Abstract
This study investigated the deep removal of complex nickel from simulated wastewater using magnetic separation and magnetic seed recycling. Nano-magnetite (Fe3O4) was used as the magnetic seed. The flocculant applied was N,N-bis-(dithiocarboxy) ethanediamine (EDTC), a highly efficient heavy metal chelating agent included in dithiocarbamate (DTC). Important investigated parameters included hydraulic retention time, magnetic seed dosage, and magnetic field strength. The study also explored the magnetic flocculation mechanism involved in the reaction. The result indicated that the residual Ni concentration was reduced to less than 0.1 mg/L from the initial concentration of 50 mg/L under optimal conditions. Magnetic seed recovery reached 76.42% after a 3-h stirring period; recycled magnetic seeds were analyzed using scanning electron microscope (SEM) and X-ray diffraction (XRD). The zeta potential results illustrated that magnetic seeds firmly combined with flocs when the pH ranged from 6.5 to 7.5 due to the electrostatic attraction. When the pH was less than 7, magnetic seeds and EDTC were also combined due to electrostatic attraction. Particle size did affect microfloc size; it decreased microfloc size and increased floc volume through magnetic seed loading. The effective binding sites between flocs and magnetic seeds increased when adding the magnetic seeds. This led the majority of magnetic flocs to be integrated with the magnetic seeds, which served as a nucleus to enhance the flocculation property and ultimately improve the nickel complex removal rate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electroplating wastewater is both complex and uncontrollable. Further, the quantity and quality of wastewater are impacted by factors such as processing conditions, production loads, production management, and water use methods. Heavy metal ions, including chromium, copper, nickel, zinc, gold, and silver, are the main contaminants in electroplating wastewater (Wang 2013). During industrial production activities, these heavy metal ions combine with complexing agents, forming different nickel complexes. Examples of these complexes include triethanolamine-nickel (TEA-Ni), tartaric acid (TA)-Ni, citric acid (CA)-Ni, sodium pyrophosphoric acid (SP)-Ni, and ethylenediaminetetraacetic acid (EDTA)-Ni. This complicates electroplating wastewater components, making it more difficult to manage and treat.
Nickel is a primary heavy metal element in electroplating wastewater. It poses a significant threat to human health, as it can accumulate in the spinal cord, brain, and viscera, causing chronic organ-specific diseases and enzyme system poisoning (Timothy et al. 1989). The Chinese Ministry of Environmental Protection has set 0.1 mg/L as a maximum allowed discharge concentration for nickel in the Discharge Standard of Electroplate Contaminant (GB21900–2008). Therefore, there is much interest in effectively wiping off complex nickel from electroplating wastewater to reach heavy metal wastewater effluent requirements. Primary methods for treating nickel-containing electroplating wastewater currently include neutralization precipitation, sulfide precipitation, ferrite process, ion exchange, electrolysis, membrane, adsorption, and flocculation.
Neutralization precipitation is a method that removes nickel in the form in Ni(OH)2. However, this method requires that the complex first be broken; the method also consumes a large mass of agents and requires significant amounts of land as well (Qi 2011). Sulfide precipitation also has these drawbacks, and sulfur ions also generate secondary pollution (He et al. 2013). Ni2+ can adhere to a ferrite lattice through an entrainment effect and other forces when a ferrite process is applied (Kozlowski and Walkowiak 2002; Chang et al. 2011). Unfortunately, this approach is very expensive due to high energy consumption (Zuo and Wang 2011). Other methods also have significant disadvantages, discouraging wide adoption. These include non-renewable for ion exchange resin and adsorption, equipment maintenance for electrolysis, and expensive materials for membrane and complicated synthetic method of flocculants (Kapoor et al. 1999; Eom et al. 2005; Shen 2005; Yan et al. 2009; Zhou and Lu 2009; Zhang and Hu 2011).
Magnetic flocculation is a method for separation based on the magnetic properties of particles. Magnetic flocs form when magnetic seeds and flocculants are added in advance (Shen et al. 2014; Wang et al. 2015). Different flocculants (aluminum sulfate, polyaluminum chloride (PAC), ferrous sulfate, polymeric ferric sulfate (PFS)) can be applied to combine colloid particles through adsorption, bridging, and sweeping. These actions thus form magnetic flocs. When magnetic seeds are added, the non-magnetic flocs become magnetic. This allows them to be separated using a magnetic separation techniques or sedimentation properties (Zhang and Yang 2012). Eventually, the magnetic flocs are separated from the solution and the pollutants are effectively removed (Nuray 2003).
