Abstract
Diesel, for its carcinogenic and teratogenic properties, is considered as an environmental hazard. The present study concentrates on diesel utilization by Stenotrophomonas pavanii DB1 under various physicochemical parameters and mass spectral analysis of the metabolites produced. Soil remediation potential of the culture for reducing diesel toxicity against Vigna radiata seed germination was also evaluated. Supplementation of the broth with ammonium sulfate, molasses and tween 80 enhanced diesel utilization (up to 66.3%). A steady decrease in diesel utilization (69.46–51.23%) was recorded with the increase in salinity from 1 to 5000 mM. Similarly, the utilization efficiency was low (up to 11.5%) when cadmium, lead and mercury salts were present but was not affected by zinc and copper salts. Liquid chromatography–mass spectrometry revealed extensive utilization (95–99%) of both the short- (C10–C20) and long-chain (C18–C30) n-alkanes. Significantly, the culture exhibited uniform utilization of various recalcitrant n-alkanes, to generate metabolites like aldehyde, ketone, fatty acids, carboxylic and dicarboxylic acids. Stenotrophomonas pavanii DB1 mediated 95% and 86% of diesel utilization in soil with and without nutrient amendment, respectively. Germination percentage of V. radiata seeds improved with the bacterial inoculation of the diesel-containing soil. Stenotrophomonas pavanii DB1 culture did not establish antagonistic effect over the resident soil bacteria. Based on the spectral analysis and soil remediation studies, it could be inferred that S. pavanii DB1 is a potential culture for bioremediation of sites polluted with diesel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diesel oil demand accounted for majority of the total oil demand during the last decade, thereby remaining as the primary fuel consumed globally. Diesel typically contains numerous alkanes and arene compounds that are moderately soluble and volatile. Therefore, the rising demand for diesel is a concern due to its deleterious effect on the environment (Palanisamy et al. 2014). Its nature of preferential binding to detritus and extreme non-polarity increases the level of pollution (Barathi and Vasudevan 2001). High concentrations of remnant diesel at the affected sites (in case of pipeline and reservoir leakages or accidental spillage) may demonstrate phytotoxic activity besides being immunotoxic, neurotoxic and carcinogenic to animals and human (Fathepure 2014).
Diesel contamination has been addressed by several physicochemical methods like evaporation, oxidation, synthetic and natural sorbent materials, barriers and skimmers, dispersing agents, gelling agents and Fenton’s reagent. But these methods are often expensive and result in sludge disposal problem (Sutton et al. 2014). Contrarily, bioremediation of diesel produces non-toxic products such as water, carbon dioxide, ammonia and has been accepted as an alternate cost-efficient method for reducing the remnant diesel concentration at the affected site (Leahy and Colwell 1990).
Microorganisms are often exploited as an effectual alternative to treat polluted sites. While there is a ubiquitous presence of diesel-degrading microorganisms, they normally form a small portion (< 1%) of the gross microbial inhabitants. Increasing number of hydrocarbon-degrading microorganisms (~ 10% of community) was reported when petroleum pollutants were available (Kostka et al. 2011). Among the microorganisms, prokaryotes (bacteria, cyanobacteria) and eukaryotes (filamentous fungi, yeasts, diatoms and algae) have been verified to have the capacity to utilize diesel (Das and Chandran 2011). Bacteria are predominant in petroleum-desecrated sites and contribute as principal degraders of the spilled oil (Rahman et al. 2003). Notably, Aeromicrobium, Acinetobacter, Burkholderia, Brevibacterium, Dietzia, Enterobacter, Gordonia, Mycobacterium, Pseudomonas, Sphingomonas, etc., are associated with diesel degradation (Daugulis and McCracken 2003).
Degradation of diesel by bacteria under natural conditions is reported to be low and is often restricted by the contaminant, nutrient availability, physical conditions (pH, salinity, temperature, etc.) or microbial competition in situ (Atlas and Cerniglia 1995). Although many diesel-utilizing bacteria have been documented, available scientific literature on physicochemical factors influencing diesel utilization by bacteria is scanty. Keeping these points in the forefront, the present study explores diesel utilization by bacteria native to diesel-contaminated soil, analyze the factors influencing diesel utilization and check the potency of the isolate to remediate diesel contamination in soil under laboratory conditions.
Materials and methods
Chemicals and reagents
Analytical grade chemicals and dehydrated media from HiMedia (Mumbai, India) were used. Diesel (density, 0.86 kg/l; cetane number, 45; sulfur, 1.5 ppm; moisture, 0.02%) was acquired from Shell petrol pump (Bangalore), and stored in dark throughout the study.
Isolation of bacteria
Soil polluted with diesel oil was sampled (1 kg) from a garage situated at Cottonpete (12.9667°N, 77.5667°E), Bangalore, India. In the laboratory, the soil was stored under aseptic condition until used. Bushnell Haas agar or BHA (composition g/l: MgSO4, 0.2; CaCl2, 0.02; KH2PO4, 1.0; K2HPO4, 1.0; NH4NO3, 1.0, FeCl3, 0.05; agar, 20; pH 7.0 ± 0.2), spiked with diesel (1% v/v), was considered for bacterial isolation. The soil sample (1 g) was diluted (dilutions 10−1 to 10−5) and plated on BHA. Plates were incubated (28 ± 2 °C, 24 h) and checked for bacterial colonies to appear. Morphologically distinct cultures were inoculated on BHA slants and preserved at 4 °C.
