Abstract
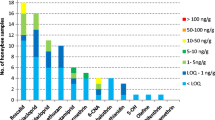
Pesticide residues in honey can negatively affect bee health. Although recent studies have detected neonicotinoid residues in honeys from around the world, little is known about how residues relate to land use and vegetation composition. To investigate potential relationships, we sampled multi-floral honey from 30 Apis mellifera hives from urban, agricultural and semi-natural habitats (SNH), identified and quantified three neonicotinoids present (clothianidin, imidacloprid and thiacloprid) using UHPLC-MS, and classified surrounding land use up to 5 km around hive sites. Neonicotinoids were most frequently detected in honeys from hives in agricultural habitats, and 70% of all samples contained at least one of the three neonicotinoid compounds. Imidacloprid was the most frequently detected neonicotinoid (found in 43% of honey samples) followed by clothianidin (40%) and thiacloprid (37%). Almost half (48%) of samples contained at least two neonicotinoids, and two of the 30 samples contained all three. Clothianidin and thiacloprid were more frequently detected in honeys from urban habitats, highlighting that exposure to pesticides does not just occur in agricultural settings. This suggests that pesticide use in urban domestic, sport and amenity contexts, given potential exposure of bees and other pollinators, needs urgent consideration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neonicotinoids are the most widely used group of insecticides in the world, and are neuroactive insecticides causing nervous stimulation at low concentrations, and receptor blockage, paralysis and death at higher concentrations (Goulson 2013). They act systemically, travelling through the plant tissue, protecting all plant parts, and providing effective pest control (Jeschke et al. 2010; Nauen et al. 2001). However, these pesticides have also been demonstrated to cause acute and chronic toxicity to bees. For example, non-lethal exposure of thiamethoxam has been found to decrease the foraging success of the Western honey bee (Apis mellifera) (Henry et al. 2012) and has been found to induce a variety of behavioural changes (Williamson and Wright 2013). The LD50 value (lethal dose 50%; the dose required to kill half the members of a tested population after a specified test duration) for honey bees varies depending on the neonicotinoid, and ranges from 3.7 to 5 ng/bee (Wood and Goulson 2017). Although thiacloprid is reported as being several orders of magnitude less toxic to honey bees (thiacloprid acute oral LD50 8.1–39 μg per bee) compared to the other neonicotinoids (imidacloprid and thiamethoxam acute oral LD50 = 0.004–0.005 μg per bee), the method of application of thiacloprid (spraying as opposed to seed dressing) may lead to higher residues in the honey bee’s food sources (Lundin et al. 2015). Concentrations as low as 0.003 mg/kg acetamiprid (Williamson et al. 2014), 0.08 mg/kg clothianidin (Brandt et al. 2016), 0.001 mg/kg imidacloprid (Brandt et al. 2016), 0.29 mg/kg thiacloprid and 0.118 mg/kg of food source thiamethoxam (Tosi and Nieh 2017) have been shown to have negative effects on honey bees when exposed orally. There is incomplete knowledge regarding the impacts of neonicotinoids on other organisms; nevertheless, there is sufficient toxicity data for a wide range of invertebrate taxa, both aquatic and terrestrial, to warrant caution (Pisa et al. 2015; Sohn et al. 2018; Basley and Goulson 2018; Gibbons et al. 2015).
Five neonicotinoids are currently approved as active substances in the EU for use in plant protection products (PPP), namely acetamiprid, clothianidin, imidacloprid, thiacloprid and thiamethoxam (The European Commission 2018). They can persist and accumulate in the environment (Goulson 2013) and have recently been found in organic soils and seeds (Guilleminot et al. 2019), demonstrating their presence even in soils to which they are not applied. Each neonicotinoid has specific properties concerning its environmental fate (persistence, biodegradability and accumulation) and specific toxicity levels. For example, the reported half-lives of neonicotinoids in soil applied as seed dressings range from 28 to 1250 days for imidacloprid (Fernández-Bayo et al. 2009; Goulson 2013; Lundin et al. 2015) and 148–6931 days for clothianidin (Goulson 2013; Lundin et al. 2015; Rexrode et al. 2003). Thiacloprid is not used in seed treatments but is common in spray application, and has a shorter half-life in soil (3–74 days) (Lundin et al. 2015; Goulson 2013; Rexrode et al. 2003) compared to clothianidin and imidacloprid.
