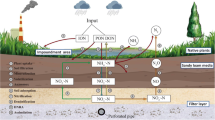
Abstract
Nitrogen migration and transformation in the stormwater bioretention system were studied in laboratory experiments, in which the effects of drying-rewetting were particularly investigated. The occurrence and distribution of nitrogen in the plants, the soil, and the pore water were explored under different drying-rewetting cycles. The results clearly showed that bioretention system could remove nitrogen efficiently in all drying-rewetting cycles. The incoming nitrogen could be retained in the topsoil (0–10 cm) and accumulated in the planted layer. However, the overlong dry periods (12 and 22 days) cause an increase in nitrate in the pore water. In addition, nitrogen is mostly stored in the plants’ stem tissues. Up to 23.26% of the inflowing nitrogen can be immobilized in plant tissues after a dry period of 22 days. In addition, the relationships between nitrogen reductase activity in the soil and soil nitrogen content were explored. The increase of soil TN content could enhance the activity of nitrate reductase. Meanwhile, the activity of hydroxylamine reductase (HyR) could be enhanced with the increase of soil NO3− content. These results provide a reference for the future development of nitrogen transformation mechanism and the construction of stormwater bioretention systems.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urban stormwater runoff is a major source of nutrient input to streams and lakes, accounting for more than 50% of the nitrogen in some water bodies (Davidson et al. 2010). Impervious surfaces (paved roadways, parking lots, sidewalks, and roofs) have expanded with increased urbanization over recent decades, resulting in an increased of pollutants washed off from paved surface to natural waters, causing serious stormwater runoff pollution (Lucke and Beecham 2011; Lucke and Nichols 2015). It has been reported that the concentrations of total nitrogen (TN) in stormwater runoff was in the range of tens to hundreds mg/L, depending on site characteristics (Jarney et al. 2014; Taylor et al. 2005). Nitrogen-polluted runoff can cause eutrophication and seriously impair aquatic ecosystems (Taylor et al. 2005). Therefore, it is necessary to remove the pollutants from rainwater for environmental protection.
Bioretention (or biofiltration) is one of the efficient stormwater control measures. It has been widely implemented on different scales from rain gardens to retention basins installed in public open space. Bioretention systems can reduce peak flows and downstream pollution loads efficiently through the combined action of soils, vegetation, and microbes (Debusk and Wynn 2011; Le Coustumer et al. 2012; Liu et al. 2014a; Mangangka et al. 2015; Yang et al. 2009). In a bioretention system, nitrogen in surface runoff undergoes a range of complex biogeochemical processes is attenuated, and then gradually stored in multiple media such as soil, plants, and microbes. Both the biotic and abiotic transformations involved in these processes are closely related to the media involved (Maltais-Landry et al. 2009).
Great variation in the water cycle of evaporation and rainfall affects the intensity and frequency of alternate drying and rewetting in such systems, affecting the plant and soil microbial communities and the functionality of the system’s physiological and biochemical processes. Depending on the climate and human activities, bioretention system will undergo frequent alternating drying and rewetting with some degree of randomness. The uncertain multiple rewetting and drying cycles can directly influence nitrogen migration and transformation in the various media involved, particularly when bioretention does not depend primarily on native soil (Canarini and Dijkstra 2015; Mikha et al. 2005). Alternate drying and rewetting can change soil moisture status, affecting the rate of nitrogen mineralization and nitrification (Guo et al. 2014). Some research suggested that the soil’s contribution to nitrogen removal is mainly through nitrification, which occurs primarily during the dry periods (Han and Cao 2013). However, the formed nitrate may then migrate with water during subsequent re-wetting, resulting in nitrogen leaching in severe cases (Fiere and Schimel 2002; Leitner et al. 2017). The biological processes involving vegetation and microorganisms are also influenced by the drying and rewetting process (Navarrogarcia et al. 2012). That also can affect the migration and bioconversion of nitrogen (Payne et al. 2014). Unfortunately, much of the scholarly work in this area has focused on the effects of a single and constant frequency of dry-wet alternation, relating antecedent dry days (ADDs) to the nitrogen removal performance of bioretention systems (Cho et al. 2011; Hatt et al. 2007; Payne et al. 2014; Subramaniam et al. 2016). Those studies have not fully explained nitrogen migration and transformation considering the development and succession of the ecosystems driven by the hydrology of multiple dry-wet cycles.
Biological transformation can change nitrogen’s form and chemistry, and determine to what extent it is bio-available for plants to assimilate. That will largely determine the actual contribution of each medium to the transport and fate of nitrogen in a bioretention system. Soil enzymes are important catalysts for some biochemical reactions in soil (Acosta-Martínez et al. 2007), and they play an important role in regulating soil nitrogen cycling. Moisture content has been identified as a main factor regulating enzyme activity (Wan et al. 2008). Therefore, the enzyme activity, which is important for the biotransformation of nitrogen, could be affected by intermittent drying and rewetting significantly. At the same time, the soil hydrodynamics during dry-wet cycles influence other environmental factors such as the role of plant roots, the characteristics of the soil’s microbial community, and the soil’s physicochemical properties. All those variations also affect and regulate the nitrogen transformation. Nitrate can be catalytically reduced to nitrite, hydroxylamine, and ammonium ion by nitrate reductase (NaR), nitrite reductase (NiR), and hydroxylamine reductase (HyR), respectively (Han and Cao 2013). All three reductases regulate biotransformation of nitrate and ammonium ion (Strohm et al. 2007). However, most of the research on enzyme activity in nitrogen transformation has focused on soils, wetlands, and other ecosystems connected hydrologically with the water source (Han and Cao 2013; Liu et al. 2007). In lined bioretention facilities, the wet-dry cycle is entirely dependent on the weather and thus somewhat random. Random wet-dry cycles would likely result in entirely different enzyme activity variations related to environmental factors and the soil’s hydrodynamics.
