Abstract
Brassinosteroids (BRs) are growth-promoting hormones that exhibit high biological activities across various plant species. BRs shield plants against various abiotic stresses. In the present study, the effect of BRs against aluminum (Al) toxicity was investigated through seed priming with 24-epibrassinolide (0.01 μM) in two different rice cultivars. BRs application was found effective in confronting plants from Al toxicity (400 μM). The rice seeds primed with BRs showed enhancement in seed germination energy, germination percentage, root and shoot length, as well as fresh and dry weight under Al-absence and Al-stressed conditions as compared to water-priming. Especially under Al stress, BRs priming promoted the growth of rice seedlings more obviously. Al toxicity significantly increased the Al contents in seedling root and shoot, as well as the MDA concentration, H2O2 production, and the activities of antioxidative enzymes including ascorbate peroxidase, catalase, and peroxidase. Meanwhile, the photosynthetic pigments of seedling reduced under Al stress. When compared to sensitive cultivar (CY-927), these modifications were more obvious in the tolerant variety (YLY-689). Surprisingly, BRs were able to alleviate the Al injury by lowering MDA and H2O2 level and increasing antioxidant activities and photosynthetic pigments under Al stress. The results on antioxidant activities were further validated by gene expression study of SOD-Cu-Zn, SOD-Fe2, CATa, CATb, APX02, and APX08. It suggested that BRs were responsible for the mitigation of Al stress in rice seedlings by inducing antioxidant activities with an effective response to other seed growth parameters and reduced Al uptake under induced metal stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa L.) is an important staple food of the developing world due to its high protein, vitamin, and mineral contents (Ahmed et al. 2021a). Almost 85% of rice is cultivated in Asia, whereby China is the world’s leading producer of rice. In southern China, rice is the main cereal crop to fulfil the nutritional requirements of underprivileged people (Ahmed et al. 2020; Huang et al. 2013). During past few years, soil properties have been changed due to rapid industrialization and excessive release of heavy metals (Ahmed et al. 2021b), such as aluminum (Al), cadmium (Cd), mercury (Hg), and lead (Pb), in environmental system (Ahmed et al. 2021c; Noman et al. 2020a; Noman et al. 2020b). In low pH soils, Al contamination has devastating impacts that catastrophically disturbed the agronomic traits, such as yield and quality, of cereal crops. It was estimated that almost 30–50% of soil is polluted with Al globally, whereby 21% of total arable land in China is Al-affected (Xu et al. 2012). Al has been found to alter the morphological (such as growth and biomass production) as well as physiological (such as oxidative stress) states of crop plants (Silva 2012). It boosts lipid peroxidation by inhibiting antioxidant enzymes including catalase, peroxidase, superoxide dismutase, and glutathione reductase, which ultimately trigger plant stress (Rout et al. 2001). It was discovered that Al toxicity can also be found in shoots that develop as a result of root system damage (Vitorello et al. 2005). Al toxicity has a number of negative effects in plants, including altered water balance, reduced stomatal conductance and photosynthetic activity, chlorosis, and necrosis of leaves (Ali et al. 2008a; Ma 2007). The excess amount of Al in soil or growth medium perturbs the entry of essential ions such as magnesium (Mg), potassium (K), calcium (Ca), manganese (Mn), and zinc (Zn) into the plant system, thereby disturbing the mineral balances and retarding the overall growth and development (Basit et al. 2021b; Manzoor et al. 2021; Mendonça et al. 2003). However, it raises proline levels, which acts as an osmoprotectant, membrane stabilizer, and ROS scavenger (Apel and Hirt 2004).
Brassinosteroids (BRs) are polyhydroxy steroidal phytohormones that have a strong ability to promote plant growth under diverse environmental conditions (Basit et al. 2021a; Latha and Vidya Vardhini 2018). 24-Epibrassinolide is the most biologically active BR compound, which is involved in a variety of developmental processes such as cell division, elongation, gene expression, and vascular differentiation and others (Basit et al. 2021a; Bergonci et al. 2014). BRs have been shown to help plants cope with a variety of stresses, including biotic and abiotic stresses (Bhandari and Nailwal 2020). Exogenously applied BRs improve the resistance mechanisms to low and high temperatures, drought, chilling, and various metal stresses. Similarly, BRs treatment improved tomato plant resistance to chromium stress by modulating physiological and molecular pathways (Jan et al. 2020). It has been discovered that BRs strengthen nitrogen fixation, secondary metabolites, and protection mechanisms in Cicer arietinum (Ali et al. 2005) and Brassica juncea under salinity and heavy metal stresses such as nickel (Ali et al. 2008b). It has also been documented that exogenous application of BRs improves the antioxidant system, photosynthesis, and growth characteristics of mung bean plants under aluminum stress (Ali et al. 2008a). Numerous studies have shown that BRs enhance plant growth under various heavy metal stresses by increasing chlorophyll contents, which play an important role in increasing photosynthetic capability, improving antioxidant system performance, boosting enzymatic activity, and upregulating stress-responsive genes [superoxide (SOD), peroxide (POD), catalase (CAT), glutathione reeducates (GR), and ascorbate peroxide (APX)] (Cominelli et al. 2008).
Hence, based on the hypothesis that BRs could boost the biomass of rice plants by reducing the uptake of Al and alleviating the oxidative stress under Al toxicity, we explored the impact of seed priming with BRs on rice seed germination, morphophysiological and biochemical parameters, nutrient acquisition, and Al uptake under Al toxicity as well as proposed the possible mechanism of Al stress mitigation in rice plants through seed priming with BRs in this study.
Materials and methods
Brassinosteroids (BRs) preparation
24-Epibrassinolide was obtained from the Shanghai Aladdin Biochemical Technology Co., Ltd. It was liquefied in sufficient ethanol, and a stock solution of 10−5 M was prepared by adding ddH2O with 0.05% Tween 20 for use as the priming reagent of BRs.
