Abstract
This study aims at examining the use of an advanced meso-scale continuous-flow photochemical reactor for the photocatalytic conversion of CO2 with water into fuel over TiO2 (P25), Ag/TiO2, and Ag/TiO2/RGO catalysts. The silver loaded photocatalysts were prepared by one-step process via hydrothermal method. The prepared photocatalysts were characterized by various characterization techniques in order to identify the morphological, chemical, physical, and optical properties. The photocatalytic activity of the as-prepared catalysts was firstly examined by the photoelectrochemical (PEC) measurements and secondly by the photocatalytic reduction of CO2 in the proposed setup. Liquid products were analyzed using gas chromatography-mass spectrometry (GC-MS) and total organic carbon (TOC) techniques. It was found that the ternary composite revealed an outstanding performance towards CO2 photocatalytic reduction, where its selectivity was directed towards methanol production. The incorporation of graphene nanosheets enhanced the photocatalytic reduction of CO2 by 3.3 and 9.4 times compared with Ag/TiO2 and bare TiO2, respectively, using the proposed photochemical reactor in a continuous mode. This study sheds the light on a novel type of a photocatalytic reactor where CO2 conversion over Ag/TiO2/RGO ternary composite was evaluated.
Graphical abstract

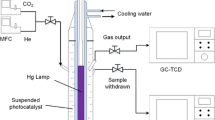
A meso-scale continuous-flow photochemical reactor
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increase of CO2 concentration is the main contributor in the global warming phenomenon. Many strategies have been recently improved to eliminate carbon emissions. Carbon capture and storage (CCS) is one of the proposed technologies used to fix CO2 level. Carbon capture step could be achieved via amine scrubbing and calcium looping while storage could be done in geological formations such as deep coal seams, oil and gas reservoirs, and underground saline aquifers (Crake et al. 2017; Xiaofeng Chang and Zheng 2015; Ola and Maroto-Valer 2015). However, the use of this strategy may lead to an increase in CO2 emissions due to the energy consumption via separation, purification, compression, transportation, and storage of CO2 (Xiaofeng Chang and Zheng 2015; Sneddon et al. 2014; Wu et al. 2017).
Carbon capture and utilization (CCU) is an interesting technique to capture CO2 then it could be converted to chemical feedstock which means utilization of CO2. Conversion of CO2 could be carried out chemically, electrochemically, or photoelectrochemically to produce various hydrocarbons (Low et al. 2017; Wang et al. 2018; Karamian and Sharifnia 2016). Among those techniques, the photocatalytic reduction of CO2 is the most promising approach that employs an infinite energy source which is solar energy to produce different valuable hydrocarbons (Low et al. 2017; Chang et al. 2016).
The photoreactor design is the most important factor influencing the efficiency of the photoreduction of CO2 and the product yield rates (M. Tahir and Amin 2013) (Tahir et al. 2015). An efficient photoreactor should include the following: (1) high (active catalyst surface area: reactor volume) ratio, (2) uniform distribution of light and its utilization, (3) superior photonic efficiency, and (4) high throughput with lower power consumption (B. Tahir et al. 2015).
Several types of reactors were used for CO2 photoconversion over the last decades such as follows: slurry, fluidized, and fixed bed reactors. Some drawbacks were reported using these reactors such as follows: inefficient light distribution, fewer catalyst recycling possibilities, product separation challenges especially with slurry reactors, and lower surface to volume ratio in case of fixed bed reactors.
Later, optical fiber photoreactors have been investigated for CO2 photoreduction. These reactors offer a uniform and efficient light to the photocatalyst surface, thus increases the efficiency of photon-conversion process. However, the optical fiber configuration cannot effectively utilize the entire reactor volume (Tahir and Amin 2013; Tahir et al. 2015). Besides, when a bundle of optical fibers is coated, fragility of the optical fibers and the durability of coatings have been described as disadvantages associated with their usage. Also, heat accumulation from a bundle of fibers may lead to deactivation of catalyst. As a result, these limitations prevent the commercial usage of the reactor (Ola and Maroto-Valer 2015).
Recently, the commonly used reactor is the monolith. It has many benefits such as the high surface to volume ratio, probability of scaling up through increasing its dimensions and channels, and controlling its structural parameters (i.e., pore-volume, pore size and surface area) (Ola and Maroto-Valer 2015). However, the main disadvantage of the monolith structure is the light distribution hindered through interconnected channels of the monolith. Moreover, the deactivation of the immobilized catalyst with continuous flow conditions also limits its use in the industry.
Tahir et al. (2015) investigated the use of monolith reactor in CO2 photoreduction by H2 in both batch and continuous modes. They found that CH4 was the main product in the batch system; however, CO is the main one in case of continuous mode. They attributed the production of CH4 in the batch process to the accumulation of products in the reaction chamber with irradiation time while in a continuous system, the continual feeding and products removal led to lower yield rates through the production of CO. Moreover, in the continuous process, they described that the catalyst partially lost its photoactivity within 4 h of irradiation time and almost deactivated after 6 h of irradiation time.
Currently, the use of the microchannel reactors provides many benefits due to their ability to be operated continuously as well as offering the option of utilizing light-emitting diodes (LED) lamps which provides extra advantage for energy saving (Heggo et al. 2016). Also, their avoidance of the previously reported limitations and drawbacks of other reactors increases their usage in many photocatalytic applications. However, most studies mentioned their usage on a lab-scale with small production quantities which could control their scale-up in the industrial processes (Loubière et al. 2016).
Thus, there is an urgent need for developing the photocatalytic reactors to meet the industrial scaling up. Besides, the design of the photochemical reactors operated in a continuous mode considers the driving force to develop more photochemical reactors.
In this study, we investigated our setup with an advanced meso-scale continuous-flow-type photochemical reactor which provides the following advantages (Josland et al. 2016): (1) continuous flow processing, (2) relatively higher production quantities comparing with other types of reactors (several grams per day) which increases the probability of the industrial scaling up (Loubière et al. 2016), (3) inner irradiation, (4) uniform light distribution, (5) lower power consumption due to the use of LED lamp, and (6) high surface area to volume ratio which enhances both photon and mass transfer (Loubière et al. 2016; Némethné-Sóvágó and Benke 2014). Moreover, it is well known that the solubility of CO2 increases with decreasing the temperature so that we introduce a cooling water bath with a continuous flow of CO2 gas into water to overcome the low solubility of CO2 with water.
The integration of the photochemical reactor with the proposed setup provides the opportunity to feed fresh catalyst particles suspended in water saturated with CO2 to be entered to the reactor by each minute simultaneously with the collection of gaseous and liquid products. Thus, there is no option for catalyst deactivation as in the other kinds of photoreactors used in CO2 reduction.