In this study, therefore, a series of laboratory experiments was designed to investigate some of the effects of alternate drying and rewetting on nitrogen migration and transformation within bioretention system. The main objectives were (1) to examine the occurrence and spatial distribution of nitrogen in multiple media (soil, plants, and pore water), (2) to investigate nitrogen’s migration and transformation bioretention systems under various drying and rewetting regimes, and (3) to deepen understanding of the influence of drying and rewetting frequency on the action of nitrogen reductases. Results will provide a theoretical basis for quantitative research on nitrogen’s behavior and fate in bioretention, which may help improve the nitrogen removal in bioretention systems.
Materials and methods
Equipment and plant
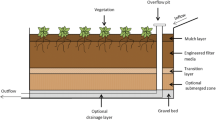
The experiments involved 126 vegetated biofilter columns constructed using PVC pipe (800 mm high, 150 mm diameter) with acrylic ponding zone (200 mm high, 150 mm diameter) placed above, as shown in Fig. 1. The columns had the three main layers shown in Fig. 1a: A 300 mm planting zone (PZ) of loamy sand composed of sandy loam soil (particles 0.02–0.2 mm in effective diameter), quartz sand (0.1–0.5 mm), and fine sand (0.05–0.15 mm) and planted with Cyperus alternifolius; a 200 mm submerged zone (SZ) of washed quartz sand (0.25–0.5 mm); and a 100 mm drainage zone of washed gravel (3–6 mm). In addition, pine woodchips without bark were mixed into the SZ to accelerate the denitrification process. Permeable geotextile was placed between the layers. All of the columns were placed in a purpose-built ventilated greenhouse, as shown in Fig. 1b.
The Cyperus alternifolius (Chinese native species) was selected due to its tolerance for both drought and waterlogging (Yu et al. 2019). Before starting the experiments, all of the planted columns were watered with dechlorinated tap water and culture suspension (purchased from Flower Market, Chongqing, China) for 5 weeks, inoculate the soil with a community of microbes, and wash out background substances.
Semi-synthetic stormwater
Semi-synthetic stormwater (consisted of actual sediment and dechlorinated tap water) was prepared before each experiment. The sediment was collected from a stormwater pond in the Nan’an district of Chongqing, China. The sediment was strained through a 1 mm sieve before being mixed with dechlorinated tap water. The target concentrations for contaminants matched those found in typical stormwater runoff from Chongqing’s mountainous urban roads, as shown in Table 1.
Stormwater dosing
According to Chongqing’s average annual rainfall volumes and interval days (data from 2011 to 2017, as shown in Fig. S1), seven ADDs conditions (1, 2, 3, 5, 7, 12, and 22 days) were selected to represent different realistic intermittent drying-wetting cycles. Twenty-two days dosing interval was selected to simulate extreme drought conditions, which could ensure maximum drying while minimizing plant death. Each group was modeled in 18 columns, and three replicates from each group were selected randomly to test the nitrogen content and state in the soil, plant, pore water, and effluent during each interval. The detail timeline of the dosing and sampling is provided as Fig. 2.
The dosing volumes were calculated as Eq. (1). The model scale stormwater volumes were 4.1 L for a 75% annual total runoff control rates (ATRCR) and 4.6 L to reflect an 80% ATRCR, which was slightly greater than sampling volume. Meanwhile, each dosing maintained at the same flow rate to ensure that the simulated rainfall at same intensity.
where SV represents volume (m3), H represents the design rainfall (mm), R is the runoff coefficient (taken as 0.85), and F represents the catchment’s area (hm2). The design rainfall for a 75% ATRCR was 19.1 mm, and 21.4 mm for an 80% ATRCR.
The collection of plants, soils, and pore waters
The plants, soils, and pore waters were sampled for one selected dosing event, while the column effluents were sampled for 5 selected dosing events over the 4-month period from September 2018 to January 2019 (shown in Fig. 2). The effluent was collected in a lidded bucket until the flow ceased. The bulk samples were then mixed thoroughly before sub-sampling for water quality analysis. Pore water samples were taken using a syringe from sampling points installed along the height of each column at depths of 10, 20, 30, 40, and 50 cm. Those samples were taken only once there was a steady flow of effluent from the column’s outlet. All of the water samples were analyzed for ammonia nitrogen (NH4+), nitrate nitrogen (NO3−), and total nitrogen (TN).