Selection of potent bacterial culture
Diesel (1% v/v) was spiked in the autoclaved modified mineral salt broth of Bhattacharya et al. (2014) taken in separate flasks, and respective bacterial culture (1 ml, 1 × 107 CFU/ml) was inoculated in the designated flasks. Uninoculated broth was considered ‘control.’ The inoculated flasks and ‘control’ were incubated (28 ± 2 °C, 120 rpm, 5 days). Following incubation, a potent isolate was chosen based on the extent of diesel utilization.
Assessment of diesel utilization by respective culture involved solvent extraction and gravimetric method (Agarry and Latinwo 2015). n-hexane (10 ml) was added to cell-free supernatant (cell cultures centrifuged at 5000 rpm for 15 min) and agitated for 3 min. The n-hexane layer was extracted and collected in sterile pre-weighed, oven-dried Eppendorf tubes. The extractant was allowed to volatilize overnight at 50 °C. The tubes were subsequently cooled and weighed on a calibrated balance (Shimadzu ELB-300) in an environmentally controlled balanced room to assess the quantity of residual diesel. Percentage of diesel utilization was calculated as:
where Wi represents the initial weight of diesel and Wr the residual weight of diesel.
Molecular identification
The genomic DNA of the selected isolate was extracted using the Bacterial Genomic DNA Isolation Kit (Chromous Biotech Pvt. Ltd., India). PCR amplification of 16S rDNA was performed with the use of universal forward and reverse 16S rDNA primers (5′-AGAGTTTGATCCTGGCTCAG-3′ and 5′-GGTTACCTTGTTACGACTT-3′, respectively). Genetic Analyzer (ABI 3500 XL Applied Biosystems, USA) and Seq Scape software (version 5.2) were used for sequencing the PCR amplicon (Bhattacharya et al. 2016). Using BLASTn, the nucleotide sequence was aligned with the other sequences available in the NCBI database (Altschul et al. 1990). The nucleotide sequence was submitted to GenBank (NCBI, USA) and provided an accession number.
Optimization of factors influencing diesel utilization
For estimating the diesel concentration that supports maximum utilization, the isolate was cultured with different diesel concentrations (0.1–5.0% v/v). Diesel utilization was studied by evaluating the effect of co-substrates (1.0% w/v: glucose, glycerol, molasses or whey); nitrogen sources (1.0% w/v: corn steep liquor, beef extract, yeast extract, tryptone, peptone, ammonium sulfate, sodium nitrate or ammonium chloride); surfactants (sodium deoxycholate, sodium lauryl sulfate [0.05% w/v], triton X-100, tween 20 or tween 80 [0.1% v/v]); salinity (NaCl: 1, 10, 100, 500, 1000, 2000, 5000 or 7000 mM); and heavy metal salts (5 mM: PbCl2, CuSO4, ZnCl2, CdCl2 or HgCl2).
Liquid chromatography–mass spectrometry analysis
The optimized broth (100 ml) containing diesel (3% w/v) was seeded with the selected isolate and incubated (28 ± 2 °C, 120 rpm) for 5 days. Uninoculated broth was considered as the abiotic control. Following incubation, the cell-free supernatant was prepared (centrifuging the broth for 15 min at 5000 rpm). The residual diesel from the cell-free supernatant was extracted with n-hexane (in equal volume). The extract was purified in a Florisil sorbent column (149–250 μm particle size, Sigma-Aldrich, Germany) and concentrated (Pacwa-Plociniczak et al. 2014).
The congeners in diesel were separated and identified using a single-quadrupole mass spectrometer (Shimadzu LCMS-2010) with an electrospray ionization interface. The ZORBAX C-18 column (50 × 4.6 mm, 1.8 μm), maintained at 50 °C, was injected with the purified sample (2 μl). Mobile phase (n-hexane with 0.01% formic acid) was used with 0.1 ml/min rate of flow. UV detector was used at 254 nm. Mass spectra were obtained by chemical ionization at atmospheric pressure and positive electron spray ionization (which gave protonated M+l values in 50–900 m/z range) using 135.0 V fragmentor voltage. Shimadzu LC–MS solution software was used for acquiring and processing the data.
The degradation efficiency was calculated by the equation given by Ghazali et al. (2004):
where Af is total area under peak for individual hydrocarbon, Ai total area under peak in abiotic control.
Phytotoxicity study and soil remediation
The diesel toxicity on germination of Vigna radiata seeds was assessed following the protocol of Vinothini et al. (2015). V. radiata seeds were selected for testing diesel toxicity because it is an important test species for the evaluation of phytotoxicity of environmental pollutants (Kannan and Upreti 2008). V. radiata is naturalized in open wastelands, roadsides, thicket margins, etc., where it may come in contact with harmful hydrocarbons. It is an erect or semi-erect legume with a well-developed taproot (a trait useful in studying effect of xenobiotics on root and shoot length).
The experimental conditions maintained are as follows: (1) control (garden soil + seeds); (2) effect of diesel (diesel-contaminated soil + seeds); (3) effect of bacterial inoculation (diesel-contaminated soil + selected bacterial suspension + seeds); (4) degradation ability in the presence of soil augmentation (diesel-contaminated soil + selected bacterial suspension + modified mineral salt broth + seeds).
