Abstract
Inflammasome activity plays a vital role in various non-microbial disease states correlated with prolonged inflammation. NLRP3 inflammasome function and IL-1β formation are augmented in obesity and several obesity-linked metabolic disorders (i.e. diabetes mellitus, hypertension, hepatic steatosis, cancer, arthritis, and sleep apnea). Also, several factors are associated with the progression of diseases viz. increased plasma glucose, fatty acids, and β-amyloid are augmented during obesity and activate NLRP3 inflammasome expression. Prolonged NLRP3 stimulation seems to play significant role in various disorders, though better knowledge of inflammasome regulation and action might result in improved therapeutic tactics. Numerous compounds that mitigate NLRP3 inflammasome expression and suppress its chief effector, IL-1β are presently studied in clinical phases as therapeutics to manage or prevent these common disorders. A deep research on the literature available till date for inflammasome in obesity was conducted using various medical sites like PubMed, HINARI, MEDLINE from the internet, and data was collected simultaneously. The present review aims to examine the prospects of inflammasome as a major progenitor in the progression of obesity via directing their role in regulating appetite.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inflammation is the major progenitor of obesity and associated comorbidities. Intake of high calorie diet induces macrophage infiltration in adipocytes, resulting in increased formation of inflammatory mediators. The microbial danger signal stimulates intracellular NLRP3 immune response, which in turn causes caspase-1 regulation and generation of inflammatory mediators, such as cytokines, IL-1β, and IL-18. Nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) are the vital constituents of the immune system, which are considered to play an important role in the development of inflammatory disorders. NLRs comprise of a class of proteins that primarily aid in the regulation of immune system (Ye and Ting 2008; Kanneganti et al. 2006; Sutterwala et al. 2006). NLRs induce inflammation by recognizing danger signals such as endogenous agents, which are formed during the inflammatory phase (Sutterwala et al. 2006; Mariathasan et al. 2006; Duewell et al. 2010). The NLRP3 protein involved in auto-inflammatory disorder is known as NLRP3 (cryopyrin). This protein upon activation, promotes recruitment and regulation of the cysteine caspase-1 in a cytosolic protein called inflammasome (Aganna 2004; Martinon 2006). The apoptosis-associated, speck-like protein, containing caspase-recruitment domain (ASC), forms a link between NLRP3 and caspase-1, via homotypic interactions, further implicating its pyrin and CARD residues as well as making it important for inflammasome regulation (Ghayur 1997; Kuida 1995; Li 1995). Stimulated caspase-1 which are substrates of associated mediators IL-1β and IL-18 permit the release of active cytokines. IL-1β contributes to the production of systemic response towards immunological disorder, by eliciting acute phase response, manifested via protein production (Dinarello 1996). However, IL-18 deficient pyrogenic activity of IL-1β is arbitrated in the development of various inflammatory mediators (Horwood 1998; Olee 1999).
Obesity is characterized by prolonged inflammation, induced through the proliferating tissue (Hotamisligil and Erbay 2008; Odegaard and Chawla 2008). Enlargement of fatty mass, accompanied by the development of adipocytes promotes macrophage infiltration inside tissues (Xu 2003; Weisberg 2003). The increased inflammation of tissue initiates assembly of cytokines which participate in progression of diabetes mellitus (Olefsky and Glass 2010; Shoelson et al. 2007). Also, IL-1β has been related with the progression of obesity-associated insulin resistance (Jager 2007; Netea 2006; Zorrilla 2007). Furthermore, overfeeding leads to stimulation of caspase-1 in adipose tissue in experimental animals (Stienstra 2010). Remarkably, NLRP3 deficiency has been reported to inhibit the progression of obesity-linked insulin resistance (Stienstra 2010). The effect of caspase-1 and ASC in the pathogenesis of high-fat diet (HFD)-induced obesity is still unclear. Chronic stimulation of inflammasome-induced caspase-1 might lead to the progression of obesity.
Cross talk between inflammasomes and inflammatory responses
The immune system functions as a host defense mechanism against the attack, mediated via microbial substances, by detecting pathogen linked molecular patterns, including microbial nucleic acid, damage-associated molecular pattern (DAMP), secreted under cellular stress conditions, consisting of ATP, and cellular mechanisms (Strowig 2012). The stimulation of immune responses to these elements is mediated through numerous types of germline-encoded pattern-recognition receptors (PRRs), comprising of toll-like receptors, and NLRs (Akira et al. 2006; Takeuchi and Akira 2010). NLRs encompass class of intracellular proteins which comprise of NOD, and a variable B-residue effector region. These proteins are categorized as Nod 1 and Nod2 receptors, and possess varied activities. The negative stimulator comprises of NLRX1 and NLRP4, while inflammasome regulators comprises of NLRP1, NLRC4 and NLRP3 (Cui 2010; Meylan et al. 2006; Moore 2011; Xia 2010; Bryan 2009). Various NLRs produce multi-protein inflammasome after regulation of DAMP pathway. NLRs enroll pro-caspases via contacting with pyrin domain (PYD) of the ASC, which is located inside nucleus, but its movement is necessary from the nucleus to the cytosol for activation of inflammasome (Dinarello 2009).
IL-1 and IL-18 produced by caspase-1, pro-IL1, and pro-IL-18 regulate inflammasome stimulation, which occurs in response to infectious molecules. IL-1β promotes T and B cell production, and leukocyte relocation, whereas IL-18 in association with IL-12 promotes induction of Th1 responses (Harrington 2005). The caspase-1 function is necessary for pyroptosis, a pro-inflammatory cell death, for the removal of microbial pathogens. IL-1 generation and pyroptosis have been implied for their therapeutic activity in various complications such as arthritis, metabolic dysregulation, and Cancer. Caspase-1 acts via several mechanism including the release of peptides, breakdown of glycolytic enzymes, inhibition of bacterial growth, and escalation of cell repair by regulating lipid uptake. The events associated with activation of cytokine and caspase-1 possess its ability to control bacterial infection. The attacking microbes avoid several phases in IL-1 and IL-18 generation to overcome the host defense mechanism. Numerous mechanisms can diminish inflammasome elicited responses. The anti-inflammatory mediator, IL-10 prevents pro-IL-1 production through the signal transducer and activator of transcription (STAT) 3 cascade, whereas regulation of STAT1 prevents IL-1 formation via inhibiting caspase-1 event. IFNγ is synthesized via CD4+ Th1 cells, which rapidly blocks IL-1 generation, which is indicated to aid during negative feedback suppression after regulation of immune responses.
