Abstract
Agrochemicals can reach freshwater bodies by drift, leaching, or runoff, where they constitute complex mixtures. Given that glyphosate and cypermethrin are within the most worldwide used pesticides, they are likely to co-occur in freshwater bodies. The aim of this study was to analyze the interaction between glyphosate and cypermethrin formulations on the cladoceran Ceriodaphnia dubia (Richard 1894) through an acute toxicity test and on a zooplankton assemblage through a mesocosm (30 L) experiment. The 24-h LC50 of both isolated pesticides and their equitoxic mixture was obtained for C. dubia. The mesocosm was performed by exposing a zooplankton assemblage to both pesticides isolated and in combination. The acute toxicity of the equitoxic mixture in C. dubia was 3 and 4 times higher than the isolated toxicity of glyphosate and cypermethrin, respectively. The total toxic units of the mixture were 0.53, indicating a synergistic interaction. In the mesocosm experiment, both pesticides also interacted causing a synergistic negative effect in Cladocera and Copepoda abundances. No interactions between pesticides were found for Rotifera; therefore, the mixture effect was considered additive. It is suggested to continue analyzing pesticide mixture effects on the basis of complementary scales of analysis to reach more environmentally relevant information.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last decades, agricultural practices have increased by expansion and intensification to satisfy growing human population demands. This increase involves the use of high amounts of pesticides associated with the production of genetically modified crops (Matson et al. 1997; Bonny 2008). Agrochemicals can reach freshwater bodies by drift, leaching, or runoff, affecting nontarget organisms (Amorós et al. 2007; Sasal et al. 2015). Their high toxicity resides in the fact that they constitute complex mixtures, because they either are applied as mixtures or converge directly in the surface waters (Akan et al. 2015; Cruzeiro et al. 2015; Etchegoyen et al. 2017). In mixtu`res, these pollutants can interact synergistically (mixture effect higher than the sum of individual effects) or antagonistically (mixture effect lower than the sum of individual effects). If contaminants do not interact, the mixture effect is additive (equal to the sum of their individual effects) (Folt et al. 1999; Piggott et al. 2015). Although current ecotoxicological regulations consider that toxicity tests should include mixture bioassays because of their representativeness, there is still poor information about the effects of pesticides in mixture on nontarget species and communities (Relyea and Hoverman 2006; Belden et al. 2007; Hasenbein et al. 2016).
Glyphosate [N-(phosphonomethyl)glycine], a broad-spectrum postemergence herbicide, is the most globally used herbicide for weed control (Baylis 2000; Annett et al. 2014). Its wide use and ubiquity in freshwater bodies make it a pseudo-persistent contaminant (Borggaard and Gimsing 2008; Primost et al. 2017). Cypermethrin [alpha-cyano-3-phenoxybenzyl ester of 2,2-dimethyl-3-(2,2-dichlorovinyl) cyclopropane carboxylic acid], a non-systematic pyrethroid, is a widely used insecticide (Friberg-Jensen et al. 2003). Thus both, glyphosate and cypermethrin, are likely to co-occur in freshwater bodies (Marino and Ronco 2005; Bonansea et al. 2013, 2017; Battaglin et al. 2014; Primost et al. 2017). The individual toxicity of glyphosate and cypermethrin to nontarget freshwater organisms has been largely documented (Friberg-Jensen et al. 2003; Kumar et al. 2010; Pérez et al. 2011; Rico-Martinez et al. 2012; Annett et al. 2014; Arias et al. 2020; Fantón et al. 2020). Nevertheless, few studies have focus on analyzing their mixture effects, with some controversies. For instance, a synergistic interaction in acute toxicity of glyphosate and cypermethrin was found in tadpoles (Rhinella arenarum) (Brodeur et al. 2014), while an antagonistic interaction of the same mixture was found in fish (Cnesterodon decemmaculatus) (Brodeur et al. 2016). These findings show that the interactions between pesticides may be different among the considered test organism, which make it necessary to study different nontarget organisms, in order to reach more complete information on how pesticide can interact on the biota.
The impacts of pesticides are frequently assessed on single-species bioassays, which provide valuable and comparable information (e.g., APHA 1998; OECD 2004). Nevertheless, these studies need to be complemented with toxicity tests covering assemblages of species, since it has been shown that pesticide effects can differ between different organization levels, as interspecific interactions and environmental factors might play an important role (Brock et al. 2014; Hasenbein et al. 2016; Barmentlo et al. 2018; Gutierrez et al. 2020a). In this sense, zooplankton community allows to easily work at different biological levels, since they have small body sizes and short generation times, responding quickly to environmental changes with high sensitivity (DeLorenzo et al. 2001; Hanazato 2001; Resh 2008).
The aim of this study was to analyze the interaction between glyphosate and cypermethrin formulations on the cladoceran Ceriodaphnia dubia (Richard 1894) through an acute toxicity test and on a zooplankton assemblage through a mesocosm experiment. These both approaches were performed on a complementary way in order to achieve a better understanding of the effects of the pesticide mixture.
Materials and methods
Selection of pesticide formulations
Two commercial formulations were employed: glyphosate: Atanor II® 43.8% w/v monopotassium salt (Atanor S.C.A., Argentina) and cypermethrin: Xiper 25® 25% w/v (40-50 cis) mixture of cis-trans isomers of alphacyano-3-phenoxybenzyl 2,2 dimethyl-3 (2,2 dichlorovinyl) cyclopropane carboxylate (UPL Argentina S.A.). The glyphosate concentrations are reported as acid equivalent (a.e.) per liter, since Atanor II® formulation presents glyphosate as monopotassium salt in order to increase water solubility (Lanctôt et al. 2014).
Experimental design
Acute toxicity test
Acute toxicity of glyphosate and cypermethrin formulations isolated and in combination was assessed in Ceriodaphnia dubia following the APHA (1998) protocol. Ceriodaphnia dubia organisms were collected in a natural reserve located in the middle Paraná River floodplain (31° 38′ 15.1″ S 60° 40′ 23.3″ W), and progressively adapted to laboratory culture conditions.
