermediates could continue to be degraded to small molecule acid, such as formic acid, acetic acid, and oxalic acid. Furthermore, highly toxic IDM transformed into less toxic products during the irradiation process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past decades, pharmaceuticals compounds have been dramatically produced and released into the environment (Li 2014). Pharmaceutical compounds have been recognized as emerging contaminants, the emergence of which in the aquatic environment is considered a serious environmental problem, because of its potential risk for the environment and human health (Luo et al. 2014; Yang et al. 2017). The major sources of pharmaceutical in aquatic systems are effluents from pharmaceutical industries, expired or surplus medicines, and excretory products from humans and animals, owing to the inefficiency of municipal sewage treatment plants (STPs) with pharmaceutical (Luo et al. 2014; Sánchez-Polo et al. 2009; Wang and Wang 2016). It has been reported that some pharmaceutical compounds are pseudo-persistent, bio-accumulative, and long-range transportable in ecosystems (Kasprzyk-Hordern et al. 2008; Subedi et al. 2012; Tanoue et al. 2015), which may accumulate through the food chain and have long-term adverse health effects in wildlife and humans (Archer et al. 2017; Xie et al. 2019).
Indomethacin (IDM) is a powerful non-steroidal anti-inflammatory drug (NSAID), which can reduce the biosynthesis of prostaglandins by inhibiting cyclooxygenase, thus producing anti-inflammatory, antipyretic, and analgesic effects (Scholz et al. 2012). IDM is widely used for its outstanding anti-inflammatory and antipyretic effects, whereas IDM is relatively stable in river water and the half-life in river water is estimated to be about 2.5 weeks, which can exist for a long time in the environment accordingly (Jiménez et al. 2017). It is present in a variety of environmental matrices, such as wastewater (Zhou et al. 2009), surface water (Rivera-Jaimes et al. 2018), tap water (Lin et al. 2016), sediment (Zhou and Broodbank 2014), and even in animals and plants (Tanoue et al. 2015). Therefore, human exposure to IDM should not be ignored. IDM is hardly removed by the typical biological treatments and the removal rate is less than 20% in the municipal sewage treatment plants (Rosal et al. 2010; Sui et al. 2010). The accumulation of IDM may affect the aquatic environment seriously. Thus, it is necessary to find an effective and environmentally friendly treatment method to effectively decompose such contaminants.
Under this background, various advanced oxidation processes (AOPs) have been developed to remove IDM, such as photocatalyst (Wang et al. 2017; Zhang et al. 2018), ozonation (Zhao et al. 2017), UV–vis/peroxydisulfate (Li et al. 2018b), ferrate (VI) (Huang et al. 2017), ferrous–peroxydisulfate (Li et al. 2017), and pulsed corona discharge (Panorel et al. 2013), which have been proved to be effective in the decomposition of IDM. However, these methods either caused secondary pollution or showed a low degree of mineralization with the high cost of treatment. Radiation-induced degradation is an emerging technology for the degradation of environmental pollutants. The electron beam (EB) can yield oxidative hydroxyl radical (•OH) and reductive hydrated electrons (e−aq) or hydrogen atoms (H•) at the same time by exciting and ionizing water molecule as given in Eq. (1) (numbers in the brackets present the radiation-chemical yields of these species per 100 eV energy, viz., G-values) (Xu et al. 2017); therefore, various organic pollutants can be degraded by oxidation and reduction schemes.
Rahil Changotra et al.(Changotra et al. 2019) found that electron beam irradiation could effectively remove ofloxacin and the transformation products did not exhibit any cytotoxicity. Xu et al. (Xu et al. 2015) studied the intermediates of atrazine and discovered that the oxidative •OH was the main species to degrade atrazine into cyanuric acid, while the reductive e−aq was crucial to the irradiation-induced degradation of cyanuric acid. Furthermore, Lyu et al. (Lyu et al. 2017) demonstrated that sym-triazine ring structure was effectively destroyed by reductive •H rather than oxidative •OH, combining density functional theory calculations with experimental observations. Irradiation technology has been proven to be a fast, clean, and efficient technique with least requirement of additional oxidizing reagents, high levels of mineralization of contaminants, and low yields of toxic intermediates (Abdel daiem et al. 2013; Wang and Chu 2016; Yu et al. 2008).
In the present work, the radiolytic degradation kinetics of IDM and the effects of absorbed dose, initial concentration, pH value, and water matrix were investigated. The degradation of IDM in the oxidation system and reduction system was assessed to obtain the contribution of active species. Based on the density functional theory, the condensed Fukui function was used to analyze the reaction sites under redox conditions(Li et al. 2018a), and LC-TOF-MS analysis was conducted for identifying degradation intermediates to give insight into the degradation mechanisms. In addition, the toxicity of IDM and degradation products were estimated by the ecological structure-activity relationship model (ECOSAR) to predict the toxicity changes during the degradation process (Liu et al. 2019). These results will provide the application of electron beam irradiation technology for the treatment of pharmaceutical wastewater.
