Abstract
Anthropogenic interference the ecosystem unavoidably changes the physical and biological environment. The biodiversity of the Amazon region has been threatened by increased agricultural production and pesticide use. Considering that monitoring pesticides in environments close to their application is one of the ways to preserve the ecosystem, this study investigated the levels of pesticide residues in different environmental compartments (soil, sediment, and water samples). Thirty-one active ingredients of pesticides of different classes were analyzed by UHPLC-MS/MS. For this purpose, we performed quarterly collections in dry and rainy seasons in the region, which helped to evaluate the impact of pesticides on the biodiversity of the study site. Sampling points were the river banks in the area of an agricultural project in Formoso do Araguaia city, Tocantins State. After analysis, we detected the following substances in the water matrix: clomazone, fluazifop-p-butyl, flutolanil, metsulfuron-methyl, propanil, and imidacloprid. Nevertheless, we did not detect any active ingredient in sediment and soil matrices. The active ingredient clomazone was present in all points in the trials, with concentrations reaching up to 0.538 μg L−1. These substances have potential for groundwater contamination. Even at low concentrations in the aquatic ecosystem, these substances can damage human populations and wildlife species, given their toxicological classification. Thus, the study showed an environmental risk of bioaccumulation and/or biomagnification in the region, which may affect environmental biodiversity as well as human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The increase in agriculture and livestock production in the Cerrado has been underpinned by the use of pesticides to ensure good productivity, raising concerns about environmental degradation and biome quality. Proximity to crop fields, mobility, and environmental persistence are all factors affecting surface water contamination by pesticides. These factors correlate with water body characteristics, such as surface, depth, and flow, and climatic conditions, such as ambient temperature, humidity, wind, and rainfall. In addition, soil physical, chemical, and biological processes are dynamic and complex (De Gerónimo et al. 2014; Azevedo et al. 2016; Mondal et al. 2018).
Pesticide pollution in aquatic environmental compartments usually occurs through routes, such as leaching, spray drift, runoff, and cotransport, and can extend far from the application site (Loewy et al. 2011; De Gerónimo et al. 2014).
The fate of pesticides in the environment is governed by different processes, such as transformation, retention, and transport, or by interactions between these processes (Vieira 2012). Pesticide transformation can occur by chemical degradation, that is, degradation of pesticide molecules by different breakdown mechanisms and factors such as light and soil pH. Another possibility is biological degradation (from organisms), being a route of pesticide removal in the environment (Dellamatrice and Monteiro 2014).
The mobility and persistence of pesticides in the environment correlate with water runoff intensity, rainfall, and environmental temperature (Azevedo et al. 2016). Retention of pesticides in the soil occurs by adsorption and desorption (Dellamatrice and Monteiro 2014).
Pesticide transport can be divided into drift, volatilization, leaching, and surface carryover. Drift is nothing more than the shift in the trajectory of pesticide drops, preventing them from reaching their target during or after application. These drops can reach large distances depending on temperature, wind, formulation type, application pressure, droplet size, and the type of pesticide (Vieira 2012).
Volatilization occurs by rapid evaporation of pesticides. As in the case of drift, the formed vapors also may reach nontarget locations. Leaching and runoff occur when rainwater follows two different paths. When water seeps into the soil and percolates deep, we have what we call leaching. This is how pesticides can reach groundwater. The tendency of a pesticide to leach depends on soil adsorption capacity, water solubility, and the soil type. These physical chemical parameters are extremely important in the study of groundwater contamination capacity. Some environmental conditions, such as porous soil, annual rainfall greater than 250 mm, and confined aquifer favor percolation (Rebelo and Caldas 2014).
Runoff is the process where water does not seep into the soil, but rather flows into rivers during or shortly after rain, thus reaching surface waters. The following factors influence this process: soil type, preservation of vegetation cover, riparian forest, agricultural management, slope relief, and incidence of rainfall (Dellamatrice and Monteiro 2014).
The environmental behavior of pesticides in the environment will depend their on physicochemical properties. Notwithstanding, factors, such as environmental characteristics and ways of use, also influence this dynamics (Amaral 2011).
The properties of greatest interest in the study of pesticides are: water solubility, adsorption coefficient (Koc), octanol/water partition coefficient (Kow), ionization constant (Ka and Kb), Henry’s law constant (KH), vapor Pressure (VP), and half-life time (DT50) (Milhome et al. 2009; Amaral 2011). These properties directly influence the transformation, removal, and transport processes described in the previous item, hence the importance of knowing them.
Dilution and low solubility of pesticides in water account for their low concentration. However, after heavy rainfall, high concentrations may occur when high doses have been applied (Dores and Lamonica-Freire 2001).
There are different mathematical modeling methods to assess the potential for pesticide contamination of water bodies. These methods differ regarding the physicochemical properties addressed, which contribute to indicating the environmental behavior of the substance, characterizing higher or lower risk of environmental contamination (Amaral 2011; Martini et al. 2012).
Some methods indicate the potential for pesticide contamination in surface waters, such as the GOSS method (GOSS 1992). Other methods relate to groundwater contamination, such as the Groundwater Ubiquity Score (GUS) (GUSTAFSON 1989) and the criteria proposed by the US Environmental Protection Agency (EPA) (Cohen et al. 1995). All these methods were used in this study.
The literature presents other methods to assess leaching potential, such as retardation factor (RF) and attenuation factor (AF), leaching index (LIX), and temperature leaching potential index (TLPI) (Amaral 2011).