Many previous studies reported the importance of graphene use in their applications (Wei et al. 2017; Zang et al. 2017; Liu et al. 2018). In order to further improve the photoconversion of CO2, RGO was incorporated with Ag/TiO2 (Akhavan and Ghaderi 2009) to prepare the ternary nanocomposite of Ag/TiO2/RGO. The superior electrical conductivity of RGO facilitates the separation of photogenerated charges which consequently enhanced the CO2 reduction.
The kind of CO2 photocatalytic reduction products depends mainly on the number of electrons formed from the semiconductor used in the system. Thus, the expected products of CO2 reduction could be formic acid and/or formaldehyde and/or methanol which could be illustrated by the following equations (Habisreutinger et al. 2013; Chang et al. 2016):
Our proposed setup together with the synthesized catalysts exhibited an outstanding performance towards CO2 photocatalytic reduction as the major product was methanol while the minor one was formaldehyde. Moreover, we have found that the amount of formed formaldehyde is too small compared to previous studies, which represents only 1.8% from the TOC.
The production of methanol has a great interest as it is easy to handle and used as an additive in gasoline and it could be converted to other useful chemicals by classic technologies (Sellaro et al. 2016). It was also reported that methanol could be used for the internal combustion engines as a source of energy by (Verhelst et al. 2019). Furthermore, it was reported by Abrishamkar and Barootkoob (2017) that formaldehyde was used as a fuel source in fuel cells.
Experimental section
Materials
TiO2 (P25) and the metal precursor of Ag (AgNO3) were purchased from (ACROS) organics company. Graphite powder and sodium sulfate (Na2SO4) were obtained from Fisher scientific, UK. All other chemicals such as iodine (99.99%) and potassium permanganate (KMnO4) were analytical grade and commercially available.
Nitric acid (HNO3) was obtained from Merck, Germany. Both of hydrochloric acid (HCl), hydrogen peroxide (H2O2) and sulfuric acid (H2SO4) were obtained from Sigma-Aldrich while both acetone (C3H6O) and ethanol (C2H5OH) were purchased from Fisher scientific, UK. All solvents were (HPLC) grade.
Samples preparation
Graphite oxide (GO) was firstly prepared then the exfoliated GO was further utilized and loaded with (TiO2) and metal nanoparticles (Ag) following the one-step approach of the hydrothermal method.
The conversion of graphite powder into (GO) was done using the modified Hummers’ method. As shown in Fig. 1 (Li et al. 2014), graphite powder was firstly oxidized by nitric acid (HNO3); later, the oxidizing by the slow addition of sulfuric acid (H2SO4) was also accomplished in a vessel immersed in an ice bath under stirring and sonication. Secondly, potassium permanganate (KMnO4) was added gradually to continue the oxidation process. The temperature was adjusted below 20 °C using the ice bath to avoid the temperature increase during the exothermic reaction. The mixture was further left for 24 h under continuous stirring to complete the oxidation process. In order to stop the reaction, hydrogen peroxide was added until a yellow-brown cake of (GO) was obtained then washed with HCl and distilled water for several times by decantation.
Schematic of GO synthesis. Copyright (Li et al. 2014)
The further synthesis of the binary (Ag/TiO2) and ternary (Ag/TiO2/RGO) composites were accomplished via hydrothermal method as follows. Firstly, both TiO2 suspended in water-ethanol (2:1) mixture and metal precursor of silver salt (AgNO3) were mixed under sonication and vigorous stirring to prepare (Ag/TiO2) composite. While, in case of the ternary composite (Ag/TiO2/RGO), the desired amount of GO (in this case, 5% of the total weight percentage of the composite) was firstly exfoliated in dist. H2O via ultrasonication till stable, suspension was formed. Then, GO solution was mixed with both the (AgNO3) solution and TiO2 suspension under sonication and vigorous stirring in water-ethanol (2:1) mixture. The obtained mixture was then transferred to a Teflon-lined autoclave and kept for 12 h at 200°C. Finally, the resulted powder was washed several times with dist. H2O and dried at 80 °C for 24 h.
Characterization of the as-prepared photocatalysts
The as-prepared nanocomposites were characterized firstly by a field-emission transmission electron microscope (FE-TEM) (JEOL JEM-2100F) to identify the shape, distribution, size, and surface morphology of the photocatalysts.
Then, further characterization of the morphology of the as-prepared composites was accomplished by the high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) on a (JEOL JEM-2100F) TEM/STEM with a spherical aberration corrector.
Moreover, the elemental analysis and the weight percentage of the samples, EDX, were accomplished by energy-dispersive X-ray spectroscopy (JEOL JEM-2100F).
Also, the phase and crystalline composition of the synthesized photocatalysts were studied by XRD pattern. The samples were firstly grounded harshly into powder prior to be analyzed. An X-ray diffractor (XRD-6100 Shimadzu, Japan) equipped with a Cu-Kα radiation source with λ = 1.55 Å was utilized to scan the 2theta in the range of 5° to 80° at a scanning pitch of 0.02°.
Fourier transform infrared spectroscopy (FTIR) was performed to identify the functional groups existed in each photocatalyst. The FTIR relies on the IR-spectrum of absorption or transmission of samples. A Vertex 70 IR spectrometer (Bruker Scientific Instruments, Germany) was employed.
The Brunauer-Emmett-Teller (BET) was performed to study the specific surface area and pore size distribution. The N2 adsorption/desorption isotherms were studied for the as-prepared nanocomposites. Brunauer-Emmett-Teller (BET) specific surface areas of the photocatalysts were obtained using Belsorp-mini II (BEL JAPAN) nitrogen adsorption instrument at 77 K. The powders were degassed prior to surface area analysis at 300°C for 3 h under vacuum.
Diffuse reflectance spectra were performed by UV-vis (Thermo Scientific, Evolution 220 coupled with ISA-220 integrated sphere). The calculations of the absorbance were obtained from reflectance data via Kubelka-Munk function.
Photoelectrochemical (PEC) measurements were performed using a potentiostat (Gamry instrument, reference 3000) in one compartment cell with a Pyrex window filled with 0.1 M Na2SO4 electrolyte. Three electrodes were utilized where the platinum rod and saturated Ag/AgCl were worked as the counter electrode (C.E) and the reference electrode (R.E), respectively. Meanwhile, the third electrode was the working electrode (W.E) fabricated from different photocatalysts powder deposited via electrophoretic deposition method (EPD) of on the surface of fluorine-doped tin oxide (FTO) glass. The light irradiation source of (Suruga Seiki-Co, Japan) UV LED lamp with a wavelength of 365 nm and intensity up to 50 mW/cm2 was utilized. The weight and the coated area of each working electrode after the deposition of several catalysts on the surface of FTO glass were ca. 4 mg and ca. 6 cm2, respectively.