Analytical methods
All water chemistry and the total suspended solids were measured using the test methods of China’s national standards (General Bureau of China National Environmental Protection 2002). Soil samples were collected from the PZ and SZ using the five-points method and removing any impurities such as broken roots. The soil water contents were determined gravimetrically after sub-samples were dried at 105 °C to a constant weight. Soil pH was measured with Hach HQ11D meter after the fresh soil had been mixed with deionized water free of CO2 at a soil/water volume ratio of 1:2.5 (Zheng et al. 2014). Organics in the soil samples were determined by potassium dichromate oxidation and subsequent spectrophotometry with a Hach DR2100 spectrophotometer. Total soil nitrogen was determined through semi-micro Kjeldahl digestion (Lu et al. 2015). Exchangeable NH4+ and NO3− were extracted with 2 M potassium chloride in a 1:10 soil/solution ratio (Chen et al. 2015; Lu et al. 2015) and determined by Nessler reagent colorimetry and ultraviolet spectrophotometry, respectively. The activities of NaR, NiR, and HyR were analyzed following the method described by Dick (Dick et al. 1996). NaR was measured at 540 nm and calculated using the reduction NO3− after 24 h of incubation. NiR was calculated using the nitrite content after 24 h of incubation using sulfanilic acid naphthylamine colorimetry. HyR was calculated based on the content of NH2OH after 5 h of incubation according to the phenanthroline and ferrous sulfate method (Weng et al. 2013).
After the soil sampling, the filters used were washed gently using flowing tap water, and then the plant roots were cleaned of soil non-destructively. The plants’ aboveground and underground biomass was analyzed after air drying. There was then a digital scan of the root system with an Epson Perfection V700 root morphology scanner. The digital images were analyzed using WinRHIZO software to quantify the root systems’ architecture, including total root height (TRH), total root area (TRA), total root volume (TRV), average root diameter (ARD), the number of root tips (RTs), branch number (BN), and crossings number (CN). The plants’ roots, stems, and leaves were then separated and weighed separately. All the separated tissues samples were dried at 80 °C for 18 h to a constant value and weighed in a crucible, and some were ground in a mortar for total nitrogen content analysis of each tissue samples.
Biotic and abiotic nitrogen immobilization was quantified in terms of immobilization ratio (IRs), shown in Eq. (2). The IR was defined as the percentage of the inflowing nitrogen accumulated in the plants’ roots, stems, and leaves and in the soil of the planting and submerged zones.
where NA is the amount of nitrogen accumulated in the soil or in plants (g), NBK is the background amount of nitrogen in the soil or in plants before the stormwater dosing (g), and Ni is the total inflow of nitrogen throughout the experimental period (g).
The significance of the differences in N concentration of the various fractions, as well as of the differences in reductase activity and soil properties among the different columns was tested using one-way analysis of variance. The significance of the observed differences in those indicators between columns given the same treatment was examined using the Student-Newman-Keuls method. Pearson correlation coefficients were calculated to examine the relationships between root system architecture, N content in the plants’ tissues, reductase activities, soil properties, and soil nitrogen content.
Results and discussion
Soil properties
Intermittent drying and rewetting process affects soil’s physicochemical properties vertically, thereby influencing the plants growth and the nitrogen transformation (Wang et al. 2017). As Table 2 shows, the soil moisture content in the SZ was higher than it in the PZ, as would be expected. The soil moisture decreased significantly as ADDs increased (shown in Table 2). The soil pH ranged from 7.01 to 7.57 and the organic content from 2.03 to 6.24 g/kg with different ADDs. It was the soil of the PZ which was weakly alkaline (7.27 to 7.57), while the pH of SZ was neutral. The organics content of the soil in the PZ was higher than that in the SZ apart from the BR1, BR6, and BR7 groups. Apart from any accumulation of nutrients, that might be attributed to the presence of the roots in the PZ. The organics content of the SZ soil in groups of BR6 and BR7 was higher than in the PZ. This was probably because the growth of roots extended into the SZ where the roots may have secreted proteins and other organic matter. Meanwhile, the death of some plants in December caused by frequent inflows and low temperatures resulted in lower organics content in the PZ than in the SZ.
Nitrogen migration in pore water
The spatial distribution of TN, NH4+, and NO3− concentrations in the soils subjected to different dry and wet cycling is shown in Fig. 3. The effluent nitrogen concentrations in all of the groups were lower than that in the influent, indicating that bioretention could remove nitrogen from the simulated storm runoff effectively. The TN concentration of the effluent was related to soil depth and ADDs. As can be seen in Fig. 3a, TN concentration tended to decrease rapidly with depth in groups BR1–BR5. However, the outlet TN concentrations of groups BR6 and BR7 were higher than those of the other groups. Longer drying could increase nitrification, resulting in high NOx load, which can be only removed in the anaerobic SZ via denitrification.
The NO3− concentration in the surface pore water (the top 10 cm) of groups BR1 to BR5 decreased in comparison to the influent water, indicating a certain accumulation of NO3− in the topsoil. The NO3− in the surface pore water of the other groups with longer ADDs increased (Fig. 3b). In group BR6, nitrate was 1.23 mg/L above that of the influent, probably because of nitrification during soil drying produced NO3−. Subsequent rewetting could cause the NO3− to migrate with the water flow deeper into the soil 10–30 cm deep, increasing the NO3− content of the pore water. The concentration of NO3− in the 10–30 cm layer of the pore water in group BR1 also increased. That was probably due to the decline of plant roots caused by continuous inflow in winter which released NO3− into the pore water. The concentrations of NO3− decreased sharply in the pore water of the SZ. This was presumably due to the presence of the solid carbon source as an electron donor which promoted denitrification of NO3− under the SZ’s hypoxic conditions. Glaister’s group (Glaister et al. 2014) has highlighted benefits of including an SZ and adding a carbon source to maintain nitrogen treatment performance even over extended dry periods.