It has been shown that adding magnetic seeds can enhance flocculation, improve floc structure, and advance floc sedimentation properties (Li 2014). This can achieve cost reductions (Li et al. 2010a, b), because the magnetic seeds can be recycled using a magnetic separation device. These advantages have led many researchers to adopt magnetic flocculation to remove turbidity, organics, salts, bacteria, and heavy metal ions from wastewater at a laboratory scale (Fang et al. 2010; Li et al. 2010a, b; Liu et al. 2012; Li 2014).
One experimental study that examined the treatment of coal-to-oil production wastewater enhanced by magnetic flocculation technology (Guan et al. 2014) found that magnetic flocculation led to better results than traditional flocculation. Adding magnetic seeds resulted in the more rapid formation of flocs at larger floc volumes, reducing sedimentation time. The water sample for experiment contained 5768 mg/L of COD and 2080 NTU of turbidity. At optimum conditions, COD and turbidity removal rates reached 56.9 and 99.7%, respectively.
Another experiment examining the removal of Ni2+ using EDTA-modified magnetic nano-particles (Chen et al. 2013) demonstrated rapid adsorption rates; the EDTA-modified magnetic nano-particles removed almost 100% of Ni2+ when the initial concentration ranged from 10 to 80 mg/L. To date, however, magnetic flocculation has rarely been applied to remove complex metal ions, including complex nickel, from electroplating wastewater.
In this study, a magnetic separation technique was first applied to remove complex nickel from wastewater; the magnetic flocculation was based on a previous experiment. Nano-magnetite (Fe3O4) was used as the magnetic seed. N,N-bis-(dithiocarboxy) ethanediamine (EDTC) was used as the flocculant, a highly efficient heavy metal-chelating agent included in dithiocarbamate (DTC). These materials directly attacked the complex heavy metal to form more stable chelates (Zhen et al. 2012; Xiao et al. 2015; Yan et al. 2015). The study systematically investigated the effects of hydraulic retention time, magnetic seed dosage, and magnetic field strength on the complex nickel removal rate. The study also explored the magnetic flocculation mechanism involved in the reaction and examined magnetic seed recycling as well. This work provides a theoretical basis for complex nickel removal from electroplating wastewater, using a magnetic separation technique.
Materials and methods
Materials
All reagents used in this study were analytical grade, obtained from commercial chemical suppliers. All solutions were prepared with deionized (DI) water (conductivity <0.1 μS/cm). The stock solutions were prepared by dissolving NiCl2·6H2O with CA, potassium sodium TA, and SP at a molar ratio of 1:1 in 1000-mL DI water. The initial Ni2+ concentrations of CA-Ni, TA-Ni, and SP-Ni were 1000 mg/L each. The pH of the solutions was adjusted using 1.0 mol/L NaOH and 1.0 mol/L HCl. The anionic polyacrylamide (APAM), with a molecular weight of approximately 3 million, was dissolved in DI water to form an APAM solution with an initial concentration of 1000 mg/L. The magnetic seeds used in the study were nano-magnetite (Fe3O4) with 99.5% 0f 20-nm spheroidal particles; the seeds were purchased from Shanghai Macklin Biochemical Co., Ltd. EDTC was prepared at laboratory scale using the synthetic method developed by Xiao et al. (2015) and dissolved in DI water to form a EDTC solution with a 10 g/L concentration.
Analytical methods
The concentration of Ni2+ was determined using Hitachi Z-2000 atomic absorption spectrophotometer (AAS). The pH values of metal ion solutions were measured using a DELTA 320 digital pH meter. The precipitant’s surface morphology was analyzed using a Hitachi Z-3400N scanning electron microscopy (SEM). The XRD was conducted using a RIGAKU ULTIA-III X-ray diffractometer. The zeta potential was determined by Brookhaven ZetaPALS. Particle size distribution was determined using an Eyetech-Comb laser particle size analyzer.