Plant materials and growth conditions
The seeds of two cultivars of Oryza sativa L. (cv. CY927 and YLY689) were obtained from the Zhejiang Nongke Seeds CO., LTD. Hangzhou, Zhejiang Province, China (Salah et al. 2015). Seeds were surface sterilized for 15 min with a 0.5% sodium hypochlorite (NaClO) solution and then washed several times with tap water before being washed three times with sterilized distilled water to remove any remaining disinfectant. Sterilized seeds were primed with 0.01-μM BRs at 15 °C in the dark for 24 h. The seeds were then dried at room temperature to their original moisture content. The seeds primed with water (H2O) and BRs without Al stress were used as the control group (CK). After seed priming, germination tests were performed. Each treatment consisted of fifty seeds that were placed in a plastic germination box (12 cm × 18 cm) and repeated three times. The seeds were then incubated for 14 days at 25 °C in a germination chamber with an 8/16 h light/darkness period (Zhang et al. 2007). The 400-μM concentration of Al was supplied to seeds with nutrients solution. The nutrient solution was made up of the following ingredients: 0.5-μM potassium nitrate (KNO3), 0.5-μM calcium nitrate (Ca(NO3)2), 0.5-μM magnesium sulfate MgSO4, 2.5-μM monopotassium phosphate (KH2PO4), 2.5-μM ammonium chloride (NH4Cl), 100-μM ferric EDTA (Fe–K–EDTA), 30-μM boric acid (H3BO3), 5-μM manganese monosulfate (MnSO4), 1-μM copper sulfate (CuSO4), 1-μM zinc sulfate (ZnSO4), and 1-μM ammonium heptamolybdate ((NH4)6Mo7O24) per 1000 mL ddH2O. The pH of the nutrients solution was adjusted to 5.0 with hydrochloric acid (HCl) and sodium hydroxide (NaOH). The Al concentration was determined using data from a primary experiment involving different Al concentrations of 0, 100, 200, 300, 400, 500, 600, 700, and 800 μM. Plant growth was slightly harmed at low aluminum concentrations (100–300 μM). Despite this, plant growth was significantly harmed by aluminum concentrations of 400 μM. Concentrations greater than 500 μM, on the other hand, were excessively toxic to the plant’s growth.
Measurement of physiological parameters
For 14 days, the number of germinated seeds was counted every day. On the 5th day of germination, total germinated seeds were counted, and germination energy was calculated. On the 14th day, the germination percentage was determined. The following formulas were used to calculate the germination index (GI), mean germination time (MGT), and vigor index (VI) (Hu et al. 2005).
Gt is the total calculated number of germinated seeds on day t, and Tt is the time conforming to Gt in days (Hu et al. 2005).
Experimental design and treatment pattern
The 2-week-old seedlings were treated with a 400-μM Al concentration after being primed with water (H2O) and 0.01-μM BRs. The experimental pattern was constituted through a completely randomized design (CRD), and the location of the pots within the growth chamber was changed every day. The plants were sampled 21 days after being treated with Al for 7 days to observe alterations morphophysiological parameters.
Plant growth investigation
The plants were harvested with intact roots and immersed in a bucket filled with water to remove any traces of media contents. The plants were divided into roots and shoots, and the lengths of the roots and shoots were measured, followed by determination of their fresh weight using electronic weighing balance. The roots and shoots were dried in an oven at 80 °C for 24 h and then weighed to determine their dry mass.
Measurement of photosynthetic pigments
Photosynthetic pigments, such as chlorophyll a and b and total chlorophyll, were investigated by following the methodology of Ahmed et al. (2021b). Briefly, fresh leaf tissues (0.2 g) were homogenized in 3 mL ethanol (95%, v/v). The supernatant was removed after centrifuging the homogenate at 5000 g for 10 min. 1 mL aliquot of the supernatant was added to 9 mL of ethanol (95% v/v). After that, the mixture was subjected to spectrophotometry to determine the absorbance at 665- and 649-nm wavelengths (Lichtenthaler and Wellburn 1983). To quantify chlorophyll pigments, the following equations were used:
The pigment concentrations were calculated in milligrams per liter of plant extract.
Measurement of metal contents in plant tissues
To determine Al contents, dried plant roots and shoots (0.2 g) for each treatment were acid digested by using 5 mL concentrated HNO3/HCLO4 (5:1, v/v) on a hot plate at 70 °C for 5 h. The digested samples were diluted with 2% HNO3 to a final volume of 10 mL and filtered through Whatman filter paper. The filtrate was used to estimate the concentrations of Al, other microelements [iron (Fe2+), zinc (Zn2+), manganese (Mn2+)], and macroelements [(calcium (Ca2+), potassium (K+), and magnesium (Mg2+)] by using atomic absorption spectrometer (iCAT-6000-6300, Thermo Scientific, USA) (Khan et al. 2013).
Measurement of MDA contents and H2O2 measurements
MDA concentration was examined in terms of production of 2-thiobarbituric acid (TBA) metabolites. Approximately 1.5 mL plant extract was homogenized in 2.5 mL of 5% TBA prepared in 5% trichloroacetic acid (TCA). The mixture was heated to 95 °C for 15 min followed by incubation on ice. After that, the supernatant was centrifuged for 10 min at 5,000g. Finally, the absorbance of the supernatant was determined at 532 nm using the abovementioned unit of spectrophotometer. To reduce nonspecific turbidity, the absorbance value of reaction mixture at 600 nm was subtracted from the value at 532 nm (Rao and Sresty 2000). The MDA concentration was measured in nmol mg−1 protein. To measure hydrogen peroxide (H2O2) concentration, the plant tissues were homogenized in phosphate buffer followed by centrifugation at 6000g. The supernatant was mixed with 0.1% titanium sulfate containing 20% (v/v) H2SO4 followed by centrifugation. The intensity of yellow color was estimated calorimetrically at 410nm using abovementioned unit of UV-vis spectrophotometer (Velikova et al. 2000). H2O2 concentration was calculated by using standard curve constructed using known concentrations of H2O2.