A stock culture of C. dubia was maintained in reconstituted freshwater (APHA 1998: 120 mg L−1 SO4Mg and CaSO4 2H2O, 192 mg L−1 NaHCO3, 8 mg L−1 KCl). The culture was settled in an incubation chamber at 22 ± 1 °C, with 16:8 light:dark regime, the culture media were weekly changed, and organisms were fed three times a week with a Scenedesmus obliquus culture.
The 24-h LC50 for each pesticide was estimated by exposing C. dubia neonates (<24 h) to 5 concentrations of each pesticide alone with the respective controls (without pesticides) (Table 1). Based on the individual LC50, an equitoxic mixture was made following Marking (1977) and 5 concentrations were tested (Table 1). A total of 4 replicates (5 neonates each) per treatment were performed in 50-mL beakers. Dissolved oxygen (DO) and pH were measured at the beginning and at the end of the experiment. Their values varied between 6 and 8 mg L−1 for DO and 7.5–8.15 for pH, being within the limits established by APHA (1998).
For preparing the final selected concentrations, three stock solutions were first prepared (glyphosate: 2580 mg L−1, cypermethrin: 13.6 mg L−1, and equitoxic mixture: 13683 mg L−1 Gly plus 0.19 mg L−1 Cyp) in distillated water. A subsample of each stock solution was conserved in darkness at −4 °C for their analytical determination. Cypermethrin was analyzed using a high-performance liquid chromatography equipment (HPLC, SHIMADZU Prominence® 20-A Series) with reverse-phase, RP18 column, and diode array detector, following EPA Method 1660 (EPA 1993) (DL: 0.03 mg L−1, QL: 0.1 mg L−1). Glyphosate was analyzed using an HPLC equipment with a conductivity detector (Waters®). An Ion Pack AG22 4 × 250 mm column, an Ion PackAS22 4 × 240 mm column, and an ion regeneration suppressor (all Dionex) were used. A mixture of sodium hydroxide (4 mM) and sodium carbonate (9 mM) was used as the mobile phase. The ion chromatography method with conductivity detection was adapted from Zhu et al. (1999) (DL: 2.52 mg L−1, QL: 8.42 mg L−1).
Mesocosm experiment
A 30-L indoor experiment was set up exposing a zooplankton assemblage to glyphosate and cypermethrin formulations isolated and in combination. Three replicates were included per treatment: control (Ctrl), glyphosate (Gly), cypermethrin (Cyp), and a mixture of both pesticides using the same individual concentrations (Mix). The exposure concentrations were Gly: 6.4 mg L−1 (± 1.9), Cyp: 0.01 mg L−1 (± 0.002), and Mix: 6.9 mg L−1 (± 1.9) of glyphosate and 0.01 mg L−1 (± 0.001) of cypermethrin.
The pesticide concentrations were selected based on the acute toxicity test preformed on C. dubia and published data (Pérez et al. 2007; Vera et al. 2010; Akan et al. 2015; Etchegoyen et al. 2017).
The zooplankton assemblage was collected in shallow lakes of the middle Paraná River floodplain with a 20-μm net, to also ensure the collection of phytoplankton as food resource. It was left for 4 days in the laboratory for its acclimation and stabilization. Laboratory conditions were 25 °C, natural photoperiod (12 h light, approximately) and permanent aeration. The zooplankton assemblage was inoculated in each plastic tank and left in the same conditions during 1 day more for stabilization. Afterward, the pesticides were carefully spiked and the experiment lasted for 7 days in the same laboratory conditions. Samples were taken at three sampling times: at days 1 (2 h after the addition of pesticides), 4, and 7.
Physicochemical parameters as pH, temperature (°C), conductivity (μS cm−1), and dissolved oxygen (mg L−1) were measured every sampling time using Hanna portable probes. Soluble reactive phosphorus, ammonium, nitrites, and nitrates were analyzed according to APHA (1998). Glyphosate and cypermethrin concentrations were also analyzed in each sampling time. Glyphosate was determined using a SHIMADZU Prominence 20A Series liquid chromatograph equipped with a fluorescence detector (SHIMADZU RF-10AxL; SHIMADZU Corporation, Kyoto, Japan) and a column (Phenomenex Luna NH2 Part No. 00G-4378-Y0) (DL: 2 μg L−1, QL: 6 μg L−1). Cypermethrin was measured through matrix solid-phase dispersion (MSPD) validated by Valenzuela-Quintanar et al. (2006) with modifications. A gas chromatograph (Agilent 6890) with a micro capture electron detector (micro-ECD) was employed and a Chrompack Capillay Column CP-Sil 5 CB (15 m, 0.53 mm, 1.5 μm) (DL: 2 μg L−1, QL: 6 μg L−1).
Quantitative zooplankton samples were taken by filtering 300 mL of water through a 45-μm net, fixed with formaldehyde 4% and colored with erythrosine. The individuals were identified and quantified using specific taxonomic keys (Ahlstrom 1940, 1943; Koste and Shiel 1989; Paggi 1995; Segers 2002, 2007; FADA 2010; Kotov et al. 2013) in 1 mL Sedgewick Rafter chamber under an optical microscope (Nikon Eclipse E-200). The whole samples were quantified.
Data analysis
Acute toxicity bioassays
A probit analysis was performed to obtain the 24-h LC50 values (Finney 1971) of each isolated pesticide. Then, the LC50 of the equitoxic mixture was calculated in order to assess the interaction between both pesticides, following Marking (1977):
where UTmix = total toxic units of the mixture (UTmix > 1 antagonism, UTmix = 1 additive, UTmix < 1 synergism); Gly = glyphosate; Cyp = cypermethrin; Mix = mixture.
Mesocosm experiment
A logarithmic transformation was applied to zooplankton abundance to fit normal distribution of data. To analyze differences in zooplankton (Cladocera, Copepoda, and Rotifera) abundance between treatments, a one-way repeated measure analysis of variance (RMANOVA) was performed with four levels: Ctrl, Gly, Cyp, and Mix. To assess the empirical interactions between pesticides, a one-way analysis of variance (ANOVA) was performed with two independent factors: glyphosate and cypermethrin; the factor levels for both were presence and absence.