Experimental procedures
EDTC synthesis
EDTC was synthesized as follows: first, 0.1 mol (6.68 mL) ethanediamine was added to an aqueous solution of 36% ethanol (40 mL) in a three-necked flask. The mixture temperature was maintained below 10 °C for 30 min with stirring. Second, carbon disulfide with a mole ratio of 2.2:1 to ethanediamine was then added dropwise, given that it is an exothermic reaction. The mixture was stirred at room temperature (25 ± 1 °C) for 3 h to maximize the conversion of ethanediamine to dithiocarbamate. The reaction mixture was then cooled to below 0 °C and crystallized. The crystal was purified using the following steps: suction filtering, washing (three times with 75% ethanol), suction filtering, and vacuum drying overnight at 40 °C. This process resulted in EDTC crystal formation (Xiao et al. 2015).
Magnetic separation experiment
Previous experiments demonstrated that the optimum conditions of complex nickel removal using magnetic flocculation coupled with EDTC were as follows: 70-mg/L magnetic seeds (350 r/min, 2 min), M EDTC/M Ni = 10 (250 r/min, 2 min), APAM at 1 mg/L (50 r/min, 3 min), and a 5-min static sedimentation time with unregulated pH (EDTC dosage was converted to a rate on mass with Ni). At these optimum conditions, the residual Ni concentrations were less than 0.1 mg/L, a significant drop from the initial concentration of 50 mg/L. The magnetic separation experiments on complex nickel in this study were conducted based on this experimental design and results.
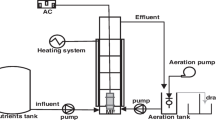
First, a volume of 100-mL simulated wastewater (CA-Ni, TA-Ni, SP-Ni) was placed in a 150-mL beaker, with an initial Ni concentration of 50 mg/L (initial pH values of 3.20, 6.27, and 9.41, respectively) at the room temperature. Then, discrete doses of magnetic seeds were added and stirred at 350 rpm for 2 min. This was followed by the addition of a discrete dose of EDTC; the mixture was then stirred at 250 rpm for 2 min. Finally, 1 mg/L APAM was added, and the mixture was stirred at 50 rpm for 3 min. The mixture was then immediately transferred to the column in the magnetic separation device, with a regulated electrical current. Samples were collected from the outlet column at different time intervals; AAS was used to calculate the removal rate. Figure 1 shows the schematic diagram of the magnetic separation device used in this study.
Magnetic seed recycle experiment
Magnetic flocs were collected using a magnetic separation device because of their magnetic properties. The magnetic flocs were soaked in sodium hydroxide solution and stirred at 300 rpm for a specific amount of time to produce a Ni enrichment solution and isolate magnetic seeds. The recycled magnetic seeds were washed at least three times using deionized water. A magnet was concurrently used to attract the magnetic seeds from the bottom of beaker for 1–2 min. The wet magnetic seeds were then dried in a vacuum drying oven, eventually resulting in a sample of dried and purified magnetic seeds. Figure 2 is a flowchart showing the magnetic seed recycling process.
Results and discussion
Effect of hydraulic retention time on complex nickel removal
This study analyzed the effect of hydraulic retention time on the nickel complex removal rate. The electrical current was maintained at 4 A throughout the experiment. Samples were collected from the outlet of the column outlet once a minute. Figure 3 shows the results.
Figure 3 shows that the removal rates of CA-Ni, TA-Ni, and SP-Ni significantly improved when the hydraulic retention time increased from 1 to 2 min. The CA-Ni increased from 95.23 to 99.54%, TA-Ni increased from 95.31 to 99.47%, and SP-Ni increased from 97.01 to 99.76%, respectively. There was no significant improvement as the hydraulic retention time increased to 3 min; the residual Ni concentration in the outflow was less than 0.1 mg/L for CA-Ni, TA-Ni, and SP-Ni. In the first 2 min, flocs became magnetic flocs by combining with magnetic seeds; magnetic flocs were drawn to the column wall and gathered at the magnetic poles, because of the magnetic property, to decrease the outflow’s Ni concentration. However, a segment of micromagnetic flocs floated in the fluid, resulting in a failure of the outflow’s Ni concentration to reach the required threshold (<0.1 mg/L). Magnetism slowly drew these micromagnetic flocs to the magnetic poles. After that, the fluid became clear, as no microflocs were observed floating in the solution and the outflow directly discharged. The magnetic flocs were steadily attracted to the column wall; the outflow water quality remained steady 3 min later.