Enzymatic antioxidants activity assay
Fresh plant tissues were homogenized in 8 mL of 50 mM potassium phosphate buffer (containing 1-mM EDTANa2 as well as 0.5% PVP W/V, pH 7.0) on ice. After that, centrifugation of the homogenate was performed at 12000rpm for 20 min at 4°С. After centrifugation, supernatant was collected in separate tube and stored at 80°С until further processing. The superoxide dismutase (SOD) activity was determined according to Giannopolitis and Ries (1977), by examining the capability of the enzyme to restrict the photochemical reduction of nitroblue tetrazolium chloride (NBT). The absorbance was measured at 560 nm, and one unit of SOD activity, represented as EU/mg protein, was shown to be the enzyme quantity required for inhibiting the rate of NBT photoreduction by up to 50% (Giannopolitis and Ries 1977). Peroxidase (POD) activity was examined according to the method of Zhang (1992) exhausting the elimination coefficient 25.5 mM−1 cm−1. The homogenization of both root and shoot fresh tissues was done in phosphate buffer (50 mM and pH 7.8) followed by centrifugation at 8000g at 25 °C. The OD of resulting mixture was measured at 470 nm. Catalase (CAT) activity was recorded as described by Aebi (1984) by the extermination constant of 39.4 mM−1 cm−1. The absorbance was calculated at 240 nm, and CAT activity was expressed as EU/mg protein, whereby for the determination of ascorbate peroxidase (APX) activity, the decrease in absorbance at 290 nm for 3 min was observed, and APX activity was expressed as EU/mg protein (Nakano and Asada 1981).
RNA extraction and gene expression analysis
Antioxidant gene profiling was performed by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR). Total RNA from plant samples was extracted using Trizol reagent as described by Sah et al. (2014). For cDNA synthesis, 1 μg of total RNA was reverse transcribed through PrimeScript™ RT reagent kit, and the resulting product was used as template for qRT-PCR. The qRT-PCR reaction was prepared in SYBR Premix Ex Taqkit (TaKaRa, Dalian, China), containing 10 mL 2× SYBR Premix Ex Taq buffer, 1-μg synthesized cDNA, and 10 μmol of each of gene-specific primers in a final volume of 20 mL (Livak and Schmittgen 2001). OsActin was used as an internal control to normalize the data to measure the relative transcript abundance for target genes. Relative expressions of target genes were calculated using the 2-∆∆CT method. Primers used for qRT-PCR are listed in Table S1.
Statistical analysis
Experimental data were analyzed using one-way analysis of variance treatments. The significance among means of different dataset was determined using least significant difference (Fisher’s LSD) test with 95% confidence level using SPSS v16.0 (SPSS, Inc., Chicago, IL, USA). Principle component analysis (PCA) and agglomerative hierarchical clustering (AHC) were performed to classify two different rice cultivars used in the current study according to their vulnerability toward Al by using XLSTAT.
Results
BRs promotes seed vigor and plant growth
The current study has demonstrated that Al toxicity (400 μM) caused a significant decrease in seed germination energy, germination percentage, vigor index, as well as germination index in both cultivars as compared to control; more reduction was observed in cultivar CY927. However, seed priming with BRs revealed a significant resistance against Al stress as compared to control (seed priming with water) in both cultivars. MGT was significantly enhanced in plants treated under Al stress as compared to untreated plants. However, seed priming with BRs reduced MGT in both cultivars (Table 1). More reduction was observed in cultivar CY927 as compared to cultivar YLY689. Current study demonstrated that 0.01-μM concentration of BRs significantly enhanced the GE, germination %, VI, and GI under metal toxicity as compared to the unprimed seeds in both cultivars (Table 1).
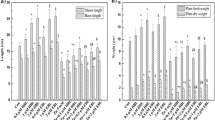
Al exposure reduced the morphological parameters of rice seedlings (Fig. 1 and Fig. 2). Plants treated with Al toxicity caused a significant decrease in root and shoot length of rice plants as compared to untreated control plants. A significant reduction was observed in seedling fresh weight of CY927 cultivar after exposure to Al stress, whereby dry weight was significantly decreased in both cultivars under Al toxicity. More reduction was observed in cultivar CY927 as compared to cultivar YLY689. It was noticed that seed priming with BRs significantly improved the shoot/root length as well as fresh/dry weight as compared to control plants without BRs treatment under Al-spiked conditions (Table 2).
Seed priming with BRs increases photosynthetic pigments
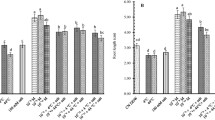
The negative effect of Al stress on photosynthetic pigment concentration was observed in both rice cultivars (Fig. 3). The present study demonstrated that Al treatment alone resulted in a significant drop in chlorophyll pigments such as Chl a and b and total chlorophyll content as compared to control. Under Al stress, both cultivars showed a decrease in photosynthetic pigments. In comparison to YLY689, the decline was more obvious in CY927. Seed priming with 0.01-μM BRs mitigated Al toxicity in both genotypes to some extent. In the case of seed priming with BRs, the total chlorophyll pigment was increased by 37.2% on average across both cultivars. Seed priming with BRs improved Chl a and b and total chlorophyll concentrations in both cultivars when compared to respective controls in both treatments without and with Al stress. In both cultivars, plants treated with BRs alone showed more photosynthetic pigments than non-treated control plants (Fig. 3).
BRs supplementation reduces Al accumulation in rice seedling
Roots are the main part that interacts with heavy metals first, as well as the primary source of nutritional solution and heavy metal uptake. Al accumulation was found to be higher in roots than in shoots (Tables 3, 4). However, Al accumulation was noticeable in the YLY689 cultivar than in the CY927 cultivar. Seed priming with BRs reduced Al content by 10.90% and 6.140% in the CY927 and YLY689 cultivars, respectively. More interestingly, when exposed to Al toxicity, the concentrations of K+, Ca2+, Fe2+, and Mn2+ in both roots and shoots reduced, whereas Zn2+ concentration increased in both cultivars (Tables 3–4). Seed priming with BRs maintained the nutritional balance of both cultivars under Al stress and significantly improved the nutrients availability such as Mn2+ and Fe2+ inside shoots of cultivar CY927 and elevated K+, Ca2+, Mn2+, and Fe2+ accessibility in shoots of YLY689 cultivar (Tables 3–4).
BRs ameliorates Al-induced oxidative stress
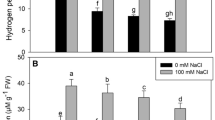
In both cultivars, the presence of Al raised MDA and H2O2 contents when compared to the control. In comparison to the YLY689 cultivar, this rise was more pronounced in CY927. The application of BRs dramatically lowered MDA levels as well as H2O2 generation in both cultivars (Fig. 4). MDA levels were found to be greater in shoots (64.70% and 55.40%) than that in roots (56% and 42%) in both CY927 and YLY689 cultivars under induced Al stress as compared to respective healthy controls, respectively. However, seed priming with BRs reduced the MDA levels in shoots (44.50% and 45%) and roots (26% and 39.70%) of both cultivars (CY927 and YLY689, respectively) as compared to corresponding controls under Al-spiked conditions.