If a significant interaction between pesticides was found, the type of interaction was determined by comparing the additive expected effect of the mixture (Exp, Formula 2) with the observed one (Obs, Formula 3). If the observed effect was lower than the expected effect of the mixture, the pesticides interacted antagonistically. If the observed and expected effects were equal, the pesticides did not interact and their combined effect was additive. If the observed effect was higher than the expected effect of the mixture, the pesticides interacted synergistically.
where Gly = abundance in glyphosate treatment; Ctrl = abundance in control treatment; Cyp = abundance in cypermethrin treatment; Mix = abundance in mixture treatment.
Results
Acute toxicity test
The 24-h LC50 obtained for each pesticide and their mixture are shown in Table 2 and the dose-response curves are shown in Fig. 1. The toxicity of both pesticide formulations in mixture was 3 and 4 times higher than their isolated toxicity, for glyphosate and cypermethrin, respectively. The UTmix was 0.53, which, being lower than 1, indicates a synergistic interaction between both pesticides.
Mesocosm experiment
The environmental variables remained constant during the experimental period: DO (7.17–7.81 mg L−1), conductivity (233–246 μS cm−1), and pH (6.8–8) and did not vary significantly among treatments (ANOVA, p = 0.668, 0.397, and 0.461 respectively).
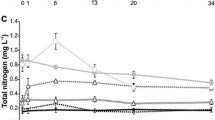
As regards nutrients, ammonium (0.008–0.5 mg L−1) and nitrates (0.32–1.36 mg L−1) did not vary significantly among treatments (ANOVA, p = 0.367 and 0.932, respectively), while nitrites and phosphates were below the detection limit (0.002 mg L−1 and 0.1 mg L−1, respectively).
The degradation rates for glyphosate were 0.096 and 0.076 mg L day−1 when it was alone or in mixture, respectively. The degradation rates for cypermethrin were 0.0005 and 0.0009 mg L day−1 when it was alone or in mixture, respectively.
At the beginning of the experiment, the zooplankton assemblage was composed by Rotifera (72%), Copepoda (22%), and Cladocera (6%). The most representative Rotifera taxa were Bdelloidea (57%) and Lecane hamata (11%); for Copepoda, Cyclopoida (45%) and Calanoida (41); and for Cladocera, Simocephalus vetulus (71%) and Coronatella monocantha (7%).
Cladocera abundance decreased marginally significantly in Cyp and Mix with respect to Ctrl and Gly, and no Cladocera were observed in Mix at day 4 and in Cyp and Mix, at day 7 (MRANOVA, p = 0.07, F = 3.3).
Copepoda abundance decreased significantly in Cyp and Mix with respect to Ctrl and Gly (MRANOVA, p < 0.001, F = 28.39).
Rotifera abundance did not vary significantly between treatments or through time (MRANOVA, p = 0.13, F = 2.55).
Both pesticides interacted significantly in Cladocera abundance at day 4 (ANOVA, p < 0.001, F = 66.76) (Fig. 2a). In this case, the additive expected value (Exp = −0.84 ind L−1Log) was lower than the observed value (Obs = −1.36 ind L−1Log) in absolute terms (Fig. 3a). This indicates that glyphosate and cypermethrin have a synergistic effect on Cladocera abundance. Although the individual effects of each pesticide were opposite (i.e., glyphosate increases Cladocera abundance and cypermethrin decreases it), a higher decrease was registered in the Mix (−1.36 ind L−1Log) than that observed in the Cyp (−1.19 ind L−1Log).
Mean expected (Exp: (Gly−Ctrl)+(Cyp−Ctrl)) and observed (Obs: Mix−Ctrl) effects values on a Cladocera, b Copepoda, and c Rotifera abundances (log ind L−1) and standard deviation. Zero indicates no difference between Ctrl and pesticide treatments, positive values indicate higher abundance in treatments than Ctrl, and negative values indicate lower abundance in treatments than Ctrl. *Significant interaction between pesticides
A significant interaction was observed between glyphosate and cypermethrin in the Copepoda abundance at day 7 (ANOVA, p < 0.001, F = 26.25) (Fig. 2b). In this case, the additive expected value (Exp = −3.19 ind L−1Log) was lower than the observed value (Obs = −3.84 ind L−1Log) in absolute terms (Fig. 3b). This indicates that glyphosate and cypermethrin have a synergistic effect on Copepoda abundance. As for Cladocera, although the individual effect of each pesticide was opposite (i.e., glyphosate increases Copepoda abundance and cypermethrin decreases it), the abundance decrease observed in Mix (−3.84 ind L−1Log) was higher than the decrease observed in Cyp (−3.62 ind L−1Log).
No interactions between pesticides were found for Rotifera abundances (ANOVA, p > 0.05); thus, the mixture effect was considered additive (Fig. 3c).