Figure 4 illustrates the effect of static sedimentation time on the complex nickel removal rate under gravity-driven natural sedimentation conditions. The Ni concentration of the supernatant remained at less than 0.1 mg/L for 5 min. Comparing the two methods of solid-liquid separation shows that magnetic separation required less time for the outflow to meet the experimental requirements. This demonstrates that magnetic separation is a promising technique due to its time-saving and cost reduction characteristics (Ritu and Mika 2010).
Effect of magnetic seed dosage on complex nickel removal
Magnetic seed dosage was an important parameter impacting non-magnetic pollutant removal using magnetic separation. The electrical current was maintained at 4 A throughout the experiment. Samples were collected from the column outlet once each minute. Figure 5 shows the results.
Figure 5 shows that there was a significant increase in the removal rates of CA-Ni, TA-Ni, and SP-Ni in the first 2 min, when the magnetic seeds ranged from 50 to 80 mg/L. The removal rate continued to increase, resulting in a Ni concentration in the outflow of less than 0.1 mg/L in the following 1 min when 70- and 80-mg/L magnetic seeds were added. This result was not seen at magnetic seed dosages of 50 and 60 mg/L. At these levels, instead of the removal rates increasing, they remained stable. This resulted in outflow concentrations that exceeded the threshold requirements (<0.1 mg/L). In the first 2 min, the Ni concentration significantly reduced; this is because the magnetic flocs were pulled to the magnetic poles, removing the pollutants.
When magnetic seed concentrations were only at 50 or 60 mg/L, some of the flocs could not be converted into magnetic flocs, because there was a lack of magnetic seeds (Zhao et al. 2012). Instead, the pollutants settled at the bottom of column due to gravity, leading to a decline in outflow quality. In contrast, when the magnetic seed concentration was higher, at 70 and 80 mg/L, there were more effective binding sites and the flocs could combine with magnetic seeds to form magnetic flocs (Li et al. 2010a, b). The flocs and magnetic materials were in full contact, wiping off all the microflocs, and efficiently removing the complex nickel.
The number of effective binding sites increased when more magnetic seeds were added, producing more magnetic flocs to draw pollutants through magnetism. This ultimately improved the removal rate. However, magnetic seed concentrations exceeding 70 mg/L did not lead to an increased removal rate. This is because all the non-magnetic flocs combined with the magnetic seeds, resulting in excess magnetic seeds failing to play the prospective role. As a result, 70 mg/L was considered to be the optimal magnetic seed dosage.
Effect of magnetic field strength on complex nickel removal
It was important to explore the effect of magnetic field strength on the complex nickel removal rate, because it was the magnetic field that generated the attractive force needed to drag the magnetic flocs toward the magnetic poles and remove the pollutants from the liquid. Table 1 shows the relationship between electricity and magnetic field strength. The magnetic field was uniformly distributed. Samples were collected from the column outlet once a minute. Figure 6 shows the results.
Figure 6 shows that removal rates improved rapidly in the first 2 min when the electrical current, representing magnetic field strength, ranged from 2 to 4 A. In the following 1 min, the removal rate of complex nickel improved slightly, reducing the Ni concentration in the outflow to less than 0.1 mg/L when the electrical current was maintained at 3.5 or 4 A. In contrast, the removal rate remained about the same with a 2- or 3-A electrical current; as a result, the outflow was unable to be discharged directly due to the higher Ni concentration. The key advantage of solid-liquid separation using magnetic separation is to guarantee the success of magnetic force compared to other forces. This is because the magnetic flocs are acted upon by magnetic force, gravity, buoyancy, and hydraulic resistance.
One research study (Kang and Yang 2011) proposed that the larger the magnetic force is, the greater chance the particles would be intercepted by the magnetic field. The electrical current, representing magnetic field strength, impacted the attraction of the magnetic flocs to the magnetic field. A stronger magnetic field led to a higher removal rate. If the magnetic field strength was not large enough, the magnetic flocs were not completely attracted around the magnetic poles. This resulted in some flocs settling at the bottom of the column and the outflow water quality failing to reach acceptable standards. The adsorption rate increased with the increased electrical current. When the magnetic field was sufficient, all the magnetic flocs were attracted to the magnetic poles. However, once the electrical current reached a certain point, the particles reached saturation and the magnetic force stopped rising (Kang and Yang 2011). It appears that the removal rate improves with the increased magnetic field strength at a certain hydraulic time and magnetic field strength range. In this study, the optimal electrical current was found to be 3.5 A.