Seed priming effect with 0.01-μM BRs on MDA contents and H2O2 production in shoots and roots of two different rice cultivars under 400-μM Al toxicity. A MDA contents in shoots of both rice cultivars, B MDA contents in roots of both rice cultivars, C H2O2 contents in shoots of both rice cultivars, D H2O2 contents in roots of both rice cultivars
Determination of antioxidant enzyme activities
Antioxidant activity was shown to be increased when Al was treated alone. A recent study found that stressed plants had higher levels of SOD, CAT, POD, and APX than control plants when exposed to 400-μM Al and that this effect was stronger in YLY689 than that in CY927 (Fig. 5). These activities were found to be more prevalent in roots than that in shoots. SOD activity was found to be 22.5% in CY927 and 43.6% in YLY689 cultivar shoots when Al was applied, whereby in roots of both cultivars, it was found in 47.7% and 58.2%, respectively. Both cultivars under combined treatment of Al and BRs showed 44.6% and 46.3% increase in shoot SOD activity and 57.4% and 58.2% increase in root SOD activity in contrast to Al-affected plants. Under independent treatment of Al, CAT activity in the shoots was found to be 45% in CY927 and 59% in YLY689. In roots, it was found to be 39% and 45%, respectively. The seed priming with BRs increased the CAT activity by 51% and 61% in shoots and by 46% and 58% in roots as compared to Al-stressed plants, respectively. Similarly, POD and APX levels were higher in the presence of Al alone, but this impact was more prevalent with BRs priming; however, POD activity was not increased significantly. However, 14.8% and 37.4% increase in POD activity was observed in shoots of CY927 and YLY689, whereby 30.2% and 46.8% boost in roots was observed in both cultivars after Al treatment, respectively (Fig. 5).
Seed priming effect with 0.01-μM BRs on SOD, CAT, APX, and POD contents in both shoots and roots of two rice cultivars under 400-μM Al toxicity. A Superoxide dismutase (SOD) in shoots of both rice cultivars, B SOD in roots of both rice cultivars, C Catalase (CAT) in shoots of both rice cultivars, D CAT in roots of both rice cultivars, E Ascorbate peroxidase (APX) in shoots of both rice cultivars, F APX in roots of both rice cultivars, G Peroxidase (POD) in shoots of both rice cultivars, H POD in roots of both rice cultivars
Determination of gene expression analysis
In both cultivars, there was a substantial difference in the expression of APX02 in both roots and shoots when compared to control. Under both independent Al stress and combined treatment of Al/BRs, the transcriptional level of APX02 was increased. The YLY689 rice cultivar had higher APX02 expression than the CY-927 rice cultivar (p < 0.01). Interestingly, the transcriptional level of APX02 in rice seedlings emerged from BRs primed seeds was found to be higher than plants under Al stress alone and supported the results of APX activity (Fig. 5). Similarly, the transcription level of APX08 was high in both roots and shoots of either cultivar when compared to control under independent and combined treatments of Al and BRs; however, the expression level of APX08 was found to be higher in the roots of YLY689 cultivar than that of CY927 cultivar (Fig. 6). Additionally, in contrast control condition, the expression levels of CATa and CATb were found to be higher in both roots and shoots of both cultivars under independent and combined treatments of Al and BRs. However, the rise was more pronounced in YLY689 cultivar than that in CY927 cultivar. Al stress also triggered the expression levels of CATa and CATb in both roots and shoots of both cultivars as compared to the non-treated control (Fig. 6). Under stressful conditions, both cultivars showed significant upregulation of SOD Cu-Zn and SOD-Fe2 genes, where YLY689 cultivar showed a greater increase. The transcriptional level of the SOD Cu-Zn and SOD-Fe2 genes was found to be higher in roots than that in shoots in both cultivars. Regardless of Al stress, the transcriptional level of the SOD Cu-Zn gene was much higher in roots than in non-treated control (Fig. 6). In contrast to seeds primed with water, seedlings primed with BRs depicted higher expression of SOD Cu-Zn and SOD-Fe2 genes. Under Al toxicity, it may be possible to modify the stress condition inside both cultivars by temporarily upregulating particular gene expression (Fig. 6). This data further confirms the BRs-based alterations in the concentrations of enzymatic antioxidants (Fig. 5). It was clearly demonstrated that BRs play a substantial role in stress tolerance in rice plants by modifying and regulating the transcriptional level of particular genes under induced Al stress.
Determination of cluster and correlation analysis between observations
Biplot graphs of PCA were generated based on physiological features of two different rice varieties to study the sensitive and tolerant groups through F1 and F2 of multiple parameters under diverse treatments, such as seeds primed with water (CY927-H2O, YLY689-H2O) and BRs (CY927-BRs, YLY689-BRs) under normal conditions and seed primed with water and BRs under Al stress (Figs. 7A, B, 8). MDA, MGT, and H2O2 were pooled together and were found to have a positive correlation. Although MGT, H2O2, and MDA had a negative relationship with VI, F/W, D/W, SL, RL, GE, and GP, they also had a negative relationship with SOD, POD, CAT, and APX in both cultivars (Fig. 7A, B). PCA analysis of both cultivars (CY927 and YLY689) demonstrated that YLY689 is a tolerant genotype, whereby CY927 is a sensitive genotype for Al stress. In CY927, the largest contribution of F1 (84.92) was observed, followed by F2 (10.20), with a total contribution of 95.12%, whereas in YLY689, the largest contribution of F1 (86.39) was observed, followed by F2 (10.69), with a total contribution of 97.09%. ACH results also confirmed the identical response of both varieties to distinct treatments (Fig. 8). It represented the close relationship between both cultivars (CY927 and YLY689) primed with BRs and primed with water under Al stress, as well as cultivars primed with water and BRs under normal condition. When compared to plants primed with water under Al toxicity, cultivars primed with BRs exhibited a close association with both controls (primed with water and BRs) (Fig. 8).
