Abstract
The speciation, behaviour, and bioavailability of released selenium (Se) from mine effluent discharge to sediments and plants were assessed. Discharged mine effluent containing 65±0.9 μg/L of total Se subsequently contaminated the exposed sediment with an average total Se concentration of 321 mg/kg as well as exposed Typha latifolia plants where 534 and 92 mg/kg were found in roots and leaves, respectively. The strategy of T. latifolia in Se phytoremediation consisted of a phytostabilization and accumulation of Se predominantly in roots. Se plant root uptake was promoted by synergistic effects of Cu, Pb, Zn, and Cd while Co, Fe, Mn, Ni, Na, K, and Mg had antagonistic effects. Se plant uptake was also governed by sediment characteristics mainly pH, total Se, and iron concentration. Se speciation results demonstrated that the most accumulated Se species by T. latifolia roots were selenite and selenomethionine with average concentrations of 2.68 and 2.04 mg/kg respectively while other Se species were the most translocated (average translocation factor of 1.89). Se speciation in roots was positively correlated with sediment pH, organic matter, electrical conductivity, and iron concentration. This study confirms deploying corrective measures for mine effluent treatment before discharge in a sediment-plant environment to protect living organisms from toxic effects. T. latifolia is recommended as a Se-hyperaccumulator to be used for mine soil phytoremediation in cold regions in Canada.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mining activities have led to the mobilization of large amounts of Se into downstream soil and plants. Sediment contamination by Se released from mining activities could ultimately enter the food chain via plant uptake and consequently induce toxicity to human, grazing animals and aquatic birds (Dos Reis et al. 2017; Zhang et al. 2014; Lopes et al. 2017). Therefore, it is crucial to assess Se transfer from mine effluent discharge to the soil-plant environment and evaluate its bioavailability based on its speciation. Indeed, Se transfer from sediments into plants and subsequently into the food chain is controlled by its speciation rather than its total concentration (Wang et al. 2013; Sakizadeh et al. 2016; Peng et al. 2017; Zolfaghari et al. 2019; Etteieb et al. 2019). Additionally, the different occurring forms of Se (elemental Se, selenite, selenide, selenate, and organic Se) have a different degree of mobility and their bioavailability in the sediment-plant interface depending on various biogeochemical processes such as sorption, oxidation-reduction, precipitation, biomethylation and complexation and on sediment’s parameters mainly pH, redox potential, organic matter, microbial activity, and competing anions (Alfthan et al. 2015; Xing et al. 2015; Saha et al. 2017; Favorito et al. 2017; Shahid et al. 2018; Zolfaghari et al. 2020). Indeed, selenate is the most mobile and bioavailable form in sediments and immediately delivered to the xylem after absorption via sulphate transporters in plants. However, selenite is highly adsorbed to clay minerals and aluminium/iron oxyhydroxides in sediment and it is more accumulated in roots and rapidly converted to organoSe compounds after the absorption via phosphate transporters (Pyrzynska 2009; Zhu et al. 2009; Sharma et al. 2010; Tolu et al. 2011). Then, selenate in plants is generally reduced to selenite and then to selenide which is then transformed to selenocysteine (SeCys) and selenomethionine (SeMet) (Li et al. 2010; Hu et al. 2018). Finally, the degree of Se accumulation in plants differs according to the plant species and can be accumulated by hundreds to thousands of mg per kg dry weight in the tissues of the hyperaccumulators plants (Wang 2010; Winkel et al. 2015). Particularly, Typha plant species were previously reported for their efficient Se phytoremediation. In fact, Azaizeh et al. (2006) reported the efficiency of Typha latifolia for Se accumulation and phytostabilization mainly in fine roots after 12 weeks of treatment. Additionally, Nattrass et al. (2019) showed that Typha angustifolia L achieved 75 % of Se removal from contaminated waters after 6 days based on Se volatilization. Yen and Saibeh (2013) also demonstrated the capability of Typha angustifolia L. for iron, copper and chromium accumulation in roots, stems and leaves after uptake from copper mine effluents.
As treated mine effluent discharge represents the initial route of Se introduction into soil-plant system, the current work aimed to assess the impact of treated mine effluent discharge on Se contamination of exposed sediment and plants. To the best of our knowledge, no reported study has been conducted on Se speciation analysis on a field scale in a mining context. Specific objectives are to i) assess total Se and Se speciation in the discharged mining effluent; ii) assess the fate and bioavailability of released Se in sediments and plants by quantifying total Se and Se speciation. The characterization of Se fate, mobility, and bioavailability in the environment is a scientific challenge. The results of this study may help mine operators and stakeholders to understand the occurrence of Se in the mining environment to select appropriate technologies and sustainable management plan before mine effluent final discharge. Se levels assessment in the sediment-plant system will support the recommendation of T. Latifolia plant species as a potential candidate for selenium-contaminated mine soils phytoremediation in the context of the cold climate of the Abitibi-Temiscamingue region in Canada.
Materials and methods
Field site sampling and analysis
The effluent sample was collected in July 2019 from a treated mine discharge in the Abitibi-Témiscamingue region in Quebec, Canada. The discharged effluent mine is usually compliant with the Mining Industry Directive 019 in terms of pH which should not be less than 6 or greater than 9.5. The concentrations of thiosalts do not induce a pH change in the recipient water bodies. Other contaminants mainly arsenic, copper, iron, nickel, lead, zinc, total cyanides, hydrocarbons, and suspended matter do not exceed the maximal acceptable limits. No toxicity is also reported for the final effluents according to the results of Oncorhynchus mykiss and Daphnia magna biotests. One liter of mine effluent was collected from the outlet and stored in a Polytetrafluoroethylene (PTFE) plastic container and transported in a cool box (4±2°C). The effluent sample was preserved by the addition of nitric acid at pH <2 for total Se analysis while for Se speciation analysis, it was kept at low temperature (4–6°C) until analysis. The effluent sample was directly analyzed for temperature, pH, redox potential, dissolved oxygen, electrical conductivity using a Hach HQ40d multiparameter. Ammonium was also directly measured by electrochemistry using a Thermo Scientific Orion Star A214. Anions analysis was performed using ion chromatography (Thermo Scientific, Dionex, ICS 5000).