According to Martini et al. (2012), models for predicting the behavior of pesticides in the environment are very useful for estimating the risk of environmental contamination of some substances. These models assist in the preliminary analysis of the choice of pesticides to be monitored. Several monitoring studies confirm the behavioral trend of various substances in different environmental compartments.
Considering that quantitative determination of pesticides is costly and that the number of active ingredients to quantify is large, these models have been a useful and widely used resource.
Many factors must be considered in classifying a target organism as vulnerable and in saying that an active ingredient will affect the biodiversity of the environment. Ecological risk assessment is a process that assists in verifying the likelihood of an environment being impacted as a result of exposure to one or more environmental stressors, such as chemicals used in agriculture (De Gerónimo et al. 2014).
The assessment of environmental risk or ecological risk of pesticides is determined by considering the concentration value and the ecological effect of an active ingredient on a living being and is determined experimentally. Because pesticides are used and synthesized to biologically affect living organisms, there is clearly a risk associated with their use, as they can reach nontarget organisms (Hanson and Starky 2011).
Environmental risk analysis is intended to help understand and predict the relationship between pesticides and their ecological effects. Ecological effects are adverse effects that may alter important structural or functional characteristics of an ecosystem. The assessment of ecological adversity caused by pesticides considers the type, intensity, and scale of the effect, as well as the recovery potential of the system (Rebelo and Caldas 2014).
Ecological risk assessment can range from qualitative judgments to quantitative probabilities. This assessment is used to decide on the risks caused, although the way these risks are estimated varies. The acceptance of this risk is determined by the risk manager, who must balance the benefits and risks of these substances (Rebelo and Caldas 2014).
In this case, risk management is nothing more than a comparison between the environmental cost of using these substances and the benefits they bring. However, this analysis is not always performed seriously, impartially, and by qualified people (Hanson and Starky 2011).
The effects of pesticides on nontarget organisms depend on exposure and sensitivity to the agent; individual characteristics of these organisms; population structure and density; interactions with other species; among other factors (Forbes et al. 2009).
Frequency of exposure also affects the toxicity of chemical compounds. Acute exposure to a single concentration may result in an immediate adverse effect on an organism. In turn, two successive cumulative exposures corresponding to the same amount of exposure of the acute exposure may have little or no effect due to organism metabolism between exposures or to organism acclimatization to the compound (Rand and Petrocelli 1985).
The biological cycle of a pesticide includes its bioconcentration in plants and animals, and its incorporation into the food chain via water or soil (Oliveira and Silva 2013).
Bioaccumulation is the process in which living beings absorb and retain substances, and biomagnification is the increase in the concentration of a given substance in the body as trophic level increases (Isherwood 2000). Concentration in body tissues can reach levels much higher than those of the environments in which these organisms inhabit (De Gerónimo et al. 2014).
Despite the common occurrence of pesticide mixtures, legislation usually considers the risk of a substance in isolation. Nevertheless, all compounds can contribute to the toxicity of the overall mixture, even if individually they occur at safe concentrations for freshwater biota (Di Lorenzo et al. 2018).
Only with scientific knowledge on the use of these substances in the Cerrado can the reduction, loss, modification, or degradation of habitats strongly affected by their use be avoided. Research can provide actions to protect the biodiversity of this biome (Azevedo et al. 2016).
Cultivation of crops near watershed areas is a risk factor for water quality. The Formoso river, object of this study, is surrounded by agricultural land. Cultivation in this area includes the use of many chemicals (fertilizers and pesticides). Considering the increase of agricultural production in Tocantins and the growth of pesticide and fertilizer use for this purpose, this study investigated the levels of pesticide residues in different environmental compartments (soil, sediment, and water samples) of the Formoso River.
Government environmental control and supervision agencies in Brazil fail to efficiently monitor pesticides in natural environments due to lack of financial, structural, and personal resources. Academic research is thus responsible for this control. Regarding the quantification of pesticides in this region and in this river, no data were found to address the evaluation of possible contamination, which shows the importance of the present study. The analysis of these substances enables a diagnosis of some different environmental compartments of the Formoso River, in the region of the Formoso River Agricultural Project.
Methods and Materials
The research for information on pesticide residue levels was performed in soil, sediment, and surface water from April 2018 to February 2019. The study included two samplings in the dry season and two in the rainy season.
Study site and sampling
According to the hydrographic division of the Brazilian National Water Agency (ANA 2018), the Formoso River basin has a drainage area of 21,328.57 km2, corresponding to approximately 7.7% of the total area of Tocantins State and 5.6% of the Araguaia river basin.
Tocantins is one of the Brazilian states that make up the Amazon Region. The Formoso River basin covers part of the territory of 21 cities in the states of Tocantins and Goiás. The economy of this basin relies on agriculture and livestock, with a strong presence of irrigated agriculture. Among other crops, rice, bean, corn, soybean, melon, and watermelon prevail on the banks of the Formoso river.
The Formoso River Irrigation Project is located within the tropical floodplain, at the western end of the Bananal basin, in a low-lying flat area in Formoso do Araguaia city, southwest of Tocantins State. It is considered the largest flooded irrigated rice project in the world. The project comprises a continuous area with subirrigation system for certified soybean production in the dry season (SILVA 2015).
According to Mattos et al. (2013), the Formoso do Araguaia region and surrounding municipalities are home to one of the largest indigenous territories in Tocantins State, with 1825 indigenous people. The area comprises villages of the Javaé, Karajá, and Avá-canoeiro peoples.