Materials and methods
Materials and reagents
IDM was purchased from TCI Reagent Co. Ltd. with >98% purity. Humic acid (HA) was purchased from International Laboratories Co. Ltd. Sodium carbonate, sodium bicarbonate, sodium nitrite, sodium nitrate, hydrogen peroxide (H2O2, 30%, w/w), and tert-butanol (TBA) of analytic grade were obtained from ANPEL Laboratory Technologies (Shanghai) Inc. All the solutions were prepared by Ultra-purewater using a Milli-Q water purification system (resistance >18.2 MU cm) from Millipore (Sartorius 611, Germany). High purity N2 and O2 (>99.999% purity) were used.
Radiolysis process
Electron beam irradiation was performed using an electron accelerator (GJ-2-II, Xianfeng electrical plant), and the electron beam energy was 1.8 MeV and the variable current was from 0 to 10 mA. The samples were sealed with 20 mL in high density polyethylene plastic bags and were placed at 30 cm from the radiation source. IDM aqueous solution was irradiated with different doses at a dose rate of 0.045kGy s−1.
Analytical methods
IDM concentration was quantitatively detected using a high-performance liquid chromatography (HPLC, Agilent 1200 series) equipped with a reversed phase column (C18 column, 150 mm×4.6 mm ×5μm). The sample injection was carried out via an autosampler with a volume of 20 μL. The VWD detector was used to determine the concentration at 254 nm. The mobile phase was a mixture of acetonitrile/ammonium acetate buffer solution (65:35 v:v, containing 5mM ammonium acetate) at a rate of 1.0 mL min−1 and the column temperature was kept at 30 °C.
The measurement of inorganic ions formed during IDM degradation was accomplished by ion chromatography (Dionex ICS1100) using hydrophilic anion exchange column IonPac AS22 (analytical, 4 mm×250 mm). The eluent was a mixture of 4.5 mM Na2CO3 and 1.4mM NaHCO3 at a flow rate of 1.2mL min−1 and the injection volume was 25 μL.
The intermediates of IDM were analyzed by HPLC-QTOF-MS/MS system, consisting of AB Sciex ExionLC AC and AB Sciex X500R QTOF. A Kinetex® C18 column (100mm×3mm, 2.6μm) was used to separate IDM and the intermediates. The mobile phase was a mixture of acetonitrile and water at the flow rate of 0.4 mL min−1. The injection volume was 5 μL and the column temperature was kept at 35 °C. Mass spectrometry analysis was carried out in the positive and negative mode with an electrospray ionization source. Data were obtained by full scan mode from 100 to 450 m/z to search for degradation products.
Computational methods
Fukui function has been extensively used to predict the reactive sites (Parr and Yang 1984). In this study, the condensed Fukui function based on the density functional theory (DFT) was applied to analyze the regioselectivity of reactive species during IDM degradation. Based on this research system, the geometry optimization and single-point energy were calculated with Gaussian 09 program using the B3LYP method with the 6–311G(d) basis set. Fukui index (fk−, f k+, f k0) corresponds to the electrophilic, nucleophilic, and radical attacking, which were obtained from Hirshfeld charges of atoms by Eqs. (2−4) (Fukui et al. 1952; Yang and Parr 1985).
where qk is the atom charge population of atom k at corresponding state and N represents the number of electrons of IDM. Meanwhile, the calculation of Hirshfeld charges was carried out by Multiwfn software (Lu and Chen 2012).
Toxicity evaluation
The acute and chronic toxicity of IDM and its transformation intermediates to three aquatic organisms (i.e., fish, daphnia, and green algae) were evaluated by ECOSAR program (Version 2.0) based on structure-activity relationships (SARs), which has been widely used and validated by the US EPA, EU, and OECD (Chen et al. 2018; Li et al. 2018b; Liu et al. 2019). The acute toxicity is expressed as 96-h EC50 value for green algae, 96-h LC50 values for the fish, and 96-h LC50 values for the daphnia. The chronic toxicity values (ChV) for these organisms were also evaluated using the same method.
Results and discussion
Degradation kinetics studies
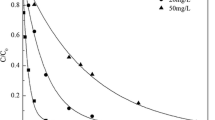
The radiolytic decompositions of IDM at four different initial concentrations (150, 200, 250, and 300μM) were studied. As shown in Fig. 1, when the absorbed dose was 1.0 kGy, the degradation rate was 97%, 93%, 87%, and 82% at the concentration of 150, 200, 250, and 300μM, respectively. It indicates that IDM could be completely removed by irradiation technology in tens of seconds. IDM in aqueous solution could be effectively and rapidly decomposed by electron beam irradiation compared to other advanced oxidation technologies such as ozonation, UV–vis/peroxydisulfate, and ferrate (VI) (Huang et al. 2017; Li et al. 2018b; Zhao et al. 2017), which commonly take tens of minutes or even hours. Furthermore, the concentration of IDM decreased exponentially with absorbed doses. Based on the data in Fig. 1, there was a linear relationship between −ln (C/C0) and absorbed dose, which was fitted with pseudo-first-order kinetics model. Dose constant kd could be calculated from Eq. (5). In order to make a comparative analysis, two indicators with large differences between 50 and 90% degradation were selected for analysis. According to kd value, the absorbed doses required for degradation of 50% (D0.5) and 90% (D0.9) can be calculated by Eqs. (6) and (7).
where C0 and C represent the concentration of IDM before and after irradiation, D the absorbed dose, and kd the dose constant.