The innate immune response is elicited through the activation of inflammatory pathways via secretion of cytokines and subsequent enrollment of macrophages at place of wound. A chief role in this response is the generation of the inflammasome, a multi-protein complex which aids stimulation of central protease, caspase 1. Caspase-1 induces maturation of the pro-inflammatory cytokines and leads to pyroptosis, a form of cell death that is elicited by microbial agents. Members of the Nod-like receptor family are vital constituents for activation of inflammasome and further associated with activation of microbial and endogenous signals by caspase-1 stimulation. By using upstream and downstream events in the stimulation process, scientists are able to evaluate the innate immune response. The nucleotide-binding domain leucine-rich repeat comprising (NLR) family of receptors are members of the innate immune system with a vital role in host defense. These molecules are known to elicit inflammatory responses to abnormal cellular conditions. NLRs serve this role upon stimulation by generating a multi-protein complex called an inflammasome. The inflammasome release cytokines such as pro-inflammatory cytokines interleukin (IL)-1β and IL-18.
The NLRP3 inflammasome primarily comprises of three phases — a protein (ASC), an effector protein (Caspase 1) and a receptor protein (NLRP3). The receptor protein is a sensor which is switched on when DAMP is activated. The ASC adaptor protein comprises of N-residue pyrin domain (PYD) and C-residue (CARD) which function as mediator between the sensor and effector protein, constituting nucleotide-binding domain, a C-residue leucine-rich repeat- substrate, and an N-residue pyrin substrate. NLRP3 acts as a receptor protein which is activated during endogenous danger signals, comprising of nucleic acids and lipo-oligosaccharides.
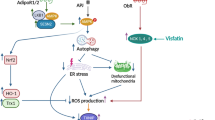
The NLRP3 inflammasome is an immune sensor which identifies a different range of dangerous compounds and functions as a metabolic danger signal, but the integrated mechanism of stimulation is not yet clear. Pro-inflammatory triglycerides such as palmitate and adipocyte-derived lyo-phosphatidylcholine stimulate NLRP3 inflammasome. The amino acids homocysteine acts as DAMP, whereas lyo-phosphatidylcholine offer secondary sensor promoting inflammasome stimulation in adipocytes and adipose tissue macrophages, initiating insulin resistance (Zhang 2018). Palmitate regulates inflammasome and IL-1β secretion via inhibiting AMP-activated protein kinase (AMPK), suppressing autophagy, inducing dysregulation of mitochondria activity and increased generation of reactive oxygen species (ROS) (Wen 2011). Alteration in ion concentration, primarily K+ efflux, Ca2+ signal, and Cl− efflux is significant, but not necessary during changes in NLRP3 activation (Yang 2019). Regulation of purinergic P2X7 receptor through enhanced ATP production with nigericin results in an intracellular decrease in K+ ions and initiate inflammasome stimulation (Camello-Almaraz 2006; Lemasters 2009; Murakami 2012). Modifications in this ion concentrations have been known to unite after signal propagation by the production of reactive oxygen species (ROS) which is appropriate for the regulation of the NLRP3 Inflammasome. Mitochondrial impairment as well as release of ROS initiates NLRP3 activation.
Subsequent activation of inflammasome takes place and promotes stimulation of zymogen pro-caspase-1 into caspase-1. Breakdown of pro-IL-1β at aspartic acid terminal is the specific target of caspase-1. Caspase-1 splits rate-limiting enzymes in the immune cells i.e., macrophages, under infection, and sepsis (Shao 2007). This is applicable to metabolic cells, as it is revealed that NLRP3 inflammasome initiate muscle glycolysis process during aging. Elimination of NLRP3 reduces age-associated cleavage and down-regulates glyceraldehyde-3-phosphate dehydrogenase in mouse muscle during aging. However, caspase-1 cleavage synthesizes IL-1β, promotes adipose tissue dysregulation, and insulin resistance via IL-1β-dependent action, as IL-18 increase insulin sensitivity and decreases body weight through AMPK.
To avoid excessive and chronic inflammation, NLRP3 inflammasome activation is stimulated via transcriptional events, directed by numerous negative stimulators which prevents persistent assembly of an inflammasome. However, it is unclear which type of negative stimulators are involved in inflammasomes activation. A study illustrates that RNA target miRNA-103, a positive stimulator of inflammasomes activation, inhibits the destruction of its target TNF receptor-associated factor 3 (TRAF3). TRAF3 prevent NFκB signaling to counteract mitophagy, following NLRP3 inflammasome formation and cytokine release in adipocytes (Zhang 2019).
IL-1β is the main effector for stimulation of LRP3 inflammasome. The biological effect of IL-1β is stimulated after the breakdown and release of cytokine mediators. Transcription of IL-1β is regulated via NF-κB activation, where inactive intracellular group of pro- IL-1β is synthesized. The stimulation of zymogen Caspase-1 is followed by a breakdown of IL-1β occurs (Monteleone et al. 2015). IL-1β, circulating in the blood, is stimulated by its interaction with soluble IL-1β receptors, which accelerates the cellular effects of IL-1β (Colotta 1993). Reduction of biologically active IL-1β to membrane-bound IL-1 receptor (IL-1R1), results in the recruitment of IL-1R protein to produce a complex at the target cell membrane. The prolonged activation of IL-1β produces adverse effects, including septic shock. The canonical action inhibits immune response to resolve infection. Also, IL-1β causes immune cell recruitment to target site by regulating the expression of adhesion proteins on monocytes i.e. intracellular adhesion molecule 1. The inflammasome and IL-1β are significant mediators of homeostasis reacting to a wide range of danger signals. Unrestrained and prolong regulation of NLRP3 inflammasome can lead to chronic inflammation and various disease events, where IL-1β has been involved. The NLRP3 inflammasome activation has been linked with cancer, atherosclerosis, adipose tissue dysregulation, and progression of diabetes mellitus, as evidently supported by various studies.
It has been investigated that NLRP3 identifies intracellular reactive oxygen species (ROS) that are generated in response to various stimulators. ROS originate from NADPH oxidases were suggested to be accountable for their activation. The second mechanism includes the disruption of lysosomal membrane of crystalline materials and peptide aggregates. Upon uptake of such substances, lysosomal wall ruptures which results in the leakage of lysosomal proteases, i.e. cathepsins B and L, into the cytosol where they elicit NLRP3 inflammasome activation. Both mechanisms are required for activation of the NLRP3 inflammasome (Figs. 1 and 2, Table 1).