Photocatalytic reduction of carbon dioxide with water
A continuous-flow photochemical reactor system (Vapourtec UV-150) was used for CO2 photoconversion into valuable organic compounds in the presence of water and light as shown in Fig. 2.
Prior to each run, the desired amount of catalyst powder was dispersed in a 65-ml distilled water bottle using ultrasonication. Then, the water bottle including the dispersed catalyst was immersed in a water bath connected to a chiller to maintain the temperature at 5°C to further ensure an efficient solubility of CO2 in the distilled water. The CO2 bubbling was performed 30 min before starting the run with a flow rate of 35 ml/min to ensure the saturation of the CO2 in the distilled water. Moreover, the continuous bubbling of CO2 was utilized while the reaction proceeds to confirm a constant concentration of soluble CO2 in water.
In order to begin the photocatalytic reduction of CO2, the reactor was turned on to allow feeding of suspension catalyst in liquid water saturated with CO2 and the light illumination. The reaction was performed under UV light-emitting diode (LED) lamp with a wavelength of 365 nm to save energy and the liquid pump was adjusted at 500 μl/min. The resulted products were divided into gaseous and liquid.
The gaseous products were collected in a gas bag purchased from (GL Sciences Inc., Japan) using He gas as a carrier then analyzed using gas chromatography (GC) (Shimadzu 2014, Japan) equipped with ShinCarbon ST micropacked column (Restek, length 2 m × ID 2mm, USA) and a thermal conductivity detector (TCD). The results revealed that there was no CO or CH4 found in the gas bag. Moreover, there was no H2 gas detected by the GC which indicates that its amount was too small to be detected or it may be completely consumed in the reaction.
Also, the use of the previously mentioned water bath allows the resulted organic vapors in the gaseous products to be condensed in the liquid phase prior to be analyzed by GC and total organic carbon (TOC) instruments.
Firstly, the liquid products were qualitatively analyzed using GC/mass spectroscopy (MS) (Thermo-Scientific with TG-5MS capillary column [length 30m, ID 0.25mm]) after filtration and extraction. Secondly, the resulted liquid samples at the end of each run were quantitatively analyzed using total organic carbon analyzer (Shimadzu, TOC-L series, Japan) (Yang et al. 2011; Chen et al. 2012; Dong et al. 2017) and the Nash method (Nash 1953) (Shehzad et al. 2018; Kometani et al. 2017) was further used to determine the formaldehyde concentration at an absorbance wavelength of 414 nm. Blank experiments were performed and neither TOC nor formaldehyde was detected.
Results and discussion
Catalysts characterization
Figure 3 shows the morphology of both the TiO2 (P25) and the as-prepared catalysts with TEM, HAADF-STEM images. In addition, Fig. 4 depicts the elemental mapping images as well as EDX images for Ag/TiO2 and Ag/TiO2/RGO photocatalysts.
As shown in Fig. 3a, the TiO2 P25 nanoparticles are spherical in shape and their particle size is ranging from 14.04 to 26.78 nm. Figure 3d shows the lattice fringes of TiO2 P25 with a spacing of 0.35 nm which is corresponding to the anatase phase (101) of TiO2.
The shiny spots seen in the HAADF-STEM images appeared in Fig. 3e represent the Ag NPs. Figure 3 c and e as well as Ag EDX mapping images in Fig. 4a show the uniform dispersion of Ag NPs on the surface of TiO2 P25 in the as-prepared 2 wt.% Ag/TiO2 catalyst. The particle size of Ag NPs was measured by TEM which is ranging from 1.33 to 4.04 nm.
Figure 3 (d, f) and 4b present the distribution of Ag NPs and RGO sheets using TEM and HAADF-STEM, and EDX mapping images respectively in the 2 wt.% Ag/TiO2/RGO ternary composite that confirm the homogenous dispersion of both elements on the surface of TiO2 P25.
Moreover, EDX and EDX mapping were further used to predict the presence of Ti, O, Ag, and C elements in the as-prepared catalysts and the weight percentage of each element. It is well known that Ti and O have come from TiO2 while C was originated from RGO sheets and the existence of Ag peaks confirms the presence of Ag NPs. The actual loading amounts with the weight percentage of each element in all catalysts are illustrated in Table 1.
The phase composition of all catalysts was examined by XRD pattern and the results are exhibited in Fig. 5. All peaks were compared with the Joint Committee on Powder Diffraction Standards (JCPDS) Card File no. 00-002-0387 and Card File no. 00-001-1292 for both tetragonal anatase and tetragonal rutile phases of TiO2, respectively. The crystal facets of the anatase phase of TiO2 were (101), (103), (004), (200), (105), (211), (204), (116), (220), and (215) which are assigned to the characteristic peaks at 2θ= 25.18°, 37.72°, 38.42°, 47.95°, 53.90°, 54.26°, 62.60°, 68.73°, 70.14°, and 74.99° of the anatase phase, respectively. While the crystal facets of the rutile phase of TiO2 were (110), (101), (200), (111), and (211) which corresponding to the characteristic peaks of rutile phase appears at 2θ= 27.32°, 36.82°, 38.82°, 41.18°, and 55.90°, respectively. The characteristic diffraction peaks of TiO2 did not change after Ag NPs loaded on the surface of TiO2 and there were no significant diffraction peaks for Ag NPs as their loading was too small to be detected with XRD detection limit.
Scherrer’s equation was used to calculate the average crystallite size of the synthesized catalysts according to the maximum three peaks of anatase and rutile phases.
where L is the crystallite size (nm), K is a constant depending on the crystallite shape (0.9 in this case), λ is the X-ray wavelength (nm), β is the full width at half maximum (FWHM), and θ is the Bragg’s angle.
The phase composition of samples was calculated using relative peak intensity of anatase and rutile (Spurr and Myers 1957) (Rajalakshmi et al. 2012).
where FA is the fraction of anatase phase and IR and IA are the intensity of the rutile (110) and anatase (101) diffraction peaks, respectively. The calculated crystallite size and phase composition for both anatase and rutile phases of all catalysts are summarized in Table 1.
As illustrated from Table 1, the percentage of rutile phase was slightly increased after the addition of Ag NPs and RGO sheets which could be assigned to temperature elevation in the hydrothermal reaction during the preparation of catalysts. The anatase to rutile transformation was also observed in previous reports during the introduction of both Ag nanoparticles and RGO sheets (Liu et al. 2017; Feng et al. 2020).
Furthermore, the crystallite size was also improved after the addition of Ag and RGO sheets which indicates the good crystallization of the as-prepared catalysts that was in consistent with the results reported by Feng et al. (2020) and Gui et al. (2015).
The examination of the functional groups and the chemical composition were performed using FTIR. Here are the FTIR spectra as exhibited in Fig. 5.
