Abstract
Bottom sediment quality from the Niterói harbor at Guanabara Bay (Rio de Janeiro, Brazil) was evaluated based on concentrations of organic phosphorus (OP) and inorganic phosphorus (IP) and bioavailability of trace metals through BCR fractionation analysis (Zn, Cu, Pb, Cr, Ni, Cd). The study area revealed elevated concentrations of fine sediments and organic matter (TOC: 2.26–7.31%). OP presented extremely elevated concentrations between 0.57 and 47.04 μmol/g, whereas IP reached a maximum concentration of 4.99 μmol/g. The anoxic bottom at the study area was confirmed by high TOC/OP values in most stations. Phosphorus enrichment index (PEI) varied between 0.07 and 2.57, pointing to ecological risk at some stations. Trace metals were mostly bonded to the bioavailable fractions (exchangeable, reducible, oxidizable), and decreasing order of mobility was Zn > Cu > Pb > Cr > Ni > Cd. The Risk Assessment Code (RAC) suggested a high risk of bioavailability for Zinc and a medium risk for the other metals. Overall, the Niterói harbor revealed poor sediment quality suggesting a strong anthropogenic pressure in the area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urbanization of coastal areas usually comes along with strong anthropogenic pressure that reduces water and sediment quality and threatens pelagic and benthic communities (Birch 2017). Large inputs of domestic and industrial effluents, containing raw sewage and all kinds of pollutants, such as organic contaminants and trace metals, arrive at coastal waters surrounding big cities worldwide. Regarding anthropogenic inputs, macronutrients and trace metals are often among the substances released in coastal urbanized environments. Nitrogen and phosphorus have a crucial role in primary production, and the excess of these substances in coastal waters, lagoons, and lakes drives eutrophication (Zhou et al. 2020). In urbanized areas, these nutrients usually reach coastal bays and estuaries through the release of untreated sewage, and some of the effects caused by eutrophication include hypoxia/anoxia, the increase of turbidity, phytoplankton blooms, and the decrease of biota diversity, among others (Alexander et al. 2017; Peyman et al. 2017; Wurtsbaugh et al. 2019). Trace metals are often present in industrial effluents, and contamination of estuaries and coastal bays by these elements has been reported for decades (Chen et al., 2016; Cukrov et al., 2011; Hamdoun et al., 2015; Karydis and Kitsiou, 2012; Qian et al., 2018; Sundararajan et al., 2017).
Harbors and shipyards are commonly located in confined environments, favoring the accumulation of fine sediments that act as sinks for contaminants (Kalnejais et al. 2010), and geochemical evaluation of sediment compartment can unveil the levels of contamination by different substances as well as ecological risks to local biota and, consequently, to the human population that lives in the surroundings. Phosphorus is the most important macronutrient limiting primary production on long time scales and the release of this element from bottom sediments affects nutrient ratios increasing eutrophication and playing a key role in the development of bottom hypoxia in marine environments (Edlund and Carman 2001; Dijkstra et al. 2016; Dan et al. 2020a). Sediments are important carriers for trace metals and can act as sources or sinks for these elements. Trace metal contamination in bottom sediments is a major concern, due to their toxicity and persistence in the environment and also bio-accumulation along the trophic chain (Saleem et al. 2015). Remobilization of metals from bottom sediments can occur due to physical factors, such as dredging, wave action, tidal movement, and biogeochemical changes, such as bioturbation, mineralization of organic matter, pH, and salinity variations (Eggleton and Thomas 2004; Durán et al. 2012). Regarding trace metals, sediment quality guidelines usually approach total metal content that does not reveal metal bioavailability or mobility. The use of the BCR technique to analyze trace metals allows the assessment of metal concentrations in bioavailable fraction and evaluation of ecological risk through the Risk Assessment Code (RAC) (Passos et al. 2010; Aguiar et al. 2018; Caraballo et al. 2018).
The health status of an aquatic system can be assessed through proper environmental monitoring, assessing the impacts caused by anthropogenic pressures to develop public policies to protect the environment as well as the economic activities that take place in coastal areas. This study aims to evaluate the quality of bottom sediments from Niterói harbor, located in an extremely polluted estuary, Guanabara Bay (Neto et al. 2006; Cordeiro et al. 2008; Abreu et al. 2016; Soares-gomes et al. 2016), and subjected to strong anthropogenic pressure through inputs of domestic effluents and from the shipyard industry in its surroundings.
Material and methods
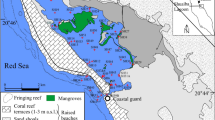
Niterói harbor is located in the east margin of Guanabara Bay (Rio de Janeiro), one of the most polluted coastal areas in southeast Brazil (Kjerfve et al. 1997; Baptista Neto et al. 2006; Aguiar et al. 2016; Soares-gomes et al. 2016; Fries et al. 2019). The Niterói Harbor has a mean depth of 7.6 m in the main channel and 9.1 m in the cargo pier with a mean tide of 1.21 m. The area is surrounded by shipyards and is known for intense naval activity (Vilela et al. 2004; Baptista Neto et al. 2005)
Bottom sediments of Niterói harbor were sampled at 11 stations in the winter of 2015 with a stainless steel Van Veen grab (Fig. 1). The samples were stored in plastic bags properly identified and immediately stored on ice until arrival at the laboratory where they were frozen at −20 °C.
In the laboratory, samples were freeze-dried and aliquots were taken for determination of grain size, total organic carbon (TOC), total nitrogen (TN), organic phosphorus (OP), inorganic phosphorus (IP), and trace metals. Samples for grain size analysis were pre-treated for the elimination of organic matter with H2O2 and carbonates with HCl 10% (Suguio 1073). Samples were then analyzed through laser scattering with the aid of a particle analyzer Malvern-series 2000, with a Hydro G dispersion unit with HCl 10%.