Magnetic seed recycling
Research by Xiao et al. (2016a, b) demonstrated that the flocs were the chelate of EDTC-Ni through infrared spectroscopy (IR) and elemental analysis. That study found that EDTC had a stronger chelating ability than three other complexing agents and could entrap Ni2+ to form a more stable chelate called EDTC-Ni (presented in Eq. (1)). M represented the complex nickel CA-Ni, TA-Ni, and SP-Ni.

The magnetic seeds were effectively dispersed using high-speed stirring and became the nuclei for the flocs. Afterwards, the magnetic flocs were separated from the liquid using the magnetic separation device shown in Fig. 1. The flocs and magnetic flocs were dried and analyzed using SEM and XRD; photos are presented in Figs. 7 and 8.
Comparing the SEM photos of flocs and magnetic flocs reveals several nano-sized spherical particles in the magnetic flocs. Further, the XRD comparative analysis showed a high level of Fe3O4 in the magnetic flocs. This confirmed that the nano-sized spherical particles observed in the SEM photos were Fe3O4 magnetic seeds. In addition, CA-Ni, TA-Ni, and SP-Ni magnetic flocs were identified as having the same peak position seen in the XRD analysis. There were also no significant differences on the microcosmic morphology seen in the SEM photos.
Comparing and analyzing the SEM and XRD results demonstrated that the magnetic flocs of the three nickel complexes contained the same substance, a mixture of flocs and magnetic seeds. Given this, CA-Ni was selected as an example to provide insights into magnetic seed recycling.
The experiment indicated that magnetic seed recovery was closely related to stirring time. Table 2 shows that the recovery reached 76.42% after 3 h of stirring time. It was also noticed that washing times had an effect on the recovery. However, it would influence the future reuse because there were more impurities in the recycled magnetic seeds with less washing times. Thus, the magnetic seeds might be washed away during the washing.
The recycled magnetic seeds were ground, so they could be reused in the next round of magnetic flocculation. The Ni concentration in the outflow for all three nickel complexes reached less than 0.1 mg/L at the optimal condition (3-min hydraulic retention time and 3.5-A electrical current). This result demonstrated that recycled magnetic seeds remained effective in enhancing flocculation (Table 3).
Figure 9 illustrates the magnetic seed recycling process. Magnetic seeds were added to form magnetic flocs; then, the magnetic flocs were soaked and stirred to recycle the magnetic seeds. To be useful at an industrial scale, there is no need to dry the magnetic seeds. For the land saving, the magnetic flocs need compression to decrease the volume. The XRD of the magnetic flocs indicated a high level of magnetite (Fe3O4) in the magnetic flocs, indicating that it was worthy of recycling. The SEM analysis and comparison of unused and recycled magnetic seeds showed no apparent variations in morphology. Even the XRD of recycled magnetic seeds showed a high magnetite (Fe3O4) level. This demonstrated that the recycled magnetic material could be applied in the next magnetic separation experiment, as it still had a high level of effective constituent. Appling the magnetic separation technique is also practical, because recycling magnetic seeds reduce the running cost.
Magnetic flocculation mechanism
Zeta potential
The zeta potentials of magnetic seeds, EDTC, non-magnetic flocs, and magnetic flocs were determined using ZetaPALS under different pH values. The non-magnetic flocs were obtained using gravity, without magnetic seeds. Figure 10 shows the results.
Zeta potentials of magnetic seeds, EDTC, non-magnetic flocs, and magnetic flocs changed from positive to negative when the pH ranged from 3 to 9. The magnetic flocs had a lower zeta potential for combining with the magnetic seeds, which carried a lower zeta potential than the non-magnetic flocs. In addition, the magnetic flocs (at pH = 6) had a lower isoelectric point than non-magnetic flocs (at pH = 7.5). Research by Prochazkova et al. (2013) suggested that particles with strong ion exchange groups have a high isoelectric point; particles with weak ion exchange groups exhibit a lower isoelectric point.