Figure 6 shows the FTIR measurements of TiO2, 2 wt.% Ag/TiO2, 2 wt.% Ag/TiO2/RGO, and GO. In case of GO spectrum, there are some characteristic peaks located at 1395, 1630, 1730, and 3400 cm−1 which belong to the stretching vibrations of O=C-O carboxyl, C=C skeleton of GO or -OH water, C=O carboxylates or ketones, and -OH water, respectively (Vasilaki et al. 2015; Y. Wang et al. 2019; M. Tahir and Amin 2017). The other functional groups are located at 1075 cm−1 which is assigned to C-O group of epoxy or alkoxy whereas 1250 cm−1 is related to the C-O-C group of epoxide or C-O-H group of phenolic. The peaks located below 1000 cm−1 are attributed to vibration of Ti-O-Ti and Ti-O-C groups. The formation of Ti-O-C absorption band confirms the reaction between TiO2 NPs and GO sheets. Moreover, comparing GO and Ag/TiO2/RGO samples, the decreased intensities of oxygen absorption bands in 2 wt.% Ag/TiO2/RGO compared to GO is further proved the reduction of GO and the formation of RGO during the hydrothermal preparation reaction. This result is consistent with the previous studies (Wang et al. 2019).
To analyze the structure of all samples, Raman spectroscopy was utilized and the spectra of photocatalysts are depicted in Fig. 7. For TiO2 photocatalyst, the most characteristic peak of TiO2 was observed at 144 cm−1 which ascribed to Eg mode, while peaks at 396, 516, and 638 cm−1 are corresponding to B1g, A1g, and Eg modes, respectively, arising from the presence of Ti-O bond in anatase phase of TiO2 (Wang et al. 2019; Naraginti and Yong 2019). The distinctive bands of GO and RGO were noticed at 1346 and 1593 cm−1 for D and G bands. D band is due to graphene-disordered structure corresponding to A1g mode while G band assigns to E2g mode from C-C bond in all hybridized carbon materials sp2. In Ag/TiO2 catalyst, it is well obvious that the intensities of the four characteristic peaks of TiO2 were increased compared with TiO2 which could be attributed to SPR effect of Ag NPs (Saquib et al. 2020).
While both the D and G bands associated to RGO along with TiO2 characteristic peaks were clearly detected in the Raman spectra of Ag/TiO2/RGO nanocomposite, however, the intensities of TiO2 peaks in that composite were decreased due to the incorporation of RGO. Comparing the ID/IG ratio of the Ag/TiO2/RGO ternary composite with GO, it was noticed that the ratio was 0.68 in GO while in the ternary composite was 0.75. The increment of ID/IG ratio in the ternary composite may assigned to the increase of the defects due to the addition of Ag and TiO2 NPs over graphene nanosheets as well as the reduction of the average size of sp2 domains owing to the use of hydrothermal reduction of GO in which the breaking of the GO layers and the formation of multilayers edges of graphene resulting in the increase of defects (Vasilaki et al. 2015; Wang et al. 2013).
In order to determine the surface area and pore volume for the synthesized catalysts, Brunauer-Emmett-Teller (BET) and Barrett-Joyner-Halendra (BJH) methods were performed, and results are depicted in Fig. 8.
As shown in Fig. 8a, the addition of Ag NPs and RGO increases the hysteresis loops which consequently increase the amount of N2 adsorbed by photocatalysts. In Table 2, the surface area and pore volume of all catalysts were exhibited. It is well noticed that the incorporation of Ag and RGO positively affected both specific surface and pore volume which attributed to the improvement of the meso-porosity. These results are consistent with previous studies (Tahir and Amin 2017; (Kočí et al. 2010). All samples are IV adsorption-desorption isotherms which is corresponding to mesoporous materials with H1 hysteresis loops.
The optical properties were analyzed in the wavelength range of 200–800 nm by UV-Vis diffuse reflectance spectra (DRS). Figure 9 shows the UV-Vis diffuse reflectance spectra of all synthesized catalysts. It could be noticed that Ag NPs and graphene samples exhibit a red shift towards the visible region. As seen in Fig. 9a, Ag sample spectra show a surface plasmon resonance (SPR) which appeared as shoulder peaks in the range of visible light region (Wang et al. 2019; Tan et al. 2015). This is a further evidence of Ag NP existence as recognized by STEM, TEM, and EDX analysis. The SPR effect of Ag nanoparticles was clearly observed at 485 nm for Ag/TiO2 sample which is comparable to reported results by Hsu and Chen (2015). However, the incorporation of RGO could extend the absorption edge with a wide absorption broadband in the range of 400–800 nm which differs than the absorption band edge of Ag/TiO2 photocatalyst (Hou et al. 2019). The band gap energies were estimated using Kubelka-Munk function vs. the photon energy as depicted in Fig. 9b. It is well clear that the Ag doping and the addition of RGO sheets contribute to the decrease of the band gap energies of catalysts compared to TiO2 that was in agreement with previous studies (Vasilaki et al. 2015; Tahir and Amin 2017; Doluel et al. 2020). The reduction of the band gap energy in the ternary composite Ag/TiO2/RGO could be attributed to the presence of Ti-O-C and/or Ti-C bonds between TiO2 and RGO as reported in Akhavan and Ghaderi (2013), Hou et al. (2019), and El-Bery et al. (2017).
Photoelectrochemical measurements
In order to examine the photoactivity of catalysts, photoelectrochemical measurements (PEC) were conducted in three-electrode cell immersed in 0.1 M Na2SO4 electrolyte. Prior to measurements, argon gas purging was performed for 30 min. The linear sweep voltammetry (LSV) was carried out to determine the magnitude of electrodes photocurrent with numerous on/off cycles while chronoamperometry (CAM) was investigated to evaluate the photocurrent density and electrodes stability. Later, electrochemical impedance (EIS) was measured to determine the interfacial resistance and charge transfer capability. Meso-porous catalyst powders were deposited using the electrophoretic deposition (EPD) method without any post-treatment such as necking or heating.
Figure 10 shows the photocurrent-potential relationship under chopped UV-LED light with a wavelength of 365 nm and the average light intensity of 50 mW/cm2. The LSV at negative applied bias gives a positive photocurrent which indicates the n-type behavior of catalysts. The reproducibility of the photocurrent generated confirms that all electrodes had an efficient response with instant illumination while the anodic photocurrent improved for those electrodes which contain Ag and RGO as shown in Fig. 10 compared with bare TiO2 electrode. Furthermore, RGO substantially enhanced the photocatalytic performance, which could be attributed to its superior electrical conductivity. This facilitates the charge separation and reduces the recombination rate at interfaces and acts as sink of the photogenerated electrons (C. Wang et al. 2014; Sun et al. 2013; Meng et al. 2014; Meng et al. 2019). In addition, it was reported that under UV-irradiation photogenerated electrons of TiO2 could reduce the remaining GO oxygenated function groups (sp2 restoration) that exist in the photocatalysts composite. This could explain the increment of the photocurrent with increasing potential and the time of irradiation in case of Ag/TiO2/RGO composite. The further deoxygenation of the partially reduced graphene oxide components under the photocatalytic reactions was considerably reported as in Williams et al. (2008) and Akhavan et al. (2010) with TiO2 semiconductor, and with ZnO as a semiconductor in Williams and Kamat (2009) and Akhavan (2010). Moreover, electron trap and SPR occurred owing to the existence of Ag NPs that could lower the recombination of electron-hole pairs. Similar observations were reported in previous studies (Ahmed et al. 2018; Arif Sher Shah et al. 2013; Chaudhary et al. 2017).