The study area is located in an agricultural frontier. The Formoso River Agricultural Project is located on the banks of the Formoso River, and has an area of 27,800 hectares, with two annual harvests. In the rainy season (October to April), the project focuses on irrigated rice. In 2018, 18,000 hectares were cultivated with rice in this region, with an average yield of 7500 kg per hectare. In the dry season (May–September), the area is mainly intended for: subirrigated soybean seed production (sanitary void), with an average yield of 3300 kg per hectare in a cultivated area of 16,000 hectares; and subirrigated watermelon production, with an average yield of 25,000 kg per hectare in an area of 2000 hectares.
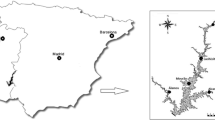
The samples were collected at seven points of the Formoso river in the Formoso River Agricultural Project region, in Formoso do Araguaia—TO. Collection points were chosen to check the influence of the project on river contamination. The following points were selected: a river point before the agricultural project, located 25 km before the start of the project region (P1); five points along the project (P2–P6); and one point after the project (P7), located 45 km away from P2. The trials were conducted in April (Trial 1—T1), during the rainy season of 2018; July (Trial 2—T2) and October (Trial 3—T3), during the dry season of 2018; and February (Trial 4—T4), during the rainy season of 2019. Table 1 shows the geographical coordinates of the sampling points. Figure 1 shows the map with the agricultural project and the points chosen for sample collection.
Area of the sampling points (SEPLAN 2012)
The choice of the monitoring months took place at the beginning of the research project. The concern was to monitor periods when climatic conditions were different, with different crops in the agricultural area.
The study region has two distinct periods of variations in rainfall and water levels, which are determining factors for the concentration of water-soluble substances.
The rainfall conditions on the days of collection were: 11.4 mm in T1, 0 mm in T2 and T3, and 0.6 mm in T4. The depth of the river reflects these climatic conditions. Only two points (P1 and P5) have depth data, as they have real-time monitoring. During the trials, the following depths were recorded: T1: 830 cm in P1, 650 cm in P5; T2: 275 cm in P1, 183 cm in P5; T3: 233 cm in P1, 158 cm in P5; T4: 413 cm in P1, 249 cm in P5 (Semarh 2017, 2018a, b, c, d).
Each sampling campaign lasted 48 h. The collection was performed by boat, at the same time in each point. Rainy season sampling took place on days without strong rain.
Quarterly trials were randomly selected considering: two samplings in the rainy season, in which the agricultural project basically focuses on irrigated rice; and two samplings in the dry season, where 70% of the project is intended for soybean seed production, and the other 30% for cultivation of beans, watermelon, and melon.
Pesticides analyzed were: 2,4-D, bentazone, cyhalofop-butyl, clomazone, chlorimuron-ethyl, chlorpyrifos, dimethoate, diuron, fention, fluazifop-p-butyl, fluroxypyr, flutolanil, imidacloprid, linuron, metalaxyl, metsulfuron-methyl, monolinuron, nicosulfuron, oxyfluorfen, penoxsulam, pyrazosulfuron-ethyl, pyridaben, pyridate, pirimiphos-methyl, profenofos, propanil, propargite, quinclorac, quizalofop-p-ethyl, saflufenacil, thiomethoxam, and tolcophos-methyl.
For the analysis of the pesticides, soil, sediment, and water samples were collected and stored according to Filizola et al. (2016) and CETESB (2011). All analyses were performed within 48 h after collection.
For soil samples, approximately 2 kg of topsoil was collected from the 0 to 20 cm layer, at 5–10 m from the river bank, in a composite sampling design. The samples were placed in plastic bags in an ice-cold environment until ready for laboratory preparation.
For sediment samples, approximately 2 kg of sediment was collected from the surface layer, at 5–10 m from the river bank, in a composite sampling design, using a modified Petersen stainless steel grab sampler. This distance from the bank chosen for collection varied as a function of river depth, which changes considerably depending on the time of year. The samples were placed in decontaminated polyethylene bottles and stored in an ice-cold environment until ready for laboratory preparation.
Water samples were collected before sediment samples, in the surface layer of the water body, at 5–10 m from the river bank due to the large difference in river depth at different collection times, but always at the same location. A simple sampling was performed and the samples were packed in new 500-mL Amber flasks, kept refrigerated until ready for laboratory preparation.
Pretreatment of Soil and Sediment Samples
Sediment and soil samples were prepared for extraction analysis by the modified QuEChERS method. Acidified acetonitrile was used as extraction solvent, and magnesium sulfate and sodium chloride were the salts used for the partitioning step. After addition of salts, the tubes were shaken and centrifuged. The extracts were cleaned by dispersive solid phase extraction (d-SPE). For the d-SPE step, magnesium sulfate, octadecylsilane (C18) sorbents, and primary secondary amine (PSA) were employed. The extracts were then shaken, centrifuged, and filtered. Prior to UHPLC-MS/MS analysis, samples were diluted five times in ultrapure water (Prestes et al. 2009).
Pretreatment of Water Samples
For pesticide analysis in water samples, solid phase extraction (SPE) was performed. For the SPE procedure, Oasis® HLB cartridges were used, in which 100 mL of sample was percolated and eluted with the acidified mixture of solvents MeOH:MeCN (1:1, v/v). Before chromatographic injection, samples were diluted twice in ultrapure water (Donato et al. 2015).