Under each set of experimental conditions, garden soil and diesel-contaminated soil (50 g each) were taken in paper cups (5 cm rim diameter). Prior to sowing, all healthy seeds of V. radiata were decontaminated using HgCl2 (0.1% w/v) and washed repeatedly with distilled water for removing traces of HgCl2. Subsequently, the seeds (4 seeds per cup) were sown in the cups. Paper cups designated to be seeded with the bacterial culture, received 1 ml of bacterial culture (1 × 107 CFU/ml). All the cups were maintained at 28 ± 2 °C for 10 days. Sterilized distilled water (1.5 ml) was added regularly to restore the moisture in soil. The germination percentage under each experimental condition was calculated as: [(number of seeds germinated/total number of seeds)] × 100. Root and shoot length were recorded (in cm) from each set up after 10 days.
Study for antagonism
Diesel-contaminated soil sample (used in the study for the isolation of diesel-utilizing bacteria) was serially diluted till 10−3 dilutions, plated on nutrient agar and incubated (28 ± 2 °C, 24 h). Morphologically dissimilar colonies were chosen and expressed as CFU/ml.
Cross-streak method was adopted to assess antagonism by the selected isolate which was streaked as a single line (central streak) on nutrient agar. The other soil isolates were perpendicularly streaked in duplicates on either side of the central streak. The plate was incubated (28 ± 2 °C, 24 h) and checked for inhibitory zone (Velho-Pereira and Kamat 2011).
Statistical analysis
Each experiment was conducted in triplicate (n = 3). The data were presented graphically [mean ± standard deviation of the mean (SD)]. Microsoft Excel 2010 was used for data analysis.
Results and discussion
Isolation, screening of potent diesel-degrading bacteria
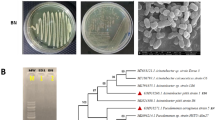
BHA spiked with diesel (1% v/v) supported the isolation of four diesel-utilizing bacteria that were designated as DB1, DB2, DB3 and DB4, respectively. Following incubation in mineral salt broth spiked with diesel, isolate DB1 demonstrated maximum utilization of diesel (27.5%) and was chosen for the study.
Molecular identification
The PCR amplicon (~ 1.5 kbp) was obtained. Using the BLASTn, the resulting nucleotide sequence (843 bp) when compared with 16S rDNA sequences in NCBI database matched with a cluster containing Stenotrophomonas sp. A similarity in sequence (99%) with Stenotrophomonas pavanii strain LMG 25348 was recorded. Hence, it was designated as Stenotrophomonas pavanii DB1. This ribosomal sequence was provided an accession number (KX249701) by GenBank (NCBI, USA).
Effect of diesel concentration
Concentration of the petroleum oil is related to its exposed surface area which in turn affects the rate of degradation. With greater area of the petroleum oil being exposed, faster degradation is facilitated. At higher concentration, the oil acts as a blanket, thereby creating oxygen limitations and disturbing the replenishment of nutrients (carbon/nitrogen/phosphorus) for the degrading organisms. In addition, at high oil concentrations the easily degradable hydrocarbons are attacked faster, leaving the recalcitrant hydrocarbons (with limited surface area and moisture retention capacity). It is usually considered that petroleum hydrocarbon concentration > 5% limits the microbial activity (Del’Arco and de Franca 2001).
When S. pavanii DB1 was exposed to different diesel concentrations, the extent of degradation was observed to be inversely proportional to the rise in diesel concentration. Beyond 3% v/v concentration of diesel, utilization was greatly reduced, and hence, this concentration was considered throughout the study. Decrease in diesel utilization at higher concentrations could possibly be a consequence of the hydrocarbon toxicity on bacterial cell membrane. Increased petroleum oil levels also facilitate anaerobic condition that inhibits the aerobic degradation process. Earlier researchers reported that diesel beyond 3% v/v proves to be increasingly deleterious to crop growth and soil microbiota (Agarry and Latinwo 2015).
Micrococcus sp., Methylobacterium sp., Pseudomonas sp., Noccardia sp., Rhodococcus sp. when inoculated in Bushnell Haas broth could degrade up to 1% v/v of petroleum hydrocarbons (2T oil, diesel, petrol and kerosene) where Pseudomonas sp. demonstrated highest biodegradation (70%) of hydrocarbons (Nilesh and Hardik 2013). The 1% hydrocarbons in the medium could possibly be higher than the endurance limit of above-mentioned bacteria (except Pseudomonas sp.), thus decelerating their growth and biodegradation. Likewise, the same concentration could probably not be formidable enough to negatively alter the growth of the Pseudomonas culture, and hence, it could degrade petroleum hydrocarbons efficiently. This accounts for the varying ability of the isolates to survive in a single concentration of crude oil.
Effect of co-substrates
Co-metabolism is the utilization of non-growth substrate(s) by any microorganism when another metabolizable compound is available. Such non-growth substrates (often referred to as co-substrates) include metabolizable compounds that result in utilization of a non-growth substrate by any microorganism (Ziagova et al. 2009). Co-substrates being growth substrates serve several purposes. First, it acts as energy source for microbial growth and maintenance. Second, it offers reducing equivalents (power), allowing utilization of non-growth substrates (Movahedin et al. 2005). The remediation process for spilled petroleum hydrocarbons is speeded by increasing aeration, by the addition of co-substrates and/or nutrients to prompt microbial metabolism or bacterial inoculations for seeding (McCarthy et al. 2004).
