Abstract
Polycyclic aromatic hydrocarbon (PAH) exposure and genetic susceptibility were conductive to genotoxic effects including gene damage, which can increase mutational probability. We aimed to explore the dose-effect associations of PAH exposure with damage of exons of epidermal growth factor receptor (EGFR) and breast cancer susceptibility gene 1 (BRCA1), as well as their associations whether modified by Flap endonuclease 1 (FEN1) genotype. Two hundred eighty-eight coke oven male workers were recruited, and we detected the concentration of 1-hydroxypyrene (1-OH-pyr) as PAH exposure biomarker in urine and examined base modification in exons of EGFR and BRCA1 respectively, and genotyped FEN1 rs174538 polymorphism in plasma. We found that the damage indexes of exon 19 and 21 of EGFR (EGFR-19 and EGFR-21) were both significantly associated with increased urinary 1-OH-pyr (both Ptrend < 0.001). The levels of urinary 1-OH-pyr were both significantly associated with increased EGFR-19 and EGFR-21 in both smokers and nonsmokers (both P < 0.001). Additionally, we observed that the urinary 1-OH-pyr concentrations were linearly associated with both EGFR-19 and EGFR-21 only in rs174538 GA+AA genotype carriers (both P < 0.001). Moreover, FEN1rs rs174538 showed modifying effects on the associations of urinary 1-OH-pyr with EGFR-19 and EGFR-21 (both Pinteraction < 0.05). Our findings revealed the linear dose-effect association between exon damage of EGFR and PAH exposure and highlight differences in genetic contributions to exon damage and have the potential to identify at-risk subpopulations who are susceptible to adverse health effects induced by PAH exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The epidermal growth factor receptor (EGFR) is one kind of the HER/ErbB family of receptor tyrosine kinases (RTKs), which includes HER1 (EGFR/ErbB1), HER2 (neu, ErbB2), HER3 (ErbB3), and HER4 (ErbB4) (da Cunha et al. 2011). It exerts critical functions of regulating epithelial tissue development and homeostasis physiologically but also drives tumorigenesis mostly of lung cancer, breast cancer, and glioblastoma pathologically (Sigismund et al. 2018). The tyrosine kinase (TK) activity of EGFR may be dysregulated by several oncogenic mechanisms, including EGFR gene mutation, increased gene copy number, and EGFR protein overexpression (da Cunha et al. 2011). Specially, epidemiologic researches have shown that the EGFR mutation is closely correlated with the non-small cell lung cancer (NSCLC) in different areas worldwide (Bircan et al. 2014; Cheng et al. 2019). Due to its overexpression and hyperactivation, EGFR has been the rational therapeutic target for human malignancies (da Cunha et al. 2011). According to current research, deletion mutations in exon 19 and point mutations at codon 858 in exon 21 are most prevalent and have been utilized as predictors of therapeutic intervention and sensitivity in lung cancer (Bircan et al. 2014; Krawczyk et al. 2016). DNA damage is associated closely with gene mutation and can increase mutational probability triggering the advent of genetic mutations (Gedik et al. 2002), and the base damage of EGFR may be a potential marker of EGFR mutation (Scalise et al. 2016). Therefore, it is of necessity to investigate the EGFR gene damage.
The involvement of EGFR in the process of the carcinogenesis is a multifactor and multistage process, which is modified not only by environment factors but also by genetic factors such as single nucleotide polymorphisms (SNPs). A published genome-wide association study (GWAS) had comprehensively reported SNPs associated with EGFR mutations based on the 10,780 never-smoking cases (Seow et al. 2017). These findings provided us with EGFR-related genetic variations. Several studies have shown that the gene polymorphisms have great significance on the EGFR mutation, which can be utilized clinically to predict cancer aggressiveness, metastatic potential, and therapeutic responsiveness of lung cancer patients (Huang et al. 2018; Lin et al. 2020). The related research has reported that the rs2910164 G allele in miR-146a decreased inhibition of the expression of EGFR, which induced the proliferation of human keratinocytes (Zhang et al. 2014). With further knowledge of EGFR tyrosine kinase inhibitors (EGFR-TKIs), which are the treatment of choice for advanced-stage NSCLC patients with mutations in EGFR, SNPs were considered to be the reason for the different outcomes varying from person to person (Perez-Ramirez et al. 2019; Winther-Larsen et al. 2019). Specially, flap endonuclease 1 (FEN1) plays a crucial role in both DNA replication and damage repair, and researches have shown that FEN1 may represent a prognostic biomarker and potential therapeutic target for NSCLC treatment (He et al. 2017; Zhang et al. 2018). Several meta-analyses have revealed that the polymorphism of FEN1 may be associated with the cancer susceptibility (Ren et al. 2015; Rezaei et al. 2016; Ying et al. 2015). Nevertheless, the role of FEN1 polymorphism in DNA damage remains unknown.
Polycyclic aromatic hydrocarbons (PAHs), as a main composition of environment tobacco smoke and PM2.5, have been of scientific concern for many years and have attracted public health attention, due to their potential for bioaccumulation and their effects of toxicity, carcinogenesis, and mutagenesis on human health (Armstrong et al. 2004; Samanta et al. 2002). In this context, several studies have revealed that urinary 1-hydroxypyrene (1-OH-Pyr) was used as a suitable indicator to assess exposure to the organic carbon compounds of PAHs from the environment and occupational settings (Jongeneelen 2001; McClean et al. 2012; Sobus et al. 2009). In addition, urinary 1-OH-Pyr was proved to be the most comprehensive carcinogenic biomarker of exposure to PAHs (Yamano et al. 2014). Therefore, the use of 1-OH-Pyr as a biomarker has been widespread due to its convenience and accessibility to evaluate the early genotoxic effects induced by PAH exposure. However, the association analysis of urinary PAH exposure and EGFR DNA damage, and whether its association is modified by gene polymorphism, remain unclear.
Here, we performed a cross-sectional study consisting of 288 male coke oven workers in an attempt to analyze the associations of urinary 1-OH-Pyr, an indicator of assessing PAH exposure, with the damage index of exon 19 and 21 of EGFR gene (EGFR-19 and EGFR-21) and their associations whether modified by FEN1 rs174538 polymorphism were further evaluated.
