Abstract
Fine particulate matter (PM2.5) is an important environmental factor affecting human health. However, most studies on PM2.5 and health have used data from fixed monitoring sites to assess PM2.5 exposure, which may have introduced misleading information on the exposure–response relationship. We aimed to assess the effect of short-term personal PM2.5 exposure on lung function in patients with chronic obstructive pulmonary disease (COPD) and asthma. To achieve this, we conducted a longitudinal panel study among 37 COPD patients and 45 asthma patients from Beijing, China. The COPD group and the asthma group completed 148 and 180 lung function tests, respectively. We found that in COPD patients, for every 10-μg/m3 increase in PM2.5 exposure at lag2, the FEV1, FVC and DLco decreased by −0.014 L (95% CI −0.025, −0.003), −0.025 L (95% CI −0.050, −0.003) and −0.089 mmol/min/kPa (95% CI −0.156, −0.023), respectively. There was also a decrease of −0.023 L/s (95% CI −0.042, −0.003) and −0.017 L/s (95% CI −0.032, −0.002) in MMEF at lag3 and lag03, respectively. In the asthma group, every 10-μg/m3 increase in PM2.5 exposure led to a reduction of −0.012 L (95% CI −0.023, −0.001), −0.042 L (95% CI −0.081, −0.003) and −0.061 L/s (95% CI −0.116, −0.004) in the FEV1, FVC and PEF at lag3, respectively. Our findings suggest that PM2.5 exposure may primarily affect both airway function and lung diffusion function in COPD patients, and airway function in asthma patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Air pollution is a major factor threatening human health worldwide. Fine particulate matter (PM2.5), which is composed of particles measuring ≤2.5 μm in aerodynamic diameter, has been recognised as the most important type of air pollutant. While PM2.5 is a serious environmental health problem all over the world, it is especially so in China, where PM2.5 has become the fourth health risk factor affecting Chinese residents (Zhou et al. 2019a). Studies have shown that PM2.5 exposure is associated with premature death and years of life lost (YLL) and that a reduction of PM2.5 concentration can reduce the loss of life of residents. A time-series study conducted in 72 Chinese cities showed that a 10-μg/m3 increase in PM2.5 was associated with an increase in YLL of 0.43 years, and that a potential life expectancy of 0.14 years could be obtained if the PM2.5 concentration was reduced in compliance with the World Health Organization’s air quality guideline (25 μg/m3) (Patz et al. 2020). PM2.5 has a large surface area and a small particle size and can absorb various toxic and harmful substances causing damage to multiple organs when entering the respiratory system via inhalation (Xing et al. 2016). Of the organ systems, the respiratory system is considered to be the most vulnerable to PM2.5.
Chronic obstructive pulmonary disease (COPD) and asthma are both chronic respiratory diseases with increasing incidence and have become significant healthcare burdens worldwide. According to the China Pulmonary Health (CPH) study, the prevalence of COPD and asthma in Chinese people aged ≥20 years was 8.6% and 4.2%, respectively (Huang et al. 2019; Wang et al. 2018). COPD and asthma are complex heterogeneous diseases that share many common risk factors, such as genetic susceptibility, smoking and air pollution (Postma et al. 2011). While active smoking is the major preventable risk factor globally, the effect of PM2.5 exposure on COPD and asthma cannot be underestimated.
Accumulated epidemiological findings have illustrated that PM2.5 exposure is responsible for the increased hospitalisation and mortality of patients with COPD and asthma (Atkinson et al. 2014; Cai et al. 2019; Cakmak et al. 2019; Fan et al. 2016; Li et al. 2016). Lung function is a good indicator of respiratory health and the severity of pulmonary disease and an early predictor of mortality. Both COPD and asthma comprise patients whose lung function have been impaired and are more vulnerable to PM2.5 exposure. Therefore, the impact of PM2.5 on lung function in these two groups of patients is of great concern. However, the existing results on the acute effects of short-time PM2.5 exposure on lung function in patients with COPD and asthma are inconsistent. For example, studies conducted in Italy (Lagorio et al. 2006) and England (Sinharay et al. 2018) found that there were negative associations between short-term exposure to PM2.5, forced expiratory volume (FEV1) and forced vital capacity (FVC) in patients with COPD. A panel study conducted in the USA found that a short-term exposure to PM2.5 was associated only with a decreased FVC but not FEV1 in COPD patients (Hart et al. 2018). However, several other epidemiological studies have indicated that there is no association between short-term exposure to PM2.5 and lung function parameters in COPD or asthma patients (de Hartog et al. 2010; Girardot et al. 2006).
Some of these inconsistencies may be due to differences in PM2.5 exposure measurement. In most of the recent epidemiological studies, PM2.5 concentration data were obtained from a central monitoring station. Such data were assumed to represent the averaged exposure of the population and were used for the analyses. However, personal PM2.5 exposure is strongly influenced by many factors, such as daily activities, lifestyle and microenvironments (Lei et al. 2016). Therefore, whether the PM2.5 concentration data of a fixed monitoring site actually represents the real exposure level has been a concern of researchers (Avery et al. 2010; Evangelopoulos et al. 2020; Sarnat et al. 2006). Moreover, some studies have also shown that using PM2.5 concentration data from a fixed monitoring site to represent personal PM2.5 exposure may lead to bias in the exposure–response relationship (Chen et al. 2019).