Biplot of principle component of 1 and 2 of the PCA extracted from results obtained from physiological data of two different rice cultivars (CY927, YLY689) under various treatments such as control primed with water (CY927-H2O, YLY689-H2O), control primed with BRs (CY927-BRs, YLY689-BRs), seed primed with BRs, and treatment under Al stress (CY927-BRs+Al, YLY689-BRs+Al), seed primed with H2O under Al stress(CY92-Al+ H2O, YLY689-Al+ H2O). Sharp angle represented positive, obtuse angle showed a negative correlation, as well as a right angle demonstrated a correlation between parameters. A Physiological parameters of rice variety CY927 illustration through Pearson’s correlation coefficients under different treatments. (I) contains POD, CAT, APX, and SOD; (II) showed GI, F/W, D/W, GE, GP, VI, and SL; (III) illustrated MDA, MGT, and H2O2; while (IV) represented RL. B Physiological parameters of rice variety YLY689 representation via Pearson’s correlation coefficients under different treatments. Distance between each circle represented the strength of correlation. (I) contains POD, CAT, APX, and SOD; (II) showed GI, F/W, D/W, GE, GP, and VI; (III) illustrated MDA, MGT, and H2O2; while (IV) represented RL and SL
Discussion
Al is the 3rd most prevalent metal in the earth’s crust, and while it is abundant, it is only minimally soluble, causing serious harm to biological systems (Bolt et al. 2020). Al is present in plant-accessible form at pH value less than 5.5, which can cause toxicity in plants, particularly the roots (Barcelo and Poschenrieder 2002). Because it has direct exposure to roots, Al toxicity affects shoot length, fresh weight, and dry weight (Table 2), causing cell elongation inhibition at an early stage and later causing damage to plant growth and development (Čiamporová 2002; Silambarasan et al. 2019). In addition to membrane permeability, Al promotes nutritional imbalance in plants by altering osmotic balance (Olivares et al. 2009). Similarly, Al treatment lowered the levels of K+, Ca2+, Fe2+, and Mn2+ in both roots and shoots in the current study (Tables 3–4). Al toxicity lowered K+, Ca2+, Fe2+, and Mn2+ concentration in rice plants, with the effect being stronger in sensitive varieties than tolerant varieties. Similarly, Al inhibited the level of K+, Ca2+, Fe2+, and Mn2+ in the roots and shoots of tomato and maize plants (Giannakoula et al. 2008; Simon et al. 1994). Consequently, photosynthetic pigments and associated processes were also disrupted (Fig. 3), resulting in a reduction in plant growth caused by Al exposure (Table 2). In comparison to seeds primed with water, seeds treated with BRs demonstrated a stronger shielding response to Al stress in rice seedlings (Tables 1–2). The concentration of Al in roots was higher than that in shoots (Tables 3–4). It could be due to the possibility that the roots are the main source of Al uptake, and the plants activate various mechanisms to minimize Al translocation from roots to shoots (Mendonça et al. 2003; Ali et al. 2008c).
Current study demonstrated that Al absorption was dramatically reduced in both cultivars, particularly in those plants primed with BRs over those primed with water. This reduction was more noticeable in tolerant variety (cv. YLY689). Furthermore, seed priming with BRs significantly maintained the nutrient balance in both cultivars. The regulation of nutrient balance was more prominent in tolerant variety under Al stress. Consequently, both rice cultivars primed with BRs showed considerably increased biomass, root length, shoot length, fresh weight, and dry weight when compared to unprimed plants, where tolerant cultivar showed more pronounced rise in morphological parameters than sensitive cultivar (Tables 2–3). BRs seed priming further boosted photosynthetic pigments within both sensitive and tolerant varieties under Al toxicity (Fig. 3). This boost in photosynthetic pigments corresponds to better stress resistance behavior and alterations in plasma membrane as well as to activation of antioxidant enzymes under Al-stressed environment (Fig. 5) (Divi and Krishna 2009). Furthermore, BRs serve as a proton pump, enhancing water uptake; regulating gene suppression and activation, protein synthesis (Nawaz et al. 2017), and nucleic acid stimulation; and triggering antioxidant enzyme activity (Khripach et al. 2003). BRs-based modifications in plant behavior play a vital role in stimulating plant growth in stressful environments. According to a report, BRs relieve plants from stress by promoting plant growth, photosynthetic pigments, and water uptake (Rajewska et al. 2016). Similarly, the ameliorating roles of BRs in reducing cadmium toxicity in cowpea plants (Santos et al. 2018), salinity stress (Anuradha and Rao 2001, 2003), zinc metal stress in B. juncea (Arora et al. 2010), heat stress (Wu et al. 2014), and heavy metal stress (Anuradha and Rao 2007; Vardhini 2016; Vázquez et al. 2013) are well-established.
The results acquired from present investigations showed antioxidant enzyme activities, such as CAT, APX, and SOD, which serve as a defense system during plant stress periods and are raised under Al stress (Fig. 5) because of elevation in ROS (Jones et al. 2006; Yamamoto et al. 2003b). These results coincide with previously reported studies that suggested increase in SOD, CAT, and APX levels in sensitive and tolerant maize after exposure to Al treatment (Liu et al. 2008; Yamamoto et al. 2003a; Yamamoto et al. 2003b). After Al exposure, the activity of SOD, POD, APX, and CAT in tea plants increased (Ghanati et al. 2005). BRs induced greater antioxidant activities in both sensitive and resistant cultivars in the present study (Fig. 5). Antioxidant activities, such as SOD, POD, and CAT, are critical, and they are boosted by BRs after Al exposure to lessen the toxicity caused by Al (Ali et al. 2008a). BRs have been discovered to improve antioxidant enzymatic activity in stress situations to ameliorate various stresses (Nawaz et al. 2017; Rattan et al. 2020) and to regulate plant normal behavior in a number of studies (Ali et al. 2008b; Sharma et al. 2007). Al stress damages the membrane, resulting in a decrease in hydrolytic enzyme activity and an increase in ROS activity (Fig. 4). An increase in ROS activity is thought to cause significant damage to the cellular structure as well as macromolecules (Halliwell 1999). Under a stress situation, the use of BRs reduced H2O2 generation as well as MDA concentration (Fig. 4) to protect the plant’s membrane from oxidative damage. It reduced the rate of superoxide radicle formation and boosted plant antioxidant activity (Anuradha and Rao 2007; Mazorra et al. 2002; Ogweno et al. 2008).