Sediment and plant sampling and analysis
Sediment and T. latifolia plant samples were collected in July 2019 from three different locations. The three sites were directly exposed to the flow of the treated mine effluent discharge. Site 1 was the nearest to the effluent discharge point and located at 100 m from the discharge basin. Site 2 and 3 were 500 m and 1 km distant from site 1 respectively. One kilogram of sediment samples was collected together with T. latifolia plants from the same locations. Samples were put in labeled Ziploc and transported at ambient temperature to the laboratory for analysis. Collected sediment samples were air-dried for 48 hours, sieved through a 150-μm stainless steel mesh and then finely ground and placed in hermetically sealed and identified plastic bags. Collected plants were rinsed first with tap water and then with deionized water to remove dust and sediment particles. Afterward, their different parts (leaves and roots) were separated. Plant leaves were dried at 60°C until constant weight while plant roots were air-dried. Once dried, leaves and roots were ground to a fine powder (< 0.05 mm) and placed in hermetic sealed and identified plastic bags and stored in the dark.
Sediment samples were analyzed for pH and conductivity by electrochemistry using a Hach HQ40d multi multi-parameter, total organic carbon (TOC) was analyzed by TOC Analyzer (Shimadzu, TOC-VCPH) and organic matter was determined by Computrac.
Se determination in treated mine effluent, sediments, and plants
Total Se analysis in effluent, sediments, and plant samples
Forty grams of effluent sample digestion was performed by adding 2 mL of concentrated HNO3 and 1 mL of concentrated HCl and heating at 105 ± 2°C for 8–14 h. One-gram sediment sample digestion was performed by adding 4 ml nitric acid and 10 ml hydrochloric acid and heating at 95°C. About 5 g of plant sample was digested by the addition of 15 ml of concentrated nitric acid, 2 ml hydrogen peroxide (30%), 2 ml concentrated hydrochloric acid and heating at 95°C. After cooling of the different digested solutions, deionized water was added and the test solution was filtered via 0.45 μm nylon membrane filter disc before introduction into the collision/reaction cell inductively coupled plasma-mass spectrometer CRC ICP-MS instrument (Agilent 7900) and analysis according to modified EPA Method 200.2, 200.3 and 200.8/6020A. Cations and heavy metals including (Al, Cd, Ca, Cr, Co, Cu, Fe, Mg, Mn, Ni, Pb, K, Na, Zn) in the effluent, sediment, and plant samples were analyzed using the same instrument.
Se speciation analysis in effluent, sediments, and plant samples
An exchangeable fraction of the sediment sample was extracted using phosphoric acid (0.1 M). Plant samples were homogenized and extracted using a methanol/enzymatic extraction procedure. Two grams of plant sample was extracted with 1 mL lipase/alpha-amylase solution and 0.5 mL protease in 25% methanol solution. Homogenized effluent sample, sediments, and plant extracts were filtered through a 0.2-μm nylon membrane filter disc and then introduced into HPLC (Agilent 1200) coupled to ICP-MS (Agilent 7700) and analyzed. Quantified species were selenite Se (IV), selenate Se (VI), and selenomethionine (SeMet). Unknown Se species consisted of a sum of all the unknown peaks observed in the chromatogram.
Quality assurance of data
For quality control, National Research Council Canada (NRC) certified reference materials (CRM) and laboratory control samples were analyzed concurrently with sediment and plant samples. The CRM used for sediment analysis was NRC Canmet Till 2 (sediment collected near Scission's Brook, New Brunswick, CA) with a total Se content of 0.361 mg/kg (0.4 mg/kg obtained concentration belonging to the range 0.11 to 0.55 mg/kg). The CRM used for plant analysis was ERM-BC210a (Wheat Flour, LGC Ltd.) containing 17.23 mg/kg of Se total (108.3% recovery). Recovery of heavy metals and cations was of 105.3% for Al, 100.6% for As, 100.2% for Cd, 103.5% for Ca, 108.6% for Cr, 105.6% for Co, 101.8% for Cu, 108.6% for Fe, 102.0% for Pb, 102.1% for Mg, 96.4% for Mn, 97.5% for Ni, 97.6% for P, 104.1% for K, 104.3% for Na, and 108.0% for Zn.
The CRMs used for selenium speciation were NRC SELM-1 (selenium-enriched yeast) containing 1314 mg/kg of selenomethionine (74.4 % recovery) and ERM-BC210a (Wheat Flour, LGC Ltd.) containing 10.49 mg/kg of selenomethionine (89.9 % recovery). Selenium species were also quantified by laboratory control sample consisting of 7.5 mg/kg of selenite, selenate, and selenomethionine (83.8%, 92.7% and 82.8% recovery respectively). The detection limits ranged between 0.01 and 0.07 for total selenium analysis and between 0.05 and 0.2 for speciation. For quality assurance, three samples were analyzed in duplicate. The relative percent difference in total Se was 1.4% while it was ranging between 1.2 and 2.9% for Se species. These relative percent differences were deemed acceptable as they were inferior to 30 % for total Se and to 20% for Se species.
Statistical analysis
Statistical correlation between total Se levels and heavy metals (Al, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, Zn) and cations (Ca, K, Mg, Na) concentrations in sediment and plant were performed using correlation matrices on Excel (Microsoft, Redmond, WA, USA). Pearson correlations were calculated using the “= CORREL (array1, array2)” function from Excel between Se concentration and metals and cations concentrations in sediment and plants. To determine synergic and antagonist effects of heavy metals and cations on Se occurrence in sediments and plant, the correlation coefficient (r) described the degree of correlation between two variables and was calculated using Eq. 1:
where x and y are the samples means AVERAGE (array1) and AVERAGE (array2).