As is shown in Fig. 3c, alternate drying and rewetting had little effect on NH4+ concentrations at any depth. In the pore water of the topsoil (0–10 cm), the NH4+ contents decreased sharply from 5.27 mg/L to less than 2.16 mg/L. That indicates that the removal of NH4+ usually resulted from the joint effects of matrix adsorption and ion exchange. Soil components bearing a negative charge accumulated NH4+ quickly in the topsoil, leading to a decline of NH4+ in the topsoil’s pore water. However, the pore-water concentration of NH4+ increased with depth from 10 to 20 cm. The results of batch shaking nitrogen desorption experiments showed that the desorption and adsorption were affected by the dry-wet cycles, shown in Fig. S2. The exchanged NH4+ in the soils was unstable and easily desorbed, resulting in increase of NH4+ concentrations in the pore water. Between 20 and 40 cm, the soil’s NH4+ content gradually decreased as the dense roots took up ammonium in preference to other inorganic nitrogen. In the SZ, however, the NH4+ concentration of the pore water increased (50–60 cm). That may have resulted from nitrate being reduced to ammonium (the DNRA process). Several studies have reported nitrogen transformations including nitrification, denitrification, DNRA, assimilation, mineralization, and anammox reactions which can occur simultaneously under wet and dry conditions (Chen et al. 2015; Lu et al. 2015). Denitrification and DNRA can coexist and compete for nitrate reduction under the anaerobic conditions caused by ponding (Chen et al. 2015; Lu et al. 2015). DNRA is thermodynamically favored over denitrification when the nitrate concentration is relatively low (Hardison et al. 2015). In that situation, NO3− in the pore water is reduced to NH4+, which leads to an increase of NH4+ content in the SZ’s pore water. Ultimately, the outlet concentration of NH4+ was lower than in the influent under the combined action of soil adsorption and plants assimilation.
Biotic nitrogen immobilization in plants
Biotic immobilization by plants is considered an important nitrogen retention process in bioretention systems. The roots could long-term take up inorganic nitrogen and a little organic nitrogen directly for the plants’ growth (Luo et al. 2011). As shown in Fig. 4, TN content in the plant tissues varied somewhat in the different groups. The TN content in the roots ranged from 2.73 to 37.0 g/kg. In the stems, the range was 3.71 to 6.86 g/kg, and in the leaves it was 7.35 to 11.90 g/kg (Fig. 4a). So stems could obtain the highest partitioning of nitrogen (52.88%) (except in group BR7 where it was in the leaves), as shown in Fig. 4b. That was probably due to the differences in nitrogen absorption, transport, and utilization in the various tissues and to the altered internal environment of the bioretention system caused by different drying-wetting cycles. The different biomass of the disparate tissues could also have created the differences in nitrogen accumulation. The large stem biomass (shown in Table S1) resulted in a greater N accumulation in stems. But among all the groups with different drying and rewetting cycles, the total amount of nitrogen in the above-ground stems and leaves was always greater than in the roots. It indicates that at least in Cyperus alternifolius, the nitrogen assimilation ability of the plant’s above-ground portion is stronger than that of the underground parts. That is consistent with the observations of previously-published work (Chen et al. 2011).
Figure 4c shows the nitrogen IR of each plant tissues in the different groups. It illustrates that nitrogen’s IR increased with increasing ADDs. The IR of group BR7 was the highest (23.26%), while that of group BR1 was the lowest (0.65%). The reason is probably that a long dry period could cause more nitrogen to accumulate in the system, and the better-growing plants assimilate more nitrogen.
Plant roots play a major role in nitrogen absorption, and their morphology is a key factor that causes differences in nitrogen assimilation (Kenobi et al. 2017). Details of the root system architectures are presented in Table S1. The results show that alternate drying and rewetting affected the total height, area, and volume of the roots. The root systems which experienced longer ADDs presented a better state of growth. Root nitrogen content was then positively correlated with the total root volume and average root diameter, while it was negatively correlated with TRH, TRA, RTs, BN, and CN, as is shown in Fig. S3. To some extent, a larger total volume of roots allows the root system and the whole plant to better assimilate nitrogen.
Abiotic nitrogen immobilization in soil
There were differences in the total nitrogen content of the soil in the planted and submerged zones, and they depended to some extent on the different groups’ drying and wetting cycles. The soil TNs of the PZs varied significantly, while there were only small differences among the SZs, as is shown in Fig. 5a. Nitrogen did accumulate in the soils to a certain extent, and the accumulation in the PZ was between 2 and 5 times than that in the SZ. This result coincides with Fig. 3a, which demonstrated that soil TN contents declined with depth. The NO3− content of the PZ and SZ varied significantly among the groups with different drying and rewetting histories (p ≤ 0.05), as Fig. 5b shows. The soil NO3− content in both the PZ and SZ (5.44 and 4.99 mg/kg, respectively) was significantly higher in group BR4 than it in the other groups (p ≤ 0.05). This indicated that more NO3− could be accumulated in bioretention system under certain drying and rewetting conditions.