Upstream regulators of NLRP3 inflammasome in adipose tissue. Priming: Following PRR activation, nuclear NF-κB translocation regulates transcription of NLRP3 inflammasome factors and its effectors (pro-IL-1β). Regulation: Various regulating components of the inflammasome lead to caspase-1 cleavage of pro-IL-1β to biologically active IL-1β. Saturated fatty acids such as palmitate can alleviate AMPK, decrease autophagy, resulting in mitochondrial degradation and ROS generation. PAMP: Pathogen-associated molecular pattern molecules; PRR: Pathogen Recognition Receptors, NFκB: Nuclear Factor Kappa B; Ca2+: Calcium Ion; AMPK: AMP activated protein kinase; P2X7: P2X purinoceptor; ASC: apoptosis-associated speck-like protein containing a caspase recruitment domain; HIF 1: Hypoxia-Inducible Factor ATP: Adenosine triphosphate; K+: Potassium Ion, PLA2G16: Phospholipase A2, group XVI
Impact of NLRP3 inflammasome on adipocytes. Stimulation of the NLRP3 Inflammasome in adipocytes is associated with homeostatic mechanisms such as adipogenesis. Stimulation of this inflammatory complex exhibits detrimental outcomes i.e. as pro-inflammatory programmed cell death (pyroptosis) and ECM remodeling leading to fibrosis. ECM: Extracellular matrix; ASC: apoptosis-associated speck-like protein containing a caspase recruitment domain; AKT: Protein kinase B
NLRP3 inflammasome in obesity induced insulin resistance
Elevated inflammasome activation has been initiated in humans and animals with diabetes mellitus (Vandanmagsar 2011). Suppression or omission of NLRP3 protects by progression of insulin resistance and beta-cell death; each of which mechanisms promotes progression of obesity-associated insulin resistance, which leads to diabetes mellitus (Vandanmagsar 2011; Lee 2013). The clinical trials done by Vandanmagsar and his co-workers (Vandanmagsar 2011) revealed that animals, fed with HFD for 6 months exhibited elevated expression and regulation of the inflammasome and IL-1β in adipose tissue and liver. Inherited deletion of NLRP3 protected mice against HFD-induced insulin resistance and reinstated aspects of insulin cascade including insulin-stimulated phosphorylation of PKB in adipose and liver. Deletion of NLRP3 decreased hepatic steatosis in HFD animals (Vandanmagsar 2011). Another research, investigated out by Stienstra 2011, demonstrates that the NLRP3 inflammasome stimulates features of obesity during HFD administration in mice.
Genomic deletion of caspase-1 exhibits reduction in weight gain and adipocyte hypertrophy in animals, administered with 50% HFD (Stienstra 2011). Obesity-related events stimulate caspase-1 activity, results in insulin sensitivity and proliferation of adipocytes (Stienstra 2010). HFD administration is identified in order to elevate caspase-1 activity in adipocytes, although treatment with a caspase-1 blocker, protected mice from HFD I induced insulin resistance (Stienstra 2010). The NLRP3 inflammasomes are known to exert cell-autonomous actions on adipocyte proliferation and absorption which elicits insulin sensitivity. The proliferation of pre-adipocytes originated from caspase1 knockout mice, showed increased insulin sensitivity (Stienstra 2010). Incubation of caspase-1 deficient pre-adipocytes with IL-1β during proliferation leads to decreased adipogenesis which in turn illustrates effects of caspase-1 (Stienstra 2010). There is high significance of the caspase-1 inflammasome in adipose tissue which shows the essential role of inflammasome in adipocytes which in turn stimulates insulin resistance.
Inflammatory mediators possess the ability to decrease insulin sensitivity, although they require certain inflammatory cytokines which in turn elicit adipose tissue remodeling and maintain the nutrient holding the ability of the proliferating tissue. Osterholmet and his co-workers investigated that RIDTg mice (an adenovirus compound which inhibits TLR4, and IL-1β facilitated signaling in adipocytes), on administration of HFD showed few adipose tissue enlargements, but a higher fat was accumulated in the liver. This shows requirement for an inflammatory response in order to control triglycerides homeostasis through the proliferation of adipose tissue. This resulted in the known effects of augmented levels of specific inflammatory cytokines, which induces insulin resistance. Significant consideration seems to be taken such as timing, interval of immune responses when associating inflammation-induced adipose tissue enlargement against inflammation-induced insulin resistance.
Free fatty acids have been correlated with an inflammatory response due to insulin resistance, where palmitate can elicit stimulation of c-Jun N terminal kinase (JNK) and IL-6 secretion (Lancaster 2018). During conditions like priming, palmitate can regulate level of NLRP3 inflammasome inside white blood cells, which in turn can result in enhanced IL-1β secretion with subsequent progression of diabetes mellitus in mice (Wen 2011). The action and mechanism of palmitate-inflammasome stimulation includes the production of reactive oxygen species which can be impaired through the suppression of AMPK, which results in decreased autophagy and mitochondrial activity. The loading of palmitate inside white blood cells can lead to lysosome degradation along with stimulation of inflammasome through cathepsin-b dependent pathway, indicating several routes of lipo-toxicity to trigger the NLRP3 inflammasome. The other lipid messengers i.e. ceramides, stimulates NLRP3 inflammasome. The administration of HFD in mice and humans depicts increased concentrations of ceramides in blood and adipose tissue which is linked with obesity. Certain ceramide species have the capability to develop insulin resistance and involve in immune responses. Irrespective of the ligands and stress responses that are involved in NLRP3 inflammasome, it is observed that elimination of NLRP3 and inflammasome can lead to enhancement in insulin sensitivity inside adipose tissue of HFD-induced mice. The cell-autonomous adipocyte encompasses NLRP3 inflammasome and IL-1β, known to support numerous aspects of adipocyte dysregulation, followed by the addition of IL-1β to 3T3-L1 adipocytes. Furthermore, human adipocytes stimulate insulin resistance followed by lower insulin activated phosphorylation of Akt/protein kinase B (PKB) and reduced insulin activated lipogenesis. Besides this, suppression of NLRP3 inflammasome factor, caspase-1 enhanced adipogenesis and insulin sensitivity in high-fat diet-induced obese animals also occurs (Table 2).
Adipose tissue inflammation and obesity
Adipose tissue is an endocrine organ which comprises of numerous cells including pre-adipocytes, immune cells, mesenchymal stem cells, and vasculature cells. The influential establishment that adipose tissue can release pro-inflammatory mediators i.e., tumor necrosis factor-alpha, generated a new perception, that adipose tissue is an endocrine and immunological organ capable of incorporating immune responses, which can change adipose tissue and systemic metabolic activity (Hotamisligil et al. 1993). It has been established that adipose tissue can release more than 500 proteins (Lehr 2012). Adipokines can cascade in an autocrine and paracrine way, triggering native variations to the adipose tissue environment, which enhances adipose cell absorption. These adipokines can also act in an endocrine way, by entering circulation and permitting the cross association with different organs, i.e. liver, muscle, and pancreas. Adipokines enhance food satiety, weight gain, inflammation, immune cell relocation and insulin sensitivity.