In this study, we extracted the relevant data when r ≥0.5 or r<−0.5
Later, regression analysis was used to determine the effect of sediment characteristics on total Se and Se speciation concentration in sediment and plant. Correlations were calculated using the LINEST function from Excel. To determine which sediment factors (independent variables) contributed most to the increase of total Se and Se specie concentration in sediments and plant (dependent variable), the determination coefficient (r2) is the square of the correlation coefficient previously calculated and which described the degree of correlation between two variables. Relevant data were extracted when the determination coefficient r2>0.5. P values <0.05 were considered statistically significant.
Results and discussion
Se occurrence in treated mine effluent discharge
Mine effluent characterization revealed its acceptability in terms of respecting the maximal limit allowed by REMM (Regulation of metal mine effluents (DORS/2002-222) concerning the pH value 7.43±0.19 (comprised between 6 and 9.5) and the concentrations of the metals mainly; zinc 0.08±0.01 mg/L (< 0.5 mg/L), copper <0.01 mg/L (< 0.3 mg/L), nickel <0.005 mg/L (<0.5 mg/L) and lead <0.01 mg/L (<0.2 mg/L). The total Se content in mine effluent was 65±0.9 μg/L with 57±6.8 μg/L identified as selenite and 7.2±0.5 μg/L identified as selenate (Table 1). The speciation results showed that the predominant Se species was selenite presenting more than 80% of the Se concentration in the final treated effluent. Based on its potential hazard, Se is expected to be placed among the high worrying priority in the mining industry. Since regulations are still under preparation, the mine effluent can be discharged into the nearby river water. However, since Se is bioaccumulative, it may pose a threat to the environment, aquatic life and the ecosystem. Environment. Climate Change-Canada (ECCC) prescribed a Se discharge limit of 1 μg/L in surface water. In the current study, the high Se level of 65.3 μg/L in mine effluent is due to strongly saline and acid drainage water resulting from the weathering and oxidation of Se-rich waste rock dump. In fact, previous studies reported that the weathering of pyrite and its leakage by the rainfall leads to the mobilization, conversion and transport of large amounts of originally immobile Se (dissolved or adsorbed) from the mine into the surrounding environment (Donner et al. 2018). Moreover, high Se levels in mine effluents can also result from the addition of extraction reagents such as cyanide. Indeed, Khamkhash et al. (2017) reported that Se concentrations at Red Dog zinc mine exceeded the standard with 12 μg/L due to the use of cyanide as flotation reagents. Furthermore, 80% of Se consisted of selenite as predominant species in the studied mine discharge with 57±6.8 μg/L. These observations revealed the presence of a reducing acidified environment upstream of the discharge point although the effort of mine managers to install a system to increase the pH to a value of 7.43±0.19. In fact, the predominance of selenite species at low pH was previously reported in the case of Sobov surface mine located near the city of Banska Stiavnica in Slovakia where 40% of Se was present as selenite in the drainage water at pH 2.18 while in Bela lake water at neutral pH, the concentration of selenite was moderate (Bujdoš et al. 2005). Martin et al. (2011) confirmed that the predominance of selenate to approximately 95% reflects the near-fully oxygenated conditions in the water. The combination of a decrease in pH and a large increase of the sulfate content also supports the hypothesis of pyrite oxidation in the overburden (Bond 2000).
Se occurrence in sediment and bioavailability in Typha latifolia plants
Total Se in sediments and plants
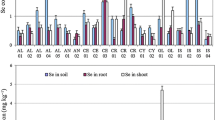
Se content in sediments was significantly correlated with Se in plant tissues with correlation coefficients (r) of 0.84 for roots and 0.97 for leaves. A significant positive relationship between Se in roots and leaves was also found with corresponding correlation coefficients of 0.7 (Table 2). This positive correlation between Se in sediments and plants confirmed the capacity of T. latifolia root system to uptake Se from sediments and to translocate and accumulate it in leaves. The increase of Se contents in T. latifolia plants was associated with the increase of Se concentration in the collected sediments from the same location. Indeed, Se sediment concentrations showed the highest levels in site 1 (342 mg/kg), site 3 (317 mg/kg) and then site 2 (304 mg/kg) and consequently Se content in plants showed the same pattern of the highest level for site 1 (813 mg/kg), then site 3 (688 mg/kg) and finally site 2 (378 mg/kg) (Fig. 1). Moreover, Se was twice more accumulated in plant roots (average of 642 mg/kg) than in sediments (average value 329 mg/kg) for site 1 and 3. Se was also significantly more accumulated in plant roots than in leaves. In fact, in site 1, Se was five times higher in roots (669 mg/kg) than that in leaves (144 mg/kg). This result was confirmed for site 2 and 3 as well since Se was accumulated in roots (317 mg/kg) five times more than in leaves (61 mg/kg) in site 2 while in site 3 Se was accumulated in roots (616 mg/kg) eight times higher than in leaves (71 mg/kg) (Fig. 1). These results confirmed the high capacity of T. latifolia for Se uptake from sediments and its accumulation predominantly in roots and then in leaves. As T. latifolia was able to accumulate more than 100 mg/kg in its roots, it could be considered as a Se hyperaccumulator plant specie while Se levels do not exceed 50 and 100 mg/kg for non-accumulators and secondary accumulators respectively (Winkel et al. 2015).