Based on this conclusion, magnetic flocs were more stable than non-magnetic flocs because of the weak ion exchange groups on the surface. The zeta potentials of magnetic flocs and non-magnetic flocs also demonstrated this fact when the pH ranged from 9 to 11. The non-magnetic flocs began to dissolve when the pH exceeded 9; however, the magnetic flocs were more stable under alkaline conditions, because the absolute value of the zeta potential of the magnetic flocs was greater than the potential of the non-magnetic flocs. Therefore, magnetic seeds enhance floc stability.
The zeta potential of EDTC was negative at pH values less than 3; the magnetic seeds gained an isoelectric point at a pH of 7. As such, magnetic seeds and EDTC were combined through electrostatic attraction (Xu et al. 2011) when the pH ranged from 3 to 7. Similary, the magnetic seeds firmly combined with non-magnetic flocs through electrostatic attraction, because the magnetic seeds had an opposite electric charge than the non-magnetic flocs, when the pH ranged from 6.5 to 7.5. Figure 11 illustrates the two ways that magnetic seeds responded.
These two ways facilitated the combination of the magnetic seeds with either EDTC or flocs to integrate the majority of magnetic flocs. The seed served as a nucleus to enhance the flocculation property and improve the complex nickel removal rate. The pH of electroplating wastewater was approximately 6 (Kabdasli et al. 2009) and rose slightly when DTC was added to the liquid. The DTC was weakly ionized, causing a slight increase of pH (presented in Eq. (2)). Therefore, no adjustments were needed to apply the magnetic flocculation to the electroplating wastewater.

Particle size analysis
The particle size distribution was determined using a Eyetech-Comb laser particle size analyzer. Figure 12 shows the results.
Development of particle size during the testing process (step 1 0–2 min, magnetic seeds (100 mg/L or 0), fast stirring (350 r/min) for 2 min; step 2 2–4 min, EDTC (M EDTC/M Ni = 10), fast stirring (250 r/min) for 2 min; step 3 4–7 min, APAM (1 mg/L), slow stirring (50 r/min) for 3 min; step 4 static sedimentation for 10 min)
Figure 12 shows that microflocs began to develop when the EDTC had a chelation reaction with the nickel complex at 2–4 min. During this 2 min, the non-magnetic floc particle sizes increased from 6.11 to 8.87 μm, while the magnetic floc particle sizes increased from 10.01 to 35.05 μm. Magnetic seeds enlarged floc size and accelerated floc formation. When APAM was added at 4 min, the particle size of non-magnetic flocs and magnetic flocs both increased rapidly, growing to 89.7 and 124.15 μm, respectively, after 7 min. The majority of the EDTC-Ni and magnetic seed mixture was suspended in the liquid at the end of step 2.
Then, APAM was added in step 3. One end of APAM absorbed one colloid; at the same time, the other end adsorbed another colloid. The polymer chain structure formed flocs of “colloid-polymer-colloid.” The EDTC-Ni and magnetic seed mixture then became unstable and began to flocculate. Furthermore, the sweep flocculation occurred while the magnetic flocs were drawn to the magnetic poles by the magnets, sweeping off the other microparticles suspended in the liquid. APAM helped enlarge the volume of magnetic flocs and also captured microparticles.
The static sedimentation started when the slow stirring was stopped after a 7-min reaction. In the first 3 min of static sedimentation, the particle size of non-magnetic flocs and magnetic flocs increased slowly and then stayed at a stable level through the end of the test. The particle size of non-magnetic flocs and magnetic flocs reached 113.2 and 150.5 μm, respectively. This demonstrated that magnetic seeds enlarged floc volume. Figure 13 presents the size distribution of non-magnetic flocs and magnetic flocs at 17 min.
With respect to size distributions, the flocs which particle size was under 100 μm took less than 20% of the total magnetic flocs but took more than 30% of the total non-magnetic flocs. In other words, there were fewer microflocs in the magnetic flocs than in the non-magnetic flocs. Adding magnetic seeds reduces microflocs and enlarges the floc volume to enhance the flocculation property.