Materials and methods
Subjects and the inclusion and exclusion criteria
This study included 295 coke oven workers who were all healthy males, aged between 19 and 32.5 years, and worked in the same coking plant in south China. They had been working at different workplaces for at least 3 months. We gained the concentrations of external total PAHs in different workplaces from coke oven plant intermittent monitoring. Briefly, the concentration of external total PAHs (mean ± SD, μg/m3) was 6.48 ± 3.89 in the coal preparation recovery workshop and 32.07 ± 23.61 in the coking oven workshop. Additionally, after acquiring written informed consent from each participant, we used the occupational health questionnaire to collect personal basic information, occupational history, medical history, and lifestyle including working years and smoking history and other data. Those who had smoked < 1 cigarettes per day for less than 1 year were regarded as nonsmokers; otherwise, subjects were viewed as smokers.
At the end (post-shift) of each work shift, we collected 20 ml of urine from each participant in 50-ml polyethylene tubes for biological detection of 1-OH-Pyr and 5 ml peripheral blood was collected into 5-ml disposable ethylenediaminetetraacetic acid (EDTA) anticoagulant tubes for detection of the gene damage index. All biological samples were stored at − 80 °C after collection until laboratory examinations. However, five subjects were excluded because of inadequate plasma sample volume and two subjects with no available urinary samples were excluded from the current analysis. So, the final study subjects in the present study consisted of 288 coke oven workers, including 151 workers from the coal preparation recovery workshop served as low-exposure group and 137 workers from the coking oven workshop served as high-exposure group (the work flow is shown in Fig. 1). The workers who are exposed to known DNA-damaging agents, such as radiotherapy and chemotherapy, in the last 3 months, were excluded. The Ethics Committee of Guangzhou Medical University approved the study (KY01-2019-02-09).
Measurement of urinary 1-OH-Pyr and urinary creatinine
We measured the concentrations of urinary 1-OH-Pyr using gas chromatography-mass spectrometry (GC/MS) following the measurement methods previously reported in detail (Kuang et al. 2013; Yang et al. 2016). The standard materials of 1-OH-Pyr (purity ≥ 98%) and the organic solvents including hexane, and acetonitrile (HPLC-grade purity) were purchased from Sigma-Aldrich and the internal standards [2H9]1-hydroxypyrene (1-OHPyr-d9) were obtained from the Toronto Research Chemicals Inc. (Ontario, Canada). Briefly, we extracted 3.0 ml of urine after thawing room temperature and determined each urinary sample in triplicate, then the mixture including 3.0 ml of urinary sample, 20 μl of 1-OHP-d9 solution, 1 ml of acetate acid buffer (0.5 M, pH 5.0), and 20 μl of β-glucuronidase/sulfatase (Sigma-Aldrich, Munich, Germany) was incubated at 37 °C overnight. In order to saturate the hydrolyzed urinary samples, we added 1.5 mg of MgSO4·7H2O. The urinary samples were extracted twice using 1.5 ml of n-hexane followed by centrifugation at 1500 rpm for 10 min. The organic extracts were treated with nitrogen, and the residue was mixed with 100 μl BSTFA followed by incubation at 90 °C for 45 min. Finally, we extracted 1 μl to inject on the GC/MS system (Agilent, Santa Clara, CA).
We quantified the urinary 1-OH-Pyr according to the retention time, peak area, and mass-to-charge ratio by a linear regression curve obtained from 1-OHPyr-d9 solutions. The limit of detection for the urinary 1-OH-Pyr was 0.1 ng/ml. The levels of urinary creatinine were measured using an automated clinical chemistry analyzer based on Jaffe’s colorimetric method. Considering the inter-individual variations in urinary 1-OH-Pyr metabolites on dilution status, the concentrations of 1-OH-Pyr were normalized to urinary creatinine and expressed as micromoles per millimole of creatine.
Determination of exon damage index of gene EGFR and BRCA1
DNA was extracted using the DNA fluorescence quantitative kit of Kangwei Century company, and the ratio of OD260/OD280 for EGFR-19, EGFR-21, and BRCA1-20 was 1.87, 1.85, and 1.88, respectively. PCR primers were designed to amplify exon 19 and 21 of EGFR gene and exon 20 of BRCA1, and β-actin sequence which was used as the control fragment. The sequence was as follows in detail: β-actin (forward: CGGGAAATCGTGCGTGACAT; reverse: GAAGGAAGGCTGGAAGAGTG); exon 19 of EGFR gene (forward: GTGCATCGCTGGTAACATCCA; reverse: AAAGGTGGGCCTGAGGTTCA); exon 21 of EGFR gene (forward: CCTCACAGCAGGGTCTTCTCTG; reverse: TGGCTGACCTAAAGCCACCTC); and exon 20 of BRCA1 gene (forward: GAGTGGTGGGGTGAGATTTTTGTC; reverse: CCTGATGGGTTGTGTTTGGTTTCT). The PCR was conducted in a 20-μl final volume containing 2×UltraSYBR mixture 10 μl, 10 μM forward primer 0.4 μl, 10 μM reverse primer 0.4 μl, and DNA 1 μl, and sterile water was used to supplement up to 10 μl. The real-time PCR protocols began with one cycle at 95 °C for 10 min followed by 45 cycles of the following: 95 °C for 10 s, 60 °C for 10 s, and 72 °C for 15 s.
The amplification process was finished with the Roche LightCycler96 real-time fluorescence quantitative PCR instrument. All PCRs were performed in triplicate for each sample. At the end of the reaction, the cycle threshold (Ct value) was automatically read out by the fluorescent PCR tape software. The more cycles needed for each sample to reach a certain amount of products, the greater the Ct value, and the smaller the initial template plate. The average amplification efficiency of PCR is proportional to 2Ct0 − Ct1; Ct1 is one specific gene to be measured and Ct0 is β-actin. In this paper, we use Δ Ct (Δ Ct = Ct1 − Ct0) to express the damage to the gene, according to the previously published measurement methods as described in detail; the level of DNA damage was expressed using the damage index (Wen et al. 2008). The higher Δ Ct value indicated the higher level of damage index.