Contents of a TN, b NO3-N, and c NH4+-N and IR of e TN, f NO3−-N, and g NH4+-N in soils. (Different capital letters refer to significant differences between different groups in the same soil layer at the p < 0.05 value, and different lowercase letters refer to significant differences between different soil layers of the same group at the p < 0.05 value.)
The results in Fig. 5c show that the highest soil NH4+ content in a PZ was 6.46 mg/kg in group BR1, which is higher than the levels in the other groups (p ≤ 0.05). Frequent inflow could reduce soil reoxygenation time, resulting in a relatively high NH4+ accumulation. Group BR1’s soil NH4+ content was greater than that in the other groups (Fig. 5c), which was consistent with observations of Morillas and his colleagues (Morillas et al. 2015). Besides, the NH4+ content in the soil of BR1was higher than that of NO3−. The soil presumably had a low redox potential caused by hypoxia during continuous inflow. That could inhibit the conversion of ammonium to nitrate and lead to NH4+ accumulation in the soil. In addition, it is also possible that the DNRA process was important in group BR1. Related research has confirmed a higher contribution from DNRA to nitrate reduction under high-moisture conditions (Chen et al. 2015). As for the BR7 group, the longer drying-rewetting cycle apparently promoted the soil’s ammonization and the NH4+ content. The soil’s NO3− content always exceeded its NH4+ content in groups BR2 to BR5, demonstrating that nitrification was favored over ammonization. Much of the ammonium was likely reduced to high-solubility nitrate, which did not accumulate in the soils through adsorption.
Regardless of the drying and rewetting cycle, the IRs of TN, NO3−, and NH4+ were always higher in the PZ than in the SZ, as is shown in Fig. 6a–c. This result further confirms that the soil in the PZ was the main retention place for incoming nitrogen in these bioretention experiments. The input nitrogen from runoff was first retained by the filtering medium in the PZ, where the root network likely provided surface area for the attachment and growth of microorganisms. That increased the microbial biomass and promoted nitrogen fixation by the microbes.
The bioretention systems’ nitrogen IR was affected by the ADDs. The longer the dry spell, the more nitrogen was immobilized. It was group BR7 that had the greatest soil immobilization of nitrogen. The IR of TN in PZ and SZ was observed highest at 36.41% and 27.66%. The same was true of its NH4+ IRs at 21.71% in the PZ and 13.08% in the SZ. That group’s NO3− IRs were, however, only 8.86% and 5.66% in the PZ and SZ respectively. It seems that prolonged drying which brings down the soil’s moisture content affects the growth of denitrifying bacteria, inhibiting denitrification and causing an accumulation of nitrogen in the soil.
Nitrogen reductase activity
Figure 7 shows that the activity of nitrogen reductases in the soils was affected by ADDs and related to depth. Different dry-wet cycles produced differences in NiR activity in the PZs. There was, however, little difference in HyR or NaR activity. Although there was no significant difference in NaR activity between the planted and submerged zones, the differences in NiR were significant (p ≤ 0.05) (except in group BR1). In groups BR2 and BR7, the HyR activities differed significantly between the PZ and the SZ (p ≤ 0.05), but no significant differences were observed in the other groups.
a NaR, b NiR, and c HyR activities in soils. (Different capital letters refer to significant differences between different groups in the same soil layer at the p < 0.05 value, and different lowercase letters refer to significant differences between different soil layers of the same group at the p < 0.05 value.)
The overall NaR activities in all of the groups were below 0.21 mg/(g·24 h) in winter. That may have been due to the lower temperature and light intensity inhibiting the reductases. In the majority of the groups, the NaR, NiR, and HyR activities were greater in the PZ than in the SZ, and the reductase concentrations decreased with depth. That is consistent with the results of related reductase studies (Gutknecht et al. 2006; Marhan et al. 2011). The plant roots and richer nutrients in the PZ promoted the growth of microbes and resulted in higher soil enzyme activity (Wang et al. 2012). In the SZ, the lower light intensity, and dissolved oxygen level presumably affected microbial metabolism, resulting in a decline in reductases. However, NiR activity in the SZ was 2.1 to 2.5 times higher than that in the PZ in groups BR2, BR3, and BR5. That indicates a relationship with soil moisture and soil nitrogen content. The ammonia content of the SZ was higher than that of the PZ in groups BR2 and BR3, and their vertical NiR activity and ammonium concentration gradients were similar. Soil nitrogen content is correlated with enzyme activity, and the higher the nitrogen content, the higher the enzyme activity (Allison et al. 2008). There was, however, no relationship between NiR activity and ammonium content in group BR5 where the soil moistures in the PZ and SZ were 25.1% and 38.5%, respectively. When the soil moisture is less than 35%, the enzyme activity is relatively high (Li et al. 2014). At the same time, the concentration of dissolved oxygen in the pore water of the SZ is low. That stimulates microbes to secrete nitrite reductase, resulting in higher NiR activity in the SZ.
