Abstract
Bottom sediments in coastal regions have been considered the ultimate sink for a number of contaminants, e.g., toxic metals. In this current study, speciation of metals in contaminated sediments of Oskarshamn harbor in the southeast of Sweden was performed in order to evaluate metal contents and their potential mobility and bioavailability. Sediment speciation was carried out by the sequential extraction BCR procedure for As, Cd, Cr, Cu, Hg, Ni, Pb, and Zn and the exchangeable (F1), reducible (F2), oxidizable (F3), and residual (R) fractions were determined. The results have shown that Zn and Cd were highly associated with the exchangeable fraction (F1) with 42–58 % and 43–46 %, respectively, of their total concentrations in the mobile phase. The assessment of sediment contamination on the basis of quality guidelines established by the Swedish Environmental Protection Agency (SEPA) and the Italian Ministry of Environment (Venice protocol for dredged sediments) has shown that sediments from Oskarshamn harbor are highly contaminated with toxic metals, especially Cu, Cd, Pb, Hg, As, and Zn posing potential ecological risks. Therefore, it is of crucial importance the implementation of adequate strategies to tackle contaminated sediments in coastal regions all over the world.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental and ecological problems due to the presence of different contaminants in terrestrial and aquatic ecosystems have been widely reported (Gao and Li 2012; Ho et al. 2012; Iannelli et al. 2012). Nowadays, special attention has been given to coastal areas where dense population and industrial activities have been causing severe environmental and ecological consequences. The pressure posed by human activities in coastal regions includes the discharge of urban/industrial stormwater runoff, domestic/industrial wastewater, diffuse pollution from agricultural fields, port/harbor-related activities like shipping, painting of boat/ship surfaces, and others. The main constraints in coastal regions are related to the extensive contamination of bottom sediments that are amongst the most exposed biotopes to anthropogenic activities (Iannelli et al. 2012). Due to extensive contamination, sediments have been considered as diffuse sources of different constituents (Baudo et al. 1990) and can be classified as potentially hazardous materials. Sediment contaminants are not necessarily immobilized in the solid-phase and remobilization to the water column can occur either due to changes in environmental conditions (e.g., pH, redox potential, ionic strength, salinity, etc.) or anthropogenic activities such as dredging, ship/boat propulsion engines, etc.
The necessity of tackling the impacts caused by contaminated sediments has been evident and a number of dredging/remediation projects have been investigated (De Jonge et al. 2012; Yin et al. 2010). In the USA, approximately 10 % of the sediments in underlying waters are contaminated, and an amount of 300 million m3 of sediments are dredged every year, among them 3–12 million m3 being highly contaminated (Mulligan et al. 2001). In Europe, approximately 100–200 million m3 of contaminated sediments are dredged yearly due to different purposes (Iannelli et al. 2012). In Sweden, 80,000 sites are currently contaminated and the remediation of 30 high-priority areas during the year of 2011 had approximate costs of SEK 635 million (ca. USD 100 million; Swedish EPA 2011). Furthermore, besides the 30 previously mentioned high-priority sites, another 16 sites were highly prioritized as remediation targets in Sweden, with the highest priority (risk class 1) given to Oskarshamn harbor situated in the Southeastern part of Sweden, due to the high contamination of bottom sediments (Kalmar Municipality 2010). Oskarshamn is located in a forested area and has been used for the exportation of wood-based materials and also for the transportation of tourists and local inhabitants to Gotland, the largest island of Sweden. The harbor basin area has approximately 1.5 km2 and contaminants have been discharged to the harbor since the mid 1850s due to industrial activities. Nowadays considerable amounts of toxic metals are deposited in the bottom sediments: 3 tons of Cd, 250 tons of Cu, 28 tons of As, 570 tons of Zn, 20 tons of Ni, and 160 tons of Pb, and organic pollutants such as dioxin, PCB, and organotin compounds (Kalmar Municipality 2010). The annual leakage from the harbor basin to the Baltic Sea is estimated as approximately 700 kg Cu, 350 kg As, 250 kg Pb, and 20 kg Cd (Kalmar Municipality 2010).
The environmental risks of heavy metals in sediments have been raising significant concern. Mobility and toxicity of metals associated with sediments are generally affected by metal speciation and sediment composition (Lin et al. 2003) and significant impacts to marine ecosystems have been reported (Snodgrass et al. 2008; Besser et al. 2009).