Personal exposure monitoring is considered as the current the ‘gold standard’ for air pollutant exposure assessment. However, few studies have been conducted to assess the effect of personal PM2.5 exposure on lung function in patients with COPD and asthma. Therefore, in this longitudinal panel study, we used a personal PM2.5 monitoring device to obtain the real-time PM2.5 exposure data of participants during daily activities and assess the effect of short-term exposure to personal PM2.5 on lung function in patients with COPD and asthma.
Methods
Study design
Two parallel longitudinal panel studies were conducted between July 2017 and August 2019—one with COPD patients and the other with asthma patients. To ensure that the patients were followed up once every season, and to include different levels of air pollution empirically, the study was designed to have follow-ups every 3 months, for a total of four visits in a year. The duration of each visit was 3 days, which was comparable with other panel studies in related fields (Bloemsma et al. 2016; de Hartog et al. 2010; Gao et al. 2020; Hart et al. 2018). Personal PM2.5 data were recorded, and questionnaires and pulmonary function tests were completed at each visit. Briefly, on the first day 10:00 of the visit, each participant was asked to wear a personal PM2.5 monitoring device. On the fourth day at 10 am, they returned the equipment, performed the pulmonary function test and completed the questionnaire survey. The survey included demographic information such as sex, age, occupation and educational levels as well as the disease course and the presence of comorbidities at the first visit. Moreover, data on medications, respiratory symptoms and number of acute exacerbations between visits were collected at each visit. All procedures were explained to the participants, and informed consent from each participant was obtained in writing. The Ethics Committee of the China–Japan Friendship Hospital approved the research protocol (2017-19). This study was conducted in accordance with the Declaration of Helsinki.
Participants
The participants were recruited from the outpatient department of the China–Japan Friendship Hospital. The inclusion criteria for the COPD patients were aged 45–75 years and physician-diagnosed COPD in line with the Global Initiatives for Chronic Obstructive Pulmonary Disease guidelines, with a ratio of FEV1 to FVC of less than 70% after post-bronchodilator spirometry (Vogelmeier et al. 2017). The inclusion criteria for the asthma patients were aged 18–75 years and physician-diagnosed asthma according to the Global Asthma Prevention Initiative guidelines, with a FEV1 reversibility of >12% and 200 mL after post-bronchodilator spirometry (Bousquet and Humbert 2015).
The exclusion criteria were designed to exclude patients whose lifestyle or complications would have had a significant impact on their lung function, or who would not be able to complete the pulmonary function tests, or all four visits. The exclusion criteria for both COPD and asthma patients were as follows: (1) currently smoking or had quit smoking for no more than 6 months; (2) the presence of complications and comorbidities, such as malignant tumours, severe cardiovascular and cerebrovascular diseases, hepatic and renal insufficiency and active tuberculosis; (3) had undergone assessments on the effects of epilepsy or psychiatric diseases; (4) had undergone chest, abdominal or eye surgeries in the last three months; (5) pregnant and lactating women.
Environmental data
We used the MicroPEM Personal Exposure Monitor (version 3.2; RTI International, USA), which has been widely used in personal PM2.5 exposure assessments, to obtain personal PM2.5 exposure data (Lei et al. 2016; Wang et al. 2017; Ye et al. 2020). The MicroPEM is a light, compact, low-noise and portable personal exposure monitoring device with a rubber tube at the top to collect the air from the wearer’s breathing area. It consists of an onboard micro-nephelometer that can simultaneously obtain real-time PM2.5 data as well as real-time temperature and humidity data through temperature and humidity sensors (Du et al. 2019).
Exposure data were recorded every 10 s with the flow rate of 0.5 L/min (calibrated with a flowmeter). The MicroPEM was placed in a small backpack, and the participant was instructed to carry it all the time and to keep the rubber tube close to the mouth and nose breathing area during the monitoring period. The wearing of the MicroPEM started at 10:00 on the first day, and the monitoring ended at 10:00 on the fourth day. All participants were instructed individually on how to use the equipment.
To assess the impact of other air pollutants on the acute effects of PM2.5 on lung function, we also collected concentrations of other air pollutants from Beijing Municipal Environmental Protection Bureau (http://www.bjepb.gov.cn/) and the monitoring site closest to the patients’ home address was selected. The 24-h mean concentrations of sulphur dioxide (SO2), nitrogen dioxide (NO2), carbon monoxide (CO) and maximum 8-h average concentration of ozone (O3) were adopted.
Pulmonary function testing
On the fourth day of the follow-up, a pulmonary function test was performed using a MasterScreen spirometer (Jaeger, Germany). Technicians involved in the study had been professionally trained, and the same spirometer was used throughout the study. Moreover, participants were asked not to change their medication before a pulmonary function test. Before the pulmonary function test, the participant’s age, sex, height, weight, temperature; atmospheric pressure; and other indicators were entered in a computer, and the corresponding predictive value was generated automatically by the computer. At least three tests were performed each time, and the time interval between each measurement was ensured to be maintained >5 min. The optimal forced expiratory flow–volume curve was taken as the final result.
The spirometry parameters were as follows: FEV1, FVC and the ratio of FEV1/FVC; diffusing capacity of the lungs for carbon monoxide (DLco); peak expiratory flow (PEF); vital capacity (VC); total lung volume (TLC); maximal mid-expiratory flow (MMEF).
Statistical analysis
Lung function, daily PM2.5 concentrations, relative humidity and temperature data were analysed using SPSS software version 22.0 (IBM Corp., Armonk, NY, USA) and expressed as means ± SDs. For the correlation analysis, since these variables are repeated measurements of individuals, and each individual served as his/her own control, we analysed the effect of PM2.5 exposure on lung function by using a linear mixed-effect (LME) model. The model allowed each person’s observations at different time points to be used as their own point of comparison, and provided the advantage of interpreting the correlations of multiple repeated measurements by including a random intercept for each person (Bondell et al. 2010).