In comparison with other carbon sources, molasses supported maximum diesel utilization (35.33%) by S. pavanii DB1 (Fig. 1a). Since molasses constitutes essential minerals (calcium, chromium, cobalt, copper, magnesium, iron, manganese, phosphorous, potassium, sodium, etc.) and is a reliable energy and carbohydrate source, it allows rapid bacterial proliferation that facilitates greater hydrocarbon utilization (Lin et al. 2016). In accordance with the present observation, Antoniou et al. (2015) showed that diesel degradation was enhanced by incorporating molasses as a carbon source. Studies also illustrated that molasses in brewery waste effluents facilitated a greater percentage of degradation as compared to other carbon sources (Agarry and Latinwo 2015). Gestel et al. (2003) while studying bioremediation of diesel-contaminated soil, spiked soil with diesel and mixed composted biowaste (1:10 ratio) to improve diesel removal.
In the present inquest, molasses at 0.1% w/v concentration showed maximum diesel utilization (41.6%), whereas at 8 and 10% w/v concentration, utilization reduced to 16%. The reason for decreased utilization of diesel at high molasses concentration possibly is related to the osmotic effect of high sugar concentrations on bacterial species and also due to Crabtree effect. Crabtree effect leads to accumulation of ethanol (a natural antiseptic that inhibits cell growth) and represses respiration by the fermentation pathway dependent on the substrate (Crabtree 1928). During anaerobic fermentation, inclusion of 3% bioagent such as diluted molasses (at 60 ml/day) would enhance diesel degradation up to 250–400% beyond what was achieved in untreated soil (Lin et al. 2016).
Effect of nitrogen supplements
Microbial utilization of xenobiotics is often challenged by the availability of mineral nutrients such as nitrogen, phosphate and potassium, thereby affecting cellular metabolism and growth. Nutrient supplementation (generally nitrogen and phosphates) stimulates the microbial growth, metabolism and thereby enhance bioremediation (Atagana et al. 2003). Ammonium sulfate favored maximum diesel utilization (43.32%) by S. pavanii DB1, while the lowest level of diesel utilization (27.55%) was reported for beef extract and tryptone supplementation. In general, inorganic nitrogen salts improved diesel utilization by S. pavanii DB1 (Fig. 1b).
While evaluation of growth conditions for diesel-degrading Pseudomonas sp. strain DRYJ3, ammonium sulfate optimally supported the degradative activity and biomass formation. Sources like l-cystine, l-glutamic acid, l-aspartic acid facilitated poor degradative activity and biomass formation. The other reason to choose ammonium sulfate as the principal nitrogen source was associated with its widespread usage as a cost-effective nitrogen source for bioremediation (Shukor et al. 2009).
Mineral salt broth supplemented with ammonium sulfate (2% w/v) showed maximum diesel utilization (49.08%) by S. pavanii DB1. Decreased diesel utilization at high ammonium sulfate concentrations may result from the osmotic imbalance arising from the diffusion of ammonium ions across bacterial cell membrane. Optimal ammonium sulfate concentration that supported maximal Pseudomonas DRYJ3 growth was 2% (w/v) (Shukor et al. 2009).
Effect of surfactants
Surfactants contain both hydrophobic and hydrophilic moiety, thus providing a ‘bridge’ between the hydrophobic hydrocarbons and the hydrophilic microbial cells. Such surface-active agents increase the bioavailability of organic contaminants by incorporation (solubilization) of the molecule into the core of micelles (hydrophobic) in solution (Bamforth and Singleton 2005).
Results revealed that as compared to the control, all the surfactants enhanced diesel utilization. Tween 80 facilitated the highest utilization (66.3%). Since all surfactants were introduced in broth at very little concentration, bacterial growth was not affected. Being non-ionic in nature, tween 80 did not disrupt the charge distribution across the cell membrane and hence the growth of S. pavanii DB1 was not affected.
Literature suggests that non-ionic surfactants (mainly polyoxyethylene sorbitan surfactants) are regularly selected for enhancing diesel degradation, pertaining to their low cytotoxicity (Cheng et al. 2008). Incorporation of tween 80 (1% v/v) to Pseudomonas culture increased the diesel degradation (up to 70% as compared to control) due to enhancement in the availability of the hydrocarbons (Nilesh and Hardik 2013).
Effect of salinity
Judging the probable fettering effect of salinity is a mandate for selecting suitable strains for the remediation of saline environment contaminated with pollutants. The concentration of salts and hydrocarbon in medium are the major factors that may disturb degradative capacity of any organism under study (Valentín et al. 2006). Determination of the effect of salinity in the present study gains importance since the literature citing such effect on diesel utilization is scanty.
Stenotrophomonas pavanii DB1 could tolerate a wider NaCl concentration. The level of diesel utilization steadily decreased with the change in salinity from 1 to 5000 mM (69.46–51.23%). Beyond 5000 mM NaCl, only 22.36% utilization was reported (Fig. 2a). Result such as this emphasizes on appropriateness of the culture particularly for oil removal on beaches, estuaries or intertidal areas where bacteria often survive under varied salinity gradients.
Exposure to particular salt concentrations can be detrimental to microbial metabolism. Higher salinity disintegrates microbial cells because of plasmolysis or cytoplasmic recession induced by an osmotic difference across the cell wall (Abou-Elela et al. 2010).