Although a number of dredging and remediation actions are focused on metals, successful projects rely on a good understanding of the behavior of contaminants when bottom sediments are dredged, brought to the surface and exposed to different environmental conditions. Despite the use of total metal concentrations as an indicator for environmental assessment, they cannot be used to assess bioavailability and the ecological/human health risks (Zhong et al. 2011). According to Ho et al. (2012), the risks posed by hazardous substances in dredged sediments are determined by their “mobile” and “available” concentrations.
Therefore, the objectives of this study were (a) to assess the speciation of toxic metals in dredged sediments of Oskarshamn harbor through sequential fractionation and (b) to carry out a preliminary assessment of the potential ecological risks. The main focus is to obtain initial knowledge to establish adequate strategies to handle contaminated dredged sediments in this harbor during transport, treatment/remediation, and final disposal/destination. Sequential fractionation of toxic metals and related bioavailability in harbor/port areas has been rarely studied in Sweden even though it can be a useful tool to establish adequate strategies for their final disposal. The results from this study will provide a better understanding of the environmental risks of metals in the sediments of the studied harbor.
Materials and methods
Study area
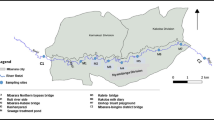
The studied harbor is located in Oskarshamn, southeastern part of Sweden at approximately 410 km south of Stockholm (Fig. 1). The harbor drainage basin has approximately 1.2 km2 and the region has a rapid industrial growth. Industries such as Ni–Fe/Ni–Cd batteries factory, copper board production, heavy machinery, shipyards, candle manufacturing, press-steel industry, hardwood/timber factory, and other anthropogenic activities have been largely contributing to the present contamination of approximately 700,000 m3 of bottom sediments. The volume of goods passing through Oskarshamn harbor is approximately 1,000,000 tons/year and approximately 400,000 passengers per year are transported to and from Gotland, the biggest island in Sweden (Kalmar Municipality 2010). Severe ecological threats to the local ecosystems have been caused by the high contamination of metals raising concerns over the last years.
Sampling procedures
Bottom sediments with depth ranging between 0 and 17 cm (0 cm = bottom surface) were randomly collected with a Van Veen grabber (total volume = 23 L) from two different sampling points (O1 and O2) in May 2012 (Fig. 1, Table 1). The choice of sampling points was done due to the proximity to the copper factory and the short distance to the Baltic Sea (located at the mouth of the basin) as it can be observed in Fig. 1. Samples were homogenized; large/coarse fractions such as gravel and plant residues were removed, and then stored in polyethylene tubes previously rinsed with diluted nitric acid (10 % v/v) and finally covered by aluminum foil paper. Transportation of samples to the laboratory was done in iceboxes followed by storage in 4 °C in the dark for further analysis.
Samples preparation
Samples were air-dried in the laboratory in a fume cupboard at room temperature (approx. 23 ± 2 °C), powdered in mortar and pestle and sieved in a 1-mm stainless-steel mesh.
Sequential extraction procedure (SEP)
The sequential extraction was carried out according to the BCR three-step procedure (Nemati et al. 2011; Rauret et al. 1999). The method determines four well defined geochemical fractions of metals in sediments: acid-soluble/exchangeable fraction (F1), reducible fraction (F2), oxidizable fraction (F3), and residual fraction (F4). All reagents used to perform the extraction were of analytical grade. Prior to the extraction procedures, all tubes and glassware were soaked in diluted nitric acid (10 % v/v) for 8 h and rinsed with ultra pure water produced by a Milli-Q™ apparatus (Millipore, 18.2 MΩ/cm resistivity). A detailed description of fractionation procedures is given as follows:
Step 1 (acid extractable/exchange fraction—bound to carbonates)
The first fraction of metals was extracted by accurately weighing 0.5 g of dry sediments, placing them in polyethylene centrifuge tubes followed by the addition of 20 mL of 0.11 M acetic acid (100 %, Scharlau Chemie S.A, Barcelona, Spain) and mechanically shaken (270 rpm) on an end-over-end shaker (Rotamix RM1) for 16 h at room temperature (approx. 23 ± 2 °C). The extracts were then separated from the residue by centrifugation for 20 min at 3,000 rpm, rinsed with distilled water, hand shaken, decanted, and finally, stored in acid-rinsed polypropylene tubes for further analysis. To continue in Step 2, residual sediments were washed with 20 mL distilled water, shaken for 15 min (Rotamix RM1), and centrifuged at 3,000 rpm. In a sequence, decantation of supernatant was carried out to eliminate any remaining particles before the next extraction step.