Samples for determination of TOC and TN were processed in an elemental analyzer Perkin Elmer® after elimination of carbonate with HCl 10% (v/v), and acetanilide was used as a standard.
Following the method described by Aspila et al. (1976), total phosphorus (TP) was extracted with HCl 1M in previously calcinated samples (550 °C) with mechanical agitation for 16 h. The extraction of inorganic phosphorus (IP) followed the same procedure without the calcination step. Organic phosphorus (OP) was obtained through the difference between total and inorganic phosphorus.
The phosphorus enrichment index (PEI) was used to estimate ecological risk concerning sedimentary P concentrations.
Ci is the sedimentary TP concentration for each sample, and Cs is the standard TP concentration (~19.4 μmol/g) established by the Department of Environmental and Energy (DOEE) above which ecological risks are expected (Mudroch and Azcue 1995, Riba et al. 2002). Total phosphorus concentrations are expected to cause ecological risks if PEI is >1.
Samples for the determination of trace metals were homogenized with a mortar and pestle and followed the BCR protocol established by Ure et al. (1993) and Rauret et al. (1999). The residual fraction was determined following the method by USEPA 3051. The determined metals (Zn, Pb, Cd, Cr, Ni, Cu) were extracted from 1.0 g of sediment according to Table 1.
The evaluation of the ecological risk was made using the Risk Assessment Code (RAC) commonly along with BCR determination (Nemati et al. 2011; Canuto et al. 2013; Sungur et al. 2014; Aguiar et al. 2020). The index considers the percentage of metal in the exchangeable fraction and classification varies from no risk to very high risk (Table 2).
The software Statistica 7.0® was used to perform principal component analysis (PCA) on sediment data.
Results and discussion
Coastal bays usually present a textural range that goes from the mixing of sand, silt, and clay or well-sorted sand representing the reworking of sediments by tidal and wave actions. In the inner portion of coastal bays, hydrodynamics is usually weaker, favoring the deposition of finer sediments (Vilela et al. 2004). The study area is located in an inner part of Guanabara Bay, with a restricted circulation that receives an anthropogenic contribution from the naval industry and domestic input especially in the proximities of station P3, where small watercourses discharge untreated sewage from Niterói city. The grain size results reflect hydrodynamic conditions with the predominance of fine fraction (silt + clay) in most stations (Fig. 2), except for P9, where sand predominated (54%). With regards to the fine fraction, silt varied from 44 to 76%, whereas clay reached a maximum of 4% at P13. Maximum concentrations of silt were registered in the most inner stations of the study area, P1, P2, P4, P6, and also P8 (Fig. 2). Grain size results corroborated the ones found by Vilela et al. (2004) and Baptista Neto et al. (2005) in the Niterói harbor and characterize a very favorable environment for the accumulation of pollutants, which are easily adsorbed by fine particles. Calcium carbonate varied from 17.76 to 26.48% (Fig. 2) being classified as litoclastic (Larssouner et al. 1982). The total organic carbon was considered elevated all over the study area, suggesting anthropogenic input (Fig. 3). The highest TOC concentration, 7.31%, was registered in station P3, and the elevated content is probably derived from the material discharged from small streams that cross part of Niterói city and discharge untreated sewage in this location. The smallest TOC concentration was found at station P13, 2.26%, still a very elevated content of organic carbon for a marine area, which usually presents concentrations of sedimentary organic matter around 0.5% (Libes 2009). Total nitrogen varied from 0.19 to 0.66%, with the highest concentration at station P3 like TOC (Fig. 2). C/N ratio varied from 10 to 12 suggesting organic matter of mixed origin tending to marine according to the classification proposed by Bader (1955). TOC concentrations corroborated the values registered in Guanabara Bay in previous studies from Vilela et al. (2003) and Carreira et al. (2004) who found values between 3.23 and 4.2 and 3.23 and 3.77%, respectively. In a study also conducted in Guanabara Bay, Carreira et al. (2002) found C/N ratios between 10.1 and 13.3, indicating organic matter of mixed origin as the results of the present study.
Being a major nutrient in marine biogeochemical cycles, phosphorus has a key role in primary production. The sources of phosphorus to the ocean are restricted to continental weathering and fluvial discharges, being the only macronutrient limited by these processes (Anderson et al. 2001). Fluvial discharges in coastal urbanized areas are usually loaded with untreated sewage causing nutrient enrichment in marine waters and eventually leading to the eutrophication process. Guanabara Bay long suffers from eutrophication caused by a severe and continuous input of domestic and industrial load, where phosphorus stands out as one of the main causes of this deleterious process (Borges et al. 2009; Aguiar et al. 2011, 2013). The predominance of fine grain sizes in the most sheltered areas of the bay favors the retention of phosphorus, and Niterói harbor is one of these sites. Total phosphorus (IP + OP) concentrations varied from 1.32 to 48.71 μmol/g, with the highest value registered at station P4, followed by P3 (38.11 μmol/g). Concentrations of IP varied between 1.09 and 4.99 μmol/g with higher concentrations as stations P7 and P13 (Fig. 4). Organic phosphorus presented much higher concentrations than IP, and the highest values were registered in the most inner stations, P4 and P3 (Fig. 4).
Previous studies in Guanabara Bay had registered IP and OP concentrations in different locations from the study area of the present study. Borges et al. (2009) have studied the evolution of sedimentary phosphorus concentrations in a core in a sheltered area in the northwestern portion of Guanabara Bay. The authors have found low levels of TP, around 9.3 μmol/g, in a period before human intervention in the drainage basin, with over 60% of total phosphorus corresponding to IP. The mentioned authors found a marked increase in TP concentrations in the core after the middle 1990s, around 38 μmol/g, where inorganic phosphorus accounted for over 90% of total phosphorus. Concentrations of TP from the present study corroborate the findings of Borges et al. (2009) in terms of total phosphorus concentrations; however, results showed contrast due to the predominance of OP over IP in the surface sediments from Niterói harbor.