Genotyping examination and high-resolution melting (HRM) analysis
We designed the primer of FEN1 (forward: CAAGTCCCTCAATGCCACTTG; reverse: TCGCATCTCCGTCTGGAACT) to detect the genotype and confirm whether wild type or mutation. Samples were assayed in duplicate using the Roche LightCycler 96 real-time fluorescence quantitative PCR instrument and analyzed using the LightCycler 96 Software version 1.1.0.1320 supplied with the LightCycler 96. Each 20 μl reaction contained 4 μl buffer, 10 mM dNTP mix 0.4 μl, 10 μM forward primer 0.4 μl, 10 μM reverse primer 0.4 μl, DNA 1 μl, Evagreen 1 μl (Biotium company number 31000), and 12.8 μl water aiming to add the system into 20 μl in all. The same touchdown PCR program and melting conditions were used for all amplicons: 95 °C for 10 min, 45 cycles of 95 °C for 10 s, 60 °C for 10 s, and 72 °C for 15 s. Data and melting curve were acquired and analyzed with the accompanying Gene Scanning software.
Statistics
Data analysis was conducted using SPSS, version 18.0 (SPSS Inc., Chicago, IL, USA). The normality of continuous variables was examined by Kolmogorov-Smirnov normality test. The level of creatinine-adjusted 1-OH-Pyr, damage index of exon 19 and 21 of gene EGFR, and damage index of exon 20 of gene BRCA1 were natural logarithm (ln) transformed to improve normality and stabilize variance. Considering that age was highly correlated with working years (r = 0.831, P < 0.001), it was not included in the multiple linear regression models. We considered working years (continuous), workplace (low exposure/high exposure), and smoking status (smokers/nonsmokers) as potential confounders, and included them in our statistical analyses unless otherwise specified. We performed Student’s t tests for continuous variables and chi-square tests for categorical variables to assess the differences of general characteristics between the low-exposure group and high-exposure group. We analyzed the between-group differences of exposure levels of urinary 1-OH-Pyr, EGFR-19, and EGFR-21, and the damage index of exon 20 of gene BRCA1 (BRCA1-20) by multivariate analysis of covariance with adjustment for working years, workplace, and smoking status. We further evaluated the associations of urinary 1-OH-Pyr (as independent variables) with the continuous value of exon damage index (as dependent variables) in the multiple linear regression models among the 288 coke oven workers. Additionally, we also categorized these 288 workers into 3 subgroups by the tertiles of the internal exposure biomarker of urinary 1-OH-Pyr (T1 ≤ 33rd percentile, T2 > 33rd percentile but ≤ 67th percentile and T3 > 67th percentile) and the multiple linear regression models were employed to explore the linear trend P value and compute the relative association coefficients (βs) and 95% confidence intervals (95% CIs) with adjustment for working years, workplace, and smoking status in T1, T2, and T3 subgroups with T1 as the reference group. The correlation coefficients (Pearson r) between the ln-transformed urinary 1-OH-Pyr and EGFR-19 and EGFR-21 were calculated by simple linear correlation analyses.
We then performed the stratified analyses by FEN1 rs174538 genotypes; considering the sample size, subjects with genotype GA and AA were combined. We conducted Hardy-Weinberg equilibrium (HWE) test with chi-square test for each SNP before the analysis. The associations between urinary 1-OH-Pyr (as independent variables) with the continuous value of exon damage index (as dependent variables) in the multiple linear regression models were estimated by βs and 95% CIs with adjustment of working years, workplace, and smoking status. Moreover, gene-environment interaction analysis was examined by introducing the interaction term [SNP× ln-transformed urinary 1-OH-Pyr (continuous)] into the confounder-adjusted linear regression model. For all associations, βs and their 95% CIs were reported to represent the estimated difference in ln-transformed dependent variables associated with a 1-SD increment in ln-transformed independent variables. Two-tailed P < 0.05 was defined as statistical significance.
Results
General characteristics of the study participants
As shown in Table 1, 288 participants were divided into the low-exposure group (n = 151) and high-exposure group (n = 137) according to their workplaces and external total PAH concentrations mentioned above (see “Study subjects” in “Materials and methods”). Among the low-exposure group or the high-exposure group, no significant differences were found between the observed genotypic frequencies and those expected under the HWE (χ2 = 2.90, P < 0.05, and χ2 = 5.36, P < 0.05, respectively). All the participants were males, and we observed no statistically significant difference between the two groups in age, working years, and FEN1 rs174538 genotype (all P > 0.05), while marginal significance was found in smoking status between the low-exposure group and the high-exposure group (P = 0.05). Furthermore, compared to the low-exposure group, we observed higher urinary 1-OH-Pyr, EGFR-19, and EGFR-21 in the high-exposure group with adjustment of potential confounders (all P < 0.05) and no significant difference was observed in BRCA1-20 between the two groups (P > 0.05). Additionally, no significant differences were found in urinary 1-OH-Pyr levels and EGFR-19, EGFR-21, and BRCA1-20 between nonsmokers and smokers (all P > 0.05, Table S1).
Dose-response relationships of urinary 1-OH-Pyr with EGFR-19 and EGFR-21
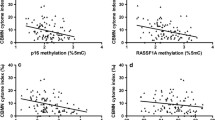
The median of the EGFR-19 in the 1st, 2nd, and 3rd urinary 1-OH-Pyr tertiles was 1.90, 2.04, and 2.23, respectively, and the median of the EGFR-21 was 1.76, 1.87, and 2.02 in the 1st, 2nd, and 3rd urinary 1-OH-Pyr tertiles, respectively. Multiple linear regression analysis was further used to estimate the covariate-adjusted associations of creatinine-standardized 1-OH-Pyr concentrations with EGFR-19 and EGFR-21, and BRCA1-20 (Table 2). We found that estimated differences in the EGFR-19 were gradually increased across tertiles of the concentrations of urinary 1-OH-Pyr after adjustment for working years (model 1) and working years, workplace, and smoking status (model 2) (both Ptrend < 0.001). Similarly, the estimated differences in EGFR-21 were also increased with each tertile increase in urinary 1-OH-Pyr levels in adjusted-model 1 and model 2 (both Ptrend < 0.001). Additionally, after multivariable adjustment, differences in both ln-transformed EGFR-19 and EGFR-21 per increment of ln-transformed urinary 1-OH-Pyr were presented as the adjusted beta coefficients (95% CI) were 0.120 (0.083–0.156) and 0.116 (0.076–0.156), respectively. After adjustment for working years (model 1) and working years, workplace, and smoking status (model 2), there were no significant changes in BRCA1-20 across urinary 1-OH-Pyr tertile (both P > 0.05). Furthermore, we also observed significantly positive correlations between urinary 1-OH-Pyr and EGFR-19 and EGFR-21 in the present study (Pearson r = 0.358 and 0.316, respectively, and both P < 0.001; Fig. 2A, B).