Step 2 (reducible fraction—bound to Fe/Mn oxides)
After pH adjustment to 1.5 with 2 mol/L nitric acid (65 %, Scharlau Chemie S.A, Barcelona, Spain), 20 mL of fresh hydroxyl ammonium chloride 0.5 M (Scharlau Chemie S.A, Barcelona, Spain) was added to the residual sediments of Step 1 and shaken (270 rpm) for 16 h at room temperature. The extraction was performed as previously described in Step 1.
Step 3 (oxidizable fraction—bound to organic matters and sulfides)
Initially, 5 mL of hydrogen peroxide 8.8 M (30 %, Scharlau Chemie S.A, Barcelona, Spain) adjusted to pH 2–3 was carefully added to the remaining sediments of Step 2 in a 50-mL centrifuge tube followed by a two-step digestion: first digestion at room temperature (approx. 23 ± 2 ° C) for 1 h and a second digestion for additional 1 h at 85 ± 2 °C in a water bath. Following the digestion step, a second addition of 5 mL pH-adjusted hydrogen peroxide was carried out; the centrifuge tube was then covered and heated at 85 ± 2 °C until it reached an approximate final volume of 2–3 mL. Samples were then removed from the water bath, cooled down to approximately 23 ± 2 ° C followed by addition of 25 mL of 1.0 M ammonium acetate (Fisher Scientific, Loughborough, Leicestershire, UK) and shake for 16 h. The extraction was performed as previously described in Step 1.
Step 4 (residual fraction—strongly associated to the crystalline structures of the minerals)
The residue from the last step was digested with a mixture of aqua regia (nitric acid: hydrochloric acid in 1:3) and hydrofluoric acid (40 %, Scharlau Chemie S.A, Barcelona, Spain) consisting of 5 mL nitric acid + 15 mL hydrochloric acid + 2 mL hydrofluoric acid. Hydrochloric acid was of analytical grade (36 % Fisher Scientific, Loughborough, Leicestershire, UK).
Total digestion and recovery percentage
Total metals contents were extracted through a total digestion procedure bringing possibilities to evaluate how accurate and consistent was the method for fractionation. For total digestion, 0.5 g of dry sediments was placed in 50 mL polypropylene tubes and filled with 10 mL mixture of aqua regia and hydrofluoric acid as previously mentioned and finally heated up to 180 ± 5 °C in a sand bath heater for 20 min (Nemati et al. 2011; Rauret et al. 1999). Following the digestion, the supernatant was taken and filtered through a Whatman filter 0.45-μm pore size (Ø110 mm, Munktell) into 50 mL polypropylene tubes. The total metal concentration was compared to the sum of the four individual fractions (F1, F2, F3, and R) and the accuracy of the experiment was evaluated through the recovery rates according to the Eq. 1 below:
Analytical methods
Prior to analysis, all tubes were stored in the refrigerator at 4 °C. Metal contents were determined by the USEPA method (modified) 200.7 (ICP-AES) and 200.8 (ICP-SFMS). The estimation of organic matter contents was based on loss on ignition (LOI, % dry weight) with different weights, temperature, and time of ignition (Luczak et al. 1997).
Quality control of the data
Quality control of the sequential extraction procedure was assured with standard samples of sediment certified reference material (SRM-CRM 701) that was purchased from the Institute for Reference Materials and Measurements (IRMM) and analyzed under identical conditions and procedures as contaminated sediments sampled in the Oskarshamn harbor (Table 2).
Results and discussion
Sediment characteristics
The total concentrations of metals (μg/g dry material) obtained through the total digestion and the sum of all fractions (F1+F2+F3+R) are presented in Table 3. The results have shown highest concentrations of Cu, Pb, and Zn in both sampling points, O1 and O2, suggesting this might be related to anthropogenic activities and the presence of different industries located in the drainage basin of the studied harbor. However, despite the assumption that main metal sources are related to land-based activities, atmospheric deposition cannot be ruled out due to an increased deposition of Cd, Cu, Pb, and Zn in Sweden in the last 150 years (Brack and Stevens 2001).