Instrumental Analysis
Analyses were performed by ultra-high performance liquid chromatography coupled with tandem mass spectrometry (UHPLC-MS/MS). The samples were analyzed using a UHPLC-MS/MS system from Waters (USA), equipped with: liquid chromatograph; Xevo-TQ triple quadrupole MS detector; electrospray ionization interface source; Peak nitrogen generator; solvent controller system (binary pump system) for high pressure operation; Acquity UPLC® BEH C18 analytical column (50 × 2.1 mm, 1.7 μm) from Waters (USA); and MassLynx 4.1 software for data acquisition (Waters, USA). Selected reactions were monitored for quantification and identification of analytes.
The mobile phases employed were (A) water:methanol (98:2, v/v) and (B) methanol, both containing 5 mmol L−1 ammonium formate and 0.1% (v/v) formic acid, with 0.225 mL min−1 flow rate and 10 µL injection volume. The gradient elution mode was [time (min), %A, %B]: [0, 95, 5], [0.25, 95, 5], [7.75, 5, 95], [8.5, 5, 95], [8.51, 95, 5], [10, 95, 5], respectively (KEMMERICH 2017).
Calibration curves were prepared in the solvent and in the white extract of the matrix with adequate linearity and coefficients of determination greater than 0.99.
Tables 2 and 4 show the limit of quantification (LOQ) and limit of detection (LOD) for water analysis, with satisfactory precision, recovery between 70 and 110%, and relative standard deviations below 19.7%. For soil and sediment analyses, recovery values were between 70 and 120%, and relative standard deviations were below 20%. The limit of quantification (LOQ) and limit of detection (LOD) are described in the discussion section.
Each analysis comprised the quality control of the method. Different LOD and LOQ were obtained depending on the sampling campaign and the active ingredient analyzed.
Results and Discussion
Pesticides in Sediment and Soil
In the analysis of soil and sediment samples, all results were below the limits of detection (LOD) and limits of quantitation (LOQ) of the method, which varied depending on the trial and active principle, as shown in Table 2. It is noteworthy that although no active ingredient was detected in the environmental compartments during the monitoring period, this does not indicate that these substances are not contaminating these compartments of the water body. It should be considered that detection of pesticides in natural (uncontrolled) environments is difficult due to the various dynamic processes involved therein (dilution, dispersion, decomposition, hydrolysis, photolysis) (Calheiros et al. 2018).
Pesticides in Water
Water analysis results showed the presence of some active ingredients, namely clomazone, fluazifop-p-butyl, flutolanil, imidacloprid, metsulfuron-methyl, and propanil (Table 3).
The results show that the dry period accounted for the highest number of substances in both soil and water samples. Trial 3 (T3) was performed in the dry period in the region and coincided with the end of the harvest of soybean for seed production in tropical lowland. At this time the river was very low, thus increasing the concentration of substances in the water, which may justify the higher number of substances in this campaign.
The study region has two distinct periods, with variations in rainfall and water levels that are determining factors for the concentration of water-soluble substances. Pesticides were detected more frequently at points where the flow and depth are smaller, thus with a trend for higher concentration, making the dry season critical to the aquatic biodiversity. Table 4 shows the LOQ and LOD values of the method for all active principles in the water matrix.
Fluazifop-p-butyl was detected at point 2 in the dry season. In that same trial (T3), flutolanil and metsulfuron-methyl were detected with values below the LOQ of the method, being detected even far from the agricultural project area (Table 5).
In the dry season, propanil (T3) was detected below the LOQ of the method. Imidacloprid (T2) was detected at a concentration of 0.065 µg L−1 before the Formoso river flows through the agricultural project, not being detected further.
At this time the river was very low, which allows the concentration of substances in water and may justify the presence of a larger number of substances found in this trial. River flow and depth varied greatly depending on the time of collection. The flow at P1 ranged from 1 m3 s−1 in T3 (dry season) to 55 m3 s−1 in T4 (rainy season), as well as the depth at P1 ranged from 233 to 414 cm, respectively, in these two trials.
At P5, in turn, the river flow ranged from 1 m3 s−1 in T3 (dry season) to 9 m3 s−1 in T4 (rainy season), and the depth ranged from 158 to 249 cm, respectively, in these two trials. Pesticides were detected more frequently in areas where the flow and depth are lower, accounting for a likely concentration thereof, placing the dry period as critical for the biodiversity of the aquatic environment.
The active ingredient clomazone was quantified at all collection points, in all monitoring trials (Table 6). Contamination by this active ingredient is critical in the river as it was already found at P1, the point before the river flows through the agricultural project. This finding indicates contamination even before the influence of the river, although it increases up to five times at P4 and P5, along the project area.
Although P1 was chosen as a control point since it is an area outside and far from the agricultural project and with a more closed riparian forest, it is located within a region surrounded by plantations, which may justify the presence of clomazone already at this sampling point. In turn, P4 and P5 are located very close to plantations and irrigation channels, contributing to the higher concentration of substances.
It is of great importance to emphasize that the active ingredients of the pesticides are more concentrated during the dry season due to lower river flow and depth. This indicates that the use of pesticides becomes more risky for the environment in this period.