To further assess the photoactivity of catalysts, Fig. 11 shows the chronoamperometry (at +0.6 vs. Ag/AgCl) for 800 s which included 60 s in dark before turning on light for 600 s then turning off light once again until the completion of the measurement. It is well seen from the figure that all samples exhibit excellent photocurrent stability; however, the ternary composite of Ag/TiO2/RGO shows the superior activity compared with the binary composite of Ag/TiO2 and bare TiO2. This confirms the results obtained in the previous section of LSV which may be assigned to the higher separation of electrons and less recombination of electron-hole pairs in the Ag/TiO2/RGO catalyst (Wang et al. 2019; Sang et al. 2019).
In order to test the charge transfer resistance of the prepared composites, EIS was recorded at dark and light conditions as depicted in Fig. 12 in 0.1 M Na2SO4 solution at a frequency ranging from 100 kHz to 0.01 Hz and at 0.0 V (vs. Ag/AgCl).
In Fig. 12, the beginning section of the intercept between the circular arc in the Nyquist plot and real impedance (Zreal) demonstrates the bulk electrolyte resistance (Re) or the equivalent series resistance (ESR) located at high-frequency region while the middle section of the arc exhibits the charge transfer resistance (RCT) located at the intermediate frequency. The smaller the arc diameter, the smaller the charge transfer resistance, and consequently effective charge transfer. This confirms the superior photocatalytic activity of the ternary composite of Ag/TiO2/RGO compared with Ag/TiO2 and bare TiO2 catalysts. The efficient separation of electrons which caused by both Ag and RGO incorporation is the reason of this enhanced activity of Ag/TiO2/RGO composite. Similar results were reported elsewhere (Chaudhary et al. 2017; Saquib et al. 2020).
Photoconversion of CO2 into organic compounds
The synthesized nanocomposites along with TiO2/RGO photocatalyst were further examined to reduce CO2 in the presence of water into valuable organic compounds in the advanced meso-scale continuous-flow-type photochemical reactor cooperated with the proposed setup. Figure 13 shows the concentrations of both TOC and formaldehyde. TOC was analyzed using total organic carbon analyzer while formaldehyde was separately analyzed by Nash method. The proposed conditions used to reduce CO2 were 30 mg of each photocatalyst weight, 30°C of the reactor temperature, 65 ml of water volume, and 500 μl/min for liquid pump flow rate which equals to 20 min of reactants’ retention time inside the reactor.
In order to further identify the produced TOC, analysis on GC/MS was performed as displayed in Fig. 14. It is evident that the major product was methanol in the aqueous phase while the minor product was formaldehyde as seen in Figs. 13 and 14. In many applications, methanol is much preferable than gaseous products because it could be easily transported and stored. Previous findings confirmed that methanol was the main product in the photocatalytic reduction of CO2 when water was used as a reductant and Ag/TiO2 as a catalyst with different reactor configurations and setups (Yu et al. 2016; Liu et al. 2014; Kočí et al. 2011). It is well obvious that the use of LED lamp to save energy as well as less catalyst utilization to produce methanol and other organic compounds in this setup emphasize the proficiency of this reactor compared with other previous setups used to reduce CO2. The formaldehyde concentration was too small as shown in Figs. 13 and 14 and the maximum concentration was achieved via Ag/TiO2/RGO which reached 0.26 mg/L.
In relation to catalysts, Ag/TiO2/RGO shows the superior photocatalytic activity towards TOC production that confirms results obtained from PEC measurements when the catalyst increases the photogenerated current compared with Ag/TiO2 and bare TiO2. The photocatalytic activity of CO2 reduction as well as photocurrent generation was found to be in the following sequence Ag/TiO2/RGO > Ag/TiO2 > TiO2.
Comparing Ag/TiO2 catalyst with bare TiO2, the enhancement of TOC productivity was assigned to Ag NP doping which act as electron trap to facilitate charge separation resulting in the increment of TOC production (Tan et al. 2015).
Moreover, the TiO2/RGO catalyst was examined in CO2 photocatalytic reduction as shown in Fig. 13 to further identify the effectiveness of the incorporation of RGO together with TiO2 semiconductor. Its result showed that a little improvement of organic carbon production (TOC) was achieved in comparison with Ag/TiO2 photocatalyst. However, the amount of formaldehyde produced in this case exceeded the produced amount of formaldehyde in case of Ag/TiO2. It was reported that the exfoliated graphene has an effective surface area ~ 2600 m2/g which promotes the traveling of electrons without scattering at mobility up to ~ 15,000 m2/V. S at room temperature. Obviously, graphene plays an essential role and acts as electrons sink or electrons bridge (Jeyalakshmi et al. 2012).
The use of graphene in the Ag/TiO2/RGO ternary composite enhanced the photocatalytic reduction of CO2 by 3.3 and 9.4 times compared with Ag/TiO2 and bare TiO2, respectively, using the proposed photochemical reactor in a continuous mode. The reason why Ag/TiO2/RGO has a superior photocatalytic activity over other catalysts is that RGO facilitates the mobility of the photoexcited electrons prior to be trapped by Ag NPs that consequently decreases the recombination of electron-hole pairs.
Comparing the results of the ternary composite of Ag/TiO2/RGO with the binary composite of TiO2/RGO, no change was found in the amount of formaldehyde while the methanol concentration was significantly increased by 2.6-fold as verified by TOC analysis. This confirms the synergistic effect of Ag NPs and RGO along with TiO2 as the main reason for improving the photocatalytic conversion of CO2 into methanol as the major product.
Proposed mechanism of CO2 photocatalytic reduction
The reduction of CO2 with water takes place according to Eqs. (6)–(12) and illustrated in Fig. 15 (Habisreutinger et al. 2013; Ran et al. 2018; Hiragond et al. 2019; Chang et al. 2016). The process begins with semiconductor excitation to produce electron-hole pairs. Later, holes are consumed to oxidize water molecules to form hydrogen ions (H+) and hydroxyl radicals (OH●) which further be react with holes to produce oxygen while electrons reduce the adsorbed CO2 on the surface of the semiconductor to form methanol and other valuable hydrocarbons. The RGO existence provides a fast mobility for electrons to facilitate separation then Ag enhance the separation process via trapping of those electrons to further be utilized in CO2 reduction and consequently produce higher carbon compounds such as formaldehyde and methanol as illustrated in Eqs. (10, 11). The last equation (Eq. (12)) exhibits the competitive reaction of reducing hydrogen instead of CO2. In this case of using the advanced meso-scale continuous-flow type photochemical reactor, the GC analysis confirms that the reactor suppresses the formation of hydrogen and induces the reduction of CO2.