Insulin is an anabolic hormone which elicits glucose absorption inside adipose tissue, elevates glycogen production inside muscle and liver, and also enhances lipogenesis in adipose tissue and liver (Taniguchi et al. 2006) (all these events are dysregulated during insulin resistance). Chief protuberances of insulin resistance, such as insulin-regulated phosphorylation eminence of insulin receptor substrate and AKT/PKB can be arbitrated in adipose tissue during obesity. An increase in the inflammatory-mediators occurs during obesity, i.e., TNFα, and IL-1β, stimulates stress kinase in order to elicit insulin resistance (Hotamisligil and Spiegelman 1994) via suppression of phosphorylation of these chief nodes (Reinhard 1997). Inflammatory and stress responses can mitigate insulin cascade through numerous serine phosphorylation sites on IRS proteins, inhibiting tyrosine phosphorylation and disrupting signal transfer (Tanti 1994). For instance, stimulation of JNK via TNF bases serine 307 phosphorylation of IRS 1 can prevent tyrosine phosphorylation (Lee 2003). This comprehensive knowledge of metal inflammation showed grip therapeutic intrusions for obesity-associated complications, but some have extended medical grip in obesity and diabetes mellitus. One vital reflection is selective insulin resistance and whether decreased insulin cascade connects to suppress metabolic response in adipocytes. Reduced insulin signal circulation can be linked with insulin resistance, where the specific metabolic effects of insulin are reduced in certain cells (Powell 2003). Various studies demonstrated that elevation in insulin resistance in adipocytes occurs when insulin stimulates glucose absorption. However, this selective insulin neither decreases insulin-regulated protein production, nor insulin inhibition of lipids breakdown.
Appropriate lipid accumulation and secretion from adipocytes are correlated with insulin sensitivity. A trademark of obesity and diabetes is fat storage in muscle, and the liver which can induce inflammation in tissues. The significance of fat deposition in adipocytes is best demonstrated by lipoatrophic A-ZIP/F-1 mice defiant in functional adipocytes. Adipose lipodystrophy in these mice results in lipid storage in the muscle, resulting in systemic insulin resistance and hepatic steatosis. Additionally, transgenic mice, which transcript lipoprotein lipase inside liver or muscle, have increased lipid deposition in the tissue with simultaneous insulin resistance. Both shreds of evidence signify the importance of lipid accumulation within adipocytes, as a defensive element in nutrient storage fractioning, where the inability to deposit fats in the adipocytes results in insulin resistance.
The secretion of fatty acids from adipose tissue takes place in resort to undesirable energy balance and regulated by inflammatory elements. Usually, insulin prevents lipolysis by regulating Akt/PKB, which further regulates phosphodiesterase and decreases intracellular cAMP levels, resulting in decreased stimulation of PKA. Moreover, insulin also alleviates the expression and regulation of lipases and adipose triglyceride lipase, which diminish lipolysis and adipocytes (Kershaw 2006). During obesity, enhanced lipolysis leads to increased circulating free fatty acids, which accords with elevated circulating inflammatory mediators (Girousse 2013). The insulin resistance linked with higher concentrations of circulating pro-inflammatory cytokines can mitigate and increase lipid extrusion from adipocytes, due to decreased insulin elicited inhibition of lipolysis and lower insulin activated lipogenesis. As the level of insulin resistance increases, the cleavage property of lipids decreases. 3T3-L1 adipocytes hardly investigate an assessable variation in the insulin inhibition of lipolysis in the presence of TNFα, which can activate lipolysis in adipocytes, is detected independently of insulin inhibition of lipolysis. Treatment of adipocytes with TNFα can elevate basal lipolysis through mitogen-activated protein kinase stimulation, which contributes to the regulation of endoplasmic reticulum stress. Bacterial constituents can involve adipocyte pathogen recognition receptors (PRRs) to induce lipolysis, which can be rectified by cofactors such as iron. The role of immune response in adipose tissue is still under research in obesity.
Knowing the association between immune and endocrine cascades in adipose tissue should help unravel dysfunction of adipocyte lipolysis that fuels ectopic accumulation, and insulin resistance in the liver.
Adipose tissue deposits and cushions fat necessities of the body, but it is also a significant organ in the generation of various adipokines that maintain whole-body energy equilibrium. Elevated concentrations of circulating fatty acids, derived from adipocyte lipolysis, are linked to inflammation and insulin resistance. Inflammation can enhance every feature of endocrine action and lipid handling in adipocytes or by cross-linking with adipose tissue inhabitant immune cells. Inflammation elicited through the NLRP3 inflammasomes plays a central role in tissue assembly during adipose tissue enlargement that takes place during obesity. Inversely, prolonged inflammation induced by effectors of the NLRP3 inflammasome can induce adipocyte dysregulation and insulin resistance (Fig. 3).
Stimulation of NLRP3 inflammasome in adipose tissue macrophages can manifest adipocyte browning and lipolysis. DAMPs stimulate the inflammasome, upregulates elements such as GDF3 and MAOA, leading to the depletion of catecholamines i.e. NE inhibiting lipolysis. In addition, generation of IL-1β by adipose white cells induce mitochondrial dysfunction, and formation of ROS. DAMPs: Damage-associated molecular patterns; GD3: Ganglioside; MAOA: Monoamine Oxidase A gene; IL-1 β: Interlukin 1 beta; UCP1: Uncoupling Protein 1; ROS: Reactive oxygen species; β3-R: Beta 3 adrenergic receptor
Expression of NLRP3 inflammasome and activity in obese subjects
The levels of IL-1β are smaller in normal individuals. Increased circulating IL-1β are studied in a few studies of obese subjects (Aygun 2005), although it is uncertain whether these levels (usually less than 100 pg/ml) can elicit physiologically significant effects. The IL-1β protein level in tissues, though, augmented in an obese patient, at concentration probable to generate physiological actions and weight loss, decreases IL-1 β mRNA transcription. IL-1β release from human lipid explants is linked with door body mass index (BMI), whereas, lipopolysaccharide-regulated IL-1β generation from pre monocytes of obese alcoholics is associated with BMI, percent body fat and waist circumference. Analogous to IL-1β, the flowing IL-18 levels are greater in overweight individuals and are reduced by weight loss; although, weight loss consequently reduces IL-18 mRNA transcription in the liver (Moschen 2011). Few kinds of research have reported the NLRP3 inflammasome phenotype in obese subjects. In another investigation, caspase-1 mRNA transcription was elevated in obese individuals, whereas NLRP3, IL-1β mRNA transcriptions did not consequently differ in healthy individuals and overweight individuals (Goossen 2012). The outcome from weight loss research is inconsistent with weight loss decreasing NLRP3 expression in the study. However, gender and adiposity variations between the cohort groups might explain this divergence, although, lately, research of adipose tissue biopsies from overweight individuals found that IL-1β generation and caspase-1 activity were greater in visceral adipose tissue (Goossen 2012). Remarkably, caspase-1 concentration was linked with CD8+T cell numbers located in adipose tissue. These verdicts are reliable with available outcomes that visceral adipose tissue is more inflammatory and indicate a promising effect for caspase-1 expression in the infiltration of CD8+ T cells in adipose tissue. Additionally, microarray study and RT-PCR of mouse adipose cell segment from adipose tissue suggest that the transcription of the NLR cascade phenotype, consisting of NLRP3 and ASC, is consequently overexpressed during obesity, indicating that obesity might prompt adipose cell inflammatory effect.