Associations of urinary 1-OH-Pyr with EGFR-19 and EGFR-21 in nonsmokers and smokers
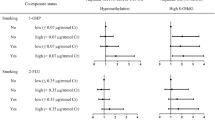
In both nonsmokers and smokers, the multiple linear regression analysis revealed that the concentrations of urinary 1-OH-Pyr were significantly associated with EGFR-19 and EGFR-21, respectively, after adjustment for working years (model 1) and working years and workplace (model 2) (Fig. 3, all P < 0.001). However, no such significant association was observed between urinary 1-OH-Pyr levels and BRCA1-20 (all P > 0.05).
Stratification analysis of the estimated difference in ln-transformed exon genetic damage index [β (95% CI)] associated with a 1-SD increase in ln-transformed exposure levels of urinary 1-OH-Pyr among 288 coke oven workers by smoking status. Note: the lines in panels represent β (95% CI) based on multivariate linear regression model 1 adjusted for working years, model 2 adjusted for working years and workplace. **P < 0.001
Interactive effects of FEN1 rs174538 with urinary 1-OH-Pyr on genetic exon damage index
We further sub-grouped the study subjects into rs174538 GA+AA and rs174538 GG genotype carriers. The multiple linear regression analysis showed that there was a significant association of levels of urinary 1-OH-Pyr with EGFR-19 in rs174538 GA+AA genotype carriers, after adjustment for working years (model 1) and working years, workplace, and smoking status (model 2) (Fig. 4, both P < 0.001). Similarly, urinary 1-OH-Pyr concentrations were associated with EGFR-21 with adjustment for working years (model 1) and working years and workplace (model 2) in these subjects with rs174538 GA+AA genotype (both P < 0.001). However, no such association was observed between urinary 1-OH-Pyr concentrations and EGFR-19 and EGFR-21 in rs174538 GG genotype carriers, after adjustment for potential confounders (all P > 0.05). Additionally, we failed to observe significant associations between urinary 1-OH-Pyr concentrations and BRCA1-21 in both GG genotype carriers and GA+AA genotype carriers (all P > 0.05).
Stratification analysis of the estimated difference in ln-transformed exon genetic damage index [β (95% CI)] associated with a 1-SD increase in ln-transformed exposure levels of urinary 1-OH-Pyr among 288 coke oven workers by FEN1 rs174538 polymorphism. Note: the lines in panels represent β (95% CI) based on multivariate linear regression model 1 adjusted for working years, model 2 adjusted for working years, workplace, and smoking status. **P < 0.001
We subsequently evaluated the interactive effects between FEN1 rs174538 genotype and urinary 1-OH-Pyr on genetic exon damage index by modeling an interaction term of continuous urinary 1-OH-Pyr × FEN1 rs174538 genotype in the covariate-adjusted linear regression models. We found rs174538 were nominally interacted with urinary 1-OH-Pyr on EGFR-19 and EGFR-21 (Pinteraction < 0.001 and P interaction = 0.031, respectively; Fig. 5). These subjects with rs174538 GA+AA genotype have greater effects of urinary 1-OH-Pyr on EGFR-19 and EGFR-21 compared to those with rs174538 GG genotype (β = 0.154 and β = 0.133 in rs174538 GA+AA genotype carriers vs. β = 0.009 and β = 0.047 in rs174538 GG genotype carriers). However, we failed to detect the interactive effect between rs174538 genotype and urinary 1-OH-Pyr on BRCA1-20.
The interaction effects of urinary 1-OH-pyr with FEN1 rs174538 polymorphism on EGFR-19 and EGFR-21. The lines in panels represent β (95% CI) based on multivariate linear regression models adjusted for working years, smoking status, and workplace; Pinteraction was calculated by entering an interaction term between urinary 1-OH-pyr (continuous) and FEN1 rs174538 polymorphism (categorical) into the confounder-adjusted linear regression models. **P < 0.001
Discussion
To the best of our knowledge, this is the first study to explore the dose-effect associations of PAH exposure with damage of exons of EGFR, as well as their associations whether modified by FEN1 rs174538 polymorphism in an occupational population. We observed significantly higher levels of urinary 1-OH-Pyr and significantly higher EGFR-19 and EGFR-21 in these individuals exposed to higher PAH levels, after adjusting for multiple covariates. Subsequently, we revealed the significant linear associations of the urinary excretion of 1-OH-Pyr with EGFR-19 and EGFR-21, respectively. More importantly, their associations were modified by FEN1 rs174538 polymorphism.
Epidemiologic studies implied that exposure to PAHs from occupational settings was significantly related to the concentrations of PAH metabolites such as urinary 1-OH-Pyr. As a major metabolite of pyrene, 1-OH-Pyr was reported to be a good biomarker for total PAH exposure and is thought to reflect PAH molecular activation (Jeng et al. 2013; McClean et al. 2012; Zhang et al. 2001). Especially, a cross-sectional study of coke oven workers in China reported that urinary 1-OH-Pyr was the most comprehensive carcinogenic indicator of exposure to PAHs (Yamano et al. 2014). In the present study, we also observed a significant difference in urinary 1-OH-Pyr levels according to environmental PAH exposure among coke oven workers and individuals with higher environmental PAH exposure had higher concentrations of urinary1-OH-Pyr. This result was consistent with previous studies on individuals including coke oven workers who were exposed to PAHs (Bin et al. 2008; Kuang et al. 2013).
During the metabolism process of PAHs, it can lead to the formation of ROS, which could lead to carcinogenesis via oxidative DNA damage (Moorthy et al. 2015). In addition, ROS can induce DNA breaks (Anandi Srinivasan et al. 2001, Kim and Jang 2018). After metabolic transformation, DNA adducts are formed, a kind of compound that carcinogenic substances form with cellular macromolecules (Nisha et al. 2017; Pratt et al. 2011). The level of specific DNA adducts is commonly considered to be a biomarker of the biologically effective dose, and if the adducts are able to induce mutations leading to cancer, they may also be recognized as biomarker of effect (Ewa and Danuta 2017). By causing DNA damage directly and forming DNA adducts, PAHs may exert great effect on gene mutation.