It is important to highlight that the concentrations of metals observed in this study were much higher in comparison to those found in other investigations carried out in Europe such as the study in a tourist harbor located in Sicily, Mediterranean sea (Petrucci et al. 2011) and in the Guardiana Estuary, Spain (Delgado et al. 2011). Furthermore, estuarine sediments in Hailing Bay, an important mariculture zone along the southern coast of China had also much lower concentrations (Zhang et al. 2012) in comparison to the studied area confirming the reasonable selection of this area as high priority for remediation by the Swedish authorities.
As observed, higher concentrations of metals were detected in the sediments from sampling site O2 in comparison to O1 with the exception of As and Pb (Table 3) suggesting this might have been caused by either contamination from surrounding areas or re-suspension of sediments due to the presence of boats/ships combined with currents and tide movements. The external conditions of the Baltic Sea have high influence over the sampling point O2 and it has been suggested by Chen et al. (2010) that the contact with waters with higher salinity can cause re-deposition of previously mobile metals. Tides and currents might have been responsible for the higher concentrations of Cu observed in O2 in comparison to O1, even though the sampling site O1 was located near the wastewater discharge from the copper factory (Fig. 1).
The results obtained through the losses on ignition (LOI) have shown 9.7 % and 11 % of organic matter in sediments in O1 and O2, respectively.
Based on the % recovery (Table 3), satisfactory accuracy for most of the metals was observed, with recovery rates ranging between 80 % and 144 %, suggesting the method to be consistent and reproducible. The relatively low recovery rates observed for Hg could be explained by reagents selectivity (Gomez Ariza et al. 2000) and volatility during several heating and oxidation steps, more precisely in the third step.
Distribution of metals in sediments
Despite the use of total metal concentrations as an indicator of potential ecological risks, an assessment of the different geochemical forms of metals in sediments brings a better understanding of mobility and bioavailability (Gao and Chen 2012). Therefore, the distribution of metals in four different fractions in contaminated sediments from Oskarshamn harbor was studied: acid soluble/exchangeable fraction (F1), reducible (F2), oxidizable (F3), and residuals (R).
The first step of sequential extraction obtains the extractable/exchangeable fraction (F1) of metals that are weakly associated to carbonates. Metals in exchangeable fraction can be released by the action of cations such as K, Ca, Mg, or (NH4) that displace metals weakly associated with organic and inorganic sites (Beckett 1989). The high mobility, bioavailability, and potential toxicity/bioaccumulation of exchangeable metals in aquatic organisms are of great concern and changes in salinity and pH increase have been reported to increase metal mobility in aquatic ecosystems (Chen et al. 2010). The results of this investigation have shown that Zn and Cd were highly associated with the mobile fraction with 42–58 % and 43–46 %, respectively, of their total concentrations (Fig. 2) raising concerns due to potential ecological constraints mainly in relation to Cd due to its well-known toxic effects to aquatic organisms. On the other hand, low % of association with the extractable fraction (F1) was observed for most of the remaining metals: Ni (15 %) > Cu (7 %) > Hg (6 %) > Pb (4.5 %) > Cr (1.5 %) ≈ As (1.5 %). These results agree with previous studies that have highlighted the potential threats caused by Zn and Cd highly associated with F1 (Delgado et al. 2011; Guevara-Riba et al. 2004; Yang et al. 2012). An investigation carried out by Vasile et al. (2010) with focus on Zn speciation during spring, summer and autumn at 11 different sites has reported up to 73 % of total Zn associated with the (F1), even though there was a slight variability throughout the different seasons. One aspect to be highlighted is that even though low % of association with the mobile fraction was observed well-known toxic metals such as Ni, Hg, Pb, Cr, and As, changes in environmental conditions could modify this scenario and potential ecological risks would still exist.