Potential for Surface and Groundwater Contamination
Table 7 shows the physicochemical properties of the active ingredients that are being used in the region for agricultural production, in addition to the results of the environmental behavior models for potential surface and groundwater contamination. Although found at low concentrations in the surface waters of Formoso river, pesticides metsulfuron-methyl, flutolanil, and imidacloprid may contaminate groundwater, as they have GUS values that indicate potential for contamination. According to EPA criteria, clomazone and propanil also fall into this group of likely groundwater contaminants. The results indicate that these active principles also need to be monitored in the water table.
GOSS values (Table 7) indicate that none of the substances has high potential for surface water/sediment contamination. Nonetheless, regarding dissolved contamination, clomazone, metsulfuron-methyl, imidacloprid, and flutolanil have a high potential for surface water contamination.
Analyses of some physicochemical properties of pesticides confirm the results of this monitoring. One example is water solubility, which is high for clomazone, metsulfuron-methyl, and imidacloprid. Fluazifop-p-butyl is the only substance that is not stable in water.
Only imidacloprid and flutolanil are persistent in soil. Furthermore, as observed for metsulfuron-methyl, these substances show slow degradation in sediment. Notwithstanding, they were not detected in these compartments.
Table 7 highlights that flutolanil and fluazifop-p-butyl are likely to bioaccumulate, which is worrisome.
This study focused on the environmental compartments of the Formoso River (surface water, soil, and sediment). Groundwater was not analyzed in the region; however, as the substances under study reached the water body, they will likely contaminate surface water. The GUS method and EPA criteria can be used for predicting groundwater contamination in the region. Table 7 shows that compounds found in the water of the Rio Formoso may be contaminating the groundwater of the region, which is of extreme concern. Only propanil and trifloxystrobin have no leaching potential according to the GUS method. Fluazifop-p-butyl is not a potential contaminant of groundwater according to EPA criteria. When considering the two contamination risk models together, fluazifop-p-butyl stands as a potential contaminant.
Ecotoxicology of Pesticides in Aquatic Environments and Ecological Risk Assessment
The toxicity of pesticides in aquatic environments can be assessed by ecotoxicological tests. Table 8 shows the ecotoxicity of the substances found in the Formoso River water. This toxicity indicates the effects that pesticides have on some aquatic organisms.
The toxicological classification of the Brazilian National Health Surveillance Agency (ANVISA) considers fluazifop-p-butyl as highly toxic, whereas clomazone, imidacloprid, and metsulfuron-methyl are moderately toxic (Table 8) (ANVISA 2017). The values help us to understand and prevent toxic effects on natural communities and are based on laboratory experiments that test for acute and/or chronic effects.
In acute toxicity, the effect is observed in a short period (0–96 h) after contact with a dose of the tested substance. In chronic toxicity, organisms are exposed for a long period, thus accounting for a prolonged effect (Maziero et al. 2016).
Effective concentration (EC) and lethal concentration (LC) values are expressed in relation to 50% of the organisms in question, referring to doses or concentrations of an agent capable of producing a response in a test organism from 24 to 96 h (Costa et al. 2008).
The fact that a substance has no toxic effect on a particular aquatic organism does not indicate that this substance is not harmful to it, because the evaluation does not indicate how these substances affect biological functions (e.g., egg reproduction, development, growth, and maturation) (Costa et al. 2008).
Substances in Table 8 are moderately toxic to most aquatic organisms, but these results refer to their isolated appearance.
Mixtures of toxic substances may lead to additive effects, when the toxicity of the mixture is equal to the sum of the individual toxicities of each substance alone, or to synergistic effects, when the toxicity of the mixture is higher than the sum of the toxicities of the substances alone (Costa et al. 2008).
The occurrence of these effects will depend on both the type of mixture and the mode of interaction between the components of the mixture.
Therefore, although toxicity was moderate and the values found in the study are low, these effects need to be considered when evaluating the presence of various substances present in the aquatic environment. This shows that the quantification of these substances and these ecotoxicity values together are important when assessing environmental risk. These interactions should be evaluated to predict the toxic effects of these pesticides on the environment (Costa et al. 2008).
Pesticide Tolerance Limits
It is noteworthy that all active ingredients are authorized for use in Brazil (ANVISA 2017). We emphasize, however, that propanil is not authorized for use in the European Union (PPDB 2018).
CONAMA Resolution No. 357, of March 17, 2005 (BRASIL 2005), does not establish limit values for the active ingredients found in Formoso river during the monitoring period of this study. Likewise, Ordinance MS No. 05, of September 28, 2017—Consolidation Ordinance MS No. 2,914, of 12/12/2011, of the Brazilian National Agency for Health Surveillance (ANVISA), also does not establish values for these substances (BRASIL 2017). The European Community, on the other hand, accepts as a maximum concentration limit the value of 0.1 μg L−1 for any pesticide in drinking water, and 0.5 μg L−1 for total residues (Armas et al. 2007).
The amount of clomazone found over the monitoring period in the sampled points exceeds by four times the maximum concentration limit of the European community.
The surface water analyzed in the region of Formoso do Araguiaia is nonpotable. Notwithstanding, this region is home to indigenous people, being one of the largest indigenous territories in Tocantins State. Mattos et al. (2013) draw attention to the worrying fact that the region of the Indigenous Lands of Parque do Araguaia shelters the Javaé, Karajá, and Avá Canoeiro peoples, who use this water for cultivation, hygiene, and food.