Role of NLRP3 inflammasome in lipolysis, adipogenesis and pyroptosis
NLRP3 stimulation has been linked to a homeostatic effect on adipose tissue consisting of adipocyte expansion and adipogenesis. Mesenchymal stem cells (MSC) that originate from adipose tissue are multipotent cells that are developed into several cell forms through ecological signals, containing adipocytes and osteocytes. NLRP3 priming and stimulation spending lipopolysaccharide and palmitic acid influenced adipogenesis by destroying osteogenesis via caspase-1 dependent action on MSC (Wang 2017). Caspase-1 stimulation elicits pro-adipogenicity phenotype i.e. peroxisome proliferator-activator receptor gamma and CCAAT — binding protein alpha (CBP-α) and ruins pro-osteogenic phenotype i.e. bone morphogenic protein 2 and run-related transcription factor 2. Stienstra investigated that caspase-1 is stimulated during adipocyte expansion in vitro (Stienstra 2010). Suppressing the NLRP3 inflammasome has proven to inhibit extracellular matrix (ECM) assembly, resulting in decreased fibrosis in mammal visceral adipocytes (Unamuno 2019). Also, data indicate that NLRP3-dependent caspase-1 stimulation takes place in hypertrophic obese adipocytes to elicit pro-inflammatory pyroptosis (Giordano 2013). The occurrence of NLRP3-dependent apoptosis might be linked to adipocyte size relative to the obese complication. This evidence indicates that stimulation of NLRP3 inflammasome distresses the life cycle of adipocyte and consequently, lipid filling bulk.
Also, the NLRP3 inflammasome plays a vital role in age-associated damages in lipid breakdown. In white adipose tissue-inhabitant macrophages, stimulation of NLRP3 inflammasome, directed the destruction of norepinephrine (NE), by the overexpression of growing differentiation factor-3 and monoamine oxidase A. Destruction of NE prohibited, catecholamine dependent, lipolytic cascade in adipocytes, decreases fatty acid, and glycerol secretion (Popa 2020). Lately, inflammasome stimulation in adaptive immunity subsidizes to age-linked lipolytic degradation (Camel 2019). Aged adipose B cells (AABs) were elevated in two-year-old mice in contrast to those of 3 months. Diminution of AABs repaired adipose tissue lipid breakdown, essential body temperature regulation during cold stress, and enhanced insulin sensitivity. Elimination of NLRP3 or management with an IL-1R inhibitor decreased AABs and reinstated lipid breakdown in aged mice. Overall, these results indicate stimulation of NLRP3 inflammasome, affecting homeostatic action and activity of adipose tissue, through the life cycle of adipocytes. The stimulation of this contributes to adipocytes growth from stem cell pioneers, enlargement and ECM restoration of adipose tissue, subsidizing to fibrosis, and consequently lipolysis and apoptosis.
Future directions
Numerous therapeutic strategies have been proposed to ameliorate conditions of the metabolic diseases, by exploring methods which target the NLRP3 inflammasome activation. These approaches focus on the production of medications to alleviate IL-1β expression; however, various other compounds that target inflammasome activity and caspase-1 activity are under production and might offer an alternative approach with different benefits. The foremost concern is alleviated tissue repair, as a consequence of inhibition of NLRP3 inflammasome activity.
Inflammasome plays a significant role in immune responses and tissue repair. Therefore, strategies are developed to target the pathogenesis of inflammasome expression, required to control a balance between decreased inflammatory activity and regulated host defense responses. The mechanism, which stimulates inflammasome and caspase-1 activation, is very complex. The caspase-1 inhibitor is the primary target for the management of inflammasome associated complications due to the greater information available for caspase-1 suppression. Caspase-1 blockers, VX-765, and VX-740 have been evaluated in clinical studies for the management of various complications. VX-765 has been tested in subject with muckle wells syndrome, which occurs due to the replication of NLRP3, where it has been known to reduce inflammatory biomarkers.
The arthritis research has been done using VX-740, which was withdrawn due to hepatic defects which were observed in experimental animals. To date, no caspase-1 blockers are present for treatment, although numerous anti-inflammatory agents can be used to prevent inflammatory responses. For instance, treatment with glyburide can often be used for the management of diabetes mellitus as it can result in reduced inflammation during diabetic complications via inhibiting IAPP activated NLRP3 inflammasome stimulation. Glyburide has been proven to inhibit NLRP3 inflammasome stimulation in response to DAMP. Glyburide prevents ATP sensitive K+ in beta cells, although NLRP3 stimulation and glyburide-induced suppression were conserved in macrophages deficient K ATP substrates, suggesting that glyburide does not prevent NLRP3 inflammasome activating via ameliorating K+ efflux. Furthermore, glyburide did not affect NLRP3 ATPase activity, indicating that glyburide acts upstream of NLRP3 inflammasome.
A better understanding of the cascades intricated in inflammasome stimulation has led to the finding of novel therapeutic targets via pre-clinical and clinical trials i.e. an ATP receptor which stimulates K+ efflux to elicit inflammasome promoted caspase-1 stimulation. Although extensive research has been done using P2RX7 blockers, it did not show any effect in decreasing inflammation. AZD9056, a P2RX7 inhibitor has been assessed in a clinical trial for the management of arthritis, but did not depict potential efficacy (Keystone 2012). The P2RX7 inhibitor, CE-224,535 also failed to show efficacy in subjects suffering from arthritis (Stock 2012). Lastly, P2RX7 modulator, GSK1482160 was examined for safety, tolerability, and pharmacokinetics effects in normal individuals, but initiation led to an inference that it was not possible to attain the resultant effect, which resulted in the end of GSK1482160 production for inflammatory disorder (Ali 2013).
Administration of weight-loss medicines to the individuals, affected with the metabolic disorder, can alleviate adiposity, enhance insulin sensitivity, and decrease inflammation in adipose tissue which is linked with decreased NLRP3 inflammasome activity. In obese rats, weight loss is triggered by decreasing the transcription of NLRP3 proteins (Shi et al. 2010). Irregular fasting in normal and HFD mice alleviated body weight, and transcription of NLRP3, IL-1β in contrast to normal mice (Di Giovine 1987). This decline in inflammation was correlated with enhanced fasting glucose. Also, weight loss elicited by regimen rectifications resulted in reduced subcutaneous white adipose mRNA expression of NLRP3 (Landis 2002). Transcriptome investigation consequently showed reduced NLRP3 inflammasome transcription linked with augmented insulin genes i.e. glucose transporter type 4, indicating that weight loss increases insulin sensitivity in adipose tissue via NLRP3associated mechanism.