Discussion
Acute toxicity test
The LC50s of the individual pesticides for C. dubia are in congruence with that recorded in the available bibliography. In relation to glyphosate formulation (Atanor II), the 24-h LC50 obtained (8.37 mg L−1) was between the medium values registered for C. dubia: 6 and 5.7 mg L−1 (Roundup 24 and 48 h, Tsui and Chu 2004); 4.84 mg L−1 (Roundup Ultramax 48 h, Reno et al. 2018); and 14.49 mg L−1 (Eskoba 48 h, Reno et al. 2015). Nevertheless, some extreme values ranged between 0.02 mg L−1 (Roundup 48 h, Ripley et al. 2002) and 415 and 707 mg L−1 (Rondeo 24 and 48 h, Tsui and Chu 2004). This variation in glyphosate toxicity among formulations shows that its toxicity may depend more on the surfactant than in the active ingredient (Tsui and Chu 2003; Reno et al. 2018). With respect to the cypermethrin formulation used here (Xiper 25), the 24-h LC50 obtained (0.16 μg L−1) was similar to the values already found for C. dubia: 0.23 μg L−1 (Pestanal 48 h, Shen et al. 2012) and 0.89 μg L−1 (active ingredient 96 h, Liu et al. 2004). In this work, it is observed that C. dubia is much more sensitive to cypermethrin (insecticide) than glyphosate (herbicide). Accordingly, several studies have shown that aquatic arthropods are highly sensitive to pyrethroids, being the 48-h LC50 for most of them less than 1 μg L−1 (Lutnicka et al. 2014). Moreover, the LC50 of cypermethrin reported in the present study (0.16 μg L−1) was lower than the maximum concentration detected in several field studies (Jergentz et al. 2005; Marino and Ronco 2005; Akan et al. 2015; Cruzeiro et al. 2015; Etchegoyen et al. 2017). Regarding glyphosate, although the lethal concentrations founded for C. dubia (8.37 mg L−1) are higher than those usually detected in field studies (e.g., Thompson et al. 2004; Peruzzo et al. 2008; Battaglin et al. 2009, 2014; Coupe et al. 2012; Bonansea et al. 2017), the environmental concentrations are known to be highly variable depending on application moment and dose, rainfalls, and different ways of direct and diffuse contamination (Götz et al. 2010; Van Gestel et al. 2011; Stehle et al. 2013). Some authors have pointed that in a worst-case scenario, the organisms can be exposed to similar concentrations than those reported in the present study (Pérez et al. 2007; Vera et al. 2010). Moreover, it should be considered that at lower concentrations, sublethal effects could be observed (e.g., Cuhra et al. 2013; Garza-León et al. 2017; Reno et al. 2018).
The lethal toxicity of the glyphosate and cypermethrin mixture on C. dubia suggested a synergistic interaction between these pesticide formulations, since the UTmix (0.53) was lower than 1 (Marking 1977). Brodeur et al. (2014) found the same synergistic interaction effect when exposing tadpoles (Rhinella arenarum) to mixtures of two pairs of formulations of glyphosate and cypermethrin in lethal toxicity tests. Nevertheless, Brodeur et al. (2016) also found an antagonistic interaction effect when exposing a fish (Cnesterodon decemmaculatus) to mixtures of the same pairs of pesticide formulations. This indicates that glyphosate and cypermethrin interaction depends on the studied organism, being this information of great interest for regulatory agencies when deciding the nontarget organisms for toxicity assessments.
Several studies concluded that only 5% of the mixtures show a synergistic effect more than twofold greater than the hypothetic additive effect (Deneer 2000; Warne 2003; Belden et al. 2007). In this way, the synergistic effect found in the present study is of great interest since the toxicity of both pesticide formulations in mixture was 3 and 4 times higher than their isolated toxicity, for glyphosate and cypermethrin, respectively.
Mesocosm experiment
Microcrustaceans, especially cladocerans, are known to be particularly sensitive to insecticides (Hanazato 1998; Van den Brink et al. 2002; Sakamoto et al. 2006). Accordingly, in our study, Cladocera as well as Copepoda showed high sensitivity in treatments with cypermethrin (Cyp and Mix). In contrast, Rotifera abundance increased in pesticide treatments, in accordance with previous studies (Hanazato 1991; Wendt-Rasch et al. 2003; Chang et al. 2005). Rotifers could have benefited by the decrease in microcrustacean abundance, since they are less competitive for food resources (Hanazato 2001). Moreover, rotifers have shorter life cycles than microcrustaceans and a faster metabolic rate (Wallace et al. 2006; Smirnov 2017), which could imply a faster detoxification, all this contributing to a higher recovery rate when facing environmental disturbances. Besides, the greater variability of functional traits of rotifers compared to microcrustaceans makes them more successful and dominant on a wide range of environmental conditions (Vogt et al. 2013; Obertegger and Flaim 2015).
As in the acute toxicity test with C. dubia, a synergistic interaction was observed between glyphosate and cypermethrin formulations for Cladocera and Copepoda abundances in the mesocosm. Although in both cases individual pesticide effects were opposite, cypermethrin being negative and glyphosate positive, the combined effect was synergistically negative. In this sense, Crain et al. (2008) assumed that synergy occurs when the mixture effect is more negative than the additive sum of the opposing individual effects. Nevertheless, this assumption could overestimate synergy when the mixture effect is less negative than the individual negative effect (Piggott et al. 2015). In the present study, the negative effect of the mixture in Cladocera and Copepoda abundances was higher than the individual negative effect of cypermethrin formulation. This indicates that there was a negative synergistic interaction between glyphosate and cypermethrin formulations.
Surfactants, solvents, or emulsifiers of the formulation may also interfere on pesticide interactions. In this sense, Brodeur et al. (2014) found differences in the magnitude of synergism between two different pairs of pesticide formulations of glyphosate and cypermethrin. This information is of great environmental concern as commercial formulations are mixtures of chemical compounds, making it difficult to predict the magnitude of their interactions.
These results are of great interest as mesocosm experiments allow to establish cause-effect relationships between contaminants and biologic responses (Brock et al. 2014), and the analyses of mixture effects on a community assemblage constitute a more ecologically relevant approach. There are several field studies analyzing zooplankton community in relation to agricultural practices (e.g., Dodson et al. 2007; Albert et al. 2010; Frau et al. 2021). Nevertheless, this community responds to several environmental factors, e.g., nutrients and conductivity (Jafari et al. 2011; Gutierrez et al. 2020b), which makes it difficult to relate their responses with pesticide contamination on the field. Therefore, it is necessary to complement these studies with medium scale analysis as mesocosms.