It is not possible to establish acceptable limits for the risk of contamination of the environment by toxic substances. For most compounds, there is no accurate information about the effects of chronic or acute exposure (Carneiro 2015). However, low the level of concern about toxicity and biodiversity will always be threatened by the action of substances outside the natural environment. The effects of one active ingredient can be synergistically potentiated in the presence of others. Studies on both this issue and the cumulative effects of pesticides are limited (Calheiros et al. 2018).
Thus, the results highlight the increasing threat to the biodiversity of the Cerrado Tocantinense, the visible degradation of the Cerrado biome, and the population and agricultural expansion in the region.
The facts that other active ingredients were not detected in this matrix and that none of the active ingredients were detected in soil and sediment do not mean that they are not present in the samples, instead they can be at concentrations below the limit of quantification of the method used in the analysis.
Conclusions
The study region comprises six active ingredients: clomazone, fluazifop-p-butyl, metsulfuron-methyl, propanil, imidacloprid, and flutolanil. The active ingredient clomazone was quantified at all points in all trials, with concentrations reaching up to 0.538 μg L−1, five times the maximum value permitted by the European Union.
These substances are contaminating the surface waters of Formoso River, because they should not be present. Their presence can cause irreversible damage to human populations and wildlife species.
This study evidenced that agricultural practices in the region are putting the environment at risk, because they are occurring in a region of enormous biodiversity (Legal Amazon) and close to indigenous lands. These practices threaten not only the environment in which these people live, but also their security.
There is a need for constant monitoring of surface and groundwater in the studied region. Pesticide analysis in the different environmental compartments of the Formoso River is essential, because these substances reduce biodiversity in these ecosystems. Only then it will be possible to implement actions that contribute to the sustainability of natural resources.
References
Amaral AB (2011) Avaliação de mananciais subterrâneos e superficiais da bacia do Córrego Sossego considerando o uso para abastecimento doméstico e irrigação – contaminação por agrotóxico. Dissertation (Master's degree in Environmental Engineering) - Technological Center, Federal University of Espírito Santo,Vitória. https://bdtd.ibict.br/vufind/Record/UFES_67f7b2974729a7cc24c9d773d7796eea Accessed 1 Aug 2018
ANA. Agência Nacional das Águas. Região Hidrográfica do Tocantins-Araguaia. https://www2.ana.gov.br/Paginas/servicos/planejamento/planoderecursos/Tocantins-Araguaia.aspx. Accessed 8 Aug 2018
ANVISA- Agência Nacional de Vigilância Sanitária- Lista de ingredientes ativos com uso autorizado e banidos no Brasil, 2017. portal.anvisa.gov.br/rss/-/asset_publisher/Zk4q6UQCj9Pn/content/id/3197746. Accessed 16 Apr 2019.
ARIADNE. Sistema de informação sobre pesticida. Comportamento ambiental. https://www.fsp.usp.br/nra/ariadne/comportamento-ambiental.php. Accessed 7 May 2019
Armas ED, Monteiro RTR, Antunes PM, Santos MADF, Camargo PB (2007) Diagnóstico espaço-temporal da ocorrência de herbicidas nas águas superficiais e sedimento do Rio Corumbataí e principais afluentes. Quim Nova 30:1119–1127. https://doi.org/10.1590/S0100-40422007000500013
Azevedo JCR, Moura ERR, Santos MM (2016) Determinação de Pesticidas na Água e Sedimento do Rio Piquiri. Rama: Revista em Agronegócio e Meio Ambiente 9(3):651–671. https://doi.org/10.17765/2176-9168.2016v9n3p651-671
BRASIL – ANVISA- Agência Nacional de Vigilância Sanitária- Portaria MS Nº 05, de 28-09-2017 - Consolidação Portaria MS Nº 2.914, de 12-12-2011 que dispõe sobre os procedimentos de controle e de vigilância da qualidade da água para consumo humano e seu padrão de potabilidade. Brasília, 2017
BRASIL - Ministério do Meio Ambiente. Resolução CONAMA Resolução N.º 357 de 17 de novembro de 2005: que dispõe sobre a classificação dos corpos de água e diretrizes ambientais para o seu enquadramento, bem como estabelece as condições e padrões de lançamento de efluentes, e dá outras providências. Brasília, 2005
Calheiros DF, Pignati WA, Pinho AP, Souza E, Lima FAN, Santos J, Pinho JS, Rosa ER (2018) Relatório Técnico Projeto: Promoção da Agroecologia e Avaliação da Contaminação por Agrotóxicos em Áreas de Proteção Ambiental na Bacia do Alto Paraguai – APA Estadual Nascentes do Rio Paraguai-Ministério da Educação, Universidade Federal de Mato Grosso -Instituto de Saúde Coletiva/Departamento de Saúde Coletiva Núcleo de Estudos Ambientais e Saúde do Trabalhador https://ecoa.org.br/wp-content/uploads/2018/05/988025221b5fb8dd47b50334964de19e.pdf Accessed 16 Apr 2019