In our LME model, the lung function parameters were approximately normally distributed (Supplementary Fig. 1) and were regarded as response variables. The PM2.5 was considered as the fixed-effect variable and the patient’s identity number was introduced as the random-effect variable. This model also used sex, age, body mass index (BMI), disease duration, past smoking status, temperature and relative humidity as fixed-effect covariates to control for potential confounding effects. For temperature and relative humidity, we conducted a linear correlation test before the regression analysis. The results showed that the influence of temperature and relative humidity on lung function was non-linearly correlated (Supplementary Table 1). Therefore, non-linear control of temperature and relative humidity was adopted in the subsequent analysis. To control the potential nonlinear relationship, we adjusted for the nonlinear and delayed effects of weather conditions on lung function by fitting natural cubic splines with a d.f. of 3 for the 3-day moving average air temperature and relative humidity.
First, we analysed the single-day lag effect and the cumulative lag effect with reference to other panel studies in related fields (Gao et al. 2020; Hart et al. 2018). We evaluated the single-day lag effect, which referred to the effect on the current day of the lung function test (lag0), then 1 day before testing (lag1), 2 days before testing (lag2) and 3 days before testing (lag3). We then evaluated the cumulative lag effect, which referred to the relationship between the moving averages (lag01, lag02 and lag03). The lag time distribution of this study is shown in Supplementary Fig. 2. Then we fitted the two-pollutant model to assess whether the effects of PM2.5 are dependent on simultaneous exposure to other air pollutants. Finally, we used the LME model to complete a stratified analysis to evaluate the effect of previous smoking history (former smoker or never smoker) on the results.
The LME model analysis was conducted using the R software (version 4.0.0) with the package ‘lmertest’. The results were expressed as the value of lung function changes and its 95% CIs for every 10-μg/m3 increase in PM2.5 concentration. The statistical significance was set at a P value of <0.05.
Results
Descriptive statistics
We included 37 patients with COPD and 45 patients with asthma. The baseline demographic and clinical characteristics are described in Table 1. Most of the participants in the COPD group were men (62.2%), whereas most of the participants in the asthma group were women (75.6%). The mean age was 63.0±9.0 and 55.7±12.3 years in the COPD group and asthma group, respectively, and the corresponding number of never smokers was 21 (56.8%) and 35 (77.8%), respectively. Comorbidities included cerebrovascular disease, hypertension, diabetes, peptic ulcer and connective tissue disease. In terms of medication use, compound preparations of inhaled corticosteroids and long-acting beta-2 agonist (ICS + LABA) were the most commonly used drugs in both groups. One month before enrolment in this study, one COPD patient (2.7%) and two asthma patients (4.4%) were treated with oral glucocorticoid therapy due to acute exacerbation.
Table 2 summarises the descriptive statistics for daily PM2.5 concentrations, meteorological variables and lung function throughout the follow-up period. The average PM2.5 concentrations during the study were 43.92 ± 42.70 μg/m3 and 46.77 ± 44.97 μg/m3 for the COPD and asthma groups, respectively, which were beyond the threshold values recommended by the World Health Organization (WHO), in which the suitable PM2.5 concentration for human health were defined as no more than 10 μg/m3 (WHO 2005). The corresponding FEV1/FVC ratios were 56.53 ± 8.14 ml and 75.82 ± 6.39 ml for the COPD and asthma groups, respectively.
As for each follow-up visit, the descriptive statistics of lung function and daily PM2.5 exposure data are shown in Supplementary Table 2 for the COPD group and Supplementary Table 3 for the asthma group.
Effects of PM2.5 exposure on lung function
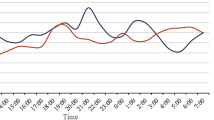
For the single-day lag model analysis in the COPD group, we found statistically significant adverse effects of personal PM2.5 exposure on FEV1, FVC, DLco and MMEF. As shown in Fig. 1, for every 10-μg/m3 increase of PM2.5 exposure in lag2, FEV1, FVC and DLco decreased by −0.014 L (95% CI −0.025, −0.003), −0.025 L (95% CI −0.050, −0.003) and −0.089 mmol/min/kPa (95% CI −0.156, −0.023), respectively. A per 10-μg/m3 increase in PM2.5 exposure at lag3 was related to a reduction in MMEF of −0.023 L/s (95% CI −0.042, −0.003). In the cumulative lag analysis, a per 10-μg/m3 increase in PM2.5 exposure was associated with a MMEF decrease of −0.017 L/s (95% CI −0.032, −0.002) at lag03.
Changes in lung function associated with every 10-μg/m3 increase in personal PM2.5 exposure of patients with chronic obstructive pulmonary disease. The x-axis represents the different time windows and the y-axis the corresponding changes (means and 95% CIs). *P <0.05. PM2.5, particulate matter with aerodynamic diameters of ≤2.5 μm; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; FEV1/FVC, ratio of forced expiratory volume in 1 s to forced vital capacity; VC, vital capacity; TLC, total lung volume; DLco, diffusing capacity of the lungs for carbon monoxide; PEF, peak expiratory flow; MMEF, maximal mid-expiratory flow.