Aguiar et al. (2013) found concentrations of IP in Jurujuba Sound (Niterói) varying from 1.61 to 15.80 μmol/g, a much higher maximum value than the maximum value in the present study (4.99 μmol/g). The mentioned authors registered OP concentrations between 0.21 and 10.06 μmol/g, much lower than the results registered in the Niterói harbor area. Carreira and Wagener (1998) found concentrations of IP and OP varying from 7.84 to 58.51 and 4.11 to 27.08 μmol/g, respectively, in the coastal area adjacent to Guanabara Bay.
Other studies concerning sedimentary phosphorus in coastal areas also presented higher contents of IP over OP such as the one from Dan et al. (2020a), who found up to 9.76 and 7.48 μmol/g of IP and OP in the South China Sea, and from Dan et al. (2020b) who registered maximum concentrations of 16.00 and 2.88 μmol/g of IP and OP, respectively, in southeast Nigeria.
Concentrations of sedimentary phosphorus in Niterói harbor contrasted with results from previous studies in different sites inside Guanabara Bay and other coastal areas regarding the predominance of OP over IP in the present study. Organic phosphorus surpassed 90% of the TP pool in several stations, especially in the most inner ones, such as P2, P3, P4, P6, and also in P8.
Bottom sediments are considered natural depositories for phosphorus and organic carbon. Usually, almost all the types of sedimentary organic matter in the aquatic environment are due to primary production in the terrestrial and marine environments. Organic phosphorus is one of the most important phases buried in sediments and directly affects the levels of dissolved inorganic phosphorus available for primary production (Anderson et al. 2001; Edlund and Carman 2001). The removal of phosphorus from the water columns is driven by primary production, and the products of this process reach bottom sediments and are incorporated into organic matter. The molar ratio TOC/OP from marine phytoplankton is close to the C/P ratio (106:1) proposed by Redfield et al. (1963). Therefore, most of the organic matter that reaches the interface water-sediment should present a C/P molar ratio close to 106:1. In this sense, TOC/OP molar ratio is a proxy that helps to understand the origin, decomposition, and preservation of sedimentary organic matter and phosphorus transformation in sediments, as well as redox conditions of sediments and interstitial waters in aquatic ecosystems. It has been reported that TOC/OP molar ratios smaller than the Redfield C/P ratio were registered in well-oxygenated environments, due to the mineralization of organic matter, whereas higher values of this ratio were found under anoxic conditions. Elevated TOC/OP usually point to terrestrial organic matter with values around 300–1300 for higher plants with soft tissues, whereas TOC/OP higher than 1300 indicates terrestrial plants with woody tissues (Dan et al. 2020b). The molar ratio TOC/OP can also be used to evaluate redox conditions from sediments and interstitial waters in aquatic environments since the burial of TOC in marine sediments is more efficient than the burial of OP under low oxygenation (Dan et al., 2020b). Despite the many uses of this proxy, TOC/OP molar ratios may present some ambiguity due to phosphorus early diagenesis. Some studies have shown that organic phosphorus is transformed and adsorbed into authigenic phosphorus compounds in shallow environments of high productivity since anaerobic bacteria cannot retain the inorganic phosphorus released (Anderson et al. 2001; Dan et al. 2020b). In the present study, results of TOC/OP exhibited a large range, from 73 to 4931 (Fig. 5), suggesting the predominance of anoxic conditions in bottom sediments in most of the stations in the Niterói harbor area, which presented values much higher than the Redfield ratio. Station P4 presented a TOC/OP molar ratio <106, suggesting an oxygenated bottom (Fig. 5); however, OP corresponded to 97% of the composition of the TP pool at this point. Some authors have found the predominance of organic phosphorus in TP pool especially in surface sediments from environments with anoxic/hypoxic bottom waters (Jilbert et al. 2011; Dijkstra et al. 2016), contradicting the results found in P4. P4 not only is one of the most inner stations, but also presents a low depth (<8 m) that might not allow mineralization of all organic matter present in the water column. It should also be considered that P4 receives the direct anthropogenic contribution of the Fonseca River, and in fact, P4 exhibited the highest concentration of OP, 47.04 μmol/g, which might have contributed to the lowering of the TOC/OP ratio, despite the elevated concentration of organic carbon (4.1%). Although the TOC/OP at P4 suggests an oxygenated bottom, it seems that the dissolved oxygen concentrations are not enough to mineralize such a large amount of organic matter.
Phosphorus enrichment index (PEI) was >1 at P3 and P4 (Fig. 5), pointing to ecological risks at the most inner stations, probably due to the heavy anthropogenic contribution of the Fonseca River that discharges in the proximities of P3 carrying untreated domestic sewage.
The concentrations of trace metals in bottom sediments from the Niterói harbor were quite elevated (Table 3). The percentage of zinc in the bioavailable fractions (%∑F1–F3) surpassed 90% in every sample, varying between 252.14 and 536.69 μg/g. Zn was mostly bonded to the first fraction (F1), which is the one that corresponds to weak associations with carbonates. Metals weakly bonded to carbonates could be displaced by ions such as Ca, K, Mg, and ammonium (Díaz-de-Alba et al. 2011; Fathollahzadeh et al. 2014), the last one, commonly present in contaminated areas by domestic effluents, through the hydrolysis of urea. Changes in salinity and pH are among the factors that can favor the release of metals bonded in the soluble fraction (F1) increasing their mobility. The most inner stations presented the higher concentrations of Zn in the soluble fraction (F1), especially P1–P6. Zinc concentrations were higher in the reducible fraction from in stations P7–P13 (Fig. 6). Oxidizable and residual fractions were the ones with lower zinc concentrations. Among the metals analyzed, Zn was the one less bonded to the residual fraction. Maximum concentrations of Zn were registered in the soluble fraction of stations P1–P3 and varied between 305.31 and 357.20 μg/g, surpassing the probable effect level (PEL) and effect range low (ERL) (Table 3).