A more precise approximation of antioxidant genes to the behavior of antioxidant enzyme activities can be obtained by studying gene expression at the transcriptional level of plants during heavy metal stress. As a result, we did the expression profiling of a number of genes related to antioxidant activity in order to evaluate both enzymatic and transcriptional responses of both rice cultivars under normal as well as stressful conditions. Higher transcriptional levels of APX02 and APX08 were recorded in the present study (Yamamoto et al. 2001). Similarly, APX expression was triggered by high levels of H2O2 in tobacco chloroplasts under heavy metal stress (Gupta et al. 1993). Moreover, significant upregulation of CAT genes (CATa and CATb) was also observed in both roots and shoots in both cultivars. Previously, no significant change in CAT gene expression was observed in leaves of Arabidopsis thaliana under varying light qualities (Cominelli et al. 2008). It could have happened because there were several allo- or isozymes present. Al, on the other hand, induces a rise in CAT gene expression as a result of protein breakdown, resulting in transcriptional upregulation. Under Al stress, gene expression of SOD-Cu-Zn and SOD-Fe2 was increased, indicating that oxidative damage inside diverse cellular compartments was produced by Al toxicity. When compared to control, the pattern of gene regulation was different because its expression was more upregulated in roots than that in shoots in both cultivars. It is possible since the plant roots are the initial site of contact with the toxicity generated by Al stress. Furthermore, superoxide production, rather than lipoxygenase activity, appears to be the primary cause of oxidative stress in roots, despite H2O2 being an important competitor in leaves. However, the knowledge regarding whether H2O2 is produced as a result of increased heavy metal content in the leaves or as a signal molecule from the roots is still elusive (Cominelli et al. 2008).
PCA is a multivariate method that is commonly used to categorize values based on biological state, quality, and origins. PCA is used to detect and categorize a big data collection into a small number of strongly associated variables (Aziz et al. 2018). Based on different treatments, agglomerative clustering hierarchy (ACH) revealed the interaction between different rice genotypes (Fig. 8). Using a combination of PCA and ACH, several treatments were used to separate the sensitive and tolerant genotypes and to show the association between various attributes based on physiological traits (Fig. 5). VI, F/W, D/W, SL, RL, GE, and GP were found to have a group and showed positive association with each other, but a negative relationship with MGT, H2O2, and MDA. On morphophysiological, biochemical, and molecular levels, this study adds to understanding the mechanistic response of both cultivars (CY927, YLY689) under Al stress, as well as seed priming with BRs under Al toxicity in metal-tolerant and metal-sensitive rice cultivars.
Conclusions
In the current study, Al has demonstrated phytotoxic effects on the physiological, antioxidant system, and molecular mechanism of rice seedlings, according to this study. Results of this study depicted that seed priming with BRs reduced the negative effects of Al on O. sativa seeds and improved both germination and early seedling growth in the presence of Al toxicity. The exposure of Al stress in rice plants boosted the antioxidant system (SOD, POD, CAT, and APX), which was substantially further activated by BRs. A transcriptional analysis of antioxidant genes in both cultivars demonstrated the similar pattern. As a result, it is possible that the improved resistance to Al in rice seedlings corresponded to the altered level of the antioxidant system. In this study, YLY689 was found to be a more resistant variety against Al stress than CY927. Furthermore, the use of BRs boosted resistance by improving plant growth, photosynthetic pigments, and other related processes in rice seedlings under Al stress.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahmed T, Shahid M, Noman M, Niazi MBK, Mahmood F, Manzoor I, Zhang Y, Li B, Yang Y, Yan C (2020) Silver nanoparticles synthesized by using Bacillus cereus SZT1 ameliorated the damage of bacterial leaf blight pathogen in rice. Pathogens 9(3):160
Ahmed T, Noman M, Luo J, Muhammad S, Shahid M, Ali MA, Zhang M, Li B (2021a) Bioengineered chitosan-magnesium nanocomposite: a novel agricultural antimicrobial agent against Acidovorax oryzae and Rhizoctonia solani for sustainable rice production. Int J Biol Macromol 168:834–845
Ahmed T, Noman M, Manzoor N, Shahid M, Hussaini KM, Rizwan M, Ali S, Maqsood A, Li B (2021b) Green magnesium oxide nanoparticles-based modulation of cellular oxidative repair mechanisms to reduce arsenic uptake and translocation in rice (Oryza sativa L.) plants. Environ Pollut 117785
Ahmed T, Noman M, Manzoor N, Shahid M, Abdullah M, Ali L, Wang G, Hashem A, Al-Arjani A-BF, Alqarawi AA (2021c) Nanoparticle-based amelioration of drought stress and cadmium toxicity in rice via triggering the stress responsive genetic mechanisms and nutrient acquisition. Ecotoxicol Environ Saf 209:111829
Ali B, Hayat S, Ahmad A (2005) Response of germinating seeds of Cicer arietinum to 28-homobrassinolide and/or potassium. Gen Appl Plant Physiol 31:55–63
Ali B, Hasan S, Hayat S, Hayat Q, Yadav S, Fariduddin Q, Ahmad A (2008a) A role for brassinosteroids in the amelioration of aluminium stress through antioxidant system in mung bean (Vigna radiata L. Wilczek). Environ Exp Bot 62:153–159