Our data showed a significantly higher damage in exon 19 and 21 of the EGFR gene in these individuals exposed to higher environmental PAHs, after adjustment for multiple covariates. Moreover, we observed a significantly linear dose-effect relationship between PAH exposure and EGFR damage in exon 19 and 21, respectively. Hence, base modification is one of the possible mechanisms that led to EGFR mutation by which the population exposed to PAHs may be associated with predisposition to lung cancer. To date, there is a lack of epidemiological study to investigate the association between PAH exposure and exon damage in the EGFR gene. It is widely known that PAHs usually co-exist at high abundance in prevalent sources of pollution such as fine particulate matter (≤ 2.5 μm in aerodynamic diameter; PM2.5) air pollution and cigarette smoking and PM2.5 and cigarette smoking were proved to be deleterious to EGFR damage and mutation of exon 19 and 21, and were closely related with the high level of EGFR mRNA expression (Han et al. 2016; Yanagawa et al. 2011). Evidences from epidemiological and laboratory studies have reported that after PM2.5 exposure, the EGFR was hypomethylated in the promoter methylation site, resulting in upregulation of gene expression. Mutations in EGFR are linked to pulmonary exposure to PM2.5 emitted from coal combustion, such as by inducing lung inflammation, elevating ROS, iNOS, EGF, and CXCL1 (Jin et al. 2017). Cigarette smoking extract also promotes EGFR signaling and ROS generation in non-small cell lung cancer cell lines (Zhang et al. 2017). Tobacco smoking was the most researched environmental factor confirmed to induce EGFR mutations (Hosgood 3rd et al. 2013, Soo et al. 2017). All of these above-mentioned scientific evidences suggested the possibility of our current association of PAH exposure with EGFR damage of exon 19 and 21 in the EGFR gene.
Besides environment factors, genetic factors such as SNPs were reported to associate with predisposition to EGFR mutation. A meta-analysis based on previous GWAS has confirmed eight known SNPs associated with EGFR mutations such as rs2736100 (TERT), rs4488809 (TP63), rs7086803 (VTI1A), rs7741164 (FOXP4), rs9387478 (ROS1/DCBLD1), and so on (Seow et al. 2017). FEN1, as a DNA repair protein, plays a crucial role in both DNA replication and damage repair and is a key enzyme in maintaining genomic instability and preventing carcinogenesis. What’s more, overexpressed FEN1 represents a prognostic biomarker and potential therapeutic target for non-small cell lung cancer treatment (Zhang et al. 2018), and FEN1 is critical for the rapid proliferation of lung cancer cells (He et al. 2017). In the recent two decades, polymorphisms of the FEN1 gene have gained attention for susceptibility of tumorigenesis or carcinogenesis (Moazeni-Roodi et al. 2019; Ying et al. 2015). Several studies focused on the associations of its major polymorphisms, namely -69G/A (rs174538) and 4150G/T (rs4246215), and different disease risk, including lung cancer (Liu et al. 2012; Yang et al. 2009). Interestingly, in the present study, we specifically examined the interaction effects of FEN1 rs174538 polymorphism and PAH exposure on damage in exon 19 and 21 of EGFR and observed significantly increased trends for damage index of exon 19 and 21 as urinary 1-OH-Pyr concentrations increased only in FEN1 rs174538 GA+AA genotype carriers but not in rs174538 GG genotype carriers. At the same time, we also found rs174538 was significantly interacted with PAH exposure on the damage index of exon 19 and 21 of EGFR, even after adjusting for potential confounders. Our data indicate that the environmental PAH exposure effects on exon damage in EGFR are stronger in FEN1 rs174538 GA+AA genotype carriers than in rs174538 GG genotype carriers, which provides us meaningful information on the role of air PAH exposure in triggering EGFR damage, even mutation. Furthermore, genetic susceptibility may partly contribute to the diverse response in EGFR mutation induced by PAH exposure. Evidence especially in lung cancer research has reported that genetic polymorphisms in FEN1 confer susceptibility to lung cancer among coke oven workers. Those with GA and GG genotype had a higher transformed value of DNA damage compared with AA genotype in rs174538 (Yang et al. 2009). Therefore, our results indicated that the functions of FEN1 were more impaired or modified with higher PAH exposure.
To the best of our knowledge, our study data is among the first to reveal the interaction effect of PAH exposure and FEN1 rs174538 polymorphism on exon damage of EGFR in an occupational male population, which imply the role of the environmental PAH exposure and genetic polymorphisms in the regulation of exon damage in EGFR and provide scientific evidence for identifying a susceptible population as well as putting forward corresponding interventions. Nevertheless, we acknowledge that the present study is exploratory and several limitations should not be neglected when interpreting our results. Firstly, because of the present cross-sectional study design, our data makes it difficult to infer the causality. However, besides urinary 1-OH-Pyr was a good exposure marker and the exon damage in EGFR was acceptable to regard as effective variable, the dose-effect associations and gene-environment interaction could be well interpreted by previous evidence to a large extent. Secondly, we just detected urinary 1-OH-Pyr instead of all urinary metabolites of PAH exposure, while like above-mentioned studies, and Kuang et al. showed urinary 1-OH-Pyr was related with nine urinary PAH metabolites including 1-hydroxynaphthalene, 2-hydroxynaphthalene, 2-hydroxyfluorene, 9-hydroxyfluorene, 1-hydroxyphenanthrene, 2-hydroxyphenanthrene, 3-hydroxyphenanthrene, 4-hydroxyphenanthrene, and 9-hydroxyphenanthrene; the Pearson correlation coefficient (r) value was ranged from 0.194 to 0.659 in smokers (all P < 0.01) and from 0.284 to 0.737 in nonsmokers (all P < 0.01). Especially, urinary 1-OH-Pyr was highly correlated to total concentration of PAH metabolites (ΣOH-PAHs), the r value between ΣOH-PAHs and 1-OH-Pyr was 0.845 in smokers (P < 0.01) and 0.880 in nonsmokers (P < 0.01), respectively (Kuang et al. 2013), which suggested 1-OH-Pyr could be an indirect suitable internal biomarker to estimate the biological effective doses of PAH exposure. Urinary metabolites had relatively short half-lives and were determined by using a spot urine instead of continuous samples, and the coke oven workers are exposed to PAHs continuously in their occupational environment, but the PAH metabolites in a single-time-point urine were also widely used as internal exposure biomarkers in the environmental epidemiological studies. Thirdly, insufficient sample size of this study to detect interaction with enough power and the lack of independent replication are also important limitations. Further investigations with larger sample–sized populations and functional studies are needed to verify the current findings and related molecular biological pathways.