For a novel isolate of Aeribacillus pallidus from a geothermal oil field under thermophilic conditions, the optimum NaCl concentration for degradation was reported to be 10 g/l, when NaCl concentration range for growth was 0–80 g/l. A steady deterioration in growth and degradation was recorded at higher concentration of NaCl, thus emphasizing the lethal effect of high salinity on microbial cells (Mnif et al. 2014).
Effect of heavy metals
Environmental presence of heavy metals may be either natural or from anthropogenic activities. Heavy metals at higher concentrations are often found in polluted sites. Heavy metal co-contamination may hamper the degradation of xenobiotics compounds in situ (Koeleman et al. 1999).
Usage of heavy metals in the present study was not undertaken to emphasize that they could enhance diesel degradation. Contrary, it was taken up to understand the degree of inhibition that the diesel utilization might suffer and also to judge the diesel utilization capacity of the bacterial culture in the presence of individual heavy metal. Diesel utilization by S. pavanii DB1 was severely affected when salts of cadmium, lead and mercury were present. Zinc and copper did not decrease the utilization efficiency drastically. Figure 2b illustrates how heavy metal salts affected the diesel utilization by S. pavanii DB1.
The presence of cadmium severely affected the diesel utilization ability of S. pavanii DB1 probably due to induction of oxidative stress. Strong affinity of mercury for thiol groups in proteins might have inactivated the enzymes associated with diesel utilization (Bartolucci et al. 2013). While studying the diesel degradation potential of a microbial community (isolated from a soil polluted by heavy metals), the heavy metals exhibited their toxicity in the following order—Hg > Cr > Cu > Cd > Ni > Pb > Zn (Riis et al. 2002).
LC–MS analysis of diesel utilization products
Individual hydrocarbons in the diesel oil (aliphatic, cycloalkanes, polycyclic aromatic hydrocarbons, etc.) differ in their vulnerability to microbial attack, where n-alkanes show moderate recalcitrance followed by branched alkanes, low molecular weight aromatics, cyclic alkanes, polyaromatic hydrocarbons in increasing order (Olajire and Essien 2014). Thus, the extent to which these hydrocarbons are removed is an important criterion in ascertaining the success of bioremediation in diesel-contaminated environments (Azubuike et al. 2016).
The mass spectrum of diesel retrieved from the abiotic control and inoculated medium is shown, respectively (Fig. 3). From the full-scan mass spectral analysis, a significant correlation was observed between the decrease in peak area of individual n-alkane (C10–C30) of diesel and their abundance in the abiotic control. Variation in relative abundance of these n-alkanes is due to the metabolic activity of S. pavanii DB1. Appearance of new peaks in the mass spectra points at generation of metabolites (possibly alicyclic and branched-chain aliphatic organic acids as well as diacids and aromatic ketones) arising from efficient utilization of these hydrocarbons by S. pavanii DB1 (Langbehn and Steinhart 1994).
The most significant observation is that besides the medium-chain n-alkanes (C12–C20), alkanes with long-chain length (C18–C30) were also effectively utilized by the bacterial culture. n-alkanes ranging from C10 to C20 were extensively utilized by S. pavanii DB1 (utilization percentage ranging between 96 and 99%), while utilization percentage for docosane, tricosane, tetracosane, pentacosane, hexacosane, heptacosane and nonacosane ranged between 95 and 97%. This phenomenon highlights that the culture was capable of utilizing diesel as a substrate and exhibited uniform utilization rates for a wide range of n-alkanes from C10 to C30. In contrast, most researchers have reported decreasing utilization rate with increasing carbon number (Balseiro-Romero et al. 2017).
Hydrocarbonoclastic bacteria are highly functional in removing hydrocarbons from polluted environments. Based on the LC–MS data, the intermediates metabolized by S. pavanii DB1 during the course of incubation were tentatively identified and the probable diesel utilization pathway was analyzed. In order to overcome the low chemical reactivity of medium- and long-chain n-alkanes in diesel, aerobic bacteria such as S. pavanii DB1 may use alkane-activating enzyme like dioxygenase to generate reactive oxygen species (n-alkyl hydroperoxides). During the aerobic utilization of these n-alkanes, the terminal alkyl group is usually oxidized to liberate a primary alcohol that further oxidized to aldehyde (e.g., anthracenecarboxaldehyde, m/z 206), and fatty acids of various chain lengths such as medium-chain fatty acid (dodecanoic acid m/z 200,207), saturated fatty acids (pentadecanoic acid, m/z 242,243; octadecanoic acid, m/z 287,284), long-chain fatty acid (octadecanedioic acid, m/z 314) are generated (Wentzel et al. 2007).
Simultaneously, detection of different carboxylic and dicarboxylic acids such as undecylic acid (m/z 186), anthracenedicarboxylic acid (m/z 266), eicosanedioic acid (m/z 342) may be due to the ω-hydroxylation of fatty acids at the both terminal alkyl groups (the ω position), rendering an ω-hydroxy fatty acid that is further converted into carboxylic and dicarboxylic acids. Beyond this point, subterminal oxidation of n-alkanes may generate secondary alcohol to be later converted to ketone [undecanone (m/z 167,170)] (Rojo 2009).
Phytotoxicity and soil remediation study
Soil contamination is considered a major problem for many industrialized nations having high population densities. Soil bioremediation mostly employs the utility of microorganisms, which either occur naturally or introduced to soil for degrading the pollutants. This technology is attracting increasing attention as an economic and safer alternative to landfilling and incineration (van Straalen 2002).