Bioconcentration, accumulation, and translocation of Se, heavy metals, and cations in sediment and Typha latifolia tissues
Besides Se, sediment and plant contamination with heavy metals mainly Al, Fe, Mn, Cr, Cu, Zn, Ni, and Pb was noticed. Equal levels in sediment and roots were found for lead (26 and 38 mg/kg respectively) and cadmium (<5 and 4.74 mg/kg respectively) while the highest manganese levels were accumulated in sediment and leaves (277 and 215 mg/kg respectively) and the highest levels of chromium (100 mg/kg), copper (185 mg/kg), zinc (1708 mg/kg), nickel (27 mg/kg), aluminium (13353 mg/kg), cobalt (9.41 mg/kg), and iron (15093 mg/kg) were accumulated in plant roots. High levels of some cations essentially calcium, magnesium, potassium and sodium were observed. Equal proportions of magnesium were observed in sediment and roots (3927 and 4113 mg/kg respectively) while calcium was predominant in sediment (20,2333 mg/kg), potassium predominated in plant leaves (28533 mg/kg) and sodium in roots (1781 mg/kg) (Fig. 1). These results demonstrated the T. latifolia roots can easily uptake Se, Co, Cu, Cr, Ni, Zn, Al, Fe, Cd, and Pb from sediment and accumulate them in roots with a small part being translocated and accumulated in leaves. Manganese tends to be easily translocated and accumulated in leaves rather than staying in roots. To compare the uptake and translocation capacity of metals in the sediment-plant system, three parameters, bio-concentration factor (BCF) in roots, bioaccumulation (BAF) factor in leaves and translocation factor (TF) to leaves were calculated. BCF was the ratio of metal concentration in roots and that in their sediments, BAF was the ratio of metal concentration in leaves and that in sediment, and TF was the ratio between metal concentration in leaves and that in roots. Results demonstrated that the most accumulated metals in T. latifolia’s roots having BCF superior to 1 were Co (7.78), Cr (3.6), Fe (3.26), Ni (2.67), Zn (2.75), Cu (1.78), Se (1.74), Al (1.64), and Pb (1.35) as ranked from highest to lowest. Cr and Ni were also well accumulated in leaves (BAF of 1.39). Mn was the easiest and greatest translocated metal from roots to leaves (TF of 1.17) (Fig. 2). Cations were accumulated both in roots and leaves with great translocation of potassium to leaves (TF of 3.46) and high accumulation there (BAF of 24) (Fig. 3). These results demonstrated the efficient phytoextraction capacity of T. latifolia for the following metals in descending order Co, Cr, Fe, Ni, Zn, Cu, Se, Al, and Pb as they were the most uptaken from sediment and then accumulated in plant roots. Se particularly was more concentrated in roots than in leaves with low translocation factor to leaves (0.17). Indeed, the Se uptake from sediment and accumulation in plant tissues is governed by its speciation and the influence of a variety of sediment factors mainly pH, redox conditions, organic matter, sediment texture, biological and microbial activity, Se contents, and competing anions (Funwie 2012; Alfthan et al. 2015; Xing et al. 2015; Saha et al. 2017; Shahid et al. 2018). Moreover, the predominant accumulation of Se in roots reflects the strategy of T. latifolia in Se phytoremediation which consisted of a phytostabilization of Se and its accumulation in roots rather than its translocation. In fact, Se and other metals mainly Al, Cd, Co, Cr, Cu, Zn, Pb, Fe, and Ni were retained in plant roots with low translocation to leaves. In particular, Se competed with manganese for being translocated to leaves as manganese was almost ten times more translocated than Se TF (1.17). Although most of the Se was excluded in roots, a small part was translocated and accumulated in leaves. Therefore, T. latifolia demonstrated its tolerance for excessive heavy metals levels and it could be considered as a potential plant species for Se phytoremediation in mine-contaminated soils. Moreover, the presence of nickel, chromium, and manganese as the most accumulated metals in the edible part of T. latifolia represents a potential risk for grazing animals and consequently a risk to human health through food chains. Thus, phytoextraction of Se with T. latifolia could be a potential approach that can be integrated with other treatment in mining industry to comply with current legislations (Xing et al. 2020; Guemiza et al. 2020; Brar et al. 2021).
Evaluation of synergistic and antagonistic effects of competitive ions on Se occurrence in sediments and for plant uptake
Correlation matrix results demonstrated that Se in sediment was positively correlated with copper (r=0.81), lead (r=0.95), and zinc (r=0.76) and it was correlated to these same metals (Cu, Pb, and Zn) in roots next to cadmium (r=0.99). In leaves, besides Cu, Pb, and Zn, and Cd, Se was positively correlated with nickel (r=0.99), chromium (r=0.97), and cobalt (r=0.71). However, Se was inversely correlated mainly with cobalt (r= −0.76) and iron (r= −0.62) in sediment and cobalt (r= −0.8), manganese (r= −0.82), nickel (r= −0.73), and iron (r= −0.67) in roots. Additionally, Se was inversely correlated with most of cations mainly sodium in sediment (r= −0.62), then sodium (r= −0.98), potassium (r= −0.94), and magnesium (r= −0.78) in roots and finally sodium (r= −0.81) and calcium (r= −0.85) in leaves (Table 3). Thus, a synergistic effect in sediment was confirmed among Se, copper, lead and zinc next to cadmium in roots and nickel, chromium and cobalt in leaves. Nevertheless, Se levels were inhibited by the occurrence of other metals like cobalt and iron in sediment next to manganese and nickel in roots while Se levels were unaffected by other metals in leaves. Whereas, antagonistic effects of Se with cations mainly sodium in sediment, next to potassium and magnesium in roots and besides calcium in leaves were identified. Therefore, Se enters in competition with some heavy metals mainly Co, Fe, Mn, and Ni and some cations like Na, K, and Mg for its uptake from sediment to root system while for accumulation in leaves, it competes only with some cations such as Ca and Na. The antagonistic effect of Mn and Fe in roots is due to the capacity of these mineral phases to adsorb Se especially selenite species under low pH and reducing environment. For cations, they usually show this antagonistic effect to Se as the plant tends to acquire cations as needed nutrients for its growth and therefore Se competes with them. Briefly, high levels of Cu, Pb, Zn, and Cd associated with low levels of Co, Fe, Mn, Ni, Na, K, and Mg promote Se plant root uptake while Se accumulation in plant leaves is only inhibited by Ca and Na while other metals like Cu, Pb, Zn, Cd, Ni, Cr, and Co had a synergistic effect on Se accumulation in plant leaves.