According to Masoud et al. (2010), during deposition processes, zinc is usually bonded to oxy-hydroxides. Salem et al. (2014), on the other hand, stated that zinc can be precipitated as ZnCO3, and this hypothesis corroborates the results of the present study that revealed Zn mostly bonded to the soluble fraction, which includes carbonates. However, zinc associated with oxy-hydroxides was the second most predominant fraction. Apart from P9 and P10, the concentrations of Zn in the soluble fraction in the remaining stations were over the threshold effect value (TEL), varying between 130.10 and 232.76 μg/g. Some stations also surpassed ERL concerning Zn in F1. In the reducible fractions, most of the stations presented Zn concentrations higher than TEL and ERL, with values between 137.50 and 234.85 μg/g. Historically, high levels of zinc in bottom sediments from Guanabara Bay have been reported in the last decades. The very elevated concentrations of Zn, especially in the soluble fraction was already observed in other parts of Guanabara Bay, most precisely in the west portion, including Rio de Janeiro Harbor, where Aguiar et al. (2018) registered concentrations as high as 220.40 ug/g in F1, however, still lower than the maximum value found in the present study, 357.20 μg/g. Cordeiro et al. (2015) also found elevated concentrations of Zn in the soluble fraction in Guanabara Bay, with a maximum value of 479 μ/g. A very high total concentration of Zn was also reported in Guanabara Bay by Aguiar et al. (2016) who found Zn concentrations up to 755 ug/g. Considering harbor areas around the world, Fathollahzadeh et al. (2014) registered high concentrations of Zn in Oskarshamn harbor (Sweden) sediments, reaching a maximum concentration of 1794 μg/g regarding the sum of the four BCR fractions (F1–F4), and 42–58% of Zn was bonded to the soluble fraction (F1), suggesting that harbor areas are prone to accumulate zinc in bottom sediments. Risk Assessment Code for Zn showed a very high risk of bioavailability in stations P1 to P6, and high risk in P7, P8, and P13 (Fig. 8). Results suggest strong anthropogenic sources in the surroundings of the study area, since this metal is usually confined close to the emission sources and widely detected in industrial and mining wastes, as well as associated with municipal wastewater, corrosion of ship hulls, and tire particles, among others (Callender and Rice 2000; Dong et al. 2012; Aguiar et al. 2016). The higher risk in the inner stations is probably due to higher contents of fine sediments and more restricted circulation. The study site, besides being a harbor area, is home to several shipyards, which certainly contributes to anthropogenic inputs of zinc and other metals.
For copper, the sum of bioavailable fractions (F1–F3) surpassed 90% in approximately 54% of the samples (Fig. 6). The oxidizable fraction (F3) was the one retaining higher concentrations of copper, corroborating the hypothesis of the high affinity of this element with organic matter (Diamond 2012). Living organisms, detritus, and coatings on mineral particles are among organic materials that can form metal complexes. Mineralization of organic material can mobilize metallic elements retained in the sediments and release soluble forms of the metals (Filgueiras et al. 2002). The soluble fraction was the one that presented the lowest concentrations of Cu; nevertheless, the TEL value was overcome in some stations (P1, P2, P4, P6), with values between 21.17 and 51.87 μg/g for F1. P1 and P4 also presented Cu concentrations higher than ERL in F1. The probable effect level (PEL) for Cu was overcome in some stations in the reducible (112.93–127.40 μg/g) and oxidizable fractions (111.94–192.56 μg/g). Stations that did not overcame PEL values in the reducible and oxidizable fractions were higher than the TEL value for copper. Effect range low (ERL) for Cu was also surpassed in every station for F2 and F3 concentrations. Elevated Cu concentrations in bottom sediments have been described at Guanabara Bay through fractionation studies by Cordeiro et al. (2015) and Aguiar et al. (2018), who found much lower values concerning BCR fractions (0.01–99.9 and 0.55–53.82 μg/g, respectively) than the results of the present study, revealing that Niterói harbor can be a hot spot of Cu contamination within Guanabara Bay.
Urban anthropogenic sources of Cu are often associated with urban runoffs, anti-fouling coatings, and diesel and fuel combustion (Warnken et al. 2004). Besides urban effluents, anti-fouling coatings could be one of the strongest sources of Cu and also Zn in the study area, due to the presence of the harbor and several shipyards. Despite presenting concentrations above the sediment guidelines (Table 3), the risk of bioavailability for Cu was considered medium for some of the most internal stations (Fig. 8), P1, P2, P4, and P5. The rest of the stations presented RAC <10% suggesting a low risk of bioavailability for Cu.
The concentration of Pb in the bioavailable fractions (F1–F3) was over 70% in every station, except for P2, which presented 54% of concentrations distributed in the bioavailable fractions (Fig. 6). Concerning fractionation, Pb was the least available element in the soluble fraction (Fig. 6). Higher concentrations of Pb were found in the reducible fraction, i.e., associated with Fe and Mn oxy-hydroxides. These secondary oxides are often found as coatings of mineral surfaces or fine particles and are known to scavenge trace metals through several mechanisms, such as adsorption, ion exchange, coprecipitation, and surface complex formation (Díaz-de-Alba et al. 2011). Under anoxic conditions, the reduction of iron and manganese can release adsorbed trace metals.