As the concentration increased from 150 to 300 Μm, the dose constant decreased from 3.620 to 1.883 kGy−1, D0.5 increased from 0.19 to 0.37 kGy, and D0.9 increased from 0.64 to 1.22 kGy. The reason for this phenomenon is that the amount of active species produced by irradiation is certain, while the high initial concentration of IDM produces many by-products which cause the competitive reaction against the parent compound. As a result, low concentration of IDM correspondingly has a higher degradation rate, and lower D0.5 and D0.9 values.
The effect of radical scavenger on IDM removal
Active species produced by ionizing radiation play a vital role in the degradation process (An et al. 2014; Lian et al. 2017; Zhang et al. 2007). The previous study has shown that •OH could react rapidly with IDM and the second-order rate of •OH with IDM is 5.51×109 M−1s−1 (Zhao et al. 2017). IDM molecule contains the reducible groups (Cl, C=O), suggesting that IDM molecule could also be degraded by e−aq and •H. To evaluate the role of •OH, e−aq and •H on IDM degradation, specific free radical degradation experiments were carried out using N2 saturated solution, O2 saturated solution, and N2 saturated solution with 0.1M tert-butanol. The experimental results were shown in the Fig. 2. In N2 saturated solution, dissolved oxygen in IDM solution is excluded by N2, and there was no scavenger in such solution, thereby •OH, e−aq, and •H could be all involved in the IDM degradation process. Since tert-butanol can effectively scavenge •OH as shown in Eq. (8) (Buxton et al. 1988), the main active species were e−aq and •H in such solution and the dose constant decreased from 1.883 to 0.325 kGy−1. According to the radiolysis experiments with different scavengers, the dose constant ratios of •OH to e−aq and •H could be calculated as:
The dose constant ratio indicates that •OH contributes most to the IDM degradation. According to the Zhao’s research, there is a fast reaction rate between IDM and •OH, which is on the magnitude of 109 M−1S−1 (Zhao et al. 2017). From the dose constant ratio, reductive radicals e−aq and •H could degrade IDM but not in a fast reaction rate relative to •OH. Due to the strong selectivity of e−aq and •H to compounds with high electron affinity group, e−aq and •H could only react with such compounds at a diffusion-controlled reaction rate (Liu et al. 2015b). In O2 saturated solution, the dose constant increased slightly from 1.883 to 2.148 kGy−1. e−aq and •H could be captured by O2 and convert into O•− 2 and HO•− 2 as shown in Eqs. (9) and (10) (Zheng et al. 2011). Wang et al. (Wang et al. 2017) found that O•− 2 could degrade IDM more effectively than e−aq. The promotion effect on IDM removal was achieved in O2 saturated solution to some extent. Considering all these circumstances, it can be concluded that •OH, e−aq and •H could be all involved in the degradation process, and oxidative radicals •OH play a vital role in IDM degradation.
Influence of environmental factors on IDM degradation
The effect of solution pH on IDM degradation
In the process of EB irradiation degradation, the key active species are affected by the solution pH, which determines the degradation efficiency. The effect of pH was investigated under four different pH values, i.e., 5, 7, 9, and 10, with absorbed doses from 0.5 to 3 kGy. Figure 3 displays the effect of pH on the degradation of IDM. At different pH values; the degradation of IDM also fitted the pseudo-first-order reaction kinetics model. At pH values of 5, 7, 9, and 10, the responding dose constants were 1.979, 1.883, 1.780, and 1.587 kGy−1, and acidic conditions contribute to the degradation of IDM.
Under acidic conditions, e−aq reacts with H+ and converts into •H (Eq. (11)) (Zheng et al. 2011), thus reducing the recombination of e−aq and •OH and increasing the concentration of •OH (Eq. (12)) (Zheng et al. 2011). Under alkaline conditions, •OH react with OH− to form O•− (Eq. (13)) (Shao et al. 2018), which reduces the concentration of •OH. Owing to the higher reactivity of •OH towards IDM, the degradation efficiency of IDM decreased at high pH levels.
The pKa of IDM is 4.5 and IDM exists in ionic forms in the studied pH range (pH 5, 7, 9, 10). The electron cloud density of the benzene ring would be increased since the negative electron on the carboxyl oxygen atom. Then the benzene ring is more susceptible to electrophilic attack of OH•, thereby enhancing the degradation of IDM. In addition, the high density of electron cloud facilitates electron transfer to accelerate the reaction (Li et al. 2018b).