Influence of sediment characteristics on total Se concentrations in sediments and plant uptake
Regression measurements of Se correlation to sediment factors demonstrated that sediment factors (pH, organic matter, total organic carbon, and sediment iron concentration) had no significant effect on sediment Se content. However, Se content in plant roots was positively correlated with Se and iron concentrations in sediment (r=0.71 and 0.88 respectively) and it showed a significant correlation with sediment pH (r=0.86) (Table 4). In fact, although Se concentration in sediment was insignificantly correlated with the mean sediment pH (7.23±0.20) (r = 0.34), Se content in plant roots was significantly correlated with sediment pH (r=0.86). The high Se uptake by T. latifolia roots following the increase of pH is due to the presence of an oxidizing and alkaline environment that helps the predominance of more soluble Se species like selenate that are easily uptaken by plant roots. However, for low pH, selenite species predominates and tends to be highly adsorbed to sediment particles and thus less bioavailable to plants (Otieno et al. 2015).
Moreover, no significant positive correlations were observed between Se concentration and both carbon level and organic matter content in the sediment (r= 0.02) meaning that carbon and organic matter content has no impact on Se accumulation in sediment. This may be due to the low content of organic matter in sediment samples (mean value of 4±0.82% and 6.9±1.42 % of total organic carbon and organic matter respectively). Besides, there is no external organic pollution source added as mine effluent has very low levels of total organic carbon (<0.001 mg/L). In addition, organic matter showed a non-significant positive correlation with Se content in roots and leaves (r=0.43 and 0.006 respectively). Other studies declared that soil organic matter shows a very poor correlation with Se in soil and plants due to the low bioavailability of organic matter-bound Se for plant uptake and presence of Se in large undecomposed organic molecules such as proteins. The increase of organic matter induces a decrease of Se plant uptake by influencing the soil pH. In fact, the increase of organic matter is associated with soil acidification and low pH through the slow release of carbon dioxide. This reducing environment subsequently favorites the conversion of Se to insoluble forms which are tightly bound to soil particles and metallic complexes (Dhillon et al. 2010). Furthermore, Se in roots had a positive correlation with sediment iron (r= 0.88) while Se in leaves was poorly correlated with sediment iron (r=0.17). Therefore, the significant impact of sediment iron was detected only on Se concentrations in plant roots and therefore on Se plant uptake. Se concentrations in soil and plants were reported to be strongly related to the pyrophosphate extractable iron content of the soil. Se in soil and especially selenite species is associated with iron as ferric selenides (Arnold 1989; Rodrigo et al. 2017).
Se Speciation in sediments and bioavailability to Typha latifolia plants
Se speciation in sediment and plants
Se speciation in sediment demonstrated the bioavailable exchangeable fraction was composed of concentrations <1 mg/kg of selenate and <1 mg/kg of selenite while the remaining part of total Se was composed of carbonate bound, mineral oxide bound, organic matter bound plus an unavailable residual fraction. In fact, in soils, the soluble Se compounds are rapidly converted back into immobile species, probably by reduction to elemental Se or inorganic selenides (Goodson et al. 2003; Bujdoš et al. 2005). The Se reduction process was encouraged by the reducing conditions existing in mine effluent discharge due to the predominance of selenite. Moreover, the bioavailable fraction of Se species in plant roots were present in the following order selenite (2.68 mg/kg)>selenomethionine (2.04 mg/kg)>unknown Se specie (1.17 mg/kg)>selenate (0.87 mg/kg) while in leaves they followed this order selenite (2.16 mg/kg)> unknown Se specie (2.1 mg/kg)> selenomethionine (0.77 mg/kg)> selenate (0.15 mg/kg) (Fig. 4). Thus, selenite and selenomethionine predominated in T. latifolia roots while in leaves selenite and unknown Se species were dominants. The predominance of selenite and organic Se species in roots is due to the aptitude of selenite to persist in roots and to be converted into organic species. The presence of selenate in lower concentration in roots is due to its higher mobility compared to selenite in roots and hence its major parts are being rapidly translocated to aerial parts of the plant. Then, the low concentration of identified selenate in leaves compared to organic Se species is due to the Se metabolism cycle in leaves consisting of reducing selenate to selenite then to organoselenide mainly selenocysteine and selenomethionine. These findings were consistent with previous studies as Wang (2010) has already reported that the most accumulated Se species in roots was SeMet, followed by selenite, then selenate. This confirms that selenate is more easily transported from root to leaf while selenite is readily transformed into organic Se after plant root uptake (Ramos et al. 2010). Li et al. (2007) have also declared that compared to selenite, organic Se (SeMet) remains the major species showing a much higher intensity in root samples due to the conversion from selenite to organic Se forms (mostly SeMet) in plant roots. In fact, SeMet was the most abundant organic Se species found in both leaf and root and its amount was approximately two folds of selenate.
Influence of sediment characteristics on Se speciation and plant uptake
Concerning the impact of sediment factors on Se speciation, only Se species (selenite, SeMet and unknown Se species) in roots were positively correlated with sediment pH, organic matter and iron concentration. Both Se species in roots and leaves were correlated to sediment electrical conductivity (Table 5). Indeed, selenite, SeMet, and unknown Se species in roots were significantly highly correlated with sediment pH (correlation coefficient of 0.52, 0.96 and 0.97 respectively). In fact, Se plant uptake is closely related to Se species solubility in the sediment which increases in presence of alkaline conditions encouraging the formation of more oxidized species, mainly selenate ions (Wang 2010).