Diesel toxicity on V. radiata seed germination and ability of S. pavanii DB1 to degrade diesel were analyzed by pot culture experiments, wherein garden soil (free from diesel contamination) supported maximum seed germination under the laboratory conditions. Seeds sown in soil befouled with diesel revealed 100% loss in germination probably because of toxicity of diesel toward seed germination. Earlier, Al-Ghazawi et al. (2005) recorded that crude oil when present decreases germination percentage of Alfalfa seeds, where 15–30% seed germinated in the presence of 500 mg/kg diesel or higher.
Seeds sown in diesel-polluted soil treated only with S. pavanii DB1 culture revealed 75% inhibition of germination. Seeds in diesel-containing soil + treated with S. pavanii DB1 + nutrient amendment, revealed 25% of germination inhibition. Reduction in germination inhibition percentage in the second case may be correlated to nutrients and surfactant availability which could have reduced the toxicity of diesel, thereby improving the germination. Length of root and shoot for the control seeds were found to be 6.0 and 7.0 cm, respectively. For soil treated with S. pavanii DB1, the root and shoot lengths were 5.0 and 3.0 cm, respectively, whereas that for soil treated with isolate and nutrients were 5.6 and 6.1 cm, respectively (Fig. 4).
Following incubation (10 days) in soil with diesel, greater degradation (95%) was mediated by S. pavanii DB1 in soil with added nutrients and surfactant than in the one without any nutrients and surfactant amendments (86%). The recovery of diesel from unaugmented soil (control) was 97%. Besides involvement of indigenous soil microflora, possible autooxidation and adsorption on soil organic matter could also be the reason for such minimal diesel degradation in the control.
Soils polluted with hydrocarbons generally contain lesser nutrients than uncontaminated soils; hence, fortification with essential nutrient sources is necessary. Besides the co-substrate and nitrogen source being available, tween 80 resulted in partitioning of diesel, thus preventing it from being tightly bound to the soil particles, and was made available for bacterial degradation (Baldrian 2008).
Study for antagonism in soil
The soil bacterial count appeared to be almost the same prior to and following the inoculation of S. pavanii DB1. Extensive colonization of S. pavanii DB1 implies that S. pavanii DB1 did not establish antagonistic interaction with the resident soil bacteria. This is due to the fact that Stenotrophomonas species are natural soil inhabitants and also act as biofertilizer. It was worth observing that unlike many soil amendment studies where the introduced bacteria suppress the existing bacterial population in the soil, introduction of S. pavanii DB1 did not show any such effect. The result from the cross-streak method for determining synergistic/antagonistic activity among microorganisms also supports the above observation (Fig. 5).
Conclusion
The present investigation highlights that S. pavanii DB1 can tolerate higher concentrations of diesel. Augmentation of the growth medium by co-substrates, nitrogen supplementation and non-ionic surfactants enhanced the diesel utilization. Salts of cadmium, lead and mercury when present in the medium severely affected the process. Moreover, S. pavanii DB1 may be used for diesel removal from environments with varied salinity gradients since the culture could tolerate a wider NaCl concentration. The mass spectrum of diesel following the activity of S. pavanii DB1 reveals that besides the medium-chain n-alkanes, long-chain alkanes were also effectively utilized to liberate aldehyde, fatty acids, ketone, carboxylic and dicarboxylic acids, etc. It is worth recognizing that improvement in diesel utilization by S. pavanii DB1 and better shoot and root growth of V. radiata were observed in soil with nutrients and surfactant amendments. Appropriateness of S. pavanii DB1 for hydrocarbon remediation in soil can be emphasized as the culture did not suppress the existing bacterial population in the soil.
References
Abou-Elela SI, Kamel MM, Fawzy ME (2010) Biological treatment of saline wastewater using a salt-tolerant microorganism. Desalination 250:1–5. https://doi.org/10.1016/j.desal.2009.03.022
Agarry S, Latinwo GK (2015) Biodegradation of diesel oil in soil and its enhancement by application of bioventing and amendment with brewery waste effluents as biostimulation–bioaugmentation agents. J Ecol Eng 16:82–91. https://doi.org/10.12911/22998993/1861
Al-Ghazawi Z, Saadoun I, Al-Shakah A (2005) Selection of bacteria and plant seeds for potential use in the remediation of diesel contaminated soils. J Basic Microbiol 45:251–256. https://doi.org/10.1002/jobm.200410503