Conclusion
Our present study showed a significant dose-effect relationship between PAH exposure and exon genetic damage for EGFR-19 and EGFR-21, respectively. Notably, exposure to PAHs may interact with FEN1 rs174538 polymorphism and further contribute to exon damage of EGFR. These findings implied the potential to identify at-risk subpopulations who are susceptible to adverse health effects attributed to PAH exposure. Further studies are warranted to validate our findings and investigate the underlying molecular mechanisms.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Anandi Srinivasan H-JL, Larry W. R, Gabriele L (2001) Production of DNA strand breaks in vitro and reactive oxygen species in vitro and in HL-60 cells by PCB metabolites. Toxicol Sci 69:92–102
Armstrong B, Hutchinson E, Unwin J, Fletcher T (2004) Lung cancer risk after exposure to polycyclic aromatic hydrocarbons: a review and meta-analysis. Environ Health Perspect 112:970–978. https://doi.org/10.1289/ehp.6895
Bin P, Leng S, Cheng J, Dai Y, Huang C, Pan Z, Niu Y, Duan H, Li H, Liu Q, Chen W, Zheng Y (2008) Association of aryl hydrocarbon receptor gene polymorphisms and urinary 1-hydroxypyrene in polycyclic aromatic hydrocarbon-exposed workers. Cancer Epidemiol Biomark Prev 17:1702–1708. https://doi.org/10.1158/1055-9965.EPI-07-2812
Bircan S, Baloglu H, Kucukodaci Z, Bircan A (2014) EGFR and KRAS mutations in Turkish non-small cell lung cancer patients: a pilot study. Med Oncol 31:87. https://doi.org/10.1007/s12032-014-0087-4
Cheng YI, Gan YC, Liu D, Davies MPA, Li WM, Field JK (2019) Potential genetic modifiers for somatic EGFR mutation in lung cancer: a meta-analysis and literature review. BMC Cancer 19:1068. https://doi.org/10.1186/s12885-019-6317-6
da Cunha SG, Shepherd FA, Tsao MS (2011) EGFR mutations and lung cancer. Annu Rev Pathol 6:49–69. https://doi.org/10.1146/annurev-pathol-011110-130206
Ewa B, Danuta MS (2017) Polycyclic aromatic hydrocarbons and PAH-related DNA adducts. J Appl Genet 58:321–330. https://doi.org/10.1007/s13353-016-0380-3
Gedik CM, Boyle SP, Wood SG, Vaughan NJ, Collins AR (2002) Oxidative stress in humans: validation of biomarkers of DNA damage. Carcinogenesis 23:1441–1446. https://doi.org/10.1093/carcin/23.9.1441
Han X, Liang WL, Zhang Y, Sun LD, Liang WY (2016) Effect of atmospheric fine particles on epidermal growth factor receptor mRNA expression in mouse skin tissue. Genet Mol Res 15. https://doi.org/10.4238/gmr.15017188
He L, Luo L, Zhu H, Yang H, Zhang Y, Wu H, Sun H, Jiang F, Kathera CS, Liu L, Zhuang Z, Chen H, Pan F, Hu Z, Zhang J, Guo Z (2017) FEN1 promotes tumor progression and confers cisplatin resistance in non-small-cell lung cancer. Mol Oncol 11:640–654. https://doi.org/10.1002/1878-0261.12058
Hosgood HD 3rd, Pao W, Rothman N, Hu W, Pan YH, Kuchinsky K, Jones KD, Xu J, Vermeulen R, Simko J, Lan Q (2013) Driver mutations among never smoking female lung cancer tissues in China identify unique EGFR and KRAS mutation pattern associated with household coal burning. Respir Med 107:1755–1762. https://doi.org/10.1016/j.rmed.2013.08.018
Huang CY, Hsieh MJ, Wu WJ, Chiang WL, Liu TC, Yang SF, Tsao TC (2018) Association of endothelial nitric oxide synthase (eNOS) polymorphisms with EGFR-mutated lung adenocarcinoma in Taiwan. J Cancer 9:2518–2524. https://doi.org/10.7150/jca.25824
Jeng HA, Pan CH, Chao MR (2013) 1-Hydroxypyrene as a biomarker for assessing the effects of exposure to polycyclic aromatic hydrocarbons on semen quality and sperm DNA integrity. J Environ Sci Health A Tox Hazard Subst Environ Eng 48:152–158. https://doi.org/10.1080/03601234.2012.716741
Jin Y, Wu W, Zhang W, Zhao Y, Wu Y, Ge G, Ba Y, Guo Q, Gao T, Chi X, Hao H, Wang J, Feng F (2017) Involvement of EGF receptor signaling and NLRP12 inflammasome in fine particulate matter-induced lung inflammation in mice. Environ Toxicol 32:1121–1134. https://doi.org/10.1002/tox.22308
Jongeneelen FJ (2001) Benchmark guideline for urinary 1-hydroxypyrene as biomarker of occupational exposure to polycyclic aromatic hydrocarbons. Ann Occup Hyg 45:3–13
Kim HJ, Jang CH (2018) Imaging DNA single-strand breaks generated by reactive oxygen species using a liquid crystal-based sensor. Anal Biochem 556:1–6. https://doi.org/10.1016/j.ab.2018.06.009
Krawczyk P, Reszka K, Ramlau R, Powrózek T, Pankowski J, Wojas-Krawczyk K, Kalinka-Warzocha E, Szczęsna A, Nicoś M, Jarosz B, Szyszka-Barth K, Bryl M, Adamowicz K, Szumiło J, Milanowski J (2016) Prevalence of rare EGFR gene mutations in nonsmall-cell lung cancer: a multicenter study on 3856 Polish Caucasian patients. Ann Oncol 27:358–359. https://doi.org/10.1093/annonc/mdv553
Kuang D, Zhang W, Deng Q, Zhang X, Huang K, Guan L, Hu D, Wu T, Guo H (2013) Dose-response relationships of polycyclic aromatic hydrocarbons exposure and oxidative damage to DNA and lipid in coke oven workers. Environ Sci Technol 47:7446–7456. https://doi.org/10.1021/es401639x