Carneiro FF (2015) Dossiê ABRASCO: um alerta sobre os impactos dos agrotóxicos na saúde. Rio de Janeiro: EPSJV; São Paulo: Expressão Popular
CETESB (2011) Guia nacional de coleta e preservação de amostras: água, sedimento, comunidades aquáticas e efluentes líquidos/Companhia Ambiental do Estado de São Paulo; Organizadores: Carlos Jesus Brandão, et al. - São Paulo: CETESB; Brasília: ANA
Cohen SZ, Wauchope RD, Klein AW, Eadsforth CV, Graney RL (1995) Offsite transport of pesticides in water: mathematical models of pesticide leaching and runoff. Pure Appl Chem 67(12):2109–2148. https://doi.org/10.1351/pac199567122109
Costa CR, Olivi P, Botta CMR, Espindola ELG (2008) A toxicidade em ambientes aquáticos: discussão e métodos de avaliação. Quím Nova 31(7):1820–1830. https://doi.org/10.1590/S0100-40422008000700038
De Gerónimo E, Aparicio VC, Bárbaro S, Portocarrero R, Jaime S, Costa JL (2014) Presence of pesticides in surface water from four sub-basins in Argentina. Chemosphere 107:423–431. https://doi.org/10.1016/j.chemosphere.2014.01.039
Dellamatrice PM, Monteiro RTR (2014) Principais aspectos da poluição de rios brasileiros por pesticidas. R Bras Eng Agríc Ambiental 18(12):1296–1301. https://doi.org/10.1590/1807-1929/agriambi.v18n12p1296-1301
Di Lorenzo T, Cifoni M, Fiasca B, Di Cioccio A, Galassi DMP (2018) Ecological risk assessment of pesticidemixtures in the alluvial aquifers of central Italy: toward more realistic scenarios for risk mitigation. Sci Total Environ 644:161–172. https://doi.org/10.1016/j.scitotenv.2018.06.345
Donato FF, Martins ML, Munaretto JS, Prestes OD, Adaime MB, Zanella R (2015) Development of a multiresidue method for pesticide analysis in drinking water by solid phase extraction and determination by gas and liquid chromatography with triple quadrupole tandem mass spectrometry. JBCS 26(10):2077–2087. https://doi.org/10.5935/0103-5053.20150192
Dores EFGC, Lamonica-Freire EM (2001) Contaminação do ambiente aquático por pesticidas. Estudo de caso: Águas usadas para consumo humano em Primavera do Leste, Mato Grosso – Análise preliminar. Quím Nova 24:27–36. https://doi.org/10.1590/S0100-40422001000100007
Filizola EF, Gomes MAF, Souza MD (2016) Manual de procedimentos de coleta de amostras em áreas agrícolas para análise da qualidade ambiental: solo, água e sedimentos. Embrapa Meio Ambiente, Jaguariúna
Forbes VE, Hommen U, Thorbek P, Heimbach F, Van Den Brink PJ, Wogram J, Thulke H, Grimm V (2009) Ecological models in support of regulatory risk assessments of pesticides: developing a strategy for the future. Environ Assess Manag 5(1):167–172. https://doi.org/10.1897/IEAM_2008-029.1
Gama AF, Oliveira AHB, Cavalcante RM (2013) Invent. de agrotóxicos e risco de cont. química dos recursos hídricos no semiárido cearense. Quím Nova 36(3):462–467. https://doi.org/10.1590/S0100-40422013000300017
Goss DW (1992) Screening procedure for soils and pesticides for potential water quality impacts. Weed Technol 6(3):701–708. https://doi.org/10.1017/S0890037X00036083
Gustafson DI (1989) Groundwater Ubiquity Score: a simple method for assessing pesticide leachability. Environ Toxicol Chem 8(4):339–357. https://doi.org/10.1002/etc.5620080411
Hanson N, Starky JD (2011) Utility of population models to reduce uncertainty and increase value relevance in ecological risk assessments of pesticides: an example based on acute mortality data for Daphnids. Integr Environ Assess Manag 8(2):262–270. https://doi.org/10.1002/ieam.272
Isherwood KF (2000) Mineral fertilizer use ande the environment, Revised. International Fertilizes Industry Association, Paris
Kemmerich M (2017) Resíduos de agrotóxicos em ameixa, maçã, pera e pêssego: desenvolvimento de métodos de análise e monitoramento. Ph.D. Thesis, Federal University Santa Maria of Santa Maria https://repositorio.ufsm.br/handle/1/12732. Accessed 12 May 2019
Loewy RM, Monza LB, Kirs VE, Savini MC (2011) Pesticide distribution in an agricultural environment in Argentina. J Environ Sci Health B 46:662–670. https://doi.org/10.1080/03601234.2012.592051
Martini LFM, Caldas SS, Bolzan CM, Bundt AC, Primel EG, Avila LA (2012) Risco de contaminação das águas de superfície e subterrâneas por agrotóxicos recomendados para a cultura do arroz irrigado. Cienc Rural 42(10):1715–1721. https://doi.org/10.1590/S0103-84782012001000001
Mattos MLB, Mattos PHC, Carniello MF, Araujo EAS (2013) Javaé native people from the Bananal Island: an analysis about development of those communities. Rev Cereus 5(3):101–116
Maziero JS, Rogero SO, Alemany A (2016) Ecotoxicological study of silver nanoparticle on Daphnia similis. J Health Sci Inst 34(3):133–139
Milhome MAL, Sousa DOB, Lima FAF, Nascimento RF (2009) Avaliação do potencial de contaminação de águas superficiais e subterrâneas por pesticidas aplicados na agricultura do Baixo Jaguaribe, CE. Eng Sanit Ambient 14(3):363–372. https://doi.org/10.1590/S1413-41522009000300010