The second fraction of extracted metals, the reducible fraction (F2), indicates the metals that are usually associated with Fe and Mn oxy/hydroxides. It was observed that Pb, Cu, and Cr were mainly associated with the reducible fraction with an average of 90 %, ≈ 80 %, and 60 % of their total contents respectively, in both sampling sites (O1 and O2; Fig. 2). The metals, Ni and Cd, had also shown considerable affinity to the reducible fraction with 36–47 % and 45–47 % of their total contents respectively (Fig. 2). High % of associations of Pb and Cu to the reducible fraction in contaminated sediments of estuaries, bays, and riverbeds has been reported in the literature (Gao and Li 2012; Ho et al. 2012; Delgado et al. 2011; Yang et al. 2012). According to Diaz-de Alba et al. (2011), high associations of Pb to the reducible phase can be related to industrial effluents, a fact that cannot be ruled out in Oskarshamn harbor. The rates of metals association with the reducible phase were considerably similar when comparing sampling sites O1 and O2. The order of metals association to the reducible fractions at sampling sites O1 and O2 were respectively: Pb (92 %) > Cu (75 %) > Cr (57 %) > As (51 %) > Zn (47 %) ≈ Cd (46 %) and Pb (93 %) > Cu (80 %) > As (66 %) > Cr (57 %) > Cd (47 %) > Zn (36 %) (Fig. 2). The large number of metals associated with the reducible fraction as observed in this current study (Fig. 2) raise concerns regarding their potential mobility in the water phase and consequent bioavailability during dredging/remediation. This once more highlights as previously mentioned that even though low % of associations with F1 were observed for toxic metals, environmental and ecological risks cannot be ruled out since metals in the reducible phase are still labile and might be released to the environment upon decomposition of the oxides under sub-oxic conditions (Delgado et al. 2011) and redox changes (Chen et al. 2010). Approximately 100 μg/g of As was associated with the reducible fraction, posing serious threats that can be caused by dissolution of Fe–Mn oxi/hydroxides in highly acidic or reducing conditions (Reczynski et al. 2004).
As illustrated in the Fig. 3, the current study has shown a dominant part of most metals associated with the reducible phase followed by the residual phase. This was statistically confirmed by the statistical analysis carried out through running a Dunnet’s multiple comparison test which has shown that whereas the average % of association with the reducible phase (F2) in both sampling sites (O1 and O2) was significantly higher (p < 0.05) in comparison to the exchangeable (F1) and oxidizable phases (F3), it was not for the residual phase (p > 0.05; Fig. 3). However, it must be highlighted that whereas considerable variability in metals distribution in both phases—reducible (F2) and residual (R)—was observed; the % of associations with the exchangeable (F1) and oxidizable phases (F3) were not as variable (Fig. 3).
The metals bound to organic matter and sulfides (F3) are extracted during the third step of the sequential fractionation (oxidizable fraction). Under oxidizing conditions, organic matter degradation can lead to a release of metals bound to this fraction. The organic fraction released during the extraction is not bioavailable due to its association with stable humic substances that release small amounts of metals slowly (Filgueiras et al. 2002). The results have shown % of association as low as 2 %, 2 %, 4 %, 11 %, 13 %, and 15 % for Zn, Hg, Cd, Cr, Cu, and Ni, respectively. The low contents of metals associated with F3 can be due to low contents of organic matter of 9.7 % and 11 % in sampling sites O1 and O2, respectively. It is important to highlight that despite the possibilities of sulfides and organic matter oxidation during sampling and consequent redistribution of different fractions, such results are realistic considering the real scenario of dredging in which sediments are taken up to the surface and oxygenation is likely to occur. It can be suggested that the potential risks posed by metals bound to organic matter (associated with F3) must be mainly considered in cases, which dredging activities are planned and sediments are brought to the surface and exposed to oxygen in the surface. However, one aspect to be emphasized in many ports/harbors with metals in the oxidizable fraction is the frequent re-suspension of sediments due to tides and boats/ships activities and consequent exposure to oxic zones, becoming then a potential environmental/ecological and human health risk.
The fourth step of the sequential extraction indicates that fraction of metals that are associated with the alumino-silicate minerals, the so-called residual fraction (R). This fraction of metals is characterized by low mobility and low probabilities of posing adverse effects to the environment. The results have shown average % of associations with the residual fraction in the following decreasing order: Hg (72 %) > As (40 %) > Cr (30 %) > Ni (26 %).
As it can be observed in the Fig. 2, the remaining elements have shown insignificant amounts associated with alumino-silicate minerals.