Overall responses
Alternate drying and rewetting can change the physiochemical properties of soil in a retention basin and also affect microbial activity. That affects the accumulation, migration, and loss of nitrogen in the basin’s soils (Fiere and Schimel 2002). Exsiccation can promote a soil’s respiration and enhance the mineralization of organics (Rey et al. 2004). In these experiments, the TN and organics content of the soils decreased with increasing ADDs, and they were positively correlated (Fig. S4). Moreover, ammonium nitrogen generated by organic degradation provided a feedstock for nitrification. Differences in soil organics also affected nitrogen accumulation under different drying and rewetting conditions. Some scholars have proposed that soil nitrogen content is determined primarily by the soil’s level of organics (Smolander and Kitunen 2002). Alternate drying and rewetting also changes the soil’s pH (Table 2), and that also affects the migration and transformation of nitrogen in the soil (Stark et al. 2012).
Soil enzymes are closely related to soil nitrogen content, and they play an important part in the nitrogen cycle. That makes research into soil enzyme activity a great significance for understanding the nitrogen cycle in bioretention systems. This study’s correlation analyses revealed that the activity of NaR could enhance with soil TN content increased. Meanwhile, the activity of HyR could enhance with the increase of soil NO3− content. As it is known, HyR activity was affected by the level of NH2OH, a target for catalytic conversion. The NH2OH came mainly from NiR conversion of NO2−. So NO2−, a participant in the first nitrogen reduction process, indirectly provides a target for a reduction involving HyR (Liu et al. 2014b; Weng et al. 2013). Meanwhile, the positive correlations among the NaR, NiR, and HyR activities indicate that NO3−, NO2−, and NH2OH are consistently and continuously being reduced, further confirming the importance of the DNRA process in bioretention systems.
Enzyme activity in the soil is closely related to moisture content; Unda’s experiments showed a significant and positive effect of soil water content on NaR and NiR activity (Unda et al. 2019). The responses of the reductases to water content were, however, different. With the increase of ADDs, soil moisture decreased and NaR activity declined. However, there were also cases where higher moisture content resulted in weak enzyme activity. The NiR activity in frequently rewetted groups was lower than in the others, indicating that frequent watering inhibits microbe growth. In addition, the poor soil aeration can eventually cause a decline in enzyme activity.
The experiments have also shown a relationship between nitrogen reductases in the soil and organics content. The concentrations and spatial distributions of the organics processed in enzymatic reactions can affect microbial activity and induce more enzymes to be secreted. They can also directly affect the rate of biochemical reactions in which soil enzymes participate. It can safely be concluded that various nitrogen transformations in bioretention systems involving nitrogen reductases can be significantly influenced by the system’s cycle of drying and rewetting.
Overall, these experiments have shown that the physiochemical properties of the soil in a bioretention system and the nitrogen reductases present affect nitrogen transformation in the system. This is what makes the research on system’s intermittent drying and rewetting cycle of great importance.
Conclusions
This study investigated the effect of drying and rewetting cycles on nitrogen migration and transformation in laboratory-scale stormwater bioretention systems. The bioretention system could remove nitrogen from artificial storm runoff effectively under any drying conditions. The top 10 cm of the planting layer played an important role in intercepting and retaining influent nitrogen. The retention of nitrogen decreased with ADDs. Cyperus alternifolius was shown to be an effective plant species, which could accumulate nitrogen in its roots, stems, and leaves, with the stem playing the most important role in nitrogen accumulation. The highest IR in the stems (23.26%) was obtained in 12 ADDs. The IRs increased with increasing ADDs. The activities of NaR, NiR, and HyR in the same soil layer differed with different ADDs (except for NaR in the PZ). There were no significant differences in NaR activity between the PZ and the SZ. In general, the soil’s physiochemical properties, nitrogen retention, and reductase activity were closely related, regardless of the drying and rewetting cycle. This study’s observations support the contention that alternate drying and rewetting affects nitrogen transformation and retention in a stormwater retention pond to some extent.
Data availability
Present study data are available with corresponding author and are available on reasonable request.