HFD-induced obese animal, preventing NLRP3 inflammasome, enhanced glucose homeostasis and insulin level. These enhancements linked to decreased inflammation in white adipose tissue, detected by IL-1β, TNF-α, IL-18 (Yagnik 2000). In obese mice, antibody therapy preventing IL-1β, enhanced insulin sensitivity (Reaven 1998). The experimental animal of diabetes mellitus, when administered with IL-1β antibody, showed elevated beta-cell mass and insulin secretion (Juraschek et al. 2013). Treatment with Anakinra, an IL-1 receptor inhibitor protein, decreased glycated hemoglobin in humans (DeMarco 2011). Together, these outcomes indicated that targeting the NLRP3 inflammasome in adipose tissue might intensely decrease inflammation, mediated through NLRP3, and consequent loss in insulin sensitivity, promoting enhancement in glucose homeostasis.
Medications including statins are used to enhance off-target effects, promoted through NLRP3 inflammasome (Henriksbo 2019). Statins are effective, cholesterol-reducing agents that subsequently decrease the risk of cardiac arrest. However, a statin elevates blood glucose and progression of diabetes mellitus, possibly through the stimulation of immune responses in adipocytes (Henriksb and Schertzer 2015). The statin remedy induces adipose tissue dysregulation and insulin resistance. The statins improved IL-1β release in bone marrow-derived macrophages through NLRP3. Suppressing caspase-1 and the NLRP3 inflammasome with diabetes agent glyburide also inhibited the statin-induced generation of IL-1β in macrophages. Statin remedy not only blocks cholesterol biosynthesis, but also the generation of isoprenoids intricated in protein prenylation cascades. The statin-dependent lowering of isoprenoids stimulates the NLRP3 inflammasome insulin resistance and consequently reduced insulin-activated lipogenesis in adipocytes. These investigations illustrate that statin-induced insulin resistance elicits adipose tissue dysregulation by NLRP3-dependent mechanism (Makkonen 1999; Mönkkönen et al. 1998; Tricarico 2018). Moreover, drugs such as glyburide that prevent NLRP3 should be arbitrated in statins to prevent user risk of producing diabetes mellitus, mitigating adverse effects (Wang 2017; Lamkanfi and Dixit 2014). Altogether, this research provides data that focuses on NLRP3 inflammasome, to enhance insulin sensitivity in adipose tissue and impair NLRP3 dependent negative adverse effects in individual approved drugs i.e. statins (Camell 2017; Abdel-Daim 2019; Vesa 2020).
Analogous to statins, bisphosphonates, which are used to treat osteoporosis and inhibit bone loss, reduce isoprenoids by suppression of farnesyl pyrophosphate synthesis. Bisphosphates elevate IL-1β release from macrophages (Finucane et al. 2015; Turpin et al. 2019; Turner et al. 2018). Although, bisphosphates do not exhibit a similar effect in diabetes mellitus, yet dysglycemia is produced in contrast to subjects consuming statins. Bisphosphonates have a greater affinity for bone tissue, although due to poor absorption, they are given via intravenous injection (Kotzbeck et al. 2018). This is significantly different from statins, the affinity of bisphosphonates for bone and distinct frequency of administration might show a difference in diabetes mellitus prevalence among individuals taking statins and bisphosphonates.
Conclusion
The appropriate stimulation of the adaptive immune response is favorable for the body to mitigate several immune and inflammatory disorders. Unevenness in the immune system might persuade several metabolic alterations at the cellular level. Cumulative evidence suggests that various cellular factors subsidize NLRP3 inflammasome stimulation that plays a chief role in adipose tissue inflammation, subsequent obesity-related tissue damage, and metabolic dysfunction. These investigations have extensive effects on several disorders, which are enhanced in obesity encompassing diabetes, cancer, and sleep apnea. Irrespective of the intricacy of the pathway, advancement has been made in the expansion of curative measures that target the NLRP3 inflammasome. The inflammasome can be responsible for augmenting IL-1β levels which can be responsible for obesity. NLRP3 inflammasome function and IL-1 formation gets amplified during obesity and several obesity-linked metabolic disorders (i.e. diabetes mellitus, hypertension, hepatic steatosis, cancer, arthritis and sleep apnea). Along with this, several factors are associated with the progression of diseases which includes higher levels of plasma glucose, fatty acids, and β-amyloid which can further activate the expression of NLRP3. The better detection of these factors can help in the regulation of inflammasome which results in improved therapeutic tactics.
Nonetheless, additional research is required to fully explicate the pathways that will augment our information on how to find exact medications and which one might result in improved therapeutic treatment. Presently, several blockers are available that target the stimulation of the NLRP3 inflammasome, which have been sustained to be beneficial in the treatment of obesity and obesity-induced insulin resistance in subjects. Apart from this, an advanced therapeutic interference in complex disorders is required to attain enhanced therapeutic outcomes. The future concern related with the same is the identification of these factors using numerous techniques. Along with this taking into consideration the implication of artificial intelligence and machine learning is placing the basis for the production of medications with a better efficiency approach.
Data availability
Not applicable
Abbreviations
- NLRP3:
-
NLR family pyrin domain containing 3
- IL:
-
interleukin
- NOD:
-
nucleotide-binding oligomerization domain
- NLRs:
-
NOD-like receptors
- ASC:
-
apoptosis-associated speck-like protein containing a caspase recruitment domain
- DAMP:
-
damage-associated molecular patter
- HFD:
-
high-fat diet
- PRRs:
-
pattern-recognition receptors
- PYD:
-
pyrin domain
- STAT :
-
signal transducer and activator of transcription
- ROS:
-
reactive oxygen species
- TRAF:
-
TNF receptor-associated factor
- P2RX7:
-
P2X purinoceptor
- JNK:
-
c-Jun N terminal kinase
- PRRs:
-
pathogen recognition receptors
- BMI:
-
body mass index
- NE:
-
norepinephrine
- AABs:
-
aged adipose B cells
- MSC:
-
mesenchymal stem cells
- ECM:
-
extracellular matrix
References
Abdel-Daim MM (2019) Applications of antioxidants in metabolic disorders and degenerative diseases: mechanistic approach. Oxidative Med Cell Longev 2019:4179676
Aganna E (2004) Allelic variants in genes associated with hereditary periodic fever syndromes as susceptibility factors for reactive systemic AA amyloidosis. Genes Immun 5(4):289–293
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124(4):783–801
Ali Z (2013) Pharmacokinetic and pharmacodynamic profiling of a P2X7 receptor allosteric modulator GSK1482160 in healthy human subjects. Br J Clin Pharmacol 75(1):197–207