Assessment of sediment contamination
Swedish environmental protection agency guidelines
A comparative evaluation between metal concentrations observed in this study and the quality guideline for contaminated sediments established by the Swedish Environmental Protection Agency (SEPA) was carried out. The SEPA has established a number of environmental quality criteria for coasts and seas (SEPA 2000) with the main purpose of enabling local and regional authorities to accurately assess the status of the environment and thus to provide proper strategies for environmental planning and management. The total concentration of metals cannot be used to assess mobility, bioavailability, and potential toxicity; therefore, the comparative evaluation was based on the reactive/labile phase (F1+F2+F3). According to the SEPA (2000), the extent of human impact can be calculated by the deviations between observed concentrations in the environment, in this case the Oskarshamn harbor, and previously established reference values representing no anthropogenic impacts. The deviations are calculated as Eq. (2) below:
The classification of deviation is based on five-level scale and whereas class 1 includes conditions with little or no deviation from the reference value, the remaining classes indicate increasing deviation levels (increasing degree of impact) up to class 5 that indicates very significant impact.
The results obtained in this study are shown in Table 4 and as it can be observed, in most cases, concentrations measured in the bottom sediments of studied harbor had either larger or very large deviations in comparison to reference values with the exception of Cr and Ni that had insignificant deviations. On the basis of the results shown in the Table 4 and the Swedish criteria for coast and seas, the deviations observed for Cu and Cd raised the most serious concerns since observed concentrations in the sampling point O2 were 134 and 40 times higher respectively than reference values. However, as previously mentioned, the results have shown that Oskarshamn harbor is seriously contaminated by all metals and urgent strategies to tackle this problem ought to be established.
Contamination factor (Cf)
According to Nemati et al. (2011), the contamination factor of metal elements brings a better understanding of the degree of risks posed by metals by estimating the relative retention time of metals in sediments and the higher the Cf value is, the lower is the retention time and higher is the risk to the environment. The contamination factor (Cf) of studied metals was calculated in this study according to Fernandes (1997). The Cf is obtained by dividing the concentrations of metals in the mobile phase (F1+F2+F3) by the concentrations in residual phase (R) as in the Eq. (3) below:
As observed in the Table 5, with the exception of Hg, all metals had higher Cf in the sampling point O2 and consequently pose higher risks to the environment, raising concerns due to its proximity to the Baltic Sea. Considering both sampling points (O1 and O2) the highest ecological/environmental concerns are related to the high proportion of Cu, Pb, Cd, and Zn in the mobile phase as indicated by their Cf values (Table 5). This result is in agreement with the very large deviations of Cu, Pb, Cd, and Zn concentrations with the background values established by the SEPA, emphasizing the environmental concerns posed by these metals. Highest contamination factors for Cd, Zn, and Pb in comparison to other metals as observed in this study have been previously reported by Nemati et al. (2011).
Venice protocol for dredged sediments
The bottom sediments of Oskarshamn harbor were also classified according to the criteria established by the Italian Ministry of Environment during dredging activities in the Venice Lagoon (Italian Ministry of Environment 1993) as shown in Table 6. The Venice Protocol determines four different disposal options on the basis of the chemical characteristic of contaminated dredged sediments: (a) class A sediments (not polluted) are dredged sediments that can be re-used for lagoon restoration; (b) class B sediments (slightly polluted) can be used to restore islands inside the lagoon as long as strategies to avoid the release of contaminants are implemented; (c) class C sediments (polluted) can be used for raising new islands but must be controlled to reduce their contact with the environment; and (d) class > C related to contaminated sediments with high concentrations that must be properly disposed off and permanently monitored.
The results have shown that studied sediments were in most cases classified as C and > C suggesting that treatment/remediation and adequate final disposal must be considered as soon as dredging takes place in order to avoid environmental, ecological and human health constraints. Another alternative based on the Venice protocol could be the use of Oskarshamn sediments as aggregates for construction materials as long as ecological/environmental and human health risks are carefully assessed. Besides, the temporary storage of these sediments in confined areas after dredging could be an alternative for further use in the expansion of the port/harbor and beach nourishment during high season in Southeastern part of Sweden. According to the Table 6, the metals Cu, Pb, and As, followed by Cd, Zn, and Hg, were those that raised highest concerns based on the Venice Protocol and dredging strategies. The results of comparative analysis to the Venice Protocol correspond well with the assessment carried out in comparison to the Swedish standards for sediments in coasts and seas as previously presented in Table 4 where most of metals in the harbor of Oskarshamn raised concerns with the exception of Cr and Ni.