For the asthma group in the single-day lag model, short-term personal PM2.5 exposure was negatively associated with FEV1, FVC and PEF, and the association was significant for all three variables at late lag (lag3). As shown in Fig. 2, for every 10-μg/m3 increase in PM2.5, exposure at lag3 was associated with a decrease in FEV1, FVC and PEF of −0.012 L (95% CI −0.023, −0.001), −0.042 L (95% CI −0.081, −0.003) and −0.061 L/s (95% CI −0.116, −0.004), respectively. For the cumulative lag analysis, a per 10-μg/m3 increase in PM2.5 exposure was associated with a PEF decrease of −0.122 L/s (95% CI −0.232, −0.011) at lag03.
Changes in lung function associated with every 10-μg/m3 increase in personal PM2.5 exposure of patients with asthma. The x-axis represents the different time windows and the y-axis the corresponding changes (means and 95% CIs). *P <0.05. PM2.5, particulate matter with aerodynamic diameters of ≤2.5 μm; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; FEV1/FVC, ratio of forced expiratory volume in 1 s to forced vital capacity; VC, vital capacity; TLC, total lung volume; DLco, diffusing capacity of the lungs for carbon monoxide; PEF, peak expiratory flow; MMEF, maximal mid-expiratory flow
Table 3 shows the results on two-pollutant models in COPD group. The associations of PM2.5 and FEV1 remained statistically significant when controlling for the effects of other air pollutants. The estimated effects of PM2.5 on FVC turned out to be statistically insignificant after adjustment of SO2 and NO2. Similarly, the estimated effects of PM2.5 on DLco and MMEF turned out to be statistically insignificant after adjustment of CO. The results on two-pollutant model in asthma group are shown in Table 4. The associations of PM2.5 and FVC remained statistically significant when controlling for the effects of other air pollutants. The estimated effects of PM2.5 on FEV1 turned out to be statistically insignificant after adjustment of NO2 and SO2. The estimated effects of PM2.5 on PEF turned out to be statistically insignificant after adjustment of SO2.
In the stratified analysis, the results showed that the effect of personal PM2.5 exposure on the lung function parameters was more evident in former smokers than never smokers (Supplementary Table 4).
Discussion
In this longitudinal panel study, we used data from the MicroPEM to explore the effect of short-term personal PM2.5 exposure on lung function in patients with COPD and asthma. We observed that short-term personal PM2.5 exposure was associated with a decreased FEV1, FVC, DLco and MMEF in patients with COPD and a lower FEV1, FVC and PEF in patients with asthma. Personal exposure monitoring is a more precise way of measuring the PM2.5 concentration that people receive daily in a microenvironment and is considered the ‘gold standard’ for air pollution exposure assessment (Lei et al. 2016). To our knowledge, this study is one of the few studies that have assessed the effect of PM2.5 in patients with COPD and asthma using personal PM2.5 exposure data.
Studies have shown that PM2.5 can cause respiratory inflammation and injury, which is related to a variety of respiratory diseases, such as chronic respiratory diseases, pneumonia, acute lower respiratory infection and lung cancer (Ciabattini et al. 2020; Gharibi et al. 2019; Liu et al. 2021; Sheikh et al. 2019; Mehta et al. 2013). COPD and asthma are the most common chronic respiratory diseases. Both patients with COPD and asthma are susceptible to air pollution. Spirometry is widely used as a non-invasive, rapid and economical detection method that can assess the severity of respiratory diseases objectively and to evaluate the impact of air pollutants on cardiopulmonary health.
Our results showed that increased personal PM2.5 exposure led to a decrease in FEV1, FVC, DLco and MMEF in patients with COPD. Moreover, increased personal PM2.5 exposure affected FEV1, FVC and PEF in asthma patients, adversely. However, no statistically significant difference between short-term PM2.5 exposure and TLC, VC and FEV1/FVC in both COPD and asthma populations was observed. FEV1 can reflect the degree of airflow limitation in the large airway, while MMEF and PEF are good indicators of small airway dysfunction (de Hartog et al. 2010; Pellegrino 2005). Our findings indicate that short-term personal PM2.5 exposure may primarily affect the airway and lung diffusion function in COPD patients, and the airway function, rather than the lung diffusion function, in asthma patients.
The degree of decrease in the FEV1 and FVC in our study was higher than that in a panel study involving 125 patients with COPD in the USA, which found that when indoor PM2.5 exposure increased by a per IQR of 5.8 μg/m3, the FEV1 and FVC decreased by 0.004 L and 0.021 L, respectively (Hart et al. 2018). The decrease in FEV1 in our study was lower than that in another panel study in Seattle, USA, which showed that for every 10-μg/m3 increase in PM2.5, the FEV1 decreased by 0.078 L and 0.046 L in COPD and asthma patients, respectively (Trenga et al. 2006). International guidelines recommend that patients with asthma have their PEF measured daily to assess asthma control and reduce acute attacks. This study found that personal PM2.5 exposure had an acute effect on PEF in asthma patients. A panel study in Seattle found a negative correlation between personal PM2.5 exposure and PEF in children with asthma, which is consistent with the results of this study (Trenga et al. 2006).
However, our results were also inconsistent with those of some previous studies. A panel study with 64 COPD patients conducted in Beijing showed that PM2.5 exposure affected the FVC% but not FEV1% (Gao et al. 2020). Another panel study conducted in Italy also found an inverse effect between PM2.5 exposure and FEV1 and FVC in COPD patients; however, no association was observed in asthma patients (Lagorio et al. 2006). Moreover, another panel study conducted in Europe showed that there was no effect of PM2.5 and the FEV1, FVC, or PEF in both asthma and COPD patients (de Hartog et al. 2010). The Oxford Street and Hyde Park study that included 60 patients with moderate to severe asthma found there were no consistent associations between PM2.5 and FEV1, FVC and MMEF (McCreanor et al. 2007). Similarly, a panel study from the UK, which included 16 patients with COPD, found that neither personal PM2.5 exposure nor PM2.5 exposure from atmospheric monitoring station was associated with FEV1 (Brauer et al. 2001). These inconsistencies may be due to the differences in exposure measurement methods, sample size, race and microenvironments.