Conclusion
The advanced meso-scale continuous-flow-type photochemical reactor exhibits an outstanding performance towards CO2 photocatalytic reduction with liquid water. GC results confirm that the reactor suppresses the production of H2, the competitive reaction, which enhances the reduction of CO2 and consequently the production of various valuable hydrocarbons via consuming less amount of catalysts.
The use of graphene increases the TOC production by 3.3 and 9.4 times compared with Ag/TiO2 and bare TiO2, respectively, using the proposed photochemical reactor in a continuous mode.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Abrishamkar M, Barootkoob M (2017) Electrooxidation of formaldehyde as a fuel for fuel cells using Fe2+-nano-zeolite modified carbon paste electrode. Int J Hydrog Energy 42(37):23821–23825. https://doi.org/10.1016/j.ijhydene.2017.04.083
Ahmed N, Ramadan M, El Rouby WMA, Farghali AA, Allam NK (2018) Non-precious co-catalysts boost the performance of TiO2 hierarchical hollow mesoporous spheres in solar fuel cells. Int J Hydrog Energy 43:21219–21230. https://doi.org/10.1016/j.ijhydene.2018.10.012
Akhavan O (2010) Graphene nanomesh by ZnO nanorod photocatalysts. ACS Nano 4(7):4174–4180. https://doi.org/10.1021/nn1007429
Akhavan O, Ghaderi E (2009) Photocatalytic reduction of graphene oxide nanosheets on TiO2 thin film for photoinactivation of bacteria in solar light irradiation. J Phys Chem C 113(47):20214–20220. https://doi.org/10.1021/jp906325q
Akhavan O, Ghaderi E (2013) Flash photo stimulation of human neural stem cells on graphene/TiO 2 heterojunction for differentiation into neurons. Nanoscale 5(21):10316–10326. https://doi.org/10.1039/c3nr02161k
Akhavan O, Abdolahad M, Esfandiar A, Mohatashamifar M (2010) Photodegradation of graphene oxide sheets by TiO2 nanoparticles after a photocatalytic reduction. J Phys Chem C 114(30):12955–12959. https://doi.org/10.1021/jp103472c
Arif Sher Shah MS, Zhang K, Park AR, Kim KS, Park NG, Park JH, Yoo PJ (2013) Single-step solvothermal synthesis of mesoporous Ag-TiO2-reduced graphene oxide ternary composites with enhanced photocatalytic activity. Nanoscale 5(11):5093–5101. https://doi.org/10.1039/c3nr00579h
Chang X, Zheng J (2015) Photocatalytic conversion of CO 2 into value-added hydrocarbon ( methanol ) with high selectivity over ZnS nanoparticles driven by 355-nm pulsed laser. Res Chem Intermed 41:739–747. https://doi.org/10.1007/s11164-013-1224-y
Chang X, Wang T, Gong J (2016) CO2 photo-reduction: insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ Sci 9(7):2177–2196. https://doi.org/10.1039/C6EE00383D
Chaudhary D, Singh S, Vankar VD, Khare N (2017) A ternary Ag/TiO2/CNT photoanode for efficient photoelectrochemical water splitting under visible light irradiation. Int J Hydrog Energy 42(12):7826–7835. https://doi.org/10.1016/j.ijhydene.2016.12.036
Chen W-Y, Shi GK, Hailey A, Tsai EST, Hammer N, Wu Z (2012) Photocatalytic conversion of carbon dioxide to organic compounds using a green photocatalyst: an undergraduate research experiment. Chem Educ 17(4):166–172. https://doi.org/10.1007/s00897122438
Crake A, Christoforidis KC, Kafizas A, Zafeiratos S, Petit C (2017) CO2 capture and photocatalytic reduction using bifunctional TiO2/MOF nanocomposites under UV–vis irradiation. Appl Catal B Environ 210:131–140. https://doi.org/10.1016/j.apcatb.2017.03.039
Doluel EC, Kartal U, Dikici T, Yurddaskal M (2020) Effect of Ag content on photocatalytic activity of Ag@TiO2/rGO hybrid photocatalysts. J Electron Mater 49(6):3849–3859. https://doi.org/10.1007/s11664-020-08102-0
Dong WH, Wu DD, Luo JM, Xing QJ, Liu H, Zou JP, Luo XB, Min XB, Liu HL, Luo SL, Au CT (2017) Coupling of photodegradation of RhB with photoreduction of CO2 over rGO/SrTi0.95Fe0.05O3−Δ catalyst: a strategy for one-pot conversion of organic pollutants to methanol and ethanol. J Catal 349:218–225. https://doi.org/10.1016/j.jcat.2017.02.004
El-Bery HM, Matsushita Y, Abdel-moneim A (2017) Fabrication of efficient TiO 2 -RGO heterojunction composites for hydrogen generation via water-splitting: comparison between RGO, Au and Pt reduction sites. Appl Surf Sci 423:185–196. https://doi.org/10.1016/j.apsusc.2017.06.130
Feng X, Pan F, Tran BZ, Li Y (2020) Photocatalytic CO2 reduction on porous TiO2 synergistically promoted by atomic layer deposited MgO overcoating and photodeposited silver nanoparticles. Catal Today 339(October 2018):328–336. https://doi.org/10.1016/j.cattod.2019.03.012
Gui MM, Wong WMP, Chai SP, Mohamed AR (2015) One-pot synthesis of Ag-MWCNT@TiO2 core-shell nanocomposites for photocatalytic reduction of CO2 with water under visible light irradiation. Chem Eng J 278:272–278. https://doi.org/10.1016/j.cej.2014.09.022
Habisreutinger SN, Schmidt-Mende L, Stolarczyk JK (2013) Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew Chem Int Ed 52(29):7372–7408. https://doi.org/10.1002/anie.201207199
Heggo D, Mohamed H, Ookawara S, Matsushita Y (2016) Process intensication of photocatalytic p-anisaldehyde synthesis by using mini batch reactor and UV-LED. J Chem Eng Jpn 49(2):130–135. https://doi.org/10.1252/jcej.15we042
Hiragond C, Ali S, Sorcar S, In SI (2019) Hierarchical nanostructured photocatalysts for co2 photoreduction. Catalysts 9(4):370. https://doi.org/10.3390/catal9040370
Hou Y, Pu S, Shi Q, Mandal S, Ma H, Xue S, Cai G, Bai Y (2019) Ultrasonic impregnation assisted in-situ photoreduction deposition synthesis of Ag/TiO2/rGO ternary composites with synergistic enhanced photocatalytic activity. J Taiwan Inst Chem Eng 104:139–150. https://doi.org/10.1016/j.jtice.2019.08.023