Ali B, Hayat S, Fariduddin Q, Ahmad A (2008b) 24-Epibrassinolide protects against the stress generated by salinity and nickel in Brassica juncea. Chemosphere 72:1387–1392
Ali M, Schiedt B, Healy K, Neumann R, Ensinger W (2008c) Modifying the surface charge of single track-etched conical nanopores in polyimide. Nanotechnology 19:085713
Anuradha S, Rao SSR (2001) Effect of brassinosteroids on salinity stress induced inhibition of seed germination and seedling growth of rice (Oryza sativa L.). Plant Growth Regul 33:151–153
Anuradha S, Rao SSR (2003) Application of brassinosteroids to rice seeds (Oryza sativa L.) reduced the impact of salt stress on growth, prevented photosynthetic pigment loss and increased nitrate reductase activity. Plant Growth Regul 40:29–32
Anuradha S, Rao S (2007) The effect of brassinosteroids on radish (Raphanus sativus L.) seedlings growing under cadmium stress. Plant Soil Environ 53:465
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Arora P, Bhardwaj R, Kanwar MK (2010) 24-Epibrassinolide induced antioxidative defense system of Brassica juncea L. under Zn metal stress. Physiol Mol Biol Plants 16:285–293
Aziz A, Mahmood T, Mahmood Z, Shazadi K, Mujeeb-Kazi A, Rasheed A (2018) Genotypic variation and genotype× environment interaction for yield-related traits in synthetic hexaploid wheats under a range of optimal and heat-stressed environments. Crop Sci 58:295–303
Barcelo J, Poschenrieder C (2002) Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: a review. Environ Exp Bot 48:75–92
Basit F, Chen M, Ahmed T, Shahid M, Noman M, Liu J, An J, Hashem A, Fahad Al-Arjani A-B, Alqarawi AA (2021a) Seed priming with brassinosteroids alleviates chromium stress in rice cultivars via improving ROS metabolism and antioxidant defense response at biochemical and molecular levels. Antioxidants 10:1089
Basit F, Liu J, An J, Chen M, He C, Zhu X, Li Z, Hu J, Guan Y (2021b) Brassinosteroids as a multidimensional regulator of plant physiological and molecular responses under various environmental stresses. Environ Sci Pollut Res:1–12
Bergonci T, Ribeiro B, Ceciliato PH, Guerrero-Abad JC, Silva-Filho MC, Moura DS (2014) Arabidopsis thaliana RALF1 opposes brassinosteroid effects on root cell elongation and lateral root formation. J Exp Bot 65:2219–2230
Bhandari S, Nailwal TK (2020) Role of brassinosteroids in mitigating abiotic stresses in plants. Biologia:1–28
Bolt A, Dincer I, Agelin-Chaab M (2020) Experimental study of hydrogen production process with aluminum and water. Int J Hydrog Energy 45:14232–14244
Čiamporová M (2002) Morphological and structural responses of plant roots to aluminium at organ, tissue, and cellular levels. Biol Plant 45:161–171
Cominelli E, Gusmaroli G, Allegra D, Galbiati M, Wade HK, Jenkins GI, Tonelli C (2008) Expression analysis of anthocyanin regulatory genes in response to different light qualities in Arabidopsis thaliana. J Plant Physiol 165:886–894
Divi UK, Krishna P (2009) Brassinosteroid: a biotechnological target for enhancing crop yield and stress tolerance. New Biotechnol 26:131–136
Ghanati F, Morita A, Yokota H (2005) Effects of aluminum on the growth of tea plant and activation of antioxidant system. Plant Soil 276:133–141
Giannakoula A, Moustakas M, Mylona P, Papadakis I, Yupsanis T (2008) Aluminum tolerance in maize is correlated with increased levels of mineral nutrients, carbohydrates and proline, and decreased levels of lipid peroxidation and Al accumulation. J Plant Physiol 165:385–396
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59:309–314
Gupta AS, Webb RP, Holaday AS, Allen RD (1993) Overexpression of superoxide dismutase protects plants from oxidative stress (induction of ascorbate peroxidase in superoxide dismutase-overexpressing plants). Plant Physiol 103:1067–1073
Halliwell B (1999) Antioxidant defence mechanisms: from the beginning to the end (of the beginning). Free Radic Res 31:261–272
Hu J, Chen G, Lo IM (2005) Removal and recovery of Cr (VI) from wastewater by maghemite nanoparticles. Water Res 39:4528–4536
Huang Z, Pan X-D, Wu P-G, Han J-L, Chen Q (2013) Health risk assessment of heavy metals in rice to the population in Zhejiang, China. PLoS One 8:e75007
Jan S, Noman A, Kaya C, Ashraf M, Alyemeni MN, Ahmad P (2020) 24-Epibrassinolide alleviates the injurious effects of Cr (VI) toxicity in tomato plants: Insights into growth, physio-biochemical attributes, antioxidant activity and regulation of Ascorbate–glutathione and Glyoxalase cycles. J Plant Growth Regul 39: 1587-1604
Jones D, Blancaflor E, Kochian L, Gilroy S (2006) Spatial coordination of aluminium uptake, production of reactive oxygen species, callose production and wall rigidification in maize roots. Plant Cell Environ 29:1309–1318
Khan MD, Mei L, Ali B, Chen Y, Cheng X, Zhu S (2013) Cadmium-induced upregulation of lipid peroxidation and reactive oxygen species caused physiological, biochemical, and ultrastructural changes in upland cotton seedlings. Biomed Res Int 2013:1–10
Khripach VA, Zhabinskii VN, Khripach NB (2003) New practical aspects of brassinosteroids and results of their ten-year agricultural use in Russia and Belarus, Brassinosteroids. Springer, pp:189–230
Latha P, Vidya Vardhini B (2018) Effect of homobrassinolide on the growth of mustard crops grown in semi-arid tropics of nizamabad. Int J Curr Res Life Sci 7:2320–2326
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Portland Press Ltd.