The available research on the association of PM2.5 and MMEF and DLco is limited. Our results are consistent with a study completed in the USA, which found that increased PM2.5 concentrations were correlated with a decreased MMEF% (−0.66; 95% CI −1.07, −0.24) in patients with asthma (Vempilly et al. 2013). The CPH study used MMEF as one of the indicators of small airway dysfunction and found that PM2.5 is a major preventable risk factor for small airway dysfunction (Xiao et al. 2020). DLco is a gas transmission measurement method that reflects the complex interaction between gas and alveolar capillaries and is one of the best predictors of emphysema and impaired lung function (Nambu et al. 2015). In addition, the decreased DLco is strongly associated with reduced physical activity, increased frequency of acute exacerbation and a higher risk of mortality in patients with COPD. DLco may be regarded as a tool for the multidimensional assessment of COPD in the future (Balasubramanian et al. 2019). Currently, there are few studies that focus on the association between PM2.5 exposure and the diffusion function in COPD or asthma patients. Further research is necessary to confirm our findings.
There are several possible biological mechanisms for the association between PM2.5 exposure and lung function. The first is the mechanism of oxidative damage. PM2.5 is a strong oxidant, which can induce oxidative stress and inflammation in the respiratory tract and lungs, leading to the apoptosis of lung epithelial cells, airway remodelling and impaired respiratory function (Xing et al. 2016; Zhao et al. 2019; Zhou et al. 2019b). Studies have also found that PM2.5 can directly stimulate the vagus nerve of the respiratory tract, causing bronchial spasm and contraction, and increasing respiratory resistance, leading to the decline of lung function. Besides, other studies have shown that PM2.5 can cause respiratory defence responses, including increased mucus secretion, impaired ciliary system clearance and bronchial hyperresponsiveness (Anderson et al. 2003). This evidence is consistent with our findings on the acute effect between PM2.5 and lung function. However, it should be noted that our research does not reveal the mechanism related to PM2.5 that may be active in these processes, so further research is needed to verify any causality.
This study had several important strengths. First, we used an individual sampler to obtain real-time individual PM2.5 exposure data that closely reflected the real exposure level and enabled us to evaluate the effect of PM2.5 exposure and lung function more accurately. Second, because smoking is an important factor affecting lung function, all patients included in our study were either non-smokers or smokers who quit smoking for more than half a year, and this decision helped in eliminating the significant confounding effect of smoking. Third, compared with other studies, we have analysed more lung function indexes, such as lung volume index TLC, airflow limitation and airway function indexes, including MMEF, PEF, FEV1 and FVC, and the lung diffusion function index DLco, which demonstrated the effect of short-term PM2.5 exposure on lung function comprehensively.
There were also some limitations to our study. First, due to the small diameter of the filter membrane of the monitoring equipment, we were not able to detect the chemical elements of PM2.5 particles. Second, in the two-pollutant model, exposure data for NO2, CO, SO2 and O3 are derived from environmental monitoring stations rather than individual monitoring. Therefore, individual monitoring of other air pollutants is needed in the future to more accurately assess the impact of air pollution on lung function.
Conclusions
Despite its limitations, our study suggested that short-term personal PM2.5 exposure can decrease the FEV1, FVC, DLco and MMEF in COPD patients and FEV1, FVC and PEF in asthma patients. The results of the two-pollutant model suggest that the effects of PM2.5 on FEV1 in COPD patients and FVC in asthma patients are relatively independent, and are not affected by other gaseous pollutants. These findings indicate that PM2.5 may primarily affect airway function and lung diffusion function in COPD patients, and airway function, rather than lung diffusion function, in asthma patients. Our findings can provide relevant information for the formulation of public health policies and the development of appropriate interventions to control air pollution, thus bringing benefits to public health. Further studies about the effect of different chemical components of PM2.5 on lung function are needed in the future.
Data availability
Data can be obtained from appropriate authors on reasonable request.