Conclusions
In this study, a magnetic separation technique was applied to remove complex nickel from wastewater using magnetic flocculation. The magnetic separation experiment resulted in a residual Ni concentration of less than 0.1 mg/L compared to the initial concentration of 50 mg/L. The experimental conditions leading to these results were 70-mg/L magnetic seeds (350 rpm, 2 min), 3-min hydraulic retention time, and 3.5-A electrical current. This was based on a previous magnetic flocculation experiment (M EDTC/M Ni = 10 (250 rpm, 2 min), APAM 1 mg/L (50 rpm, 3 min)). Magnetic seed recovery reached 76.42% after 3 h of stirring, and the recycled magnetic seeds were still effective in enhancing flocculation. The magnetic seeds firmly combined with EDTC or flocs through electrostatic attraction. This decreased the microflocs and increased floc volume under optimum conditions. The magnetic seeds were firmly combined with EDTC or flocs to integrate majority of magnetic flocs with magnetic seeds as the nucleus to enhance the flocculation property and improve the removal rate of complex nickel.
References
Chang JX, Wang SF, Chen X (2011) Research on the treatment of Ni2+-containing wastewater by ferrite co-precipitation process. Industrial Water Treatment 31(3):46–47 (in Chinese)
Chen JY, Hao YM, Chen M (2013) Rapid and efficient removal of Ni2+ from aqueous solution by the one-pot synthesized EDTA-modified magnetic nanoparticles. ENVIRON SCI POLLUT R 21:1671–1679
Eom TH, Lee CH, Kim JH, Lee CH (2005) Development of an ion exchange system for plating wastewater treatment. Desalination 180(1):163–172
Guan DX, Yuan LM, Jin XY, He D (2014) Treatment of coal-to-oil production wastewater enhanced by magnetic flocculation technology. ENVIRON SCI TECHNOL 37(9):141–144 (in Chinese)
He XW, Hu JL, Li JW, Zhang JJ, Wang JB, Ge P (2013) Treatment of wastewater containing lead by sodium sulfide precipitation. Chinese Journal of Environmental Engineering 7(4):1394–1398 (in Chinese)
Kabdasli I, Arslan T, Hancıa TÖ, Alatona IA, Tünay O (2009) Complexing agent and heavy metal removals from metal plating effluent by electrocoagulation with stainless steel electrodes. J Hazard Mater 165(1–3):838–845
Kang XH, Yang YL (2011) An experimental study on removal of copper ions from industrial wastewater by magnetic flocculation. Industrial Water and Wastewater 42(3):24–27 (in Chinese)
Kapoor A, Viraraghavan T, Cullimore DR (1999) Removal of heavy metals using the fungus Aspergillus niger. BIORESOURCE TECHNOL 70:95–104
Kozlowski CA, Walkowiak W (2002) Removal of chromium (VI) from aqueous solutions by polymer inclusion membranes. Water Res 36(19):4870–4876
Li JX (2014) Application of magnetic coagulation process for slightly polluted river water treatment. Chinese Journal of Environmental Engineering 8(7):2901–2905 (in Chinese)
Li SQ, Wang MF, Zhu ZA, Wang Q, Zhang X, Song HQ, Cang DQ (2010a) Application of superconducting HGMS technology on turbid wastewater treatment from converter. Sep Purif Technol 84:56–62
Li YR, Wang J, Zhao Y, Luan ZK (2010b) Research on magnetic seeding flocculation for arsenic removal by superconducting magnetic separation. Sep Purif Technol 73:264–270
Liu D, Wang P, Wei GR, Dong WB, Hui F (2012) Removal of algal blooms from freshwater by the coagulation-magnetic separation methond. ENVIRON SCI POLLUT R 20:60–65
Fang M, Mishima F, Akiyama Y, Nishijima S (2010) Fundamental study on magnetic separation of organic dyes in wastewater. Physica C 470:1827–1830
Nuray K (2003) Magnetic separation of ferrihydrite from wastewater by magnetic seeding and high-gradient magnetic separation. Miner Process 71:45–54
Prochazkova G, Podolova N, Safarik L et al (2013) Physicoshemical approach to freshwater microalgae harvesting with magnetic particles. COLLOID SURFACE B 112:213–218
Qi YS (2011) The treatment of electroplating effluent with low content of complex copper and complex nickel. Shandong University (in Chinese)
Ritu DA, Mika S (2010) Water purification using magnetic assistance: a review. J Hazard Mater 180:38–49