The effect of H2O2 on IDM degradation
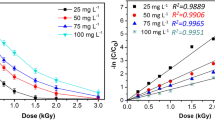
Hydrogen peroxide can promote the generation of •OH. It has been reported in many studies that the addition of H2O2 produces additional •OH to enhance irradiation degradation of pollutants (Liu et al. 2016; Liu and Wang 2013). Therefore, the effect of H2O2 (1–100 mM) on the degradation of 300 μM IDM was investigated (Fig. 4). Interestingly, proper concentration of H2O2 played a synergistic role, while excessive H2O2 lead to an antagonistic effect in the degradation of IDM.
When H2O2 concentration is relatively low, H2O2 mainly reacts with e−aq and •H to form •OH by Eqs. (14) and (15) (Liu et al. 2016). Obviously, due to the increase of •OH concentration, the removal efficiency increased with the addition of H2O2 concentration. The dose constant increased from 1.883 to 2.582 kGy−1 as the concentration of H2O2 increased from 0 to 10 mM. However, as the concentration of H2O2 was further increased, the removal efficiency was reduced. The reason is that the scavenging effect by the high concentration of H2O2 became more significant. H2O2 reacts with •OH to produce peroxide radicals, which is less reactive than the •OH, and peroxide radicals would also react with •OH (Eqs. (16) and (17)) (Liu et al. 2016). Therefore, with the addition of H2O2, the degradation efficiency of IDM increased first, and then decreased after reaching the maximum. An optimum level of H2O2 addition is necessary, and a 10 mM H2O2 is suggested in removing IDM under these experimental conditions.
The effects of inorganic ions and humic acid on IDM degradation
There are many anions and humic acids in natural water or wastewater, which may compete for the active species produced by irradiation, affecting the degradation efficiency of IDM. The effect of 100 mM various inorganic anions (NO−3, NO−2, CO2−3, HCO− 3, SO− 3) and three different concentrations of humic acid (10 ppm, 50 ppm, 100 ppm) on the degradation of 300 μM IDM were investigated and the results were shown in Fig. 5. The degradation process follows pseudo-first-order kinetics. Through a significant analysis of the degradation rate at 2 kGy, 100 mM of each anion has a significant difference in the impact of radiation degradation (p<0.05). The addition of inorganic anions causes the decrease of dose constant in varying degrees. The inhibitory effect of NO−2 was the most pronounced and the dose constant decreased from 1.883 to 0.126 kGy−1. Since the prompt reactions between NO−2 and active species (Eqs. (18–20)) (Liu et al. 2015a), active species would be rapidly removed, resulting in a 93.3% reduction in the dose constant. NO−3 could react with e−aq at a rate of 9.7×109 M−1s−1 (Eq. (21)) (Liu et al. 2015a), which is an effective scavenger for e−aq. Although NO−3 does not react with •OH, HNO3 could be achieved by NO−3 in acidic conditions and scavenge the •OH to some extent (Eqs. (22) and (23)) (Zheng et al. 2011). As a result, the inhibitory effect of NO−3 was less marked as that of NO−2 on IDM degradation. CO2−3 and HCO−3 can also react with the active species produced by irradiation, as shown in Eqs. (25−29) (Liu et al. 2015a). However, the inhibitory effect of CO2−3 on the degradation of IDM was more obvious than that HCO−3. On the one hand, the removal rate of •OH by HCO−3 is slower than that of CO2−3, so the inhibitory effect of HCO−3 is weak. On the other hand, HCO−3 can react with •OH to produce •CO−3. The redox potential of •CO−3 is 1.78 V, which may effectively oxidize IDM. Reductive SO−3 can scavenge •OH with a high rate constant of 5.5×109 (Eq. (30)) (Buxton et al. 1988). Hence, the addition of SO−3 reduces the concentration of •OH, which resulted in a great decrease in dose constant. The order of influence of various anions on degradation is as follows: NO−2 > SO−3 > NO−3 > CO2−3 > HCO−3. There is no significant difference with 10 ppm HA in the degradation rate of IDM at a dose of 2kGy (P=0.83). When the concentration of HA is more than 50 ppm, there is a significant difference on IDM degradation rate at a dose of 2kGy (P<0.05). As the addition of HA increased, the inhibition effect is strengthened. There are various functional groups on the surface of HA and HA would compete for •OH inevitably (Li et al. 2017). These results also indicated that •OH was more effective in degradation of IDM.
Degradation pathways of IDM
Irradiation-induced degradation involves a complex redox process with both nucleophilic and electrophilic reactions, including the participation of •OH, e−aq, and •H. The condensed Fukui function, based on the change of the electron density, is considered to be useful in elucidating the stereoselectivity and reaction mechanism qualitatively (Fukui et al. 1954; Oláh et al. 2002; Yang and Mortier 1986). Accordingly, the condensed Fukui function was adopted in providing deeper insights into IDM degradation. Optimized structure of IDM is presented in Fig. 6 and the calculated values of fukui index (f 0, f −, f +) are listed and sorted in descending order in Table 1. The higher the f value, the greater the possibility of reaction at positions.