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Antoniou E, Fodelianakis S, Korkakaki E, Kalogerakis N (2015) Biosurfactant production from marine hydrocarbon-degrading consortia and pure bacterial strains using crude oil as carbon source. Front Microbiol 6:274. https://doi.org/10.3389/fmicb.2015.00274
Atagana HI, Haynes RJ, Wallis FM (2003) Optimization of soil physical and chemical conditions for the bioremediation of creosote-contaminated soil. Biodegradation 14:297–307. https://doi.org/10.1023/A:1024730722751
Atlas RM, Cerniglia CE (1995) Bioremediation of petroleum pollutants—diversity and environmental aspects of hydrocarbon biodegradation. Bioscience 45:332–338. https://doi.org/10.2307/1312494
Azubuike CC, Chikere CB, Okpokwasili GC (2016) Bioremediation techniques-classification based on site of application: principles, advantages, limitations and prospects. World J Microbiol Biotechnol 32:180. https://doi.org/10.1007/s11274-016-2137-x
Baldrian P (2008) Wood-inhabiting ligninolytic basidiomycetes in soils: ecology and constraints for applicability in bioremediation. Fungal Ecol 1:4–12. https://doi.org/10.1016/j.funeco.2008.02.001
Balseiro-Romero M, Gkorezis P, Kidd PS, Van Hamme J, Weyens N, Monterroso C, Vangronsveld J (2017) Characterization and degradation potential of diesel-degrading bacterial strains for application in bioremediation. Int J Phytoremediat 19:955–963. https://doi.org/10.1080/15226514.2017.1337065
Bamforth SM, Singleton I (2005) Bioremediation of polycyclic aromatic hydrocarbons: current knowledge and future directions. J Chem Technol Biotechnol 80:723–736. https://doi.org/10.1002/jctb.1276
Barathi S, Vasudevan N (2001) Utilization of petroleum hydrocarbons by Pseudomonas fluorescens isolated from a petroleum-contaminated soil. Environ Int 26:413–416. https://doi.org/10.1016/S0160-4120(01)00021-6
Bartolucci S, Contursi P, Fiorentino G, Limauro D, Pedone E (2013) Responding to toxic compounds: a genomic and functional overview of Archaea. Front Biosci 18:165–189. https://doi.org/10.2741/4094
Bhattacharya S, Das A, Prashanthi K, Palaniswamy M, Angayarkanni J (2014) Mycoremediation of benzo[a]pyrene by Pleurotus ostreatus in the presence of heavy metals and mediators. 3 Biotech 4:205–211. https://doi.org/10.1007/s13205-013-0148-y
Bhattacharya S, Das A, Samadder S, Rajan SS (2016) Biosynthesis and characterization of a thermostable, alkali-tolerant chitinase from Bacillus pumilus JUBCH08 displaying antagonism against phytopathogenic Fusarium oxysporum. 3 Biotech 6:87. https://doi.org/10.1007/s13205-016-0406-x
Cheng KY, Lai KM, Wong JW (2008) Effects of pig manure compost and non-ionic- surfactant Tween 80 on phenanthrene and pyrene removal from soil vegetated with Agropyron elongatum. Chemosphere 73:791–797. https://doi.org/10.1016/j.chemosphere.2008.06.005
Crabtree HG (1928) The carbohydrate metabolism of certain pathological overgrowths. Biochem J 22:1289–1298. https://doi.org/10.1042/bj0221289
Das N, Chandran P (2011) Microbial degradation of petroleum hydrocarbon contaminants: an overview. Biotechnol Res Int 2011:941810. https://doi.org/10.4061/2011/941810
Daugulis AJ, McCracken RM (2003) Microbial degradation of high and low molecular weight polyaromatic hydrocarbons in a two-phase partitioning bioreactor by two strains of Sphingomonas sp. Biotechnol Lett 25:1441–1444. https://doi.org/10.1023/A:1025007729355
Del’Arco JP, de Franca FP (2001) Influence of oil contamination levels on hydrocarbon biodegradation in sandy sediment. Environ Pollut 110:515–519. https://doi.org/10.1016/S0269-7491(00)00128-7
Fathepure BZ (2014) Recent studies in microbial degradation of petroleum hydrocarbons in hypersaline environments. Front Microbiol 5:1–16. https://doi.org/10.3389/fmicb.2014.00173
Gestel KV, Mergaert J, Swings J, Coosemans J, Ryckeboer J (2003) Bioremediation of diesel oil-contaminated soil by composting with biowaste. Environ Pollut 125:361–368. https://doi.org/10.1016/S0269-7491(03)00109-X
Ghazali FM, Rahman RNZA, Salleh AB, Basri M (2004) Biodegradation of hydrocarbons in soil by microbial consortium. Int Biodeterior Biodegrad 54:61–67. https://doi.org/10.1016/j.ibiod.2004.02.002
Kannan A, Upreti RK (2008) Influence of distillery effluent on germination and growth of mung bean (Vigna radiata) seeds. J Hazard Mater 153:609–615. https://doi.org/10.1016/j.jhazmat.2007.09.004
Koeleman M, vd Laak WJ, Ietswaart H (1999) Dispersion of PAH and heavy metals along motorways in the Netherlands: an overview. Sci Total Environ 235:347–349. https://doi.org/10.1016/S0048-9697(99)00253-3
Kostka JE, Prakash O, Overholt WA, Green SJ, Freyer G, Canion A, Delgardio J, Norton N, Hazen TC, Huettel M (2011) Hydrocarbon-degrading bacteria and the bacterial community response in Gulf of Mexico beach sands impacted by the Deepwater Horizon oil spill. Appl Environ Microbiol 77:7962–7974. https://doi.org/10.1128/AEM.05402-11
Langbehn A, Steinhart H (1994) Determination of organic acids and ketones in contaminated soils. J High Resolut Chromatogr 17:293–298. https://doi.org/10.1002/jhrc.1240170502