Shen YL (2005) The study of treating wastewater containing heavy metal ions by the DDTC and composite flocculants. Xichang University (in Chinese)
Shen ZP, Mei RW, Wei YF (2014) Pilot research on treating wastewater by magnetic flocculation. Environ Eng 32:367–368 (in Chinese)
Timothy PC, Dorothy ML, Elizabeth TS (1989) Toxicity and careinogenieity of nickel compounds. Critieal Reviewsin Toxieology 19:341–384
Wang HK (2013) Treatment of electroplating wastewater containing heavy metal. Plating and Finishing 35(3):75–78 (in Chinese)
Wang J, Yang SS, Jia H, Liu LL, Wen ZW, Yang G, Guo SN, Bai JD (2015) Magnetic flocculation technology in turbidity removal of high turbidity seawater for desalination pretreatment. Journal of Tianjin Polytechnic University 34(4):18–21 (in Chinese)
Xiao X, Sun SY, Yan PF, Qiu YQ, Ye MY (2015) The structure characterization of highly-efficient heavy metal chelating agent EDTC and its characteristics of acid complex copper removal. Acta Sci Circum Stantiae 36(2):537–543 (in Chinese)
Xiao X, Qiu YQ, Sun SY, Ye MY, Yan PF, Ye ZW (2016a) Research on the characteristics of tracecomplex nickel ions deep removal by N, N′-bis-(dithiocarboxy) ethylenediamine. Acta Sci Circum Stantiae 36(9):3251–3257 (in Chinese)
Xiao X, Ye MY, Yan PF, Qiu YQ, Sun SY, Ren J, Dai YK, Han DJ (2016b) Disodium N,N-bis-(dithiocarboxy)ethanediamine: synthesis, performance, and mechanism of action toward trace ethylenediaminetetraacetic acid copper (II). Environ Sci Pollut Res 23:19696–19706
Xu L, Guo C, Wang F, Zheng S, Liu CZ (2011) A simple and rapid harvesting method for microalgae by in situ magnetic separation. BIORESOURCE TECHNOL 102:10047–10051
Yan L, Yu XJ, Li SQ (2009) Treatment of spent electroplating nickel bath by electrolytic method. Journal of Shenyang Jianzhu University (Natural Science) 25(4):762–766 (in Chinese)
Yan PF, Sun SY, Ye MY, Xiao X, Liu JY (2015) Research on the efficiency and mechanism of low-concentration Ni removal from electroplating wastewater by the heavy metal capturing agent with thiol. Acta Sci Circum Stantiae 35(9):2833–2839 (in Chinese)
Zhang SF, Hu XE (2011) Application of pulse electrodeposition to nickel wastewater treatment. Environmental Science and Management 36(11):91–94
Zhang Z, Yang YL (2012) Experimental study on advanced treatment of coking wastewater by magnetic flocculation technology. Industrial water and wastewater 43(2):25–29 (in Chinese)
Zhao Y, Xi BD, Li YR, Wang MF, Zhu Z, Xia XF, Zhang LY (2012) Removal of phosphate from wastewater by using open gradient superconducting magnetic separation as pretreatment for high gradient superconducting magnetic separation. Sep Purif Technol 86:255–261
Zhen HB, Xu Q, Hu YY, Cheng JH (2012) Characteristics of heavy metals capturing agent dithiocarbamate (DTC) for treatment of ethylene diamine tetraacetic acid-Cu (EDTA-Cu) contaminated wastewater. J CHEM ENG 209:547–557
Zhou XL, Lu JT (2009) Characterization and properties of modified coal fly ash adsorbent for removal of nickel (II) ions from solution. Clean Coal Technology 16(2):97–100
Zuo M, Wang XJ (2011) Treatment of wastewater containing nickel, chromium, zinc and copper by ferrite process. Electroplating and Finishing 30(7):48–50 (in Chinese)
Acknowledgements
This research was supported by the National Natural Science Foundation of Guangdong Province (No. 2015A030308008), Science and Technology Planning Project of Guangdong Province (No. 2016A040403068), and Special Fund of Department of Environmental Protection of Guangdong Province (2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Angeles Blanco
Electronic supplementary material
ESM 1
(DOCX 844 kb)
Rights and permissions
About this article
Cite this article
Qiu, Y., Xiao, X., Ye, Z. et al. Research on magnetic separation for complex nickel deep removal and magnetic seed recycling. Environ Sci Pollut Res 24, 9294–9304 (2017). https://doi.org/10.1007/s11356-017-8563-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8563-y