References
Anderson HR, Atkinson RW, Bremner SA, Marston L (2003) Particulate air pollution and hospital admissions for cardiorespiratory diseases: are the elderly at greater risk? Eur Respir J Suppl 40:39s–46s. https://doi.org/10.1183/09031936.03.00402203
Atkinson RW, Kang S, Anderson HR, Mills IC, Walton HA (2014) Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: a systematic review and meta-analysis. Thorax 69(7):660–665. https://doi.org/10.1136/thoraxjnl-2013-204492
Avery CL, Mills KT, Williams R, McGraw KA, Poole C, Smith RL et al (2010) Estimating error in using ambient PM2.5 concentrations as proxies for personal exposures: a review. Epidemiology 21(2):215–223. https://doi.org/10.1097/EDE.0b013e3181cb41f7
Balasubramanian A, MacIntyre NR, Henderson RJ, Jensen RL, Kinney G, Stringer WW et al (2019) Diffusing capacity of carbon monoxide in assessment of COPD. Chest 156(6):1111–1119. https://doi.org/10.1016/j.chest.2019.06.035
Bloemsma LD, Hoek G, Smit LAM (2016) Panel studies of air pollution in patients with COPD: systematic review and meta-analysis. Environ Res 151:458–468. https://doi.org/10.1016/j.envres.2016.08.018
Bondell HD, Krishna A, Ghosh SK (2010) Joint variable selection for fixed and random effects in linear mixed-effects models. Biometrics 66(4):1069–1077. https://doi.org/10.1111/j.1541-0420.2010.01391.x
Bousquet J, Humbert M (2015) GINA 2015: the latest iteration of a magnificent journey. Eur Respir J 46(3):579–582. https://doi.org/10.1183/13993003.01084-2015
Brauer M, Ebelt ST, Fisher TV, Brumm J, Petkau AJ, Vedal S (2001) Exposure of chronic obstructive pulmonary disease patients to particles: respiratory and cardiovascular health effects. J Expo Anal Environ Epidemiol 11(6):490–500. https://doi.org/10.1038/sj.jea.7500195
Cai J, Peng C, Yu S, Pei Y, Liu N, Wu Y, Fu Y, Cheng J (2019) Association between PM2.5 exposure and all-cause, non-accidental, accidental, different respiratory diseases, sex and age mortality in Shenzhen, China. Int J Environ Res Public Health 16(3):401. https://doi.org/10.3390/ijerph16030401
Cakmak S, Hebbern C, Vanos J, Crouse DL, Tjepkema M (2019) Exposure to traffic and mortality risk in the 1991–2011 Canadian Census Health and Environment Cohort (CanCHEC). Environ Int 124:16–24. https://doi.org/10.1016/j.envint.2018.12.045
Chen XC, Chow JC, Ward TJ, Cao JJ, Lee SC, Watson JG, Lau NC, Yim SHL, Ho KF (2019) Estimation of personal exposure to fine particles (PM2.5) of ambient origin for healthy adults in Hong Kong. Sci Total Environ 654:514–524. https://doi.org/10.1016/j.scitotenv.2018.11.088
Ciabattini M, Rizzello E, Lucaroni F, Palombi L, Boffetta P (2020) Systematic review and meta-analysis of recent high-quality studies on exposure to particulate matter and risk of lung cancer. Environ Res 10:110440. https://doi.org/10.1016/j.envres.2020.110440
de Hartog JJ, Ayres JG, Karakatsani A, Analitis A, Brink HT, Hameri K et al (2010) Lung function and indicators of exposure to indoor and outdoor particulate matter among asthma and COPD patients. Occup Environ Med 67(1):2–10. https://doi.org/10.1136/oem.2008.040857
Du Y, Wang Q, Sun Q, Zhang T, Li T, Yan B (2019) Assessment of PM2.5 monitoring using MicroPEM: a validation study in a city with elevated PM2.5 levels. Ecotoxicol Environ Saf 171:518–522. https://doi.org/10.1016/j.ecoenv.2019.01.002
Evangelopoulos D, Katsouyanni K, Keogh RH, Samoli E, Schwartz J, Barratt B et al (2020) PM2.5 and NO2 exposure errors using proxy measures, including derived personal exposure from outdoor sources: a systematic review and meta-analysis. Environment International 137:105500. https://doi.org/10.1016/j.envint.2020.105500
Fan J, Li S, Fan C, Bai Z, Yang K (2016) The impact of PM2.5 on asthma emergency department visits: a systematic review and meta-analysis. Environ Sci Pollut Res Int 23(1):843–850. https://doi.org/10.1007/s11356-015-5321-x
Gao N, Xu W, Ji J, Yang Y, Wang ST, Wang J, Chen X, Meng S, Tian X, Xu KF (2020) Lung function and systemic inflammation associated with short-term air pollution exposure in chronic obstructive pulmonary disease patients in Beijing, China. Environ Health 19(1):12. https://doi.org/10.1186/s12940-020-0568-1
Gharibi H, Entwistle MR, Ha S, Gonzalez M, Brown P, Schweizer D, Cisneros R (2019) Ozone pollution and asthma emergency department visits in the Central Valley, California, USA, during June to September of 2015: a time-stratified case-crossover analysis. J Asthma 56(10):1037–1048. https://doi.org/10.1080/02770903.2018.1523930
Girardot SP, Ryan PB, Smith SM, Davis WT, Hamilton CB, Obenour RA, Renfro JR, Tromatore KA, Reed GD (2006) Ozone and PM2.5 exposure and acute pulmonary health effects: a study of hikers in the Great Smoky Mountains National Park. Environ Health Perspect 114(7):1044–1052. https://doi.org/10.1289/ehp.8637
Hart JE, Grady ST, Laden F, Coull BA, Koutrakis P, Schwartz JD, Moy ML, Garshick E (2018) Effects of indoor and ambient black carbon on pulmonary function among individuals with COPD. Environ Health Perspect 126(12):127008. https://doi.org/10.1289/EHP3668
Huang K, Yang T, Xu J, Yang L, Zhao J, Zhang X, Bai C, Kang J, Ran P, Shen H, Wen F, Chen Y, Sun T, Shan G, Lin Y, Xu G, Wu S, Wang C, Wang R, Shi Z, Xu Y, Ye X, Song Y, Wang Q, Zhou Y, Li W, Ding L, Wan C, Yao W, Guo Y, Xiao F, Lu Y, Peng X, Zhang B, Xiao D, Wang Z, Chen Z, Bu X, Zhang H, Zhang X, An L, Zhang S, Zhu J, Cao Z, Zhan Q, Yang Y, Liang L, Tong X, Dai H, Cao B, Wu T, Chung KF, He J, Wang C (2019) Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. The Lancet 394(10196):407–418. https://doi.org/10.1016/S0140-6736(19)31147-X