Hsu KC, Chen DH (2015) Highly sensitive, uniform, and reusable surface-enhanced raman scattering substrate with TiO2 interlayer between Ag nanoparticles and reduced graphene oxide. ACS Appl Mater Interfaces 7(49):27571–27579. https://doi.org/10.1021/acsami.5b08792
Jeyalakshmi V, Mahalakshmy R, Krishnamurthy KR, Viswanathan B (2012) Titania based catalysts for photoreduction of carbon dioxide: role of modifiers. Indian J Chem - Section A Inorgan, Physical, Theor Anal Chem 51(9–10):1263–1283
Josland S, Mumtaz S, Oelgemöller M (2016) Photodecarboxylations in an advanced meso-scale continuous-flow photoreactor. Chem Eng Technol 39(1):81–87. https://doi.org/10.1002/ceat.201500285
Karamian E, Sharifnia S (2016) On the general mechanism of photocatalytic reduction of CO2. J CO2 Util 16:194–203. https://doi.org/10.1016/j.jcou.2016.07.004
Kočí K, Matějů K, Obalová L, Krejčíková S, Lacný Z, Plachá D, Čapek L, Hospodková A, Šolcová O (2010) Effect of silver doping on the TiO2 for photocatalytic reduction of CO2. Appl Catal B Environ 96(3–4):239–244. https://doi.org/10.1016/j.apcatb.2010.02.030
Kočí K, Zatloukalová K, Obalová L, Krejčíková S, Lacný Z, Čapek L, Hospodková A, Šolcová O (2011) Wavelength effect on photocatalytic reduction of CO2 by Ag/TiO2 catalyst. Cuihua Xuebao/Chin J Catal 32(5):812–815. https://doi.org/10.1016/s1872-2067(10)60199-4
Kometani N, Hirata S, Chikada M (2017) Photocatalytic reduction of CO2 by Pt-loaded TiO2 in the mixture of sub- and supercritical water and CO2. J Supercrit Fluids 120:443–447. https://doi.org/10.1016/j.supflu.2016.05.031
Li J, Zeng X, Ren T, van der Heide E (2014) The preparation of graphene oxide and its derivatives and their application in bio-tribological systems. Lubricants 2(3):137–161. https://doi.org/10.3390/lubricants2030137
Liu E, Kang L, Wu F, Sun T, Hu X, Yang Y, Liu H, Fan J (2014) Photocatalytic reduction of CO2 into methanol over Ag/TiO2 nanocomposites enhanced by surface plasmon resonance. Plasmonics 9(1):61–70. https://doi.org/10.1007/s11468-013-9598-7
Liu L, Zhang Y, Zhang B, Feng Y (2017) A detailed investigation on the performance of dye-sensitized solar cells based on reduced graphene oxide-doped TiO2 photoanode. J Mater Sci 52(13):8070–8083. https://doi.org/10.1007/s10853-017-1014-9
Liu X, Xu T, Li Y, Zang Z, Peng X, Wei H, Zha W, Wang F (2018) Enhanced X-ray photon response in solution-synthesized CsPbBr3 nanoparticles wrapped by reduced graphene oxide. Sol Energy Mater Sol Cells 187(August):249–254. https://doi.org/10.1016/j.solmat.2018.08.009
Loubière K, Oelgemöller M, Aillet T, Dechy-cabaret O, Prat L (2016) Continuous- flow photochemistry: a need for chemical engineering. Chem Eng Process Process Intensif 104:120–132. https://doi.org/10.1016/j.cep.2016.02.008
Low J, Cheng B, Yu J (2017) Surface modification and enhanced photocatalytic CO 2 reduction performance of TiO 2: a review. Appl Surf Sci 392:658–686. https://doi.org/10.1016/j.apsusc.2016.09.093
Meng D, Yang S, Guo L, Li G, Ge J, Huang Y, Bielawski CW, Geng J (2014) The enhanced photothermal effect of graphene/conjugated polymer composites: photoinduced energy transfer and applications in photocontrolled switches. Chem Commun 50(92):14345–14348. https://doi.org/10.1039/c4cc06849a
Meng D, Fan J, Ma J, Du SW, Geng J (2019) The preparation and functional applications of carbon nanomaterial/conjugated polymer composites. Compos Commun 12(December 2018):64–73. https://doi.org/10.1016/j.coco.2018.12.009
Naraginti S, Yong YC (2019) Enhanced detoxification of p-bromophenol by novel Zr/Ag-TiO 2 @rGO ternary composite: degradation kinetics and phytotoxicity evolution studies. Ecotoxicol Environ Saf 170(December 2018):355–362. https://doi.org/10.1016/j.ecoenv.2018.12.001
Nash T (1953) The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J 55(3):416–421. https://doi.org/10.1042/bj0550416
Némethné-Sóvágó J, Benke M (2014) Microreactors: a new concept for chemical synthesis and technological feasibility. Mater Sci Eng 39(2):89–101
Ola O, Maroto-Valer MM (2015) Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J Photochem Photobiol C: Photochem Rev 24:16–42. https://doi.org/10.1016/j.jphotochemrev.2015.06.001
Rajalakshmi K, Jeyalakshmi V, Krishnamurthy KR, Viswanathan B (2012) Photocatalytic reduction of carbon dioxide by water on titania: role of photophysical and structural properties. Indian J Chem - Section A Inorgan, Physical, Theor Anal Chem 51(3):411–419
Ran J, Jaroniec M, Qiao SZ (2018) Cocatalysts in semiconductor-based photocatalytic CO2 reduction: achievements, challenges, and opportunities. Adv Mater 30(7):1–31. https://doi.org/10.1002/adma.201704649
Sang L, Lei L, Burda C (2019) Electrochemical fabrication of rGO-embedded Ag-TiO2 nanoring/nanotube arrays for plasmonic solar water splitting. Nano-Micro Lett 11(1):1–16. https://doi.org/10.1007/s40820-019-0329-2
Saquib M, Kaushik R, Halder A (2020) Photoelectrochemical Activity of Ag coated 2D-TiO2/RGO heterojunction for hydrogen evolution reaction and environmental remediation. Chem Select 5(21):6376–6388. https://doi.org/10.1002/slct.202000843
Sellaro M, Bellardita M, Brunetti A, Fontananova E, Palmisano L, Drioli E, Barbieri G (2016) CO2 conversion in a photocatalytic continuous membrane reactor. RSC Adv 6(71):67418–67427. https://doi.org/10.1039/c6ra06777h
Shehzad N, Tahir M, Johari K, Murugesan T, Hussain M (2018) A critical review on TiO2 based photocatalytic CO2 reduction system: strategies to improve efficiency. J CO2 Util 26(May):98–122. https://doi.org/10.1016/j.jcou.2018.04.026