Conclusion
In the present study, synergistic interactions between glyphosate and cypermethrin formulations were found in both the single-specie toxicity test (C. dubia) and the mesocosm experiment with a zooplankton assemblage. It is suggested to continue analyzing pesticide mixture effects, particularly encompassing complementary scales of analysis to reach more environmentally relevant ecotoxicological information.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahlstrom EH (1940) A revision of the rotatorian genera Brachionus and Platyias, with descriptions of one new species and two new varieties. Bull AMNH 77:143–184
Ahlstrom EH (1943) A revision of the rotatorian genus Keratella with descriptions of three new species and five new varieties. Bull Am Mus Nat Hist 80:411–457
Akan JC, Battah N, Waziri M, Mahmud MM (2015) Organochlorine, organophosphorus and pyrethroid pesticides residues in water and sediment samples from River Benue in Vinikilang, Yola, Adamawa State, Nigeria using gas chromatography-mass spectrometry equipped with electron capture detector Organochlori. Am J Environ Prot 3:164–173. https://doi.org/10.12691/env-3-5-2
Albert MR, Chen G, MacDonald GK, Vermaire JC, Bennett EM, Gregory-Eaves I (2010) Phosphorus and land-use changes are significant drivers of cladoceran community composition and diversity: an analysis over spatial and temporal scales. Can J Fish Aquat Sci 67:1262–1273. https://doi.org/10.1139/F10-058
Amorós I, Alonso JL, Romaguera S, Carrasco JM (2007) Assessment of toxicity of a glyphosate-based formulation using bacterial systems in lake water. Chemosphere 67:2221–2228. https://doi.org/10.1016/j.chemosphere.2006.12.020
Annett R, Habibi HR, Hontela A (2014) Impact of glyphosate and glyphosate-based herbicides on the freshwater environment. J Appl Toxicol 34:458–479. https://doi.org/10.1002/jat.2997
APHA (American Public Health Association) (1998) Standard methods for the examination of water and wastewater, 20th edn. American Water Works Association, Water Environment Federation, Washington, D.C
Arias M, Bonetto C, Mugni H (2020) Sublethal effects on Simocephalus vetulus (Cladocera: Daphnidae) of pulse exposures of cypermethrin. Ecotoxicol Environ Saf 196:110546. https://doi.org/10.1016/j.ecoenv.2020.110546
Barmentlo SH, Schrama M, Hunting ER, Heutink R, van Bodegom PM, de Snoo GR, Vijver MG (2018) Assessing combined impacts of agrochemicals: aquatic macroinvertebrate population responses in outdoor mesocosms. Sci Total Environ 631–632:341–347. https://doi.org/10.1016/j.scitotenv.2018.03.021
Battaglin WA, Meyer MT, Kuivila KM, Dietze JE (2014) Glyphosate and its degradation product AMPA occur frequently and widely in U.S. soils, surface water, groundwater, and precipitation. J Am Water Resour Assoc 50:275–290. https://doi.org/10.1111/jawr.12159
Battaglin WA, Rice KC, Focazio MJ, Salmons S, Barry RX (2009) The occurrence of glyphosate, atrazine, and other pesticides in vernal pools and adjacent streams in Washington, DC, Maryland, Iowa, and Wyoming, 2005-2006. Environ Monit Assess 155:281–307. https://doi.org/10.1007/s10661-008-0435-y
Baylis AD (2000) Why glyphosate is a global herbicide: strengths, weaknesses and prospects. Pest Manag Sci 56:299–308. https://doi.org/10.1002/(SICI)1526-4998(200004)56:4<299::AID-PS144>3.0.CO;2-K
Belden JB, Gilliom RJ, Lydy MJ (2007) How well can we predict the toxicity of pesticide mixtures to aquatic life? Integr Environ Assess Manag 3:364–372. https://doi.org/10.1897/1551-3793(2007)3[364:HWCWPT]2.0.CO;2
Bonansea RI, Amé MV, Wunderlin DA (2013) Determination of priority pesticides in water samples combining SPE and SPME coupled to GC-MS. A case study: Suquía River basin (Argentina). Chemosphere 90:1860–1869. https://doi.org/10.1016/j.chemosphere.2012.10.007
Bonansea RI, Filippi I, Wunderlin DA, Marino DJG, Amé MV (2017) The fate of glyphosate and AMPA in a freshwater endorheic basin: an ecotoxicological risk assessment. Toxics 6:1–13. https://doi.org/10.3390/toxics6010003
Bonny S (2008) Genetically modified glyphosate-tolerant soybean in the USA: adoption factors, impacts and prospects-a review. Sustain Agric 28:257–272. doi:https://doi.org/10.1007/978-90-481-2666-8_17, Genetically Modified Glyphosate-Tolerant Soybean in the USA: Adoption Factors, Impacts and Prospects - A Review
Borggaard OK, Gimsing AL (2008) Glyphosate: a once-in-a-century herbicide. Pest Manag Sci 63:1100–1106. doi:10.1002/ps
Brock TCM, Hammers-Wirtz M, Hommen U, Preuss TG, Ratte HT, Roessink I, Strauss T, van den Brink PJ (2014) The minimum detectable difference (MDD) and the interpretation of treatment-related effects of pesticides in experimental ecosystems. Environ Sci Pollut Res 22:1160–1174. https://doi.org/10.1007/s11356-014-3398-2
Brodeur JC, Malpel S, Anglesio AB, Cristos D, D’andrea MF, Poliserpi MB (2016) Toxicities of glyphosate- and cypermethrin-based pesticides are antagonic in the ten spotted livebearer fish (Cnesterodon decemmaculatus). Chemosphere 155:429–435. https://doi.org/10.1016/j.chemosphere.2016.04.075
Brodeur JC, Poliserpi MB, D’Andrea MF, Sánchez M (2014) Synergy between glyphosate- and cypermethrin-based pesticides during acute exposures in tadpoles of the common South American Toad Rhinella arenarum. Chemosphere 112:70–76. https://doi.org/10.1016/j.chemosphere.2014.02.065
Chang KH, Sakamoto M, Hanazato T (2005) Impact of pesticide application on zooplankton communities with different densities of invertebrate predators: an experimental analysis using small-scale mesocosms. Aquat Toxicol 72:373–382. https://doi.org/10.1016/j.aquatox.2005.02.005
Coupe RH, Kalkhoff SJ, Capel PD, Gregoire C (2012) Fate and transport of glyphosate and aminomethylphosphonic acid in surface waters of agricultural basins. Pest Manag Sci 68:16–30. https://doi.org/10.1002/ps.2212