Aygun AD (2005) Proinflammatory cytokines and leptin are increased in serum of prepubertal obese children. Mediat Inflamm 3:180–183
Benetti E (2016) Empagliflozin protects against diet-induced NLRP-3 inflammasome activation and lipid accumulation. J Pharmacol Exp Ther 359(1):45–53
Bryan NB (2009) Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. J Immunol 182(5):3173–3182
Camel CD (2019) Aging induces an Nlrp3 inflammasome-dependent expansion of adipose B cells that impairs metabolic homeostasis. Cell Metab 30(6):1024–1039.e6
Camell CD (2017) Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nat 550(7674):119–123
Camello-Almaraz C (2006) Mitochondrial reactive oxygen species and Ca2+ signaling. Am J Phys Cell Phys 291(5):C1082–C1088
Chi Y (2015) Apelin inhibits the activation of the nucleotide-binding domain and the leucine-rich, repeat-containing family, pyrin-containing 3 (NLRP3) inflammasome and ameliorates insulin resistance in severely burned rats. Surgery 157(6):1142–1152
Colotta F (1993) Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Sci 261(5120):472–475
Cui J (2010) NLRC5 negatively regulates the NF-κB and type I interferon signaling pathways and antiviral immunity. Cell 141(3):483–496
DeMarco MA (2011) Obesity and younger age at gout onset in a community-based cohort. Arthritis Care Res 63(8):1108–1114
Di Giovine FS (1987) Interleukin 1 (IL 1) as a mediator of crystal arthritis. Stimulation of T cell and synovial fibroblast mitogenesis by urate crystal-induced IL 1. J Immunol 138(10):3212–3218
Dinarello CA (1996) Biologic basis for interleukin-1 in disease. Blood 87(6):2095–2147
Dinarello CA (2009) Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27:519–550
Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E (2010) NLRP3 inflamasomes are required for atherogenesis and activated by cholesterol crystals that form early in disease. Nat 464:1357–1361
Finucane OM, Lyons CL, Murpy AM, Reynolds CM, Klinger C, Healy NP, Cookie AA, Coll RC, Mccallan L, Nilaweera KN, Reilley ME, Tierney AC, Morine MJ, Daza AL, Miranda JL, Connor DP, Neil LA, Mccgillicudy F, Roche FM (2015) Monounsaturated fatty acid-enriched high-fat diets impede adipose NLRP3 inflammasome-mediated IL-1β secretion and insulin resistance despite obesity. Diabetes 64(6):2116–2128
Ghayur T (1997) Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN- γ production. Nat 386:619–623
Giordano A (2013) Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J Lipid Res 54(9):2423–2436
Girousse A (2013) Partial inhibition of adipose tissue lipolysis improves glucose metabolism and insulin sensitivity without alteration of fat mass. PLoS Biol 11(2):e1001485
Goossen GH (2012) Expression of NLRP3 inflammasome and T cell population markers in adipose tissue are associated with insulin resistance and impaired glucose metabolism in humans. Mol Immunol 50(3):142–149
Harrington LE (2005) Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immun 6(11):1123–1132
Henriksb BD, Schertzer JD (2015) Is immunity a mechanism contributing to statin-induced diabetes? Adipocyte 4(4):232–238
Henriksbo BD (2019) Statins promote interleukin-1β-dependent adipocyte insulin resistance through lower prenylation, not cholesterol. Diabetes 68(7):1441–1448
Horwood NJ (1998) Interleukin 18 inhibits osteoclast formation via T cell production of granulocyte macrophage colony-stimulating factor. J Clin Invest 101(3):595–603
Hotamisligil GS, Erbay E (2008) Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol 8(12):923–934
Hotamisligil GS, Spiegelman BM (1994) Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes 43(11):1271–1278
Hotamisligil GS, Shargill S, Spiegelman M (1993) Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259(5091):87–91
Jager J (2007) Interleukin-1 beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrin 148(1):241–251
Juraschek P, Miller ER, Gelber AC (2013) Body mass index, obesity, and prevalent gout in the United States in 1988-1994 and 2007-2010. Arthritis Care Res 65(1):127–132
Kanneganti TD (2006) Critical role for cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem 281(48):36560–36568
Kanneganti TD, Ozören N, Malapel MB, Amer A, Park LH, Franchi L, Whitfield J, Barchet W, Marco Colonna M, Vandenabeele P, Bertin J, Coyle A, Grant EP, Akira S, Núñez G (2006) Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440:233–236
Kershaw EE (2006) Adipose triglyceride lipase: function, regulation by insulin, and comparison with adiponutrin. Diabetes 55(1):148–157
Keystone EC (2012) Clinical evaluation of the efficacy of the P2X7 purinergic receptor antagonist AZD9056 on the signs and symptoms of rheumatoid arthritis in patients with active disease despite treatment with methotrexate or sulphasalazine. Ann Rheum Dis 71(10):1630–1635
Kotzbeck P, Giordano A, Mondini E, Severi I, Venema W, Cecchini MP, Kershaw EE, Barbatelli G, Haemmerle G, Zechner R, Cinti S (2018) Brown adipose tissue whitening leads to brown adipocyte death and adipose tissue inflammation. J Lipid Res 59(5):784–794
Kuida K (1995) Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Sci 267(5206):2000–2003
Lamkanfi M, Dixit VM (2014) Mechanisms and functions of inflammasomes. Cell 157(5):1013–1022
Lancaster GI (2018) Evidence that TLR4 is not a receptor for saturated fatty acids but mediates lipid-induced inflammation by reprogramming macrophage metabolism. Cell Metab 27(5):1096–1110.e5
Landis RC (2002) Safe disposal of inflammatory monosodium urate monohydrate crystals by differentiated macrophages. Arthritis Rheum 46(11):3026–3033
Larsen CM (2007) Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 356(15):1517–1526
Latz E (2010) The inflammasomes: mechanisms of activation and function. Curr Opin Immunol 22(1):28–33
Lee YH (2003) c-Jun N-terminal Kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J Biol Chem 278(5):2896–2902
Lee HM (2013) Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes 62(1):194–204
Lehr S (2012) Identification and validation of novel adipokines released from primary human adipocytes. Mol Cell Proteomics 11(1):M111.010504
Lemasters JJ (2009) Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta 1787(11):1395–1401
Li P (1995) Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell 80(3):401–411
Li H, Ambade A, Re F (2009) Cutting edge: necrosis activates the NLRP3 inflammasome. J Immunol 183(3):1528–1532
Makkonen N (1999) Contrasting effects of alendronate and clodronate on RAW 264 macrophages: the role of a bisphosphonate metabolite. Eur J Pharm Sci 8(2):109–118
Mariathasan S, Weiss DS, Newton K, Jacqueline McBride J, O'Rourke K, Girma R, Lee WP, Weinrauch Y, Monack DM, Dixit VM (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nat 440:228–232
Martinon F (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nat 440:237–241
Masters SL (2010) Activation of the Nlrp3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol 11(10):897–904
Meylan E, Tschopp J, Karin M (2006) Intracellular pattern recognition receptors in the host response. Nat 442:39–44