The reaction transformation products were identified by LC-QTOF-MS. Detailed data are summarized in Table S1. Based on identifying the degradation products and theoretical calculations, the possible degradation pathways of IDM treated by electron beam irradiation was proposed in Fig. 7.
The amide bond was cleaved by the attack of •OH, and produce P1, P2, which is in line with the condensed Fukui function that strong oxidizing •OH can usually attacks position sites with high f 0 values (C (10) and O (11)) and rapidly oxidize organic compounds (De Vleeschouwer et al. 2007). The same pathway was reported for the degradation of IDM using other advanced oxidation technologies (Li et al. 2017; Li et al. 2018b; Wang et al. 2017). Subsequently, P1 can be further attacked by •OH and generate P3. Similarly, due to the high f 0 value of C (8), C (8) on benzene ring was likely to be attacked by •OH to form hydroxylated IDM (P4). As an electron donating group, •OH could activate the aromatic rings and promote electrophilic attack by increasing electron cloud density, leading to further decomposition. •OH would prefer to attack Cl (16) with the highest f 0 value, but chlorine is not easily replaced due to the conjugation effect of chlorine with benzene rings. As a result, a small part of chlorine could be eventually substituted by hydroxyl group to form P5.
The position sites with high f – values could also be attacked by electrophilic •OH through electron extraction (An et al. 2010). C(8), C(6), C(19), and O(2) were found to be highest f – values, showing that C(8), C(6), C(19), and O(2) should be the first site and the electron would be extracted in such sites. When •OH attacked C19 on the indole ring, the indole ring of IDM was opened and the branched chain was removed to form P6, then the amide bond of P6 was broken, forming P7. Besides, high electron-cloud density C19, C21 double bond could be hydroxylated through addition mechanism, with the resultant hydroxylated product of P8. The removal of methoxyl group is due to electrophilic attack of •OH, which leads to the formation of P9. The radical adduct P10 was formed by an •OH addition route. It is noteworthy that the initially formed oxygenated species of IDM were not only hydroxylated IDM but also indole-2,3-epoxide (P11). Such an epoxide is unstable at room temperature, which results in the opening of the ring and decarboxylation of the epoxide, thereby yielding P12 (Li et al. 2005).
For high electron affinity groups, such as carbonyl, nitro, cyano, and halogen atoms, e−aq and •H can react with these groups by addition reactions; as a result, e−aq and •H tend to react with atoms with high f + values (Buxton et al. 1988; Liu et al. 2015b). The chlorine on the aromatic ring and the carbonyl group on the amide bond showed high vulnerability to be attacked by nucleophilic species through nucleophilic addition. IDM was dechlorinated to form P13 and nucleophilic addition took place on the amide bond to form P14 on account of the reduction mechanism of e−aq and •H.
The obtained products agree with the results of active sites predicted by the condensed Fukui function. Most intermediates are produced by •OH. Reductive active species e− aq and •H generated by irradiation process can also react with IDM (Liu et al. 2015b). Hence, electron beam irradiation technology has a wide application for molecules with different functional groups in both oxidation and reduction systems. Overall, the main degradation pathways of IDM can be attributed to cleavage of amide bond, •OH addition, demethoxylation, indole ring opening, dechlorination, and the reduction of the carbonyl group on amide bond during the electron beam irradiation process.
Most of degraded compounds contained indole or chlorobenzene rings. Intermediates could continue to be degraded to small molecular with increasing dose. After that, short-chain carboxylic acids including formic acid, acetic acid, and oxalic acid were generated by ring-opened products, as shown in Fig. 8a. Chlorinated organic compounds are generally considered to have environmental risks and should be taken seriously (Li et al. 2017). At a low absorbed dose of 2 kGy, the dechlorination rate can reach 30% (Fig. 8b), and it means that the dechlorination reaction pathway contributes about 1/3 of the IDM degradation, which is mainly attributed to the fact that chlorine could be easily attacked by •OH, e−aq, and •H. With the increase of dose, more than 60% of chlorine can be removed at 5 kGy, and 90% of chlorine can be removed at 10 kGy, which may play an important role in the removal of toxicity. In conclusion, the initial degradation of IDM generated some intermediates by electron beam irradiation. With the increase of absorbed dose, these intermediates continued to degrade into short-chain carboxylic acids, which were eventually mineralized into carbon dioxide and water.
Toxicity assessment
ECOSAR program was employed to predict the toxicity of IDM and transformation products to three different aquatic organisms (fish, daphnia, and green algae). The Globally Harmonized System of Classification and Labelling of Chemicals classified the predicted toxicity into four levels, as is shown in Table S2 (Secretariat UNECfE 2009). The acute and chronic toxicities were presented in Table 2. As can be seen, the acute toxicity of IDM was classified as toxic to fish and green algae, harmful to Daphnid. However, chronic toxicity indicates that IDM is very toxic for three aquatic organisms. It is reported that the toxicity of chlorinated compounds depends on the number of chlorine groups and aromatic rings (Chen et al. 2016; del Castillo et al. 2012). It could be found that the toxicity of products generated by the cleavage of the amide bond decreased effectively. P2 does not exhibit acute toxicity. Additionally, the toxicity of IDM would be decreased whether chlorine on benzene ring was substituted by hydroxyl or removed by reduction. The effect of demethoxy on the toxicity of IDM was not profound. Lower toxicity products (P8, P11) were formed when •OH attacked the position 2 of indole ring. The toxicity of P6 to fish was lower than IDM, while the opposite was observed that the chronic toxicity to green algae was very high, which was nearly nine times more toxic to green algae. Fortunately, P6 was formed at the beginning of the reaction and it could be further degraded to low toxicity P7. The results indicate that the toxicity of IDM decrease after treatment with electron beam irradiation, thus reducing its environmental threat.