Fractionation of Pb of the present study corroborates the ones found in previous studies in Guanabara Bay by Cordeiro et al. (2015), who registered over 80% of Pb bonded to F2, and Aguiar et al. (2018), who found approximately 45% of Pb in the same fraction. In the reducible fraction (F2), stations P4 and P8 surpassed the PEL value, with concentrations of 152.21 and 153.35 μg/g respectively. Still in the reducible fraction, all the other stations, except for P2, presented values higher than TEL and ERL, varying between 51.11 and 95.37 μg/g. The concentrations of Pb in the oxidizable fraction were higher than TEL in stations P5 to P13, with values between 32.99 and 39.05 μg/g. Despite overcoming some of the sediment guidelines, the risk of bioavailability for Pb was considered low, since RAC <10% in every sample (Fig. 8).
Approximately half of the samples presented over 60% of Cr concentrations distributed in the first three fractions (Fig. 7), concerning bioavailability. Chromium presented the lowest concentrations in the soluble fraction and the maximum values in the oxidizable one (Fig. 7). All the concentrations concerning the four fractions were below the TEL value for Cr (Table 3). The residual fraction retained most of the Cr concentrations, after the oxidizable one. Nickel also presented concentrations below the TEL value in every station and fractions (Table 3). The lowest concentrations of Ni were registered in the soluble fraction and the highest in the residual fraction (Fig. 7). Both Cr and Ni results were lower than the ones registered in Guanabara Bay in previous studies by Cordeiro et al. (2015) and Aguiar et al. (2018). Considering bioavailability, RAC suggested medium risk for Cr in every station. For Ni, the risk of bioavailability was considered low, except for P1 with RAC suggesting high risk (Fig. 8).
Values of Cd were higher in the residual fraction, and approximately 27% of the samples presented over 50% of Cd concentrations distributed in the bioavailable fractions (Fig. 7). Cadmium concentrations, however, were more elevated in the residual fraction.
The most concerning issue regarding Cd was that all concentrations of every fraction surpassed the probable effect level (Table 3). Concentrations of Cd were also higher than ERL, mostly in the reducible fraction, which presented concentrations between 0.60 and 2.47 μg/g. The higher concentration of Cd among the bioavailable fractions was registered in the oxidizable fraction of P1, 33.01 μg/g. Compared to Cd results obtained through BCR by Cordeiro et al. (2015) in Guanabara Bay (0.005–80.3 μg/g), the results of the present study were much lower. The Risk Assessment Code suggested a medium risk of bioavailability for Cd in most of the stations (Fig. 8).
Based on the bioavailable fractions (F1–F3), the decreasing order of mobility of the studied elements in Niterói harbor bottom sediments is Zn > Cu > Pb > Cr > Ni > Cd.
Principal component analysis (PCA) was made using the sum of the four fractions for each metal and revealed a total variance of 54.84% for two factors (Fig. 9). The first factor corresponded to 37.96% suggesting that TOC was the most significant component influencing the variance (Table 4) and showing direct correlation with Zn, Cu, Ni, and OP. Direct correlation between Zn and Cu suggests a common origin, which is probably anti-fouling paints from ships. The second axis showed clay as the most significant component, showing its direct correlation with Cr and Pb. Station P3 appeared detached from the others (Fig. 9), probably reflecting the highest content of TOC (7.31%), which may be related to the proximity with the mouth of the Fonseca River in the study area. Stations P7, P9, and P10 were the ones with the lowest concentrations of clay, whereas P5 and P13 were the ones with the lowest content of carbonates (Fig. 9). The last group including most of the inner stations (P1, P2, P4, P6), and also P8, presented very similar contents of silt and very high concentrations of organic phosphorus (except P1).
Conclusions
The evaluation of bottom sediments from Niterói harbor revealed a poor quality due to anthropogenic impact, not only through the harbor operations, but also to the presence of shipyards, in the surroundings of the study area, and domestic inputs that arrive through the Fonseca River. Restricted circulation leads to the accumulation of fine sediments that act as sinks for all kinds of pollutants that arrive at the study area. The predominance of organic phosphorus over the inorganic form of the nutrient revealed the massive input of organic matter, that along with restricted circulation, limits the mineralization processes, also reflected by the elevated concentrations of total organic carbon. As for metal contamination, most trace metal concentrations are bonded to the bioavailable fractions that, under certain physicochemical changes, can release the soluble form of the metals. Zn posed the highest threat of bioavailability with RAC values above 30% at almost every station. The other elements presented mostly medium risk regarding the more immediate bioavailability through the exchangeable fraction. The decreasing order of mobility based on the bioavailable fractions was Zn > Cu > Pb > Cr > Ni > Cd. Results corroborated the results of bottom sediment quality of Guanabara Bay reported in previous studies, suggesting an ecological risk to biota and its associated economic activities such as fishing and mariculture.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abreu IM, Cordeiro RC, Soares-Gomes A, Abessa DMS, Maranho LA, Santelli RE (2016) Ecological risk evaluation of sediment metals in a tropical Euthrophic Bay, Guanabara Bay, Southeast Atlantic. Mar Pollut Bull 109:435–445. https://doi.org/10.1016/j.marpolbul.2016.05.030