Mönkkönen JJ, Similä J, Rogers J (1998) Effects of tiludronate and ibandronate on the secretion of proinflammatory cytokines and nitric oxide from macrophages in vitro. Life Sci 62(8):PL95–PL102
Monteleone M, Stow JL, Schroder K (2015) Mechanisms of unconventional secretion of IL-1 family cytokines. Cytokine 74(2):213–218
Moore CB (2011) NLRX1 is a regulator of mitochondrial antiviral immunity. Nature 451:573–577
Moschen AR (2011) Adipose and liver expression of interleukin (IL)-1 family members in morbid obesity and effects of weight loss. Mol Med 17(7-8):840–845
Murakami T (2012) Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A 109(28):11282–11287
Nakahira K (2011) Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 12(3):222–230
Netea MG (2006) Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med 12(6):650–656
Odegaard JI, Chawla A (2008) Mechanisms of macrophage activation in obesity-induced insulin resistance. Nat Clin Pract Endocrinol Metab 4(11):619–626
Olee T (1999) IL-18 is produced by articular chondrocytes and induces proinflammatory and catabolic responses. J Immunol 162(2):1096–1100
Olefsky JM, Glass CK (2010) Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 72:219–246
Popa AR (2020) Risk factors for adiposity in the urban population and influence on the prevalence of overweight and obesity. Exp Ther Med 20(1):129–133
Powell DJ (2003) Ceramide disables 3-phosphoinositide binding to the Pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol Cell Biol 23(21):7794–7808
Reaven GM (1998) Role of insulin resistance in human disease. Diabetes 37(12):1595–1607
Reinhard C (1997) Tumor necrosis factor alpha-induced activation of c-jun N-terminal kinase is mediated by TRAF2. EMBO J 16(5):1080–1092
Rissanen A (2012) Effect of anti-IL-1β antibody (Canakinumab) on insulin secretion rates in impaired glucose tolerance or type 2 diabetes: results of a randomized, placebo-controlled trial. Diabetes Obes Metab 14(12):1088–1096
Shao W (2007) The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J Biol Chem 282(50):36321–36329
Shi Y, Mucsi AD, Ng G (2010) Monosodium urate crystals in inflammation and immunity. Immunol Rev 233(1):203–217
Shoelson SE, Herrero L, Naaz A (2007) Obesity, inflammation, and insulin resistance. Gastroenterol 132(6):2169–2180
Stienstra R (2010) The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab 12(6):593–605
Stienstra R (2011) Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A 108(37):15324–15329
Stock TC (2012) Efficacy and safety of CE-224,535, an antagonist of P2X7 receptor, in treatment of patients with rheumatoid arthritis inadequately controlled by methotrexate. J Rheumatol 39(4):720–727
Strowig T (2012) Inflammasomes in health and disease. Nat 481:278–286
Sutterwala FS, Ogura Y, Szczepanik M, Tejero ML, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galán JE, Askenase PW, Flavel RA (2006) Critical role for NALP3/CIAS1/cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24(3):317–327
Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140(6):805–820
Taniguchi CM, Emanuelli B, Kahn R (2006) Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 7(2):85–96
Tanti JF (1994) Serine/threonine phosphorylation of insulin receptor substrate 1 modulates insulin receptor signaling. J Biol Chem 269(8):6051–6057
Tricarico PM (2018) Alendronate treatment induces IL-1B expression and apoptosis in glioblastoma cell line. Inflammopharmacol 26(1):285–290
Turner N, Xin Y, Hamish DT, Brenna O, Amanda EB, Elysha NT, Corrine EF, Hemna G, Teo JD, McEwen HP, Timothy AC, Stephen MB, Abhirup D, Greg MK, Clinton RB, Kyle LH, Fath T, Carsten SP, Gregory JC, Magdalene KM, Jonathan CM, Anthony S (2018) Don A selective inhibitor of ceramide synthase 1 reveals a novel role in fat metabolism. Nat Commun 9:3165
Turpin SM, Hammerschmidt P, Chen W, Jais A, Timper K, Awazawa V, Brodesser S, Bruining JC (2019) CerS1-Derived C 18:0 Ceramide in skeletal muscle promotes obesity-induced insulin resistance. Cell Rep 26(1):1–10.e7
Unamuno X (2019) NLRP3 inflammasome blockade reduces adipose tissue inflammation and extracellular matrix remodeling. Cell Mol Immunol
Vandanmagsar B (2011) The NALP3/NLRP3 inflammasome instigates obesity-induced autoinflammation and insulin resistance. Nat Med 17(2):179–188
Vesa CM (2020) Current data regarding the relationship between type 2 diabetes mellitus and cardiovascular risk factors. Diagnostics 10(5):314
Wang X (2015) Sodium butyrate alleviates adipocyte inflammation by inhibiting NLRP3 pathway. Sci Rep 5
Wang L (2017) NLRP3 inflammasome activation in mesenchymal stem cells inhibits osteogenic differentiation and enhances adipogenic differentiation. Biochem Biophys Res Commun 484(4):871–877
Weisberg SP (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112(12):1796–1808
Wen H (2011) Fatty acid-induced NLRP3-PYCARD inflammasome activation interferes with insulin signaling. Nat Immunol 12(5):408–415
Xia X (2010) NLRX1 negatively regulates TLR-induced NF-κB signaling by targeting TRAF6 and IKK. Immunity 34(6):843–853
Xu H (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112(12):1821–1830
Yagnik DR (2000) Noninflammatory phagocytosis of monosodium urate monohydrate crystals by mouse macrophages. Implications for the control of joint inflammation in gout. Arthritis Rheum 43(8):1779–1789
Yang Y (2019) Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis 10(2):128
Ye Y (2017) SGLT-2 inhibition with dapagliflozin reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic cardiomyopathy in mice with type 2 diabetes. Further augmentation of the effects with saxagliptin, a DPP4 inhibitor. Cardiovasc Drugs Ther 31(2):119–132
Ye Z, Ting P (2008) NLR, the nucleotide-binding domain leucine-rich repeat containing gene family. Curr Opin Immunol 20(1):3–9
Zhang SY (2018) Adipocyte-derived lysophosphatidylcholine activates adipocyte and adipose tissue macrophage nod-like receptor protein 3 inflammasomes mediating homocysteine-induced insulin resistance. EBioMedicine 31:202–216
Zhang Z (2019) circARF3 Alleviates mitophagy-mediated inflammation by targeting miR-103/TRAF3 in mouse adipose tissue. Mol Ther Nucleic Acids 14:192–203
Zorrilla EP (2007) Interleukin-18 controls energy homeostasis by suppressing appetite and feed efficiency. Proc Natl Acad Sci U S A 104(26):11097–11102
Author information
Authors and Affiliations
Contributions
AS and TB: Conceived the study and wrote the first draft of the paper; IK, SS and NS: Data compilation; LA: Proof read
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
All the authors have approved the manuscript for publication.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sehgal, A., Behl, T., Kaur, I. et al. Targeting NLRP3 inflammasome as a chief instigator of obesity, contributing to local adipose tissue inflammation and insulin resistance. Environ Sci Pollut Res 28, 43102–43113 (2021). https://doi.org/10.1007/s11356-021-14904-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14904-4