References
Acosta-Martínez V, Cruz L, Sotomayor-Ramírezb D, Pérez-Alegríac L (2007) Enzyme activities as affected by soil properties and land use in a tropical watershed. Appl Soil Ecol 35(1):35–45
Allison SD, Czimczik CI, Treseder KK (2008) Microbial activity and soil respiration under nitrogen addition in Alaskan boreal forest. Glob Chang Biol 14:1156–1168
Canarini A, Dijkstra FA (2015) Dry-rewetting cycles regulate wheat carbon rhizodeposition, stabilization and nitrogen cycling. Soil Biol Biochem 81:195–203
Chen L, Li J, Zhong C, Wang X, An L (2011) Treatment of breeding wastewater using constructed wetland with plant bed. Chin J Environ Eng 5(7):1542–1547 (in Chinese)
Chen Z, Ding W, Xu Y, Müller C, Rütting T, Yu H, Fan J, Zhang J, Zhu T (2015) Importance of heterotrophic nitrification and dissimilatory nitrate reduction to ammonium in a cropland soil: Evidences from a 15N tracing study to literature synthesis. Soil Biol Biochem 91:65–75
Cho KW, Yoon MH, Song KG, Ahn KH (2011) The effects of antecedent dry days on the nitrogen removal in layered soil infiltration systems for storm run-off control. Environ Technol 32:747–755
Davidson EA, Savage KE, Bettez ND, Marino R, Howarth RW (2010) Nitrogen in runoff from residential roads in a coastal area. Water Air Soil Pollut 210:3–13
Debusk KM, Wynn TM (2011) Storm-water bioretention for runoff quality and quantity mitigation. J Environ Eng 137:800–808
Dick RP, Breakwell DP, Turco RF (1996) Soil enzyme activities and biodiversity measurements as integrative microbiological indicators. Methods Assess Soil Qual:247–271
Fiere N, Schimel JP (2002) Effects of drying-rewetting frequency on soil carbon and nitrogen transformations. Soil Biol Biochem 34(6):777–787
General Bureau of China National Environmental Protection (2002) Water and wastewater monitoring and analysis method, 4th edn. China Environmental Science Press, Beijing (in Chinese)
Glaister BJ, Fletcher TD, Cook PL, Hatt BE (2014) Co-optimisation of phosphorus and nitrogen removal in stormwater biofilters: the role of filter media, vegetation and saturated zone. Water Sci Technol 69:1961–1969
Guo X, Drury CF, Yang X, Reynolds D, Fan R (2014) The extent of soil drying and rewetting affects nitrous oxide emissions, denitrification, and nitrogen mineralization. Soil Sci Soc Am J 78(1):194–204
Gutknecht JLM, Goodman RM, Balser TC (2006) Linking soil process and microbial ecology in freshwater wetland ecosystems. Plant Soil 289:17–34
Han J, Cao X (2013) Effects of drying-rewetting alternation on nitrogen dynamics in a typical coastal wetland: a simulation study. Environ Sci 34(6):2383–2389 (in Chinese)
Hardison AK, Algar CK, Giblin AE, Rich JJ (2015) Influence of organic carbon and nitrate loading on partitioning between dissimilatory nitrate reduction to ammonium (DNRA) and N2 production. Geochim Cosmochim Acta 164:146–160
Hatt BE, Fletcher TD, Deletic A (2007) Hydraulic and pollutant removal performance of stormwater filters under variable wetting and drying regimes. Water Sci Technol 56:11–19
Jarney FJ, Olson AF, Miller JJ, Tovell BC (2014) Nitrogen and phosphorus in runoff from cattle manure compost windrows of different maturities. J Environ Qual 43(2):671–680
Kenobi K, Atkinson JA, Wells DM, Gaju O, De Silva JG, Foulkes MJ, Dryden IL, Wood ATA, Bennett MJ (2017) Linear discriminant analysis reveals differences in root architecture in wheat seedlings by nitrogen uptake efficiency. J Exp Bot 68(17):4969–4981
Le Coustumer S, Fletcher TD, Deletic A, Barraud S, Poelsma P (2012) The influence of design parameters on clogging of stormwater biofilters: a large-scale column study. Water Res 46(20):6743–6752
Leitner S, MinixhoferI P, Inselsbacher E, Keiblinger KM, Zimmermann M, Zechmeister-Boltenstern S (2017) Short-term soil mineral and organic nitrogen fluxes during moderate and severe drying-rewetting events. Appl Soil Ecol 114:28–33
Li Y, Yuan X, Zhu H (2014) Simulation study on effects of soil moisture contents on nitrogen transformation and enzyme activities in black soil. Chin J Soil Sci 45(4):903–908 (in Chinese)
Liu C, Lu J, Li H (2007) Landward changes of soil enzyme activities in a tidal flat wetland of the Yangtze River Estuary and correlations with physico-chemical factors. Acta Ecol Sin 27(9):3663–3669
Liu J, Sample D, Bell C, Guan Y (2014a) Review and research needs of bioretention used for the treatment of urban stormwater. Water 6:1069–1099
Liu S, Vereecken H, Brüggemann N (2014b) A highly sensitive method for the determination of hydroxylamine in soils. Geoderma 232:117–122
Lu W, Zhang H, Min J, Shi W (2015) Dissimilatory nitrate reduction to ammonium in a soil under greenhouse vegetable cultivation as affected by organic amendments. J Soils Sediments 15(5):1169–1177
Lucke T, Beecham S (2011) Field investigation of clogging in a permeable pavement system. Build Res Inf 39(6):603–615
Lucke T, Nichols PWB (2015) The pollution removal and stormwater reduction performance of street-side bioretention basins after ten years in operation. Sci Total Environ 536:784–792
Luo FL, Nagel KA, Scharr H, Zeng B, Schurr U, Matsubara S (2011) Recover dynamics of growth, photosynthesis and carbohydrate accumulation after de-submergence: a comparison between two wetland plants showing escape and quiescence strategies. Ann Bot 107(1):49–63