Crain CM, Kroeker K, Halpern BS (2008) Interactive and cumulative effects of multiple human stressors in marine systems. Ecol Lett 11:1304–1315. https://doi.org/10.1111/j.1461-0248.2008.01253.x
Cruzeiro C, Pardal MÂ, Rocha E, Rocha MJ (2015) Occurrence and seasonal loads of pesticides in surface water and suspended particulate matter from a wetland of worldwide interest—the Ria Formosa Lagoon, Portugal. Environ Monit Assess 187. https://doi.org/10.1007/s10661-015-4824-8
Cuhra M, Traavik T, Bøhn T (2013) Clone- and age-dependent toxicity of a glyphosate commercial formulation and its active ingredient in Daphnia magna. Ecotoxicology 22:251–262. https://doi.org/10.1007/s10646-012-1021-1
DeLorenzo ME, Scott GI, Ross PE (2001) Toxicity of pesticides to aquatic microorganisms: a review. Environ Toxicol Chem 20:84–98. https://doi.org/10.1002/etc.5620200108
Deneer JW (2000) Toxicity of mixtures of pesticides in aquatic systems. Pest Manag Sci 56:516–520. https://doi.org/10.1002/(SICI)1526-4998(200006)56:6<516::AID-PS163>3.0.CO;2-0
Dodson SI, Everhart WR, Jandl AK, Krauskopf SJ (2007) Effect of watershed land use and lake age on zooplankton species richness. Hydrobiologia 579:393–399. https://doi.org/10.1007/s10750-006-0392-9
EPA (Environmental Protection Agency) (1993) Method 1660 The Determination of Pyrethrins and Pyrethroids in Municipal and Industrial Wastewater
Etchegoyen M, Ronco A, Almada P, Abelando M, Marino DJ (2017) Occurrence and fate of pesticides in the Argentine stretch of the Paraguay-Paraná basin. Environ Monit Assess 189:63. https://doi.org/10.1007/s10661-017-5773-1
FADA (Freshwater Animal Diversity Assessment) (2010) Crustacea-Copepoda Checklist.
Fantón N, Bacchetta C, Rossi A, Gutierrez MF (2020) Effects of a glyphosate-based herbicide on the development and biochemical biomarkers of the freshwater copepod Notodiaptomus carteri (Lowndes, 1934). Ecotoxicol Environ Saf 196:110501. https://doi.org/10.1016/j.ecoenv.2020.110501
Finney DJ (1971) Probit analysis. 333. London, U.K.: Cambridge University Press.
Folt CL, Chen CY, Moore MV, Burnaford J (1999) Synergism and antagonism among multiple stressors. Limnol Oceanogr 44:864–877. https://doi.org/10.4319/lo.1999.44.3_part_2.0864
Frau D, Gutierrez MF, Regaldo L, Saigo M, Licursi M (2021) Plankton community responses in Pampean lowland streams linked to intensive agricultural pollution. Ecol Indic 120:1–12. https://doi.org/10.1016/j.ecolind.2020.106934
Friberg-Jensen U, Wendt-Rasch L, Woin P, Christoffersen K (2003) Effects of the pyrethroid insecticide, cypermethrin, on a freshwater community studied under field conditions. I. Direct and indirect effects on abundance measures of organisms at different trophic levels. Aquat Toxicol 63:357–371. https://doi.org/10.1016/S0166-445X(02)00201-1
Garza-León CV, Arzate-Cárdenas MA, Rico-Martínez R (2017) Toxicity evaluation of cypermethrin, glyphosate, and malathion, on two indigenous zooplanktonic species. Environ Sci Pollut Res 24:18123–18134. https://doi.org/10.1007/s11356-017-9454-y
Götz CW, Stamm C, Fenner K, Singer H, Schärer M, Hollender J (2010) Targeting aquatic microcontaminants for monitoring: exposure categorization and application to the Swiss situation. Environ Sci Pollut Res 17:341–354. https://doi.org/10.1007/s11356-009-0167-8
Gutierrez MF, Molina FR, Frau D, Mayora G, Battauz Y (2020a) Interactive effects of fish predation and sublethal insecticide concentrations on freshwater zooplankton communities. Ecotoxicol Environ Saf 196:110497. https://doi.org/10.1016/j.ecoenv.2020.110497
Gutierrez MF, Simões NR, Frau D, Saigo M, Licursi M (2020b) Responses of stream zooplankton diversity metrics to eutrophication and temporal environmental variability in agricultural catchments. Environ Monit Assess 192:1–17. https://doi.org/10.1007/s10661-020-08766-5
Hanazato T (1991) Effects of repeated application of carbaryl on zooplankton communities in experimental ponds with or without the predator Chaoborus. Environ Pollut 74:309–324. https://doi.org/10.1016/0269-7491(91)90078-B
Hanazato T (1998) Response of a zooplankton community to insecticide application in experimental ponds: a review and the implications of the effects of chemicals on the structure and functioning of freshwater communities. Environ Pollut 101:361–373. https://doi.org/10.1016/S0269-7491(98)00053-0
Hanazato T (2001) Pesticide effects on freshwater zooplankton: an ecological perspective. Environ Pollut 112:1–10. https://doi.org/10.1016/S0269-7491(00)00110-X
Hasenbein S, Lawler SP, Geist J, Connon RE (2016) A long-term assessment of pesticide mixture effects on aquatic invertebrate communities. Environ Toxicol Chem 35:218–232. https://doi.org/10.1002/etc.3187
Jafari N, Nabavi SM, Akhavan M (2011) Ecological investigation of zooplankton abundance in the river Haraz, northeast Iran: impact of environmental variables. Arch Biol Sci 63:785–798. https://doi.org/10.2298/ABS1103785J
Jergentz S, Mugni H, Bonetto C, Schulz R (2005) Assessment of insecticide contamination in runoff and stream water of small agricultural streams in the main soybean area of Argentina. Chemosphere 61:817–826. https://doi.org/10.1016/j.chemosphere.2005.04.036
Koste W, Shiel RJ (1989) Rotifera from Australian inland waters. IV. Colurellidae (Rotifera: Monogononta). Transactions of the Royal Society of South Australia. 113(3):119–143 https://doi.org/10.15560/12.4.1942
Kotov AL, Forró NM, Korovchinsky, Petrusek A (2013) World Checklist of Freshwater Cladocera Species. Available online at http://fada.biodiversity.be/group/show/17
Kumar A, Sharma B, Pandey RS (2010) Toxicological assessment of pyrethroid insecticides with special reference to cypermethrin and λ-cyhalothrin in freshwater fishes. Int J Biol Med Res 3:315–325