Due to the wide application in both agriculture and horticulture, persistence in soil and water (Silva et al. 2019; Casado et al. 2019) and uptake by non-target plants (e.g. wild plants and succeeding crops) (Krupke et al. 2012; Botías et al. 2015), neonicotinoids are bioavailable to bees for most of the year (van der Sluijs et al. 2013; Hladik et al. 2018). This has resulted in the presence of neonicotinoids in honey. Residues of neonicotinoids have been found in honey samples from many countries around the world (Austria (Tanner and Czerwenka 2011), France (Paradis et al. 2014; Lambert et al. 2013), Poland (Bargańska et al. 2013) and the USA (Mullin et al. 2010)). A recent worldwide survey of honey from all continents has shown that these pesticides can be found around the world, indicating their global prevalence (Mitchell et al. 2017a). Different national regulations have established maximum levels of pesticide residues (maximum residue levels (MRLs)—levels determined to be of concern to human health) permitted in honey (Al-Waili et al. 2012). However, a lack of consistency in MRLs between countries causes problems in international marketing and trade (Al-Waili et al. 2012). While MRLs are mostly observed in food trade, their legal enforcement is rare (Wyckhuys et al. 2020). In Europe, current MRLs for clothianidin and imidacloprid are 0.05 mg/kg and the MRL for thiacloprid is 0.2 mg/kg. The pesticide levels found in honey samples vary considerably and are usually below the MRLs authorised for human consumption (Raghunandan and Basavarajappa 2013; Mitchell et al. 2017a), unlike the pesticide residues detected in plant-based foods (Wyckhuys et al. 2020). However, while the levels found in honey are usually below MRLs for human health, they can be higher than the concentrations which, as discussed above, have been found to adversely impact bee health (Mitchell et al. 2017a).
Since honey bees forage within certain distances of their colony, i.e. are central place foragers, and forage in a radius up to 10 km (with an average of 5.5 km around their hives) (Beekman and Ratnieks 2000), it is likely that pesticide residues in honey vary according to the prevailing land use in the area surrounding a hive site. For example, in a landscape that contains large proportions of pesticide-treated crops and arable weeds attractive to insects, bees may be more likely to collect contaminated nectar and pollen (Wood et al. 2019). Conversely, in a landscape dominated by grasslands or semi-natural habitats, where pesticide use is reduced, bees are less likely to be exposed to contaminated food. European landscapes are dominated by agriculture, two-thirds of this being arable crops; hence, the potential for exposure is high. The country with the highest proportion of land devoted to agriculture is Ireland, but here grasslands dominate (Copernicus 2018), and very few crops attractive to bees are grown. However, since the agricultural landscape of Ireland is highly dynamic, with parcels moving in and out of grassland and arable crops frequently (Zimmermann et al. 2016), and semi-natural areas exist mostly as fragments within agriculturally dominated landscapes, the potential for bee exposure to persistent chemicals, including neonicotinoids, increases (Zimmermann and Stout 2016).
Clothianidin, imidacloprid and thiamethoxam were identified by EFSA (European Food Safety Authority) as posing a risk to bees in 2013, and more recently, in 2018, the risks were reviewed and confirmed by EFSA (European Food Safety Authority) 2018). Consequently, the use of these three compounds has been restricted in the EU. However, these three active ingredients were, until 2013, approved for use on crops grown in enclosed systems, winter cereals and in grass management in Ireland and were frequently used. In addition, thiacloprid was also widely used (Table S1) on both soft fruit and vegetable crops in Ireland; however, only one report exists for PPP usage on soft fruit, published in 2014 (Department of Agriculture Food and the Marine 2014), and only one report exists for its use on vegetable crops, published in 2011 (Department of Agriculture Food and the Marine 2011). Despite the limited amount of information on pesticide inputs in Ireland, we hypothesise that since thiacloprid has been used on crops that are attractive to bees, we expect to find residues of this compound in our honey samples. Conversely, imidacloprid has only been reported for use on fodder turnip and fodder swede crops (crops that are not usually visited by bees) and we therefore expect residues in honey to be absent or very low. The aims of the present study were therefore to:
-
(i)
identify and quantify three neonicotinoids (clothianidin, imidacloprid and thiacloprid) in Irish honeys (thiamethoxam was not included because it is no longer approved for use in Ireland).