Lagorio S, Forastiere F, Pistelli R, Iavarone I, Michelozzi P, Fano V et al (2006) Air pollution and lung function among susceptible adult subjects: a panel study. Environ Health 5;5:11. https://doi.org/10.1186/1476-069X-5-11
Lei X, Xiu G, Li B, Zhang K, Zhao M (2016) Individual exposure of graduate students to PM2.5 and black carbon in Shanghai, China. Environ Sci Pollut Res Int 23(12):12120–12127. https://doi.org/10.1007/s11356-016-6422-x
Li MH, Fan LC, Mao B, Yang JW, Choi AMK, Cao WJ, Xu JF (2016) Short-term exposure to ambient fine particulate matter increases hospitalizations and mortality in COPD. Chest 149(2):447–458. https://doi.org/10.1378/chest.15-0513
Liu L, Liu C, Chen R, Zhou Y, Meng X, Hong J, Cao L, Lu Y, Dong X, Xia M, Ding B, Qian L, Wang L, Zhou W, Gui Y, Zhang X (2021) Associations of short-term exposure to air pollution and emergency department visits for pediatric asthma in Shanghai, China. Chemosphere 263:127856. https://doi.org/10.1016/j.chemosphere
McCreanor J, Cullinan P, Nieuwenhuijsen MJ, Stewart-Evans J, Malliarou E, Jarup L et al (2007) Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med 357(23):2348–2358. https://doi.org/10.1056/NEJMoa071535
Mehta S, Shin H, Burnett R, North T, Cohen AJ (2013) Ambient particulate air pollution and acute lower respiratory infections: a systematic review and implications for estimating the global burden of disease. Air Qual Atmos Health 6(1):69–83. https://doi.org/10.1007/s11869-011-0146-3
Nambu A, Zach J, Schroeder J, Jin GY, Kim SS, Kim YI, Schnell C, Bowler R, Lynch DA (2015) Relationships between diffusing capacity for carbon monoxide (DLCO), and quantitative computed tomography measurements and visual assessment for chronic obstructive pulmonary disease. Eur J Radiol 84(5):980–985. https://doi.org/10.1016/j.ejrad.2015.01.010
Patz JA, Qi J, Ruan Z, Qian Z, Yin P, Yang Y et al (2020) Potential gains in life expectancy by attaining daily ambient fine particulate matter pollution standards in mainland China: a modeling study based on nationwide data. PLOS Medicine 17(1):e1003027. https://doi.org/10.1371/journal.pmed.1003027
Pellegrino R (2005) Interpretative strategies for lung function tests. European Respiratory Journal 26(5):948–968. https://doi.org/10.1183/09031936.05.00035205
Postma DS, Kerkhof M, Boezen HM, Koppelman GH (2011) Asthma and chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 183(12):1588–1594. https://doi.org/10.1164/rccm.201011-1796PP
Sarnat SE, Coull BA, Schwartz J, Gold DR, Suh HH (2006) Factors affecting the association between ambient concentrations and personal exposures to particles and gases. Environ Health Perspect 114(5):649–654. https://doi.org/10.1289/ehp.8422
Sheikh A, Tian Y, Liu H, Wu Y, Si Y, Li M et al (2019) Ambient particulate matter pollution and adult hospital admissions for pneumonia in urban China: a national time series analysis for 2014 through 2017. PLOS Medicine 16(12):e1003010. https://doi.org/10.1371/journal.pmed.1003010
Sinharay R, Gong J, Barratt B, Ohman-Strickland P, Ernst S, Kelly FJ, Zhang J(J), Collins P, Cullinan P, Chung KF (2018) Respiratory and cardiovascular responses to walking down a traffic-polluted road compared with walking in a traffic-free area in participants aged 60 years and older with chronic lung or heart disease and age-matched healthy controls: a randomised, crossover study. The Lancet 391(10118):339–349. https://doi.org/10.1016/s0140-6736(17)32643-0
Trenga CA, Sullivan JH, Schildcrout JS, Shepherd KP, Shapiro GG, Liu LJ et al (2006) Effect of particulate air pollution on lung function in adult and pediatric subjects in a Seattle panel study. Chest 129(6):1614–1622. https://doi.org/10.1378/chest.129.6.1614
Vempilly J, Abejie B, Diep V, Gushiken M, Rawat M, Tyner TR (2013) The synergetic effect of ambient PM2.5 exposure and rhinovirus infection in airway dysfunction in asthma: a pilot observational study from the Central Valley of California. Exp Lung Res 39(10):434–440. https://doi.org/10.3109/01902148.2013.840693
Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, Frith P, Halpin DMG, López Varela MV, Nishimura M, Roche N, Rodriguez-Roisin R, Sin DD, Singh D, Stockley R, Vestbo J, Wedzicha JA, Agusti A (2017) Strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 Report. Am J Respir Crit Care Med 195(5):557–582. https://doi.org/10.1111/resp.13012
Wang C, Cai J, Chen R, Shi J, Yang C, Li H, Lin Z, Meng X, Liu C, Niu Y, Xia Y, Zhao Z, Li W, Kan H (2017) Personal exposure to fine particulate matter, lung function and serum club cell secretory protein (Clara). Environmental Pollution 225:450–455. https://doi.org/10.1016/j.envpol.2017.02.068
Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, Kang J, Ran P, Shen H, Wen F, Huang K, Yao W, Sun T, Shan G, Yang T, Lin Y, Wu S, Zhu J, Wang R, Shi Z, Zhao J, Ye X, Song Y, Wang Q, Zhou Y, Ding L, Yang T, Chen Y, Guo Y, Xiao F, Lu Y, Peng X, Zhang B, Xiao D, Chen CS, Wang Z, Zhang H, Bu X, Zhang X, An L, Zhang S, Cao Z, Zhan Q, Yang Y, Cao B, Dai H, Liang L, He J (2018) Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. The Lancet 391(10131):1706–1717. https://doi.org/10.1016/S0140-6736(18)30841-9
WHO (2005) WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide. Available online:http://apps.who.int/iris/bitstream/10665/69477/1/WHO_SDE_PHE_OEH_06.02_eng.pdf (Accessed on 24 Dec 2020).