Leahy JG, Colwell RR (1990) Microbial degradation of hydrocarbons in the environment. Microbiol Rev 54:305–315
Lin Y, Lay JJ, Shieh WK (2016) Diesel degradation in soil catalyzed by the addition of a bioagent. Int J Environ Sci Technol 13:551–560. https://doi.org/10.1007/s13762-015-0889-8
Mccarthy K, Walker L, Vigoren L, Bartel J (2004) Remediation of spilled petroleum hydrocarbons by in situ landfarming at an Arctic Site. Cold Reg Sci Technol 40:31–39. https://doi.org/10.1016/j.coldregions.2004.05.001
Mnif S, Sayadi S, Chamkha M (2014) Biodegradative potential and characterization of a novel aromatic-degrading bacterium isolated from a geothermal oil field under saline and thermophilic conditions. Int Biodeterior Biodegrad 86:258–264. https://doi.org/10.1016/j.ibiod.2013.09.015
Movahedin H, Shokoohi R, Parvaresh A, Hajia M, Jafari AJ (2005) Evaluating the effect of glucose on phenol removal efficiency and changing the dominant microorganisms in a serial combined biological system. Pak J Biol Sci 8:1491–1494. https://scialert.net/abstract/?doi=pjbs.2005.1491.1494
Nilesh PK, Hardik P (2013) Isolation and screening of hydrocarbon degrading bacteria from soil near Kadi (Gujarat) region. Int J Res BioSciences 2:10–16
Olajire AA, Essien JP (2014) Aerobic degradation of petroleum components by microbial consortia. J Pet Environ Biotechnol 5:195. https://doi.org/10.4172/2157-7463.1000195
Pacwa-Płociniczak M, Płaza GA, Poliwoda A, Piotrowska-Seget Z (2014) Characterization of hydrocarbon-degrading and biosurfactant-producing Pseudomonas sp. P-1 strain as a potential tool for bioremediation of petroleum-contaminated soil. Environ Sci Pollut Res Int 21:9385–9395. https://doi.org/10.1007/s11356-014-2872-1
Palanisamy N, Ramya J, Kumar S, Vasanthi NS, Chandran P, Khan S (2014) Diesel biodegradation capacities of indigenous bacterial species isolated from diesel contaminated soil. J Environ Health Sci Eng 12:142. https://doi.org/10.1186/s40201-014-0142-2
Rahman KS, Rahman TJ, Kourkoutas Y, Petsas I, Marchant R, Banat IM (2003) Enhanced bioremediation of n-alkane in petroleum sludge using bacterial consortium amended with rhamnolipid and micronutrients. Bioresour Technol 90:159–168. https://doi.org/10.1016/S0960-8524(03)00114-7
Riis V, Babel W, Pucci OH (2002) Influence of heavy metals on the microbial degradation of diesel fuel. Chemosphere 49:559–568. https://doi.org/10.1016/S0045-6535(02)00386-7
Rojo F (2009) Degradation of alkanes by bacteria. Environ Microbiol 11:2477–2490. https://doi.org/10.1111/j.1462-2920.2009.01948.x
Shukor MY, Hassan NA, Jusoh AZ, Perumal N, Shamaan NA, MacCormack WP, Syed MA (2009) Isolation and characterization of a Pseudomonas diesel-degrading strain from Antarctica. J Environ Biol 30:1–6
Sutton NB, Langenhoff AA, Lasso DH, van der Zaan B, van Gaans P, Maphosa F, Smidt H, Grotenhuis T, Rijnaarts HH (2014) Recovery of microbial diversity and activity during bioremediation following chemical oxidation of diesel contaminated soils. Appl Microbiol Biotechnol 98:2751–2764. https://doi.org/10.1007/s00253-013-5256-4
Valentín L, Feijoo G, Moreira MT, Lema JM (2006) Biodegradation of polycyclic aromatic hydrocarbons in forest and salt marsh soils by white-rot fungi. Int Biodeterior Biodegrad 58:15–21. https://doi.org/10.1016/j.ibiod.2006.04.002
van Straalen NM (2002) Assessment of soil contamination: a functional perspective. Biodegradation 13:41–52. https://doi.org/10.1023/A:1016398018140
Velho-Pereira S, Kamat NM (2011) Antimicrobial screening of actinobacteria using a modified cross-streak method. Indian J Pharm Sci 73:223–228. https://doi.org/10.4103/0250-474X.91566
Vinothini C, Sudhakar S, Ravikumar R (2015) Biodegradation of petroleum and crude oil by Pseudomonas putida and Bacillus cereus. Int J Curr Microbiol App Sci 4:318–329
Wentzel A, Ellingsen TE, Kotlar HK, Zotchev SB, Throne-Holst M (2007) Bacterial metabolism of long chain n-alkanes. Appl Microbiol Biotechnol 76:1209–1221. https://doi.org/10.1007/s00253-007-1119-1
Ziagova M, Kyriakou G, Liakopoulou KM (2009) Co-metabolism of 2, 4-dichlorophenol and 4-Cl-m-cresol in the presence of glucose as an easily assimilated carbon source by Staphylococcus xylosus. J Hazard Mater 163:383–390. https://doi.org/10.1016/j.jhazmat.2008.06.102
Acknowledgments
We extend our sincere gratitude to the management of JAIN (Deemed-to-be University) for providing the research facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: M. Abbaspour.
Rights and permissions
About this article
Cite this article
Bhattacharya, S., Das, A., Srividya, S. et al. Prospects of Stenotrophomonas pavanii DB1 in diesel utilization and reduction of its phytotoxicity on Vigna radiata. Int. J. Environ. Sci. Technol. 17, 445–454 (2020). https://doi.org/10.1007/s13762-019-02302-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-019-02302-w