Conclusions
In the present study, chemical analysis and speciation of As, Cd, Cr, Cu, Hg, Ni, Pb, and Zn in forms of carbonate/exchangeable/extractable (F1), reducible (F2), oxidizable (F3), and residual (R) fractions, followed by total metal digestion in contaminated sediments from the Oskarshamn harbor were investigated. The studied harbor has been negatively impacted by different anthropogenic activities as indicated by high concentrations of metals such as Cu, Pb, Cd, Zn, Hg, and As. Furthermore the combination of high concentrations as observed and high % of association to the non-residual fraction (F1+F2+F3) mainly considering Cu, Zn, Pb, and Cd, raise serious concerns in terms of necessities for adequate strategies during dredging activities. On the basis of the Swedish criteria established for coastal areas and seas, Cu and Cd were those metals, which require more attention, even though most metals had much higher concentrations in comparison to those established by the Swedish Authorities as reference values. Furthermore, based on the Venice Protocol for dredged sediments, the studied sediments were in most cases classified as C and >C suggesting that treatment/remediation and adequate final disposal must be considered as soon as dredging takes place in order to avoid environmental, ecological and human health constraints. Another alternative could be the use of these contaminant sediments as aggregates for the construction industry. Further studies are recommended in order to have a better understanding on the feasibility of recovering different metals and technologies that can be applied in a cost-effective way in real scenario.
References
Baudo R, Giesy JP, Muntau H (1990) Sediments: Chemistry and toxicity of in-place pollutions. CRC Press, Lewis Publishers, Michigan
Beckett PHT (1989) The use of extractants in studies of trace metals in soils, sewage sludges, and sludge-treated soils. In: Stewater BA (ed) Advances in soil science. Springer-Verlag, New York, pp 143–171
Besser J, Brumbaugh W, Allert A, Poulton B, Schmitt C, Ingersoll C (2009) Ecological impacts of lead mining on Ozark streams: toxicity of sediment and pore water. Ecotoxicol Environ Saf 72:516–526
Brack K, Stevens RL (2001) Historical pollution trends in a disturbed, estuarine sedimentary environment, SW Sweden. Environ Geol 40:1017–1029
Chen C, Lu J, Ye M, Wang Y, Lu H (2010) Metal and metalloid contaminant availability in Yundang Lagoon Sediments. Xiamen Bay, China, after 20 years continuous rehabilitation. J Hazard Mater 175:1048–1055
De Jonge M, Belpaire C, Geeraerts C, De Cooman W, Blust R, Bervoets L (2012) Ecological impact assessment of sediment remediation in a metal-contaminated lowland river using translocated zebra mussels and resident macroinvertebrates. Environ Pollut 171:99–108
Delgado J, Barba-Brioso C, Nieto JM, Boski T (2011) Speciation and ecological risk of toxic elements in estuarine sediments affected by multiple anthropogenic contributions (Guadiana saltmarshes, SW Iberian Peninsula): I. Surficial sediments. Sci Total Environ 409:3666–3679
Diaz-de Alba M, Galindo-Riaño MD, Casanueva-Marenco MJ, García-Vargas M, Kosore CM (2011) Assessment of the metal pollution, potential toxicity and speciation of sediment from Algeciras Bay (South of Spain) using chemometric tools. J Hazard Mater 190:177–187
Fernandes HM (1997) Heavy metal distribution in sediments and ecological risk assessment: the role of diagenetic processes in reducing metal toxicity in bottom sediments. Environ Pollut 97:317–325
Filgueiras AV, Lavilla I, Bendicho C (2002) Chemical sequential extraction for metal Partitioning in environmental solid samples. J Environ Monitor 4:823–857
Gao X, Chen C-TA (2012) Heavy metal pollution status in surface sediments of the coastal Bohai Bay. Water Res 46:1901–1911
Gao X, Li P (2012) Concentration and fractionation of trace metals in surface sediments of intertidal Bohai Bay, China. Mar Pollut Bull 64:1529–1536
Gomez Ariza JL, Giraldez I, Sanchez-Rodas D, Morales E (2000) Selectivity assessment of a sequential extraction procedure for metal mobility characterization using model phases. Talanta 52:545–554