Aguiar VM d C, Neto JAB, Rangel CM (2011) Eutrophication and hypoxia in four streams discharging in Guanabara Bay, RJ, Brazil, a case study. Mar Pollut Bull 62:1915–1919. https://doi.org/10.1016/j.marpolbul.2011.04.035
Aguiar VMC, Abuchacra PFF, Baptista Neto JA (2013) Biogeochemistry of Jurujuba sound concerning phosphorus dynamics, Rio de Janeiro, Brazil. J Coast Res 65:1–6. https://doi.org/10.2112/SI65-001.1
Aguiar VM d C, Lima MN, Abuchacra RC et al (2016) Ecotoxicology and environmental safety ecological risks of trace metals in Guanabara Bay , Rio de Janeiro , Brazil: an index analysis approach. Ecotoxicol Environ Saf 133:306–315. https://doi.org/10.1016/j.ecoenv.2016.07.012
Aguiar VMC, Abuchacra PFF, Neto JAB, de Oliveira AS (2018) Environmental assessment concerning trace metals and ecological risks at Guanabara Bay, RJ. Brazil. Environ Monit Assess 190:448. https://doi.org/10.1007/s10661-018-6833-x
Aguiar VM d C, Neto JAB, Quaresma V d S et al (2020) Bioavailability and ecological risks of trace metals in bottom sediments from Doce river continental shelf before and after the biggest environmental disaster in Brazil: the collapse of the Fundão dam. J Environ Manage 272:111086. https://doi.org/10.1016/j.jenvman.2020.111086
Alexander TJ, Vonlanthen P, Seehausen O (2017) Does eutrophication-driven evolution change aquatic ecosystems? Philos Trans R Soc B Biol Sci 372:20160041. https://doi.org/10.1098/rstb.2016.0041
Anderson LD, Delaney ML, Faul KL (2001) Carbon to phosphorus ratios in sediments: Implications for nutrient cycling. Global Biogeochem Cycles 15:65–79. https://doi.org/10.1029/2000GB001270
Aspila KI, Agemain H, Chau A (1976) A semi-automated method for the determination of inorganic, organic and total phosphorus in sediments. Analyst 101:187–197
Bader RG (1955) Carbon and nitrogen relations in surface and subsurface marine sediments. Geochim Cosmochim Acta 7:205–211
Baptista Neto JA, Crapez M, McAlister JJ, Vilela CG (2005) Concentration and bioavailability of heavy metals in sediments from Niterói Harbour (Guanabara Bay/S.E. Brazil). J Coast Res 21:811–817. https://doi.org/10.2112/012-NIS.1
Baptista Neto JA, Gingele FX, Leipe T, Brehme I (2006) Spatial distribution of heavy metals in surficial sediments from Guanabara Bay: Rio de Janeiro, Brazil. Environ Geol 49:1051–1063. https://doi.org/10.1007/s00254-005-0149-1
Birch GF (2017) Determination of sediment metal background concentrations and enrichment in marine environments – A critical review. Sci Total Environ 580:813–831. https://doi.org/10.1016/j.scitotenv.2016.12.028
Borges AC, Sanders CJ, Santos HLR, Araripe DR, Machado W, Patchineelam SR (2009) Eutrophication history of Guanabara Bay (SE Brazil) recorded by phosphorus flux to sediments from a degraded mangrove area. Mar Pollut Bull 58:1750–1754. https://doi.org/10.1016/j.marpolbul.2009.07.025
Callender E, Rice KC (2000) The urban environmental gradient: anthropogenic influences on the spatial and temporal distributions of lead and zinc in sediments. Environ Sci Technol 34:232–238. https://doi.org/10.1021/es990380s
Canuto FAB, Garcia CAB, Alves JPH, Passos EA (2013) Mobility and ecological risk assessment of trace metals in polluted estuarine sediments using a sequential extraction scheme. Environ Monit Assess 185:6173–6185. https://doi.org/10.1007/s10661-012-3015-0
Caraballo MA, Serna A, Macías F, Pérez-López R, Ruiz-Cánovas C, Richter P, Becerra-Herrera M (2018) Uncertainty in the measurement of toxic metals mobility in mining/mineral wastes by standardized BCR®SEP. J Hazard Mater 360:587–593. https://doi.org/10.1016/j.jhazmat.2018.08.046
Carreira RS, De Wagener ALR (1998) Speciation of sewage derived phosphorus in coastal sediments from Rio de Janeiro, Brazil. Mar Pollut Bull 36:818–827. https://doi.org/10.1016/S0025-326X(98)00062-9
Carreira RS, Wagener ALR, Readman JW, Fileman TW, Macko SA, Veiga Á (2002) Changes in the sedimentary organic carbon pool of a fertilized tropical estuary, Guanabara Bay, Brazil: an elemental, isotopic and molecular marker approach. Mar Chem 79:207–227. https://doi.org/10.1016/S0304-4203(02)00065-8
Carreira RS, Wagener ALR, Readman JW (2004) Sterols as markers of sewage contamination in a tropical urban estuary (Guanabara Bay, Brazil): space-time variations. Estuar Coast Shelf Sci 60:587–598. https://doi.org/10.1016/j.ecss.2004.02.014
Chen C-W, Ju Y-R, Chen C-F, Dong C-D (2016) Evaluation of organic pollution and eutrophication status of Kaohsiung Harbor, Taiwan. Int Biodeterior Biodegradation 113:318–324. https://doi.org/10.1016/j.ibiod.2016.03.024