-
(ii)
determine if there is an association between their occurrence and the land use composition of the landscape surrounding the hive.
Methods
Honey sampling
Thirty Irish honey samples from 30 hive sites across the island of Ireland were collected directly from beekeepers in 2014 (Fig. 1). Once collected, all honey samples were stored in amber containers in the fridge set to 4 °C. The hive sites were grouped into one of three habitat categories (agriculture (n = 10), semi-natural habitats (SNH) (n = 10) and urban (n = 10)) based on the proportions of land cover surrounding each of the hives, according to the CORINE 2012 data series (Copernicus 2018; Environmental Protection Agency 2021). The percentage of each habitat surrounding the hive sites to a 5-km radius ranged from 29 to 97% for agriculture, 4–54% for SNH and 40–95% for urban across the 30 hive sites. Honeys were harvested either in June or July.
Neonicotinoid analysis
Neonicotinoid identification and quantification was determined by ultra-high performance liquid chromatography (UHPLC), coupled with mass spectrometry detection (hereafter referred to as LC-MS) following the method of Mitchell et al. (2017b). Further details are given below.
Reagents and standards
All salts, solvents and standards were analytical or LC-MS grade. LC-MS grade water, acetonitrile (ACN), formic acid, ammonium formate, all salts used for QuEChERS and each of the standards (clothianidin, imidacloprid and thiacloprid) were obtained from Sigma-Aldrich, Ireland. ISOLUTE primary secondary amine (PSA) bulk phase was purchased from Biotage, Sweden. Isotopically labelled standards (clothianidin-D3, imidacloprid-D4 and thiacloprid-D4) were obtained from QMX Laboratories, UK. Millipore Millex syringe filters with hydrophobic PTFE membrane (pore size 0.22 μm and 20 mm diameter) and low-adsorption LC-MS-certified vials and conical inserts were purchased from Sigma-Aldrich. XBridge UPLC BEH column 4.6 × 100 mm i.d., 3.5 μm particle size using XBridge BEH C18 VanGuard Cartridge (3.5 μL 3.9 × 5 mm) and VanGuard cartridge holder were all purchased from Waters Chromatography Ireland.
Sample preparation
Honey samples were prepared using a QuEChERS protocol described previously (Paradis et al. 2014; Mitchell et al. 2017b). Specifically, 2.5 g of honey was weighed in a 15-mL polypropylene tube (Sparks Lab Supplies Ltd., Ireland) to which 9 mL of H2O:ACN (50:50, v/v) and 20 μL of a 500 ng/mL internal standard (IS) methanolic solution containing the 3 labelled neonicotinoids were added. Honey was dissolved by manual agitation and ultrasonication for 10 min and the resulting solution was transferred into a 15-mL tube containing the extraction salts (2 g magnesium sulphate (MgSO4), 0.5 g sodium chloride, 0.5 g sodium citrate hydrate and 0.25 g sodium citrate sesquihydrate). One milliliter of H2O:ACN (50:50, v/v) was added to the first tube, and after a brief agitation period, the remaining solution was also transferred to the extraction salts tube. The mixture was vigorously shaken by hand for approximately 2 min and centrifuged at 4000g for 10 min. The upper phase (approx. 4.5 mL) was collected in a second 15-mL tube containing the purification salts (0.15 g MgSO4 and 0.1 g PSA). After vigorous shaking for 1 min, the tube was centrifuged at 4000g for 10 min and the supernatant (4.5 mL) recovered and dispensed into three 1.5-mL Eppendorf tubes (Lennox Laboratory Supplies, Ireland). The solution was evaporated until dry in a CentriVap centrifugal evaporator (Labconco, Thermo Scientific, Ireland) thermostated at 40 °C and the dried residue that remained was re-suspended in 0.25 mL of methanol (MeOH) 25%. The tubes were vortexed and filtered through 20-mm PTFE hydrophilic syringe filters (Sigma-Aldrich) into LC-MS vials containing 250 μL conical inserts. The final concentration of IS was 40 ng/mL.