Lin CH, Yang PJ, Lin SH, Yeh KT, Tsao TC, Chen YE, Lin SH, Yang SF (2020) Association between EGFR gene mutation and antioxidant gene polymorphism of non-small-cell lung cancer. Diagnostics (Basel) 10. https://doi.org/10.3390/diagnostics10090692
Liu L, Zhou C, Zhou L, Peng L, Li D, Zhang X, Zhou M, Kuang P, Yuan Q, Song X, Yang M (2012) Functional FEN1 genetic variants contribute to risk of hepatocellular carcinoma, esophageal cancer, gastric cancer and colorectal cancer. Carcinogenesis 33:119–123. https://doi.org/10.1093/carcin/bgr250
McClean MD, Osborn LV, Snawder JE, Olsen LD, Kriech AJ, Sjodin A, Li Z, Smith JP, Sammons DL, Herrick RF, Cavallari JM (2012) Using urinary biomarkers of polycyclic aromatic compound exposure to guide exposure-reduction strategies among asphalt paving workers. Ann Occup Hyg 56:1013–1024. https://doi.org/10.1093/annhyg/mes058
Moazeni-Roodi A, Ghavami S, Ansari H, Hashemi M (2019) Association between the flap endonuclease 1 gene polymorphisms and cancer susceptibility: an updated meta-analysis. J Cell Biochem 120:13583–13597. https://doi.org/10.1002/jcb.28633
Moorthy B, Chu C, Carlin DJ (2015) Polycyclic aromatic hydrocarbons: from metabolism to lung cancer. Toxicol Sci 145:5–15. https://doi.org/10.1093/toxsci/kfv040
Nisha AR, Hazilawati H, Mohd Azmi ML, Noordin MM (2017) DNA damage and adduct formation in immune organs of developing chicks by polycyclic aromatic hydrocarbons. Toxicol Mech Methods 27:215–222. https://doi.org/10.1080/15376516.2016.1273432
Perez-Ramirez C, Canadas-Garre M, Molina MA, Cabeza Barrera J, Faus-Dader MJ (2019) Impact of single nucleotide polymorphisms on the efficacy and toxicity of EGFR tyrosine kinase inhibitors in advanced non-small cell lung cancer patients. Mutat Res 781:63–70. https://doi.org/10.1016/j.mrrev.2019.04.001
Pratt MM, John K, MacLean AB, Afework S, Phillips DH, Poirier MC (2011) Polycyclic aromatic hydrocarbon (PAH) exposure and DNA adduct semi-quantitation in archived human tissues. Int J Environ Res Public Health 8:2675–2691. https://doi.org/10.3390/ijerph8072675
Ren H, Ma H, Ke Y, Ma X, Xu D, Lin S, Wang X, Dai ZJ (2015) Flap endonuclease 1 polymorphisms (rs174538 and rs4246215) contribute to an increased cancer risk: evidence from a meta-analysis. Mol Clin Oncol 3:1347–1352. https://doi.org/10.3892/mco.2015.617
Rezaei M, Hashemi M, Sanaei S, Mashhadi MA, Hashemi SM, Bahari G, Taheri M (2016) FEN1 -69G>A and +4150G>T polymorphisms and breast cancer risk. Biomed Rep 5:455–460. https://doi.org/10.3892/br.2016.738
Samanta SK, Singh OV, Jain RK (2002) Polycyclic aromatic hydrocarbons: environmental pollution and bioremediation. Trends Biotechnol 20:243–248. https://doi.org/10.1016/s0167-7799(02)01943-1
Scalise JR, Pocas RC, Caneloi TP, Lopes CO, Kanno DT, Marques MG, Valdivia JC, Maximo FR, Pereira JA, Ribeiro ML, Priolli DG (2016) DNA damage is a potential marker for TP53 mutation in colorectal carcinogenesis. J Gastrointest Cancer 47:409–416. https://doi.org/10.1007/s12029-016-9846-0
Seow WJ, Matsuo K, Hsiung CA, Shiraishi K, Song M, Kim HN, Wong MP, Hong YC, Hosgood HD III, Wang Z, Chang IS, Wang JC, Chatterjee N, Tucker M, Wei H, Mitsudomi T, Zheng W, Kim JH, Zhou B, Caporaso NE, Albanes D, Shin MH, Chung LP, An SJ, Wang P, Zheng H, Yatabe Y, Zhang XC, Kim YT, Shu XO, Kim YC, Bassig BA, Chang J, Ho JCM, Ji BT, Kubo M, Daigo Y, Ito H, Momozawa Y, Ashikawa K, Kamatani Y, Honda T, Sakamoto H, Kunitoh H, Tsuta K, Watanabe SI, Nokihara H, Miyagi Y, Nakayama H, Matsumoto S, Tsuboi M, Goto K, Yin Z, Shi J, Takahashi A, Goto A, Minamiya Y, Shimizu K, Tanaka K, Wu T, Wei F, Wong JYY, Matsuda F, Su J, Kim YH, Oh IJ, Song F, Lee VHF, Su WC, Chen YM, Chang GC, Chen KY, Huang MS, Yang PC, Lin HC, Xiang YB, Seow A, Park JY, Kweon SS, Chen CJ, Li H, Gao YT, Wu C, Qian B, Lu D, Liu J, Jeon HS, Hsiao CF, Sung JS, Tsai YH, Jung YJ, Guo H, Hu Z, Wang WC, Chung CC, Lawrence C, Burdett L, Yeager M, Jacobs KB, Hutchinson A, Berndt SI, He X, Wu W, Wang J, Li Y, Choi JE, Park KH, Sung SW, Liu L, Kang CH, Hu L, Chen CH, Yang TY, Xu J, Guan P, Tan W, Wang CL, Sihoe ADL, Chen Y, Choi YY, Hung JY, Kim JS, Yoon HI, Cai Q, Lin CC, Park IK, Xu P, Dong J, Kim C, He Q, Perng RP, Chen CY, Vermeulen R, Wu J, Lim WY, Chen KC, Chan JKC, Chu M, Li YJ, Li J, Chen H, Yu CJ, Jin L, Lo YL, Chen YH, Fraumeni JF Jr, Liu J, Yamaji T, Yang Y, Hicks B, Wyatt K, Li SA, Dai J, Ma H, Jin G, Song B, Wang Z, Cheng S, Li X, Ren Y, Cui P, Iwasaki M, Shimazu T, Tsugane S, Zhu J, Jiang G, Fei K, Wu G, Chien LH, Chen HL, Su YC, Tsai FY, Chen YS, Yu J, Stevens VL, Laird-Offringa IA, Marconett CN, Lin D, Chen K, Wu YL, Landi MT, Shen H, Rothman N, Kohno T, Chanock SJ, Lan Q (2017) Association between GWAS-identified lung adenocarcinoma susceptibility loci and EGFR mutations in never-smoking Asian women, and comparison with findings from Western populations. Hum Mol Genet 26:454–465. https://doi.org/10.1093/hmg/ddw414