Cordeiro LGSM, Carreira RS, Wagener ALR (2008) Geochemistry of fecal sterols in a contaminated estuary in southeastern Brazil. Org Geochem 39:1097–1103. https://doi.org/10.1016/j.orggeochem.2008.02.022
Cordeiro RC, Machado W, Santelli RE, Figueiredo AG Jr, Seoane JCS, Oliveira EP, Freire AS, Bidone ED, Monteiro FF, Silva FT, Meniconi MFG (2015) Geochemical fractionation of metals and semimetals in surface sediments from tropical impacted estuary (Guanabara Bay, Brazil). Environ Earth Sci 74:1363–1378. https://doi.org/10.1007/s12665-015-4127-y
Cukrov N, Frančišković-Bilinski S, Hlača B, Barišić D (2011) A recent history of metal accumulation in the sediments of Rijeka harbor, Adriatic Sea, Croatia. Mar Pollut Bull 62:154–167. https://doi.org/10.1016/j.marpolbul.2010.08.020
Dan SF, Lan W, Yang B, Han L, Xu C, Lu D, Kang Z, Huang H, Ning Z (2020a) Bulk sedimentary phosphorus in relation to organic carbon, sediment textural properties and hydrodynamics in the northern Beibu Gulf, South China Sea. Mar Pollut Bull 155:111176. https://doi.org/10.1016/j.marpolbul.2020.111176
Dan SF, Liu S-M, Yang B (2020b) Geochemical fractionation, potential bioavailability and ecological risk of phosphorus in surface sediments of the Cross River estuary system and adjacent shelf, South East Nigeria (West Africa). J Mar Syst 201:103244. https://doi.org/10.1016/j.jmarsys.2019.103244
Diamond RL (2012) Characterizing dissolved organic matter and Cu2 + , Zn2 + , Ni2 + , and Pb2 + binding in salt water and implications for toxicity. Wilfrid Laurier University
Díaz-de-Alba M, Galindo-Riãno MD, Casanueva-Marenco MJ et al (2011) Assessment of the metal pollution, potential toxicity and speciation of sediment from Algeciras Bay ( South of Spain) using chemometric tools. J Hazard Mater 190:177–187
Dijkstra N, Slomp CP, Behrends T (2016) Vivianite is a key sink for phosphorus in sediments of the Landsort Deep, an intermittently anoxic deep basin in the Baltic Sea. Chem Geol 438:58–72. https://doi.org/10.1016/j.chemgeo.2016.05.025
Dong C-D, Chen C-F, Chen C-W (2012) Contamination of zinc in sediments at river mouths and channel in northern Kaohsiung Harbor, Taiwan. Int J Environ Sci Dev 3:517–521. https://doi.org/10.7763/IJESD.2012.V3.278
Durán I, Sánchez-Marín P, Beiras R (2012) Dependence of Cu, Pb and Zn remobilization on physicochemical properties of marine sediments. Mar Environ Res 77:43–49. https://doi.org/10.1016/j.marenvres.2012.02.001
Edlund G, Carman R (2001) Distribution and diagenesis of organic and inorganic phosphorus in sediments of the Baltic proper. Chemosphere 45:1053–1061. https://doi.org/10.1016/S0045-6535(01)00155-2
Eggleton J, Thomas KV (2004) A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environ Int 30:973–980. https://doi.org/10.1016/j.envint.2004.03.001
Fathollahzadeh H, Kaczala F, Bhatnagar A, Hogland W (2014) Speciation of metals in contaminated sediments from Oskarshamn Harbor, Oskarshamn, Sweden. Environ Sci Pollut Res 21:2455–2464. https://doi.org/10.1007/s11356-013-2173-0
Filgueiras AV, Lavilla I, Bendicho C (2002) Chemical sequential extraction for metal partitioning in environmental solid samples. J Environ Monit 4:832–857
Fries AS, Coimbra JP, Nemazie DA, Summers RM, Azevedo JPS, Filoso S, Newton M, Gelli G, de Oliveira RCN, Pessoa MAR, Dennison WC (2019) Guanabara Bay ecosystem health report card: science, management, and governance implications. Reg Stud Mar Sci 25:100474. https://doi.org/10.1016/j.rsma.2018.100474
Hamdoun H, Van-Veen E, Basset B et al (2015) Characterization of harbor sediments from the English Channel: assessment of heavy metal enrichment, biological effect and mobility. Mar Pollut Bull 90:273–280. https://doi.org/10.1016/j.marpolbul.2014.10.030
Jilbert T, Slomp CP, Gustafsson BG, Boer W (2011) Beyond the Fe-P-redox connection: preferential regeneration of phosphorus from organic matter as a key control on Baltic Sea nutrient cycles. Biogeosciences 8:1699–1720. https://doi.org/10.5194/bg-8-1699-2011
Kalnejais LH, Martin WR, Bothner MH (2010) The release of dissolved nutrients and metals from coastal sediments due to resuspension. Mar Chem 121:224–235. https://doi.org/10.1016/j.marchem.2010.05.002
Karydis M, Kitsiou D (2012) Eutrophication and environmental policy in the Mediterranean Sea: a review. Environ Monit Assess 184:4931–4984. https://doi.org/10.1007/s10661-011-2313-2
Kjerfve B, Ribeiro CHA, Dias GTM, Filippo AM, da Silva Quaresma V (1997) Oceanographic characteristics of an impacted coastal bay: Baía de Guanabara, Rio de Janeiro, Brazil. Cont Shelf Res 17:1609–1643. https://doi.org/10.1007/s11023-007-9060-8.COPYRIGHT
Larssouner C, Bouysse P, Aufret JP (1982) The superficial sediments of the English channel and its western approach. Sedimentology 29:851–864
Libes SM (2009) Introduction to marine biogeochemistry, 2nd edn. Elsevier
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31
Masoud MS, Said OT, Zokm GE, Shreadah MA (2010) Speciation of Fe, Mn and Zn in surficial sediments from the Egyptian Red Sea Coasts. Chem Speciat Bioavailab 22:257–269
Mudroch A, Azcue JM (1995) Manual of aquatic sediment sampling. Lewis Publishers
Nemati K, Bakar NKA, Abas MR, Sobhanzadeh E (2011) Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia. J Hazard Mater 192:402–410. https://doi.org/10.1016/j.jhazmat.2011.05.039