Sneddon G, Greenaway A, Yiu HHP (2014) The potential applications of nanoporous materials for the adsorption , separation , and catalytic conversion of carbon dioxide. Adv Energy Mater 4(10):1–19. https://doi.org/10.1002/aenm.201301873
Spurr RA, Myers H (1957) Quantitative analysis of anatase-rutile mixtures with an X-ray diffractometer. Anal Chem 29(5):760–762. https://doi.org/10.1021/ac60125a006
Sun J, Xiao L, Meng D, Geng J, Huang Y (2013) Enhanced photoresponse of large-sized photoactive graphene composite films based on water-soluble conjugated polymers. Chem Commun 49(49):5538–5540. https://doi.org/10.1039/c3cc40563j
Tahir M, Amin NS (2013) Photocatalytic CO2 reduction and kinetic study over In/TiO 2 nanoparticles supported microchannel monolith photoreactor. Appl Catal A Gen 467:483–496. https://doi.org/10.1016/j.apcata.2013.07.056
Tahir M, Amin NAS (2017) Photo-induced CO2 reduction by hydrogen for selective CO evolution in a dynamic monolith photoreactor loaded with Ag-modified TiO2 nanocatalyst. Int J Hydrog Energy 42(23):15507–15522. https://doi.org/10.1016/j.ijhydene.2017.05.039
Tahir B, Tahir M, Amin NS (2015) Performance analysis of monolith photoreactor for CO2 reduction with H2. Energy Convers Manag 90:272–281. https://doi.org/10.1016/j.enconman.2014.11.018
Tan LL, Ong WJ, Chai SP, Mohamed AR (2015) Noble metal modified reduced graphene oxide/TiO2 ternary nanostructures for efficient visible-light-driven photoreduction of carbon dioxide into methane. Appl Catal B Environ 166–167:251–259. https://doi.org/10.1016/j.apcatb.2014.11.035
Vasilaki E, Georgaki I, Vernardou D, Vamvakaki M, Katsarakis N (2015) Ag-loaded TiO 2 /reduced graphene oxide nanocomposites for enhanced visible-light photocatalytic activity. Appl Surf Sci 353:865–872. https://doi.org/10.1016/j.apsusc.2015.07.056
Verhelst S, Turner JW, Sileghem L, Vancoillie J (2019) Methanol as a fuel for internal combustion engines. Prog Energy Combust Sci 70:43–88. https://doi.org/10.1016/j.pecs.2018.10.001
Wang P, Tang Y, Dong Z, Chen Z, Lim TT (2013) Ag-AgBr/TiO2/RGO nanocomposite for visible-light photocatalytic degradation of penicillin G. J Mater Chem A 1(15):4718–4727. https://doi.org/10.1039/c3ta01042b
Wang C, Meng D, Sun J, Memon J, Huang Y, Geng J (2014) Graphene wrapped TiO2 based catalysts with enhanced photocatalytic activity. Adv Mater Interfaces 1(4):1–8. https://doi.org/10.1002/admi.201300150
Wang B, Chen W, Song Y, Li G, Wei W, Fang J, Sun Y (2018) Recent progress in the photocatalytic reduction of aqueous carbon dioxide. Catal Today 311(October):23–39. https://doi.org/10.1016/j.cattod.2017.10.006
Wang Y, Zhang M, Fang L, Yang H, Zuo Y, Gao J, He G, Sun Z (2019) A multifunctional Ag/TiO 2 /reduced graphene oxide with optimal surface-enhanced Raman scattering and photocatalysis. J Am Ceram Soc 102(7):4000–4013. https://doi.org/10.1111/jace.16273
Wei J, Zang Z, Zhang Y, Wang M, Du J, Tang X (2017) Enhanced performance of light-controlled conductive switching in hybrid cuprous oxide/reduced graphene oxide (Cu_2O/rGO) nanocomposites. Opt Lett 42(5):911–914. https://doi.org/10.1364/ol.42.000911
Williams G, Kamat PV (2009) Graphene-semiconductor nanocomposites: excited-state interactions between ZnO nanoparticles and graphene oxide. Langmuir 25(24):13869–13873. https://doi.org/10.1021/la900905h
Williams G, Seger B, Kamt PV (2008) TiO2-graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano 2(7):1487–1491. https://doi.org/10.1021/nn800251f
Wu J, Huang Y, Ye W, Li Y (2017) CO2 reduction: from the electrochemical to photochemical approach. Adv Sci 4(11):1–29. https://doi.org/10.1002/advs.201700194
Yang X, Xiao T, Edwards PP (2011) The use of products from CO2 photoreduction for improvement of hydrogen evolution in water splitting. Int J Hydrog Energy 36(11):6546–6552. https://doi.org/10.1016/j.ijhydene.2011.02.116
Yu B, Zhou Y, Li P, Tu W, Li P, Tang L, Ye J, Zou Z (2016) Photocatalytic reduction of CO2 over Ag/TiO2 nanocomposites prepared with a simple and rapid silver mirror method. Nanoscale 8(23):11870–11874. https://doi.org/10.1039/c6nr02547a
Zang Z, Zeng X, Wang M, Hu W, Liu C, Tang X (2017) Tunable photoluminescence of water-soluble AgInZnS–graphene oxide (GO) nanocomposites and their application in-vivo bioimaging. Sensors Actuators B Chem 252:1179–1186. https://doi.org/10.1016/j.snb.2017.07.144
Acknowledgements
The first author is grateful to the Egypt-Japan University of Science and Technology (E-JUST), especially Prof. Dr. Ahmed H. El-Shazly, for providing the support and supervision to complete this work.
Funding
The analysis of all samples as well as all experiments was conducted in the laboratories of Egypt-Japan University of Science and Technology (E-JUST).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Samar Nabil, Ahmed Hammad, and Haitham El-Bery. The supervision, discussion of results, and suggestion of the research point were performed by prof. Elsayed A. Shalaby and prof. Ahmed H. El-Shazly. The first draft of the manuscript was written by Samar Nabil and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The manuscript does not involve the use of any animal or human data or tissue.
Consent for publication
The manuscript does not contain data from any individual person.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ricardo Torres-Palma
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nabil, S., Hammad, A.S., El-Bery, H.M. et al. The CO2 photoconversion over reduced graphene oxide based on Ag/TiO2 photocatalyst in an advanced meso-scale continuous-flow photochemical reactor. Environ Sci Pollut Res 28, 36157–36173 (2021). https://doi.org/10.1007/s11356-021-13090-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13090-7