Conclusions
Electron beam irradiation could effectively decompose IDM in aqueous solution in tens of seconds, and the degradation process followed a pseudo-first-order kinetic model. Radiolysis studies with different radical scavenger demonstrated that •OH was the main species for the decomposition of IDM. The possible degradation pathways of IDM treated by electron beam irradiation was proposed. Active sites with high condensed Fukui function interpreted that cleavage of amide bond, •OH addition, demethoxylation, indole ring opening, dechlorination, and the reduction of the carbonyl group on amide bond took place on IDM molecule under the attack of active species, and mainly intermediates were obtained by the attack of •OH. ECOSAR analysis showed that the overall toxicity of IDM and degradation products decreased during EB irradiation process.
Data availability
All date generated or analyzed during this study are included in this article and its supplementary information files.
References
Abdel Daiem MM, Rivera-Utrilla J, Ocampo-Pérez R, Sánchez-Polo M, López-Peñalver JJ (2013) Treatment of water contaminated with diphenolic acid by gamma radiation in the presence of different compounds. Chemical Engineering Journal 219:371–379
An T, Yang H, Li G, Song W, Cooper WJ, Nie X (2010) Kinetics and mechanism of advanced oxidation processes (AOPs) in degradation of ciprofloxacin in water. Applied Catalysis B: Environmental 94:288–294
An T, Gao Y, Li G, Kamat PV, Peller J, Joyce MV (2014) Kinetics and mechanism of •OH mediated degradation of dimethyl phthalate in aqueous solution: experimental and theoretical studies. Environmental Science & Technology 48:641–648
Archer E, Petrie B, Kasprzyk-Hordern B, Wolfaardt GM (2017) The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere 174:437–446
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O− in Aqueous Solution). Journal of Physical and Chemical Reference Data 17:513–886
Changotra R, Guin JP, Khader SA, Varshney L, Dhir A (2019) Electron beam induced degradation of ofloxacin in aqueous solution: kinetics, removal mechanism and cytotoxicity assessment. Chemical Engineering Journal 356:973–984
Chen J, Qu R, Pan X, Wang Z (2016) Oxidative degradation of triclosan by potassium permanganate: kinetics, degradation products, reaction mechanism, and toxicity evaluation. Water Research 103:215–223
Chen J, Xu X, Pan X, Yao J, Li C, Qu R, Wang Z (2018) Mechanism insights into the oxidative degradation of decabromodiphenyl ethane by potassium permanganate in acidic conditions. Chemical Engineering Journal 332:267–276
De Vleeschouwer F, Van Speybroeck V, Waroquier M, Geerlings P, De Proft F (2007) Electrophilicity and nucleophilicity index for radicals. Organic Letters 9:2721–2724
Del Castillo I, Hernández P, Lafuente A, Rodríguez-Llorente ID, Caviedes MA, Pajuelo E (2012) Self-bioremediation of cork-processing wastewaters by (chloro)phenol-degrading bacteria immobilised onto residual cork particles. Water Research 46:1723–1734
Fukui K, Yonezawa T, Shingu H (1952) A Molecular orbital theory of reactivity in aromatic hydrocarbons. The Journal of Chemical Physics 20:722–725
Fukui K, Yonezawa T, Nagata C, Shingu H (1954) Molecular orbital theory of orientation in aromatic, heteroaromatic, and other conjugated molecules. The Journal of Chemical Physics 22:1433–1442
Huang J, Wang Y, Liu G, Chen P, Wang F, Ma J, Li F, Liu H, Lv W (2017) Oxidation of indometacin by ferrate (VI): kinetics, degradation pathways, and toxicity assessment. Environmental Science and Pollution Research 24:10786–10795
Jiménez JJ, Sánchez MI, Pardo R, Muñoz BE (2017) Degradation of indomethacin in river water under stress and non-stress laboratory conditions: degradation products, long-term evolution and adsorption to sediment. Journal of Environmental Sciences 51:13–20
Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ (2008) The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Research 42:3498–3518
Li WC (2014) Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environmental Pollution 187:193–201
Li M, Conrad B, Maus RG, Pitzenberger SM, Subramanian R, Fang X, Kinzer JA, Perpall HJ (2005) A novel oxidative degradation pathway of indomethacin under the stressing by hydrogen peroxide. Tetrahedron Letters 46:3533–3536
Li R, Kong J, Liu H, Chen P, Liu G, Li F, Lv W (2017) A sulfate radical based ferrous-peroxydisulfate oxidative system for indomethacin degradation in aqueous solutions. RSC Advances 7:22802–22809