Additionally, only Se species in roots and mainly selenite, SeMet and unknown Se species were significantly correlated with sediment organic matter (correlation coefficient of 0.91, 0.92 and 0.65 respectively). Indeed, organic Se species increase in the presence of high organic matter content in the sediment (Supriatin et al. 2016). Organic matter contributes to Se immobilization and increasing the availability of Se to plants (Garcia Moreno et al. 2013). Moreover, selenite, SeMet, and selenate in roots were positively correlated to electrical conductivity (0.96, 0.52, and 0.83 respectively) as well as selenite and SeMet in leaves (0.59 and 0.97 respectively). Furthermore, only SeMet and unknown Se species concentrations in roots were positively correlated with an iron concentration in sediment (0.94 and 0.98 respectively). Thus, the concentrations of SeMet and other Se species increase in roots when the iron level increases in sediment. This is due to the adsorption of inorganic species by high levels of iron in sediment rather than organic species which become more bioavailable for plant uptake by roots. Indeed, selenite is the most adsorbed species by iron oxide especially under a reducing environment while above pH 7-8, the solubility of Se increases and selenite is broken down and released into the soil solution. In the presence of high iron concentrations, insoluble iron hydroxide - selenite complexes can be found (Wang 2010).
Influence of Se speciation on Se bioavailability for plant uptake, translocation, and accumulation
The uptaken Se species by T. latifolia roots were selenite, Selenate, SeMet, and unknown Se species. Selenite and SeMet particularly were the most accumulated in plant roots with average concentrations of 2.68 and 2.04 mg/kg respectively. Then, the calculation of the translocation factor for each species from roots to leaves demonstrated that the most translocated species (TF> 1) were selenite (average TF of 1.1) and unknown Se species (average TF of 1.89) which represent as well the most accumulated species in leaves with average concentrations of 2.16 and 2.1 mg/kg respectively (Fig. 5). Selenite, selenate, and SeMet have usually been reported as the most bioavailable species in sediment for plant uptake and their presence in plant tissues confirms their solubility, mobility and bioavailability (Otieno et al. 2015). Nothstein et al. (2016) have previously confirmed that selenite accumulated more in roots than in shoots. Organic Se was also more dominant in the lower parts of cereal roots. In fact, selenate is taken up actively by the sulphate transporter and readily transported via the xylem into plant shoots and leaves, while selenite is rapidly transformed into organic Se in the roots. Thus, the presence of high levels of selenite, SeMet, and other unknown Se species in our root samples could be justified by the conversion of selenite in the root into methylated or unmethylated organic forms stored in high levels in the roots and which could be present as a free amino acid, such as MeSeCys or incorporated into proteins. The low level of selenate in roots is also due to its reduction to selenite. Then, organic Se compound may be transported as selenomethionine (SeMet) and other organic species to aerial parts exclusively via the phloem, whereas selenate was transported via xylem. In leaves, high levels of selenite are due to the selenate reduction in the chloroplasts which produces selenite under the effect of ATP sulfurylase (APS) and APS reductase (APR) enzymes (Gupta and Gupta 2017). Subsequently, selenite is reduced to selenide using glutathione (GSH), which is later converted into selenocysteine under the action of cysteine synthase enzyme coupled with O-acetylserine (Wallenberg et al. 2010). Finally, selenocysteine is transformed into selenomethionine (SeMet) under the sequential action of the cystathionine-Ɣsynthase (CƔS), cystathionine-b-lyase and methyl synthase enzymes (Gupta and Gupta 2017). Huerta et al. (2003) and Li et al. (2010) confirmed that the main absorbed Se species by plants are selenate, selenite, and organic Se. In particular, Selenate ion is the most readily taken up by plants using the absorption mechanism which is similar to that for sulphate ions. Uptake of organic Se compounds likely occurs via the same mechanisms as the uptake of corresponding sulfur analogs while selenite uptake occurs via passive diffusion, and is inhibited by phosphate. Oram et al. (2011) have also confirmed that once taken up by roots, absorbed selenite species are quickly metabolized to organic Se which are not translocated to shoots. Selenate is actively taken up by a sulfate transport protein and is transported to the shoot as selenate, where it is reduced to various Se biomolecules.
Conclusion
Although mine effluent was compliant with REMM standards in terms of pH, zinc, copper, nickel, and lead, total Se was reported at a high level of 65±0.9 μg/L from which 57±6.8 μg/L was identified as selenite. This discharged effluent subsequently contaminated the surrounding sediment and plant environment with high levels of total Se reaching an average concentration of 321 mg/kg in sediment, 534 mg/kg in plant roots and 92 mg/kg in plant leaves. These results confirmed the high aptitude of T. latifolia for Se uptake from sediment and its accumulation predominantly in roots and then in leaves. Se particularly was more concentrated in roots with low translocation factor to leaves (0.17). This reflects the mechanism of Se phytostabilization in roots which usually succeed to mitigate Se levels when achieved by a high-biomass plant species like T. latifolia. The increase of Se plant root uptake was promoted by high levels of Cu, Pb, Zn, and Cd in association with low levels of Co, Fe, Mn, Ni, Na, K, and Mg while Se accumulation in plant leaves was only inhibited by Ca and Na. Moreover, pH, total Se and iron concentration influenced Se plant uptake. Furthermore, Se speciation results demonstrated that selenite and selenomethionine predominated in T. latifolia roots with average concentrations of 2.68 and 2.04 mg/kg respectively. In addition, the most translocated species from roots to leaves (TF> 1) were unknown Se species (average TF of 1.89) which represent as well the most accumulated species in leaves with a concentration of 2.1 mg/kg. Finally, sediment factors had an impact only on Se species (selenite, SeMet, and unknown Se species) in roots. Further studies on selenium fate assessment in the real soil-plant system in relation to animal and human health are required. This study will urge stakeholders to minimize the exposure of water, soil, and plant environment to the discharge of mine effluents to prevent the accumulation of toxic levels of Se in plants and subsequently avoid poisonous effects for grazing animals and the possible introduction of Se in higher trophic levels. Based on this study results, T. Latifolia is recommended as a potential Se- hyperaccumulator plant species to be used for the phytoremediation of Se-rich mine soils in cold climate regions in Canada.