Maltais-Landry G, Maranger R, Brisson J, Chazarenc F (2009) Nitrogen transformations and retention in planted and artificially aerated constructed wetlands. Water Res 43(2):535–545
Mangangka I, An L, Egodawatta P, Goonetilleke A (2015) Performance characterisation of a stormwater treatment bioretention basin. J Environ Manag 150:173–178
Marhan S, Philippot L, Bru D, Rudolph S, Franzaring J, Högy P, Fangmeier A, Kandeler E (2011) Abundance and activity of nitrate reducers in an arable soil are more affected by temporal variation and soil depth than by elevated atmospheric [CO2]. FEMS Microbiol Ecol 76(2):209–219
Mikha MM, Rice CW, Milliken GA (2005) Carbon and nitrogen mineralization as affected by drying and wetting cycles. Soil Biol Biochem 37(2):339–347
Morillas L, Durán J, Rodríguez A, Roales J, Gallardo A, Lovett LM, Groffman PM (2015) Nitrogen supply modulates the effect of changes in drying-rewetting frequency on soil C and N cycling and greenhouse gas exchange. Glob Chang Biol 21(10):3854–3863
Navarrogarcia F, Casermeiro MA, Schimel JP (2012) When structure means conservation: effect of aggregate structure in controlling microbial responses to rewetting events. Soil Biol Biochem 44(1):1–8
Payne EGI, Fletcher TD, Cook PLM, Deletic A, Hatt BE (2014) Processes and drivers of nitrogen removal in stormwater biofiltration. Crit Rev Environ Sci Technol 44:796–846
Rey A, Petsikos C, Jarvis PG, Grace J (2004) Effect of temperature and moisture on rates of carbon mineralization in a Mediterranean oak forest soil under controlled and field conditions. Eur J Soil Sci 56(5):589–599
Smolander A, Kitunen V (2002) Soil microbial activities and characteristics of dissolved organic C and N in relation to tree species. Soil Biol Biochem 34(5):651–660
Stark S, Eskelinen A, Mnnisto MK (2012) Regulation of microbial community composition and activity by soil nutrient availability, soil pH, and herbivory in the Tundra. Ecosystems 15(1):18–33
Strohm TO, Griffin B, Zumft WG, Schink B (2007) Growth yields in bacterial denitrification and nitrate ammonification. Appl Environ Microbiol 73(5):1420–1424
Subramaniam D, Mather P, Russell S, Rajapakse J (2016) Dynamics of nitrate-nitrogen removal in experimental stormwater biofilters under intermittent wetting and drying. J Environ Eng 142(3):04015090
Taylor GD, Fletcher TD, Wong THF, Breen PF, Duncan HP (2005) Nitrogen composition in urban runoff—implications for stormwater management. Water Res 39:1982–1989
Unda J, Martínez-Santos M, Ruiz-Romera E, Lechuga-Crespo JL (2019) Implications of denitrification in the ecological status of an urban river using enzymatic activities in sediments as an indicator. J Environ Sci 75:255–268
Wan Z, Song C, Guo Y, Wang L, Huang J (2008) Effects of water gradients on soil enzyme activity and active organic carbon composition under Carex lasiocarpa marsh. Acta Ecol Sin 28(12):5980–5986
Wang B, Xu S, Liu GB, Zhang GH, Li G, Ren ZP (2012) Changes in soil nutrient and enzyme activities under different vegetations in the Loess Plateau area, Northwest China. Catena 92:186–195
Wang Y, Jensen CR, Liu F (2017) Nutritional responses to soil drying and rewetting cycles under partial root-zone drying irrigation. Agric Water Manag 179:254–259
Weng B, Xie X, Yang J, Liu J, Lu H, Yan C (2013) Research on the nitrogen cycle in rhizosphere of Kandelia obovata under ammonium and nitrate addition. Mar Pollut Bull 76:227–240
Yang H, Florence DC, McCoy EL, Dick WA, Grewal PS (2009) Design and hydraulic characteristics of a field-scale bi-phasic bioretention rain garden system for storm water management. Water Sci Technol 59(9):1863–1872
Yu XH, Chen Y, Ren PP, Zheng S, Cheng QH (2019) Selection and comprehensive assessment of plants in bioretention system. Chin J Environ Eng 13(7):1634–1644
Zheng YL, Hou LJ, Newell S, Liu M, Zhou JL, Zhao H, You LL, Cheng XL (2014) Community dynamics and activity of ammonia-oxidizing prokaryotes in intertidal sediments of the Yangtze Estuary. Appl Environ Microbiol 80:408–419
Funding
The authors are grateful for the financial support provided by the Natural Science Foundation of China (51709024), the Science and Technology Research Program of Chongqing Science and Technology Commission (cstc2017jcyjAX0292), and Venture & Innovation Support Program for Chongqing Overseas Returnees (cx2017065).
Author information
Authors and Affiliations
Contributions
RC, XY: data curation and formal analysis. SZ, SY: literature search. YC, ZL: editing and review. All authors wrote the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• N could be removed by bioretention systems under extreme drying/rewetting condition
• The N transformation under multiplicity drying/rewetting conditions was clarified
• The increase of soil TN content could enhance the activity of nitrate reductase
• Hydroxylamine reductase activity could be enhanced with the increase of soil NO3− content
Supplementary Information
ESM 1
(DOCX 574 kb)
Rights and permissions
About this article
Cite this article
Chen, Y., Chen, R., Liu, Z. et al. Nitrogen process in stormwater bioretention: the impact of alternate drying and rewetting on nitrogen migration and transformation. Environ Sci Pollut Res 28, 43803–43814 (2021). https://doi.org/10.1007/s11356-021-13802-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13802-z