UHPLC-MS/MS analysis
The analysis of neonicotinoids in honey was performed on a Dionex Ultimate 3000 RSLC (Thermo Scientific) coupled to a LTQ XL mass spectrometer (Thermo Scientific). Neonicotinoid separation was achieved on an XBridge UPLC BEH column 4.6 × 100 mm i.d., 3.5 um particle size, Waters) using a column oven at 25 °C and a flow rate of 0.3 mL/min. Mobile phase A consisted of 5 mM ammonium formate with 0.05% formic acid and mobile phase B comprised ACN with 0.05% formic acid. The following gradient programme was used: linear increase from 10 to 80% B in 15.0 min, 80–98% B in 0.1 min, holding at 98% B for 2 min and returning to initial conditions at 10% B in 2.0 min. The injection volume was 5 μL. The flow was deviated from the mass spectrometer from 0.0 to 5.0 min using a 6-port diverter Valco valve on the mass spectrometer.
For directed LC-MS/MS, an inclusion list on the LTQ XL (Xcalibur 2.1 software release 2.1, March 31, 2011) comprising of m/z, collision-induced dissociation (CID) fragmentation energy and retention time values for the three neonicotinoids and their associated labelled standard (retention times were determined from previous experiments using standards) (Table S2) were generated.
The following parameters were used for inclusion list-dependent acquisition on the LTQ XL mass spectrometer. MS detection was performed in positive electrospray mode using the following parameters: capillary voltage 5 kV, capillary temperature 275 °C, sheath and auxiliary gas flow (N2) ten and three (arbitrary units), tube lens voltage 55 V. The analysis was carried out in the ultra-zoom single ion monitoring (SIM) mode in positive mode profile. The intensity threshold for triggering of detected peaks was set to ten, isolation width (m/z) 1.6, activation time of 90 ms and collision energy was specified at 32% for all list members. The repeat count was set to six. Neonicotinoids were identified and quantified according to the corresponding spectral characteristics: mass, tandem mass spectrum, characteristic retention time.
Detection, quantification and validation
The identification of eluted components was achieved by comparing the retention time of reference standards with the honey samples. Neonicotinoids were detected based on (a) the characteristic fragmentation pattern and (b) calibration. For detection based on fragmentation, quantitation was carried out in scan mode by monitoring the response for a specific ion in an analyte’s mass spectrum. In many cases, this ion, termed the “quantifier” ion, is the most abundant in the spectrum. Other lesser abundant ions may also be monitored to aid in confident identification of the analyte. These are often termed “qualifier” ions. Neonicotinoids were quantified by internal calibration using calibration solutions prepared in MeOH 25% at 0.25, 0.5, 1, 10, 50 and 200 μg/L. The linearity range of the analytes, the limit of detection (LOD), the limit of quantification (LOQ) and the recoveries for three concentration levels were determined using a three-point calibration curve (0.25, 0.5, 1 μg/L). Above 1 μg/L, the response departed from linearity. A validation assay was performed using methanol blanks (n = 2) before each batch was analysed. Validation parameters are presented in Table S3. Three replicates of each honey sample were analysed. Thermo Xcalibur 2.2.0 (Thermo Scientific) was employed to process the data.