Data availability
The data that support the findings of this study are available from the corresponding author on request.
References
Alfthan G, Eurola M, Ekholm P, Venäläinen ER, Root T, Korkalainen K, Hartikainen H, Salminen P, Hietaniemi V, Aspila P, Aro A (2015) Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J Trace Elem Med Biol 31:142–147
Arnold L (1989) The Influence of Geochemistry on Selenium in Soils and Plants of England and Wales. University of London, Dissertation
Azaizeh H, Salhani N, Sebesvari Z, Shardendu S, Emons H (2006) Phytoremediation of selenium using subsurface-flow constructed wetland. Int J Phytoremediation 8(3):187–198
Bond M (2000) Characterization and control of selenium releases from mining in the Idaho phosphate region. Dissertation, University of Idaho
Brar KK, Magdouli S, Etteieb S, Zolfaghari M, Fathollahzadeh H, Calugaru L, Komtchou SP, Tanabene R, Brar SK (2021) Integrated bioleaching-electrometallurgy for copper recovery - A critical review. J Clean Prod 291:125257
Bujdoš M, Muľová A, Kubova J, Medveď J (2005) Selenium fractionation and speciation in rocks, soils, waters and plants in polluted surface mine environment. Environ Geol 47:353–360
Dhillon KS, Dhillon SK, Dogra R (2010) Selenium accumulation by forage and grain crops and volatilization from seleniferous soils amended with different organic materials. Chemosphere 78:548–556
Donner MW, Cuss CW, Poesch M, Sinnatamby RN, Shotyk W, Siddique T (2018) Selenium in surface waters of the lower Athabasca River watershed: Chemical speciation and implications for aquatic life. Environ Pollut 243:1343–1351
Dos Reis AR, El-Ramady H, Santos EF, Gratao PL, Schomburg L (2017) Overview of selenium deficiency and toxicity worldwide: affected areas, selenium-related health issues, and case studies. In: Pilon-Smits E, Winkel L, Lin ZQ (eds) Selenium in plants. Plant ecophysiology. Springer, Cham, pp 209–230
Etteieb S, Magdouli S, Zolfaghari M, Kaur Brar S (2019) Monitoring and analysis of selenium as an emerging contaminant in mining industry: A critical review. Sci Total Environ 698:134339
Favorito JE, Luxton TP, Eick MJ, Grossl PR (2017) Selenium speciation in phosphate mine soils and evaluation of a sequential extraction procedure using XAFS. Environ Pollut 229:911–921
Funwie AV (2012) Effect of Soil Properties on Availability and Mobility of Selenium. Dissertation, University of Gent
Garcia Moreno R, Burdock R, Díaz Álvarez MC, Crawford JW (2013) Managing the selenium content in soils in semiarid environments through the recycling of organic matter. Appl Environ Soil Sci 2013:283468
Goodson CC, Parker DR, Amrhein C, Zhang Y (2003) Soil selenium uptake and root system development in plant taxa differing in Se-accumulating capability. New Phytol 159:391–401
Guemiza K, Saffar T, Gmar M, Magdouli S, Foudhaili T (2020) Thermal Desorption and Incineration- he Handbook of Environmental Remediation: Classic and Modern Techniques. Royal Society of Chemistry, Cambridge
Gupta M, Gupta S (2017) An overview of selenium uptake, metabolism, and toxicity in plants. Front Plant Sci 7:2074
Hu Z, Cheng Y, Suzuki N, Guo X, Xiong H, Ogra Y (2018) Speciation of Selenium in Brown Rice Fertilized with Selenite and Effects of Selenium Fertilization on Rice Proteins. Int J Mol Sci 19:3494
Huerta VD, Reyes LH, Marchante-Gayon JM, Sanchez MLF, Sanz-Medel A (2003) Total determination and quantitative speciation analysis of selenium in yeast and wheat flour by isotope dilution analysis ICP-MS. J Anal At Spectrom 18:1243–1247
Khamkhash A, Srivastava V, Ghosh T, Akdogan G, Ganguli R, Aggarwal S (2017) Mining-related selenium contamination in Alaska, and the state of current knowledge. Minerals 7:46
Li HF, McGrath SP, Zhao F (2007) Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol 178:92–102
Li HF, Lombi E, Stroud JL, Mcgrath SP, Zhao FJ (2010) Selenium Speciation in Soil and Rice: Influence of Water Management and Se Fertilization. J Agric Food Chem 58:11837–11843
Lopes G, Ávila FW, Guilherme LRG (2017) Selenium behavior in the soil environment and its implication for human health. Ciênc Agrotec 41(6):605–615
Martin AJ, Simpson S, Fawcett S, Wiramanaden CIE, Pickering IJ, Belzile N, Chen YW, London J, Wallschläger D (2011) Biogeochemical Mechanisms of Selenium Exchange between Water and Sediments in Two Contrasting Lentic Environments. Environ Sci Technol 45(7):2605–2612
Nattrass M, McGrew NR, Morrison JI, Baldwin BS (2019) Phytoremediation of selenium-impacted water by aquatic macrophytes. JASMR 8(1):69–79
Nothstein AK, Eiche E, Riemann M, Nick P, Winkel LH, Göttlicher J, Steininger R, Brendel R, Brasch M, Konrad G, Neumann T (2016) Tracking Se assimilation and speciation through the rice plant–nutrient competition, toxicity and distribution. PLoS One 11:e0152081
Oram L, Strawn DG, Moller G (2011) Chemical Speciation and Bioavailability of Selenium in the Rhizosphere of Symphyotrichum eatonii from Reclaimed Mine Soils. Environ Sci Technol 45(3):870–875
Otieno SB, Jayne TS, Muyanga M (2015) The effects of Soil Chemical Characteristics on Accumulation of Native Selenium by Zea Mays Grains in Maize Belt in Kenya. Int J Appl Sci Technol 5(1):1163–1167