Li F, Du P, Liu W, Li X, Ji H, Duan J, Zhao D (2018a) Hydrothermal synthesis of graphene grafted titania/titanate nanosheets for photocatalytic degradation of 4-chlorophenol: solar-light-driven photocatalytic activity and computational chemistry analysis. Chemical Engineering Journal 331:685–694
Li R, Kong J, Liu H, Chen P, Su Y, Liu G, Lv W (2018b) Removal of indomethacin using UV–vis/peroxydisulfate: kinetics, toxicity, and transformation pathways. Chemical Engineering Journal 331:809–817
Lian L, Yao B, Hou S, Fang J, Yan S, Song W (2017) Kinetic study of hydroxyl and sulfate radical-mediated oxidation of pharmaceuticals in wastewater effluents. Environmental Science & Technology 51:2954–2962
Lin T, Yu S, Chen W (2016) Occurrence, removal and risk assessment of pharmaceutical and personal care products (PPCPs) in an advanced drinking water treatment plant (ADWTP) around Taihu Lake in China. Chemosphere 152:1–9
Liu Y, Wang J (2013) Degradation of sulfamethazine by gamma irradiation in the presence of hydrogen peroxide. J Hazard Mater 250-251:99–105
Liu N, Wang T, Zheng M, Lei J, Tang L, Hu G, Xu G, Wu M (2015a) Radiation induced degradation of antiepileptic drug primidone in aqueous solution. Chemical Engineering Journal 270:66–72
Liu X, Zhang T, Wang L, Shao Y, Fang L (2015b) Hydrated electron-based degradation of atenolol in aqueous solution. Chemical Engineering Journal 260:740–748
Liu N, Lei Z-D, Wang T, Wang J-J, Zhang X-D, Xu G, Tang L (2016) Radiolysis of carbamazepine aqueous solution using electron beam irradiation combining with hydrogen peroxide: Efficiency and mechanism. Chemical Engineering Journal 295:484–493
Liu H, Yao J, Wang L, Wang X, Qu R, Wang Z (2019) Effective degradation of fenitrothion by zero-valent iron powder (Fe0) activated persulfate in aqueous solution: kinetic study and product identification. Chemical Engineering Journal 358:1479–1488
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. Journal of Computational Chemistry 33:580–592
Luo Y, Guo W, Ngo HH, Nghiem LD, Hai FI, Zhang J, Liang S, Wang XC (2014) A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Science of The Total Environment 473-474:619–641
Lyu G, Shi G, Tang L, Fang H, Wu M (2017) Mechanism of degradation of a nitrogenous heterocycle induced by a reductive radical: decomposition of a sym-triazine ring. Physical Chemistry Chemical Physics 19:9354–9357
Oláh J, Van Alsenoy C, Sannigrahi AB (2002) Condensed fukui functions derived from stockholder charges: assessment of their performance as local reactivity descriptors. The Journal of Physical Chemistry A 106:3885–3890
Panorel I, Preis S, Kornev I, Hatakka H, Louhi-Kultanen M (2013) Oxidation of aqueous pharmaceuticals by pulsed corona discharge. Environmental Technology 34:923–930
Parr RG, Yang W (1984) Density functional approach to the frontier-electron theory of chemical reactivity. Journal of the American Chemical Society 106:4049–4050
Rivera-Jaimes JA, Postigo C, Melgoza-Alemán RM, Aceña J, Barceló D, López de Alda M (2018) Study of pharmaceuticals in surface and wastewater from Cuernavaca, Morelos, Mexico: Occurrence and environmental risk assessment. Science of The Total Environment 613-614:1263–1274
Rosal R, Rodríguez A, Perdigón-Melón JA, Petre A, García-Calvo E, Gómez MJ, Agüera A, Fernández-Alba AR (2010) Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation. Water Research 44:578–588
Sánchez-Polo M, López-Peñalver J, Prados-Joya G, Ferro-García MA, Rivera-Utrilla J (2009) Gamma irradiation of pharmaceutical compounds, nitroimidazoles, as a new alternative for water treatment. Water Research 43:4028–4036
Scholz M, Blobaum AL, Marnett LJ, Hey-Hawkins E (2012) Ortho-carbaborane derivatives of indomethacin as cyclooxygenase (COX)-2 selective inhibitors. Bioorganic & medicinal chemistry 20:4830–4837
Secretariat UNECfE (2009) Globally harmonized system of classification and labelling of chemicals (GHS). United Nations Publications
Shao H-Y, Wu M-h, Deng F, Xu G, Liu N, Li X, Tang L (2018) Electron beam irradiation induced degradation of antidepressant drug fluoxetine in water matrices. Chemosphere 190:184–190
Subedi B, Du B, Chambliss CK, Koschorreck J, Rüdel H, Quack M, Brooks BW, Usenko S (2012) Occurrence of pharmaceuticals and personal care products in german fish tissue: A National Study. Environmental Science & Technology 46:9047–9054
Sui Q, Huang J, Deng S, Yu G, Fan Q (2010) Occurrence and removal of pharmaceuticals, caffeine and DEET in wastewater treatment plants of Beijing, China. Water Research 44:417–426