Lanctôt C, Navarro-Martín L, Robertson C, Park B, Jackman P, Pauli BD, Trudeau VL (2014) Effects of glyphosate-based herbicides on survival, development, growth and sex ratios of wood frog (Lithobates sylvaticus) tadpoles. II: Agriculturally relevant exposures to Roundup WeatherMax® and Vision® under laboratory conditions. Aquat Toxicol 154:291–303. https://doi.org/10.1016/j.aquatox.2014.05.025
Liu W, Gan J, Werner I (2004) Isomer selectivity in aquatic toxicity and biodegradation of cypermethrin Environmental Toxicology View project Pelagic Organism Decline View project. Artic J Agric Food Chem. 52:6233–6238. https://doi.org/10.1021/jf0490910
Lutnicka H, Fochtman P, Bojarski B, Ludwikowska A, Formicki G (2014) The influence of low concentration of cypermethrin and deltamethrin on phyto- and zooplankton of surface waters. Folia Biol 62:251–257. https://doi.org/10.3409/fb62_3.251
Marino D, Ronco A (2005) Cypermethrin and chlorpyrifos concentration levels in surface water bodies of the Pampa Ondulada, Argentina. Bull Environ Contam Toxicol 75:820–826. https://doi.org/10.1007/s00128-005-0824-7
Marking L (1977) Method for assessing additive toxicity of chemical mixtures. In: Mayer FL, Hamelink JL (eds) Aquatic toxicology and hazard evaluation. American Society for Testing and Materials (ASTM), pp 99–108
Matson PA, Parton WJ, Power AG, Swift MJ (1997) Agricultural intensification and ecosystem properties. Science (80- ) 277:504–509. https://doi.org/10.1126/science.277.5325.504
Obertegger U, Flaim G (2015) Community assembly of rotifers based on morphological traits. Hydrobiologia 753:31–45. https://doi.org/10.1007/s10750-015-2191-7
OECD (2004) OECD Guideline for Testing of Chemicals - Daphnia sp., Acute Immobilization Test 202. Paris, France
Paggi JC (1995) Cladocera. Ecosystems of continental waters. Methodology for Their Study. eds. Lopretto, E., and G. Tell Ed. Sur, La Plata, Argentina, In, pp 909–951
Pérez GL, Torremorell A, Mugni H, Rodriguez P, Vera MS, Nascimento MD et al (2007) Effects of the herbicide roundup on freshwater microbial communities: a mesocosm study. Ecol Appl 17:2310–2322. https://doi.org/10.1890/07-0499.1
Pérez GL, Vera MS, Miranda LA (2011) Effects of herbicide glyphosate and glyphosate-based formulations on aquatic ecosystems. In: Kortenkamp A (ed) Herbicides and environment. In Tech, pp 343–368
Peruzzo PJ, Porta AA, Ronco AE (2008) Levels of glyphosate in surface waters, sediments and soils associated with direct sowing soybean cultivation in north pampasic region of Argentina. Environ Pollut 156:61–66. https://doi.org/10.1016/j.envpol.2008.01.015
Piggott JJ, Townsend CR, Matthaei CD (2015) Reconceptualizing synergism and antagonism among multiple stressors. Ecol Evol 5:1538–1547. https://doi.org/10.1002/ece3.1465
Primost JE, Marino DJG, Aparicio VC, Costa JL, Carriquiriborde P (2017) Glyphosate and AMPA, “pseudo-persistent” pollutants under real-world agricultural management practices in the Mesopotamic Pampas agroecosystem, Argentina. Environ Pollut 229:771–779. https://doi.org/10.1016/j.envpol.2017.06.006
Relyea R, Hoverman J (2006) Assessing the ecology in ecotoxicology: a review and synthesis in freshwater systems. Ecol Lett 9:1157–1171. https://doi.org/10.1111/j.1461-0248.2006.00966.x
Reno U, Doyle SR, Momo FR, Regaldo L, Gagneten AM (2018) Effects of glyphosate formulations on the population dynamics of two freshwater cladoceran species. Ecotoxicology 27:784–793. https://doi.org/10.1007/s10646-017-1891-3
Reno U, Gutierrez MF, Longo M, Vidal E, Regaldo L, Negro A, Mariani M, Zalazar C, Gagneten AM (2015) Microcrustaceans: biological models to evaluate a remediation process of glyphosate-based formulations. Water Air Soil Pollut 226:349. https://doi.org/10.1007/s11270-015-2616-y
Resh VH (2008) Which group is best? Attributes of different biological assemblages used in freshwater biomonitoring programs. Environ Monit Assess 138:131–138. https://doi.org/10.1007/s10661-007-9749-4
Richard J (1894) Cladocera collected by Dr. Theod. Barrois in Pelestine, in Syria and in Egypt. Biological review of the north of France, Lille 6:360–378 (In French)
Rico-Martinez R, Arias-Almeida JC, Pérez-Legaspi IA, Alvardo-Flores J, Retes-Pruneda JL (2012) Adverse effects of herbicides on freshwater zooplankton. Herbic - Prop Synth Control Weeds. https://doi.org/10.5772/33558
Ripley BJ, Davis KC, Carter BJ, Simovich MA (2002) Toxicity of Malathion and Roundup ® to the San Diego fairy shrimp. Transactions of the Western Section of the Wildlife Society 38:13–21
Sakamoto M, Chang KH, Hanazato T (2006) Inhibition of development of anti-predator morphology in the small cladoceran Bosmina by an insecticide: impact of an anthropogenic chemical on prey-predator interactions. Freshw Biol 51:1974–1983. https://doi.org/10.1111/j.1365-2427.2006.01628.x
Sasal MC, Demonte L, Cislaghi A, Gabioud EA, Oszust JD, Wilson MG, Michlig N, Beldoménico HR, Repetti MR (2015) Glyphosate loss by runoff and its relationship with phosphorus fertilization. J Agric Food Chem 63:4444–4448. https://doi.org/10.1021/jf505533r
Segers H (2002) The nomenclature of Rotifera: annotated checklist of valid family and genus-group names. Journal of Natural History. 36:631–640. https://doi.org/10.1080/00222930010024573
Segers H (2007) Annotated checklist of the rotifers (Phylum Rotifera) with notes on nomenclature, taxonomy and distribution. Zootaxa 1564:1–104 https://doi.org/10.11646/zootaxa.1564.1.1
Shen MF, Kumar A, Ding SY, Grocke S (2012) Comparative study on the toxicity of pyrethroids, α-cypermethrin and deltamethrin to Ceriodaphnia dubia. Ecotoxicol Environ Saf 78:9–13. https://doi.org/10.1016/j.ecoenv.2011.07.018
Smirnov NN (2017) Physiology of the Cladocera, 2nd edn Academic Press, 418.