Peng Q, Wang M, Cui Z, Huang J, Chen C, Guo L et al (2017) Assessment of bioavailability of selenium in different plant-soil systems by diffusive gradients in thin-films (DGT). Environ Pollut 225:637–643
Pyrzynska K (2009) Selenium speciation in enriched vegetables. Food Chem 114:1183–1191
Ramos SJ, Faquin V, Guilherme LRG, Castro EM, Ávila FW, Carvalho GS, Bastos CEA, Oliveira C (2010) Selenium biofortification and antioxidant activity in lettuce plants fed with selenate and selenite. Plant Soil Environ 56(12):584–588
Rodrigo S, Santamaria O, Perez-Izquierdo L, Poblaciones MJ (2017) Arsenic and selenium levels in rice fields from south-west of Spain: influence of the years of monoculture. Plant Soil Environ 63(4):184–188
Saha U, Fayiga A, Sonon L (2017) Selenium in the soil-plant environment: a review. Int J Appl Agric Sci 3:1–18
Sakizadeh M, Sharafabadi FM, Shayegan E, Ghorbani H (2016) Concentrations and Soil-To-Plant Transfer Factor of Selenium in Sediment and Plant Species from an Arid Area. IOP Conf. Series: Earth and Environmental. Science 44(5):052027
Shahid M, Niazi NK, Khalid S, Murtaza B, Bibi I, Rashid MI (2018) A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ Pollut 234:915–934
Sharma S, Bansal A, Dhillon SK, Dhillon KS (2010) Comparative effects of selenate and selenite on growth and biochemical composition of rapeseed (Brassica napus L.). Plant Sediment 329:339–348
Supriatin S, Weng L, Comans RNJ (2016) Selenium-rich dissolved organic matter determines selenium uptake in wheat grown on Low-selenium arable land soils. Plant Soil 408(1):73–94
Tolu J, Le Hécho I, Bueno M, Thiry Y, Potin-Gautier M (2011) Selenium speciation analysis at trace level in soils. Anal Chim Acta 684:126–133
Wallenberg M, Olm E, Hebert C, Björnstedt M, Fernandes AP (2010) Selenium compounds are substrates for glutaredoxins: a novel pathway for selenium metabolism and a potential mechanism for selenium-mediated cytotoxicity. Biochem J 429:85–93
Wang X (2010) Selenium Speciation in Arabidopsis Thaliana. Dissertation, Norwegian University of Life Sciences
Wang J, Li H, Li Y, Yu J, Yang L, Feng F et al (2013) Speciation, distribution, and bioavailability of soil selenium in the Tibetan Plateau Kashin–Beck disease area—a case study in Songpan County, Sichuan Province, China. Biol Trace Elem Res 156:367–375
Winkel L, Vriens B, Jones G, Schneider L, Pilon-Smits E, Bañuelos G (2015) Selenium cycling across soil-plant-atmosphere interfaces: a critical review. Nutrients 7:4199–4239
Xing K, Zhou S, Wu X, Zhu Y, Kong J, Shao T et al (2015) Concentrations and characteristics of selenium in soil samples from Dashan Region, a selenium-enriched area in China. Soil Sci Plant Nutr 61:889–897
Xing D, Magdouli S, Zhang J, Koubaa A (2020) Microbial remediation for the removal of inorganic contaminants from treated wood: Recent trends and challenges. Chemosphere 258:127429
Yen LV, Saibeh K (2013) Phytoremediation Using Typha Angustifolia L. for Mine Water Effluence Treatment: Case Study of Ex-Mamut Copper Mine, Ranau, Sabah. Borneo. Science 33:16–22
Zhang H, Feng X, Jiang C, Li Q, Liu Y, Gu C, Shang L, Li P, Lin Y, Larssen T (2014) Understanding the paradox of selenium contamination in mercury mining areas: High sediment content and low accumulation in rice. Environ Pollut 188:27–36
Zhu YG, Pilon-Smits EA, Zhao FJ, Williams PN, Meharg AA (2009) Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci 14:436–442
Zolfaghari M, Magdouli S, Komtchou SP, Tanabene R (2019) Investigation on the Efficiency of Electro-coagulation Process for Treatment of Different Selenium Species, 13th PARIS Int'l Conference on Agricultural, Chemical, Biological & Environmental Sciences (PACBES-19) March 14-16 2019 Paris (France)
Zolfaghari M, Magdouli S, Komtchou SP, Tanabene R, Martial R, Saffar T (2020) Pragmatic strategy for the removal of ammonia from gold mine effluents using a combination of electro-coagulation and zeolite cation exchange processes: A staged approach. J Water Process Eng 37:101512
Funding
This work was partly supported by Mitacs-Acceleration Postdoctoral Research Fellowship (Ref FR32800) and we also appreciate the support from the Centre technologique des résidus industriels (CTRI) for providing facilities and financial support.
Author information
Authors and Affiliations
Contributions
Conceptualization: [Selma Etteieb]; Methodology: [Selma Etteieb, Simon Pierre Komtchou]; Formal analysis and investigation: [Selma Etteieb, Simon Pierre Komtchou]; Writing - original draft preparation: [Selma Etteieb]; Writing-review and editing: [Selma Etteieb, Sara Magdouli, Satinder Kaur Brar]; Funding acquisition: [Sara Magdouli, Satinder Kaur Brar]; Resources: [Selma Etteieb, Mehdi Zolfaghari, Rayan Tanabene, Kamalpreet Kaur Brar, luliana Laura Calugaru]; Supervision: [Sara Magdouli, Satinder Kaur Brar]
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Severine Le Faucheur
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Etteieb, S., Magdouli, S., Komtchou, S.P. et al. Selenium speciation and bioavailability from mine discharge to the environment: a field study in Northern Quebec, Canada. Environ Sci Pollut Res 28, 50799–50812 (2021). https://doi.org/10.1007/s11356-021-14335-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14335-1