Tanoue R, Nomiyama K, Nakamura H, Kim J-W, Isobe T, Shinohara R, Kunisue T, Tanabe S (2015) Uptake and tissue distribution of pharmaceuticals and personal care products in wild fish from treated-wastewater-impacted streams. Environmental Science & Technology 49:11649–11658
Wang J, Chu L (2016) Irradiation treatment of pharmaceutical and personal care products (PPCPs) in water and wastewater: an overview. Radiation Physics and Chemistry 125:56–64
Wang J, Wang S (2016) Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. Journal of environmental management 182:620–640
Wang F, Chen P, Feng Y, Xie Z, Liu Y, Su Y, Zhang Q, Wang Y, Yao K, Lv W, Liu G (2017) Facile synthesis of N-doped carbon dots/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of indomethacin. Applied Catalysis B: Environmental 207:103–113
Xie H, Hao H, Xu N, Liang X, Gao D, Xu Y, Gao Y, Tao H, Wong M (2019) Pharmaceuticals and personal care products in water, sediments, aquatic organisms, and fish feeds in the Pearl River Delta: occurrence, distribution, potential sources, and health risk assessment. Science of The Total Environment 659:230–239
Xu G, Yao J-z, Tang L, Yang X-y, Zheng M, Wang H, Wu M-h (2015) Electron beam induced degradation of atrazine in aqueous solution. Chemical Engineering Journal 275:374–380
Xu Z, Zhang X, Huang N, Hu H-y (2017) Oxidation of benzalkonium chloride by gamma irradiation: kinetics and decrease in toxicity. Journal of Radioanalytical and Nuclear Chemistry 312:631–637
Yang W, Mortier WJ (1986) The use of global and local molecular parameters for the analysis of the gas-phase basicity of amines. Journal of the American Chemical Society 108:5708–5711
Yang W, Parr RG (1985) Hardness, softness, and the fukui function in the electronic theory of metals and catalysis. Proceedings of the National Academy of Sciences 82:6723–6726
Yang Y, Ok YS, Kim K-H, Kwon EE, Tsang YF (2017) Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: a review. Science of The Total Environment 596-597:303–320
Yu S, Lee B, Lee M, Cho I-H, Chang S-W (2008) Decomposition and mineralization of cefaclor by ionizing radiation: kinetics and effects of the radical scavengers. Chemosphere 71:2106–2112
Zhang S-J, Jiang H, Li M-J, Yu H-Q, Yin H, Li Q-R (2007) Kinetics and mechanisms of radiolytic degradation of nitrobenzene in aqueous solutions. Environmental Science & Technology 41:1977–1982
Zhang Q, Chen P, Zhuo M, Wang F, Su Y, Chen T, Yao K, Cai Z, Lv W, Liu G (2018) Degradation of indometacin by simulated sunlight activated CDs-loaded BiPO4 photocatalyst: roles of oxidative species. Applied Catalysis B: Environmental 221:129–139
Zhao Y, Kuang J, Zhang S, Li X, Wang B, Huang J, Deng S, Wang Y, Yu G (2017) Ozonation of indomethacin: kinetics, mechanisms and toxicity. Journal of Hazardous Materials 323:460–470
Zheng BG, Zheng Z, Zhang JB, Luo XZ, Wang JQ, Liu Q, Wang LH (2011) Degradation of the emerging contaminant ibuprofen in aqueous solution by gamma irradiation. Desalination 276:379–385
Zhou J, Broodbank N (2014) Sediment-water interactions of pharmaceutical residues in the river environment. Water Research 48:61–70
Zhou JL, Zhang ZL, Banks E, Grover D, Jiang JQ (2009) Pharmaceutical residues in wastewater treatment works effluents and their impact on receiving river water. Journal of Hazardous Materials 166:655–661
Funding
This work was financially supported by the National Natural Science Foundation of China (Nos. 11975147, 12075148, 12075146, and 41773121), National Key Research and Development Project (No. 2020YFC1808200), and Project supported by Science and Technology Commission of Shanghai Municipality (20010500300).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Yu Duan: data curation, writing-original draft preparation. Wei Zhou: investigation, writing-reviewing and editing. Haiyang Shao: conceptualization, investigation. Zhibo Zhang: investigation, validation. Wenyan Shi: methodology, investigation. Gang Xu: conceptualization, methodology. All authors read the approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Weiming Zhang
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Duan, Y., Zhou, W., Shao, H. et al. Electron beam induced degradation of indomethacin in aqueous solution: kinetics, degradation mechanism, and toxicity assessment. Environ Sci Pollut Res 29, 19283–19294 (2022). https://doi.org/10.1007/s11356-021-16348-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16348-2