Xiao D, Chen Z, Wu S, Huang K, Xu J, Yang L, Xu Y, Zhang X, Bai C, Kang J, Ran P, Shen H, Wen F, Yao W, Sun T, Shan G, Yang T, Lin Y, Zhu J, Wang R, Shi Z, Zhao J, Ye X, Song Y, Wang Q, Hou G, Zhou Y, Li W, Ding L, Wang H, Chen Y, Guo Y, Xiao F, Lu Y, Peng X, Zhang B, Wang Z, Zhang H, Bu X, Zhang X, An L, Zhang S, Cao Z, Zhan Q, Yang Y, Liang L, Liu Z, Zhang X, Cheng A, Cao B, Dai H, Chung KF, He J, Wang C, Wang C, He J, Xu J, Yang L, Xu Y, Zhang X, Bai C, Kang J, Ran P, Shen H, Wen F, Huang K, Yao W, Shan G, Yang T, Lin Y, Wu S, Zhu J, Wang R, Shi Z, Zhao J, Ye X, Song Y, Yang D, Wang Q, Hou G, Zhou Y, Li W, Ding L, Wan C, Yang T, Wang H, Chen Y, Guo Y, Xiao F, Lu Y, Peng X, Zhang B, Xiao D, Wang Z, Bu X, Zhang H, Zhang X, An L, Zhang S, Cao Z, Zhan Q, Yang Y, Cao B, Dai H, Liang L, Wang C, Tong X, Wu T, Kan H, Chen R, Cai H, Xiong W, Zhang P, Li Y, Niu W, Chen CS, Xu G, Zhang X, Gu X, Dong F, Liu Z, Cheng A, Pei Z, Niu H, Huang K, Chen S, Chung KF, Chen Z (2020) Prevalence and risk factors of small airway dysfunction, and association with smoking, in China: findings from a national cross-sectional study. The Lancet Respiratory Medicine 8(11):1081–1093. https://doi.org/10.1016/s2213-2600(20)30155-7
Xing YF, Xu YH, Shi MH, Lian YX (2016) The impact of PM2.5 on the human respiratory system. J Thorac Dis 8(1):E69–E74. https://doi.org/10.3978/j.issn.2072-1439.2016.01.19
Ye W, Saikawa E, Avramov A, Cho S-H, Chartier R (2020) Household air pollution and personal exposure from burning firewood and yak dung in summer in the eastern Tibetan Plateau. Environmental Pollution 263:114531. https://doi.org/10.1016/j.envpol.2020.114531
Zhao J, Li M, Wang Z, Chen J, Zhao J, Xu Y et al (2019) Role of PM2.5 in the development and progression of COPD and its mechanisms. Respir Res 20(1):120. https://doi.org/10.1186/s12931-019-1081-3
Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W et al (2019a) Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 394(10204):1145–1158. https://doi.org/10.1016/S0140-6736(19)30427-1
Zhou T, Hu Y, Wang Y, Sun C, Zhong Y, Liao J, Wang G (2019b) Fine particulate matter (PM2.5) aggravates apoptosis of cigarette-inflamed bronchial epithelium in vivo and vitro. Environ Pollut 248:1–9. https://doi.org/10.1016/j.envpol.2018.11.054
Acknowledgements
We would like to thank all the study participants from the China–Japan Friendship Hospital. We thank Yong Li for performing the pulmonary function tests on the patients. The authors would like to thank all the study participants for their dedicated participation.
Funding
This work was supported by the National Key Research and Development Project of China (grant number 2016YFC0206502), the National Nature Science Foundation of China (grant numbers 81970043, 91643115) and the CAMS Innovation Fund for Medical Sciences (CIFMS) (grant number 2018-I2M-1-001).
Author information
Authors and Affiliations
Contributions
T.Y. and C.W. proposed this study and revised the manuscript. R.D., N.H. and T.Y. completed participants’ recruitment and follow-up. R.D. wrote the original draft. H.N., T.Y. and K.H. helped perform the analysis with constructive discussions. H.C. and C.C. collected samples and did quality control. All authors revised the report and approved the final version before submission.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of the China–Japan Friendship Hospital (2017-19). Written informed consent was obtained from each participant.
Consent for publication
Not applicable.
Competing interests
Not applicable.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 4869 kb)
Rights and permissions
About this article
Cite this article
Duan, R., Niu, H., Yu, T. et al. Adverse effects of short-term personal exposure to fine particulate matter on the lung function of patients with chronic obstructive pulmonary disease and asthma: a longitudinal panel study in Beijing, China. Environ Sci Pollut Res 28, 47463–47473 (2021). https://doi.org/10.1007/s11356-021-13811-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13811-y