Guevara-Riba A, Sahuquillo A, Rubio R, Rauret G (2004) Assessment of metal mobility in dredged harbour sediments from Barcelona (Spain). Sci Total Environ 321:241–255
Ho HH, Swennen R, Cappuyns V, Vassilieva E, Gerven TV, Tran TV (2012) Potential release of selected trace elements (As, Cd, Cu, Mn, Pb and Zn) from sediments in Cam River-mouth (Vietnam) under influence of pH and oxidation. Sci Total Environ 435–436:487–498
Iannelli R, Bianchi V, Macci C, Peruzzi E, Chiellini C, Petroni G, Masciandaro G (2012) Assessment of pollution impact on biological activity and structure of seabed bacterial communities in the Port of Livorno (Italy). Sci Total Environ 426:56–64
Italian Ministry of the Environment (1993) Protocollo d’intesa—Criteri di sicurezza ambientale per gli interventi di escavazione, trasporto e impiego dei fanghi estratti dai canali di Venezia, 8th April 1993 (in Italian)
Kalmar Municipality (2010) Regionalt program för efterbehandling av förorenade områden i Kalmar län 2011–2015 (in Swedish)
Lin JG, Chen SY, Su CR (2003) Assessment of sediment toxicity by metal speciation in different particle-size fractions of river sediment. Water Sci Technol 47:233–241
Luczak C, Janquin M, Kupka A (1997) Simple standard procedure for the routine determination of organic matter in sediments. Hydrobiologia 345:87–94
Mulligan CN, Yong RN, Gibbs BF (2001) An evaluation of technologies for the heavy metal remediation of dredged sediments. J Hazard Mater 85:145–163
Nemati K, Abu Bakar NK, Abas MR, Sobhanzadeh E (2011) Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia. J Hazard Mater 192:402–410
Petrucci E, Montanaro D, Merli C (2011) Sequential extraction analysis provides decision-making tools for the use of contaminated sediments. Chem Ecol 27:107–118
Rauret G, López-Sánchez JF, Sahuquillo A, Rubio R, Davidson C, Ure A, Quevauviller P (1999) Improvement of the BCR three-step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J Environ Monit 1:57–61
Reczynski W, Posmyk G, Nowak K (2004) Dynamics of arsenic containing compounds sorption on sediments. J Soils Sediments 4:95–100
Snodgrass JW, Casey RE, Joseph D, Simon JA (2008) Microcosm investigations of stormwater pond sediment toxicity to embryonic and larval amphibians: variation in sensitivity among species. Environ Pollut 154:291–297
Swedish EPA (2000) Environmental quality criteria—coasts and seas
Swedish EPA (2011) Lägesbeskrivning av efterbehandlingsarbetet i landet år 2011. http://www.naturvardsverket.se/upload/stod-i-miljoarbetet/rattsinformation/yttranden/2012/Lagesbeskr-ebh-2011-regeringen-20120413.pdf/. Accessed 2 Aug 2013 (in Swedish)
Vasile DG, Nicolau M, Vladescu L (2010) Zinc speciation in sediments from a polluted river, as an estimate of its bioaccessibility. Environ Monit Assess 160:71–81
Yang Y, Chen F, Zhang L, Liu J, Wu S, Kang M (2012) Comprehensive assessment of heavy metal contamination in sediment of the Pearl River Estuary and adjacent shelf. Mar Pollut Bull 64:1947–1955
Yin K, Viana P, Zhao X, Rockne K (2010) Characterization, performance modeling, and design of an active capping remediation project in a heavily polluted urban channel. Sci Total Environ 408:3454–3463
Zhang W, Liu X, Cheng H, Zheng EY, Hu Y (2012) Heavy metal pollution in sediments of typical mariculture zone in South China. Mar Pollut Bull 64:712–720
Zhong X-I, Zhou S-I, Zhu Q, Zhao Q (2011) Fraction distribution and bioavailability of soil heavy metals in the Yangtze River Delta—A case study of Kunshan City in Jiangsu Province, China. J Hazard Mater 198:13–21
Acknowledgments
This work was supported by NOVA FoU (Center for University Studies, Research and Development). Special thanks are given to Dr. Roland Engkvist for crucial help during sampling events. Ms Sara Gunnarsson and Jean-Christophe De Bortoli are gratefully acknowledged for their assistance and help in laboratory works.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Céline Guéguen
Rights and permissions
About this article
Cite this article
Fathollahzadeh, H., Kaczala, F., Bhatnagar, A. et al. Speciation of metals in contaminated sediments from Oskarshamn Harbor, Oskarshamn, Sweden. Environ Sci Pollut Res 21, 2455–2464 (2014). https://doi.org/10.1007/s11356-013-2173-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-2173-0