Mondal R, Mukherjee A, Biswas S, Kole RK (2018) GC-MS/MS determination and ecological risk assessment of pesticides in aquatic system: a case study in Hooghly River basin in West Bengal, India. Chemosphere 206:217–230. https://doi.org/10.1016/j.chemosphere.2018.04.168
Oliveira A, Silva N (2013) Determinação da concentração de metais em águas do Córrego Barbado, Cuiabá – MT. R Gest Sust Ambient Florianópolis 2(1):47–63. https://doi.org/10.19177/rgsa.v2e1201347-63
PPDB -THE PESTICIDE PROPERTIES DATABASE Other product constituents Insecticides-Herbicides-Fungicides. University of Hertfordshire, funded by UK National Sources and the EU-funded FOOTPRINT project. 2018. https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/118.htm#trans. Accessed 8 Apr 2019
Prado GL (2013) Avaliação da susceptibilidade à contaminação de corpos hídricos, em áreas de cultivo e do entorno (reservas indígenas), pelo uso de agrotóxicos. Ph.D. thesis, Federal University of Tocantins, Palmas. https://repositorio.uft.edu.br/bitstream/11612/260/1/Liana%20Bezerra%20Dias%20de%20Lima%20-%20Dissertação.pdf Accessed 13 May 2019
Prestes OD, Friggi CA, Adaime MB, Zanella R (2009) QuEChERS – um método moderno de preparo de amostra para determinação multirresíduo de pesticidas em alimentos por métodos cromatográficos acoplados à espectrometria de massas. Quim Nova 32(6):1620–1634. https://doi.org/10.1590/S0100-40422009000600046
Rand GM, Petrocelli SR (1985) Fundamentals of aquatic toxicology: methods and application . Hemisphere Publishing, Washington
Rebelo R, Caldas ED (2014) Avaliação de risco ambiental de ambientes aquáticos afetados pelo uso de agrotóxicos. Quím Nova 37(7):1199–1208. https://doi.org/10.5935/0100-4042.20140165
SEMARH- Secretaria do Meio Ambiente e Recurso Hídricos- Governo do Tocantins. Boletim de qualidade de água- Boletim 2017. https://semarh.to.gov.br/diretorias/diretoria-de-planejamento-e-recursos-hidricos/boletim-de-qualidade-de-agua/-boletim-anual-2017-/. Accessed 1 May 2019
SEMARH- Secretaria do Meio Ambiente e Recurso Hídricos- Governo do Tocantins. Boletim de qualidade de água- Boletim Trimestral 1/2018a. https://central3.to.gov.br/arquivo/416996/. Accessed 1 May 2019
SEMARH- Secretaria do Meio Ambiente e Recurso Hídricos- Governo do Tocantins. Boletim de qualidade de água- Boletim Trimestral 2/2018b. https://central3.to.gov.br/arquivo/412019/. Accessed 1 May 2019
SEMARH- Secretaria do Meio Ambiente e Recurso Hídricos- Governo do Tocantins. Boletim de qualidade de água- Boletim Trimestral 3/2018c. https://central3.to.gov.br/arquivo/416998/. Accessed 1 May 2019
SEMARH- Secretaria do Meio Ambiente e Recurso Hídricos- Governo do Tocantins. Boletim de qualidade de água- Boletim Trimestral 4/2018d. https://central3.to.gov.br/arquivo/427447/. Accessed 1 May 2019
SEPLAN- Secretaria do Planejamento e Orçamento – Governo do Estado do Tocantins– Base de Dados Geográficos do Estado do Tocantins-2012 https://www.sefaz.to.gov.br/zoneamento/bases-vetoriais/base-de-dados-geograficos-do-tocantins-atualizacao-2012/ Accessed 28 May 2019
Silva AJR (2015) Sensoriamento remoto como subsídio para a gestão agrícola: estudo de caso do Projeto de Irrigação Rio Formoso - Formoso do Araguaia-TO. Dissertation (Master's degree in Technology of Sustainable Processes). Federal Institute of Science and Technology Education of Goiás, Goiânia. https://www.ifg.edu.br/attachments/article/5213/Dissertação_Processos%20Sustentáveis_Alberto%20José%20Rezende%20Silva.pdf. Accessed 27 May 2019
Vieira E (2012) Impacto ambiental em área com aplicação de agrotóxicos no município de Brotas, SP. Thesis (Ph.D. in Agronomy) - Faculty of Agronomic Sciences, Paulista State University, Botucatu. https://repositorio.unesp.br/handle/11449/105418. Accessed 22 May 2019
Acknowledgements
To the Federal University of Tocantins and its Postgraduate Program in Biodiversity and Biotechnology – BIONORTE, and to the Environmental Chemistry and Biofuels Laboratory (LAPEQ-UFT). The present study was performed with the support of the Coordination of Improvement of Higher Education Personnel Brazil - (CAPES) - Financing Code [001].
Author information
Authors and Affiliations
Contributions
PMG, main author of the article, participated in all stages, from planning to final writing of the text. AMSP, R de SD, and L da SG helped in sample collection and preparation for analysis. DBM helped in writing and formatting the article and translating the text into English. EAG and JEC da S were co-supervisor and supervisor, respectively, supervising all experimental and theoretical work and reviewing the article.
Corresponding author
Rights and permissions
About this article
Cite this article
Guarda, P.M., Pontes, A.M.S., Domiciano, R.S. et al. Assessment of Ecological Risk and Environmental Behavior of Pesticides in Environmental Compartments of the Formoso River in Tocantins, Brazil. Arch Environ Contam Toxicol 79, 524–536 (2020). https://doi.org/10.1007/s00244-020-00770-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-020-00770-7