Stehle S, Knäbel A, Schulz R (2013) Probabilistic risk assessment of insecticide concentrations in agricultural surface waters: a critical appraisal. Environ Monit Assess 185:6295–6310. https://doi.org/10.1007/s10661-012-3026-x
Thompson DG, Wojtaszek BF, Staznik B, Chartrand DT, Stephenson GR (2004) Chemical and biomonitoring to assess potential acute effects of Vision® herbicide on native amphibian larvae in forest wetlands. Environ Toxicol Chem 23:843–849. https://doi.org/10.1897/02-280
Tsui MTK, Chu LM (2003) Aquatic toxicity of glyphosate-based formulations: comparison between different organisms and the effects of environmental factors. Chemosphere 52:1189–1197. https://doi.org/10.1016/S0045-6535(03)00306-0
Tsui MTK, Chu LM (2004) Comparative toxicity of glyphosate-based herbicides: aqueous and sediment porewater exposures. https://doi.org/10.1007/s00244-003-2307-3
Valenzuela-Quintanar A, Armenta-Corral R, Moreno Villa E, Gutiérrez-Coronado L, Grajeda-Cota P, Orantes-Arenas C (2006) Optimización y validación de un método de dispersión de matriz en fase sólida para la extracción de plaguicidas organofosforados en hortalizas. Rev la Fac Agron La Univ del Zulia 23:464–474
Van den Brink PJ, Hartgers EM, Gylstra R, Bransen F, Brock TCM (2002) Effects of a mixture of two insecticides in freshwater microcosms: I. Fate of chlorpyrifos and lindane and responses of macroinvertebrates. Ecotoxicology 11:165–180. https://doi.org/10.1023/A:1015470731330
Van Gestel CA, Jonker M, Kammenga JE, Laskowski R, Svendsen C (2011) Mixture toxicity: linking approaches from ecological and human toxicology. CRC Press, Taylor and Francis Group, New York, USA, SETAC
Vera MS, Lagomarsino L, Sylvester M, Pérez GL, Rodríguez P, Mugni H, Sinistro R, Ferraro M, Bonetto C, Zagarese H, Pizarro H (2010) New evidences of Roundup® (glyphosate formulation) impact on the periphyton community and the water quality of freshwater ecosystems. Ecotoxicology 19:710–721. https://doi.org/10.1007/s10646-009-0446-7
Vogt RJ, Peres-Neto PR, Beisner BE (2013) Using functional traits to investigate the determinants of crustacean zooplankton community structure. Oikos 122:1700–1709. https://doi.org/10.1111/j.1600-0706.2013.00039.x
Wallace RL, Snell TW, Ricci C, Nogrady T (2006) Rotifera. Biology, ecology and systematics. In: Wallace RL, Snell TW, Ricci C, Nogrady T (eds) Guides to the identification of the microinvertebrates of the continental waters of the world, 2nd edn. SPB Academic Publishing, Amsterdam
Warne MSJ (2003) A review of the ecotoxicity of mixtures, approaches to, and recommendations for, their management. In: Langley A, Gilbey M, Kennedy B (eds) Proceedings of the Fifth National Workshop on the Assessment of Site Contamination. National Environment Protection Council Service Corporation, Adelaide, Australia
Wendt-Rasch L, Pirzadeh P, Woin P (2003) Effects of metsulfuron methyl and cypermethrin exposure on freshwater model ecosystems. Aquat Toxicol 63:243–256. https://doi.org/10.1016/S0166-445X(02)00183-2
Zhu Y, Zhang F, Tong C, Liu W (1999) Determination of glyphosate by ion chromatography. J Chromatogr A 850:297–301. https://doi.org/10.1016/S0021-9673(99)00558-0
Acknowledgements
We thank N. Romero for the assistance on the laboratory and S.V. Kergaravat for the assistance during the organism’s collection.
Funding
This research was supported by the National University of the Littoral (Research Project CAI+D 2016: PI 50120150100001LI) and the National Agency for Scientific and Technological Promotion (PICT-2016-1605).
Author information
Authors and Affiliations
Contributions
VSA contributed to the data curation, formal analysis, investigation, methodology, and writing of the original draft. MFG contributed to the investigation, data curation, funding acquisition, methodology, and writing—review and editing of the manuscript. UR contributed to the study design and writing—review and editing of the manuscript. AP and SG made the analytical determination of pesticides. AMG contributed to the conceptualization, funding acquisition, investigation, methodology, project administration, supervision, and writing—review and editing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Andrade, V.S., Gutierrez, M.F., Reno, U. et al. Synergy between glyphosate and cypermethrin formulations on zooplankton: evidences from a single-specie test and a community mesocosm experiment. Environ Sci Pollut Res 28, 26885–26894 (2021). https://doi.org/10.1007/s11356-021-12619-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12619-0