Liu Q, Yang J, He L, Li Y, Zheng S (2008) Effect of aluminum on cell wall, plasma membrane, antioxidants and root elongation in triticale. Biol Plant 52:87–92
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. methods 25:402–408
Ma JF (2007) Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. Int Rev Cytol 264:225–252
Manzoor N, Ahmed T, Noman M, Shahid M, Nazir MM, Ali L, Alnusaire TS, Li B, Schulin R, Wang G (2021) Iron oxide nanoparticles ameliorated the cadmium and salinity stresses in wheat plants, facilitating photosynthetic pigments and restricting cadmium uptake. Sci Total Environ 769:145221
Mazorra LM, Nunez M, Hechavarria M, Coll F, Sánchez-Blanco MJ (2002) Influence of brassinosteroids on antioxidant enzymes activity in tomato under different temperatures. Biol Plant 45:593–596
Mendonça RJD, Cambraia J, Oliveira JAD, Oliva MA (2003) Aluminum effects on the uptake and utilization of macronutrients in two rice cultivars. Pesqui Agropecu Bras 38:843–848
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nawaz F, Naeem M, Zulfiqar B, Akram A, Ashraf MY, Raheel M, Shabbir RN, Hussain RA, Anwar I, Aurangzaib M (2017) Understanding brassinosteroid-regulated mechanisms to improve stress tolerance in plants: a critical review. Environ Sci Pollut Res 24:15959–15975
Noman M, Ahmed T, Hussain S, Niazi MBK, Shahid M, Song F (2020a) Biogenic copper nanoparticles synthesized by using a copper-resistant strain Shigella flexneri SNT22 reduced the translocation of cadmium from soil to wheat plants. J Hazard Mater 398:123175
Noman M, Shahid M, Ahmed T, Tahir M, Naqqash T, Muhammad S, Song F, Abid HMA, Aslam Z (2020b) Green copper nanoparticles from a native Klebsiella pneumoniae strain alleviated oxidative stress impairment of wheat plants by reducing the chromium bioavailability and increasing the growth. Ecotoxicol Environ Saf 192:110303
Ogweno JO, Song XS, Shi K, Hu WH, Mao WH, Zhou YH, Yu JQ, Nogués S (2008) Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicon esculentum. J Plant Growth Regul 27:49–57
Olivares E, Peña E, Marcano E, Mostacero J, Aguiar G, Benítez M, Rengifo E (2009) Aluminum accumulation and its relationship with mineral plant nutrients in 12 pteridophytes from Venezuela. Environ Exp Bot 65:132–141
Rajewska I, Talarek M, Bajguz A (2016) Brassinosteroids and response of plants to heavy metals action. Front Plant Sci 7:629
Rao KM, Sresty T (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci 157:113–128
Rattan A, Kapoor D, Kapoor N, Bhardwaj R, Sharma A (2020) Brassinosteroids regulate functional components of antioxidative defense system in salt stressed maize seedlings. J Plant Growth Regul, 1-11
Rout G, Samantaray S, Das P (2001) Aluminium toxicity in plants: a review. Agronomie 21:3–21
Sah SK, Kaur G, Kaur A (2014) Rapid and reliable method of high-quality RNA extraction from diverse plants. Am J Plant Sci 5:3129–3139
Salah SM, Yajing G, Dongdong C, Jie L, Aamir N, Qijuan H, Weimin H, Mingyu N, Jin H (2015) Seed priming with polyethylene glycol regulating the physiological and molecular mechanism in rice (Oryza sativa L.) under nano-ZnO stress. Sci. Rep 5(1):1–14
Santos L, Batista B, Lobato A (2018) Brassinosteroids mitigate cadmium toxicity in cowpea plants. Photosynthetica 56:591–605
Sharma R, Duveiller E, Ortiz-Ferrara G (2007) Progress and challenge towards reducing wheat spot blotch threat in the Eastern Gangetic Plains of South Asia: is climate change already taking its toll? Field Crop Res 103:109–118
Silambarasan S, Logeswari P, Cornejo P, Kannan VR (2019) Role of plant growth–promoting rhizobacterial consortium in improving the Vigna radiata growth and alleviation of aluminum and drought stresses. Environ Sci Pollut Res 26:27647–27659
Silva S (2012) Aluminium toxicity targets in plants. J Bot
Simon L, Smalley T, Jones JB Jr, Lasseigne F (1994) Aluminum toxicity in tomato. Part 1. Growth and mineral nutrition. J Plant Nutr 17:293–306
Vardhini BV (2016) Brassinosteroids are potential ameliorators of heavy metal stresses in plants, Plant Metal Interaction. Elsevier, pp. 209-237
Vázquez MN, Guerrero YR, González LM, de la Noval WT (2013) Brassinosteroids and plant responses to heavy metal stress. An overview. Open J Metal 3:34–41
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Vitorello VA, Capaldi FR, Stefanuto VA (2005) Recent advances in aluminum toxicity and resistance in higher plants. Braz J Plant Physiol 17:129–143
Wu X, Yao X, Chen J, Zhu Z, Zhang H, Zha D (2014) Brassinosteroids protect photosynthesis and antioxidant system of eggplant seedlings from high-temperature stress. Acta Physiol Plant 36:251–261
Xu F, Li G, Jin C, Liu W, Zhang S, Zhang Y, Lin X (2012) Aluminum-induced changes in reactive oxygen species accumulation, lipid peroxidation and antioxidant capacity in wheat root tips. Biol Plant 56:89–96
Yamamoto Y, Kobayashi Y, Matsumoto H (2001) Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol 125:199–208
Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K (2003a) Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301:640–643
Yamamoto Y, Kobayashi Y, Devi SR, Rikiishi S, Matsumoto H (2003b) Oxidative stress triggered by aluminum in plant roots. The Dynamic Interface between Plants and the Earth. Springer, Roots, pp 239–243
Zhang X (1992) The measurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system. Research methodology of crop physiology. Agriculture Press, Beijing, 208-211
Zhang S, Hu J, Zhang Y, Xie X, Knapp A (2007) Seed priming with brassinolide improves lucerne (Medicago sativa L.) seed germination and seedling growth in relation to physiological changes under salinity stress. Aust J Agric Res 58:811–815
Funding
This research was supported by the Key Research and Development Program of Zhejiang Province (No. 2019C02011), Zhejiang Provincial Natural Science Foundation (No. LY21C130006), National Natural Science Foundation of China (No. 32072127), and Hainan Provincial Science and Technology Plan-Sanya Yazhou Bay Science and Technology City Joint Project (No. 320LH032). Dabeinong Funds for Discipline Development and Talent Training in Zhejiang University and Collaborative Innovation Center for Modern Crop Production co-sponsored by Province and Ministry (CIC-MCP).
Author information
Authors and Affiliations
Contributions
FB, JH, and GY are involved in the conceptualization, and GY and FB in design experiment. FB and AJ performed the experiment and wrote the manuscript. HC, LZ, and LJ assisted in writing and editing the manuscript. CM, JH, and ZX perform statistical analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Basit, F., Liu, J., An, J. et al. Seed priming with brassinosteroids alleviates aluminum toxicity in rice via improving antioxidant defense system and suppressing aluminum uptake. Environ Sci Pollut Res 29, 10183–10197 (2022). https://doi.org/10.1007/s11356-021-16209-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16209-y