Neto JAB, Gingele FX, Leipe T, Brehme I (2006) Spatial distribution of heavy metals in surficial sediments from Guanabara Bay: Rio de Janeiro, Brazil. Environ Geol 49:1051–1063. https://doi.org/10.1007/s00254-005-0149-1
Passos E d A, Alves JC, dos Santos IS et al (2010) Assessment of trace metals contamination in estuarine sediments using a sequential extraction technique and principal component analysis. Microchem J 96:50–57. https://doi.org/10.1016/j.microc.2010.01.018
Peyman N, Tavakoly Sany SB, Tajfard M, Hashim R, Rezayi M, Karlen DJ (2017) The status and characteristics of eutrophication in tropical coastal water. Environ Sci Process Impacts 19:1086–1103. https://doi.org/10.1039/C7EM00200A
Qian W, Gan J, Liu J, He B, Lu Z, Guo X, Wang D, Guo L, Huang T, Dai M (2018) Current status of emerging hypoxia in a eutrophic estuary: the lower reach of the Pearl River Estuary, China. Estuar Coast Shelf Sci 205:58–67. https://doi.org/10.1016/j.ecss.2018.03.004
Rauret G, López-Sánchez JF, Sahuquillo A, Rubio R, Davidson C, Ure A, Quevauviller P (1999) Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J Environ Monit 1:57–61. https://doi.org/10.1039/a807854h
Redfield AC, Ketchum BH, Richards FA (1963) The influence of organisms on the composition of seawater. In the Sea. New York, In, pp 26–77
Riba I, Delvalls TA, Forja JM (2002) Evaluating the heavy metal contamination in sediments from the Guadalquivir estuary after the Aznalcóllar mining spill (SW Spain): a multivariate analysis approach. Environ Monit Assess 77:191–207
Saleem M, Iqbal J, Shah MH (2015) Geochemical speciation, anthropogenic contamination, risk assessment and source identification of selected metals in freshwater sediments - a case study from Mangla Lake, Pakistan. Environ Nanotechnology, Monit Manag 4:27–36. https://doi.org/10.1016/j.enmm.2015.02.002
Salem DMSA, Khaled A, El Nemr A, El-Sikaily A (2014) Dalia_et_al_2004.pdf. Egypt J Aquat Res 40:349–362
Soares-gomes A, Gama BAP, Neto JAB et al (2016) An environmental overview of Guanabara Bay , Rio de Janeiro. Reg Stud Mar Sci 8:319–330. https://doi.org/10.1016/j.rsma.2016.01.009
Suguio K (1073) Introdução à Sedimentologia, 1st edn. Edgard Blucher/EDUSP, São Paulo
Sundararajan S, Khadanga MK, Kumar JPPJ, Raghumaran S, Vijaya R, Jena BK (2017) Ecological risk assessment of trace metal accumulation in sediments of Veraval Harbor, Gujarat, Arabian Sea. Mar Pollut Bull 114:592–601. https://doi.org/10.1016/j.marpolbul.2016.09.016
Sungur A, Soylak M, Yilmaz S, Özcan H (2014) Determination of heavy metals in sediments of the Ergene River by BCR sequential extraction method. Environ Earth Sci 72:3293–3305. https://doi.org/10.1007/s12665-014-3233-6
The National Oceanic and Atmospheric Administration (NOAA) (2012). Available in: <http://response.restoration.noaa.gov/environmental-restoration/environmentalassessment-tools/squirt-cards.html
Ure AM, Quevaullier P, Muntau H, Griepink B (1993) Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities. Int J Enviromental Anal Chem 51:135–151. https://doi.org/10.1080/03067319308027619
USEPA (2007). United States Environmental Protection Agency – USEPA Microwave assisted acid digestion of sediments sludge, soils, and oils. EPA 3051a. 30p. https://www.epa.gov/sites/production/files/2015-12/documents/3051a.pdf
Vilela CG, Sanjinés AES, Jr ROG et al (2003) Search for bioindicators of pollution in the Guanabara Bay integrations of ecologic patterns. Anuário do Inst Geociências 26:25–35
Vilela CG, Batista DS, Baptista-Neto JA et al (2004) Benthic foraminifera distribution in high polluted sediments from Niterói Harbor (Guanabara Bay), Rio de Janeiro, Brazil. An Acad Bras Cienc 76:161–171. https://doi.org/10.1590/S0001-37652004000100014
Warnken J, Dunn RJK, Teasdale PR (2004) Investigation of recreational boats as a source of copper at anchorage sites using time-integrated diffusive gradients in thin film and sediment measurements. Mar Pollut Bull 49:833–843. https://doi.org/10.1016/j.marpolbul.2004.06.012
Wurtsbaugh WA, Paerl HW, Dodds WK (2019) Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. Wiley Interdiscip Rev Water 6:1–27. https://doi.org/10.1002/wat2.1373
Zhou Y, Wang L, Zhou Y, Mao X (2020) Eutrophication control strategies for highly anthropogenic influenced coastal waters. Sci Total Environ 705:135760. https://doi.org/10.1016/j.scitotenv.2019.135760
Funding
The authors received financial support from Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Contributions
Valquíria Maria de Carvalho Aguiar: formal analysis, writing, review, and editing.
José Antônio Baptista Neto: Data acquisition, writing, review, and editing.
Estefan Monteiro da Fonseca: Writing, review, and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Xianliang Yi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Carvalho Aguiar, V.M., Neto, J.A.B. & da Fonseca, E.M. Assessment of bottom sediment quality in Niterói harbor (Brazil, South America) through ecological indexes concerning nutrients and trace metals. Environ Sci Pollut Res 28, 62292–62305 (2021). https://doi.org/10.1007/s11356-021-15173-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15173-x