Sigismund S, Avanzato D, Lanzetti L (2018) Emerging functions of the EGFR in cancer. Mol Oncol 12:3–20. https://doi.org/10.1002/1878-0261.12155
Sobus JR, McClean MD, Herrick RF, Waidyanatha S, Nylander-French LA, Kupper LL, Rappaport SM (2009) Comparing urinary biomarkers of airborne and dermal exposure to polycyclic aromatic compounds in asphalt-exposed workers. Ann Occup Hyg 53:561–571. https://doi.org/10.1093/annhyg/mep042
Soo RA, Kubo A, Ando M, Kawaguchi T, Ahn MJ, Ou SI (2017) Association between environmental tobacco smoke exposure and the occurrence of EGFR mutations and ALK rearrangements in never-smokers with non-small-cell lung cancer: analyses from a prospective multinational ETS registry. Clin Lung Cancer 18:535–542. https://doi.org/10.1016/j.cllc.2017.01.005
Wen W, Che W, Lu L, Yang J, Gao X, Wen J, Heng Z, Cao S, Cheng H (2008) Increased damage of exon 5 of p53 gene in workers from an arsenic plant. Mutat Res 643:36–40. https://doi.org/10.1016/j.mrfmmm.2008.06.005
Winther-Larsen A, Fynboe Ebert EB, Meldgaard P, Sorensen BS (2019) EGFR gene polymorphism predicts improved outcome in patients with EGFR mutation-positive non-small cell lung cancer treated with erlotinib. Clin Lung Cancer 20:161–166 e1. https://doi.org/10.1016/j.cllc.2019.02.011
Yamano Y, Hara K, Ichiba M, Hanaoka T, Pan G, Nakadate T (2014) Urinary 1-hydroxypyrene as a comprehensive carcinogenic biomarker of exposure to polycyclic aromatic hydrocarbons: a cross-sectional study of coke oven workers in China. Int Arch Occup Environ Health 87:705–713. https://doi.org/10.1007/s00420-013-0913-6
Yanagawa N, Tamura G, Oizumi H, Endoh M, Sadahiro M, Motoyama T (2011) Inverse correlation between EGFR mutation and FHIT, RASSF1A and RUNX3 methylation in lung adenocarcinoma: relation with smoking status. Anticancer Res 31:1211–1214
Yang M, Guo H, Wu C, He Y, Yu D, Zhou L, Wang F, Xu J, Tan W, Wang G, Shen B, Yuan J, Wu T, Lin D (2009) Functional FEN1 polymorphisms are associated with DNA damage levels and lung cancer risk. Hum Mutat 30:1320–1328. https://doi.org/10.1002/humu.21060
Yang B, Deng Q, Zhang W, Feng Y, Dai X, Feng W, He X, Huang S, Zhang X, Li X, Lin D, He M, Guo H, Sun H, Yuan J, Lu J, Hu FB, Zhang X, Wu T (2016) Exposure to polycyclic aromatic hydrocarbons, plasma cytokines, and heart rate variability. Sci Rep 6:19272. https://doi.org/10.1038/srep19272
Ying N, Wang S, Xu H, Wang Y (2015) Association between FEN1 polymorphisms -69G>A and 4150G>T with susceptibility in human disease: a meta-analysis. Iran J Public Health 44:1574–1579
Zhang J, Ichiba M, Hara K, Zhang S, Hanaoka T, Pan G, Yamano Y, Takahashi K, Tomokuni K (2001) Urinary 1-hydroxypyrene in coke oven workers relative to exposure, alcohol consumption, and metabolic enzymes. Occup Environ Med 58:716–721. https://doi.org/10.1136/oem.58.11.716
Zhang W, Yi X, Guo S, Shi Q, Wei C, Li X, Gao L, Wang G, Gao T, Wang L, Li C (2014) A single-nucleotide polymorphism of miR-146a and psoriasis: an association and functional study. J Cell Mol Med 18:2225–2234. https://doi.org/10.1111/jcmm.12359
Zhang L, Li J, Hu J, Li D, Wang X, Zhang R, Zhang H, Shi M, Chen H (2017) Cigarette smoke extract induces EGFR-TKI resistance via promoting EGFR signaling pathway and ROS generation in NSCLC cell lines. Lung Cancer 109:109–116. https://doi.org/10.1016/j.lungcan.2017.05.011
Zhang K, Keymeulen S, Nelson R, Tong TR, Yuan YC, Yun X, Liu Z, Lopez J, Raz DJ, Kim JY (2018) Overexpression of flap endonuclease 1 correlates with enhanced proliferation and poor prognosis of non-small-cell lung cancer. Am J Pathol 188:242–251. https://doi.org/10.1016/j.ajpath.2017.09.011
Funding
This study was supported by the Natural Science Foundation of Guangdong Province (grant number 2018A030313606) and by the National Natural Science Foundation of China (grant numbers 81903381, 81803189, and 81860572), and Guangzhou Key Laboratory Fund (201905010004).
Author information
Authors and Affiliations
Contributions
We thank all individuals who volunteered to participate in this study. M.Y., Q.H., J.T., Y.H., and S.C. collected the samples and established the database and conducted the experiments. M.Y, Y.Z., and Y.B. designed and carried out the study. S.C., Y.H., and B.Y. conducted the data analysis and drafted the manuscript. Y.B. revised the manuscript. All the authors had access to the data and reviewed and approved the final submitted manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Kunming Medical University.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOC 31 kb)
Rights and permissions
About this article
Cite this article
Chen, S., He, Y., Yan, M. et al. The interaction effects of FEN1 rs174538 polymorphism and polycyclic aromatic hydrocarbon exposure on damage in exon 19 and 21 of EGFR gene in coke oven workers. Environ Sci Pollut Res 28, 60692–60703 (2021). https://doi.org/10.1007/s11356-021-15013-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15013-y