Landscape analysis
Landscape composition was quantified to a 5-km radius (based on upper end average foraging distances in Beekman and Ratnieks 2000) around each of the 30 hive sites using the CORINE (Co-ordinated Information of the Environment) 2012 land cover classification system (CLC). CLC is a common classification system of geo-spatial information that is available for all European Member States. It is based on satellite images and categorises land cover into 44 classes within three hierarchal levels (Central Statistics Office 2016). The minimum map unit is 25 ha (241). The 2012 data were selected for use because this dataset was closest to the honey harvest dates. Land cover was classified to levels 1, 2 and 3 of the hierarchal classes (1–3). The relationships between the detection and concentration of target analytes and the percentage of 11 land covers were assessed. These land covers include artificial surfaces (level 1), continuous urban fabric (assigned when urban structures and transport networks are dominating the surface area. > 80% of the land surface is covered by impermeable features like buildings, roads and artificially surfaced areas, level 3), artificial non-agricultural vegetated areas (areas voluntarily created for recreational use, includes green or recreational and leisure urban parks, sport and leisure facilities, level 2), agricultural areas (level 1), non-irrigated arable land (cultivated land parcels under rainfed agricultural use for annually harvested non-permanent crops, normally under a crop rotation system, including fallow lands within such crop rotation, level 3), pastures (lands that are permanently used (at least 5 years) for fodder production, includes natural or sown herbaceous species, unimproved or lightly improved meadows and grazed or mechanically harvested meadows, level 3), heterogeneous agricultural areas (areas of annual crops associated with permanent crops on the same parcel, annual crops cultivated under forest trees, areas of annual crops, meadows and/or permanent crops which are juxtaposed, landscapes in which crops and pastures are intimately mixed with natural vegetation or natural areas, level 2), land principally occupied by agriculture (areas principally occupied by agriculture, interspersed with significant natural or semi-natural areas (including forests, shrubs, wetlands, water bodies, mineral outcrops) in a mosaic pattern, level 3), forest and semi-natural areas (level 1), broad-leaved forest (vegetation formation composed principally of trees, including shrub and bush understorey, where broad-leaved species predominate. level 3), and wetlands (level 1) (Copernicus 2020).
Statistical analysis
Fishers exact tests were used to compare the proportions of honey samples containing clothianidin, imidacloprid and thiacloprid and the proportions where at least one, two or all three neonicotinoids were detected according to habitat type (agriculture, SNH and urban). Mann-Whitney U tests were used to compare differences in the area of each of the 11 land covers according to whether each of the three neonicotinoids was present or not. Eleven land covers were selected for analysis on the basis of their presence in at least 60% of the hive sites. Only imidacloprid was quantifiable, as the concentrations of clothianidin and thiacloprid fell below the limits of quantification (LOQ). The relationships between the concentration of imidacloprid and percentage of 11 land covers were assessed where possible (8 samples). Correlations between land cover and imidacloprid concentration were investigated using Spearman’s rank-order correlation because data were not normally distributed. All analyses were carried out in R-3.2.5.
Results
Separation of the three neonicotinoids can be seen in Fig. S1. The results presented here have been generated using (a) detection based on fragmentation and (b) detection and quantification based on calibration. Subsequently, the relationship between the presence and concentration of each of the three neonicotinoids with habitat type and separately, with land cover, was assessed.
Detection based on fragmentation
Analysis of blank samples revealed no traces of the pesticides studied. Three neonicotinoid peaks were structurally identified by comparing retention times, ion trap mass spectrometry data and fragment ions with those of the reference substances. The mass spectra and characteristic fragmentation pattern of each of the three neonicotinoids are illustrated in Figs. 2, 3, and 4 and documented in Table 1. The fragmentation pattern proposed here is similar to those reported previously (Xie et al. 2011; Mitchell et al. 2017b; Hou et al. 2019; Campillo et al. 2013).
Imidacloprid was the most frequently detected neonicotinoid (found in 13, 43%, of honey samples) followed by clothianidin (12, 40%) and thiacloprid (11, 37%). The results show that 70% of all analysed samples contained at least one neonicotinoid (clothianidin, imidacloprid and thiacloprid). At least two neonicotinoids were detected in 48% of the samples and two of the 30 samples contained all three neonicotinoids.
Detection and quantification based on calibration
Using detection based on calibration (i.e. LODs), the number of honey samples where imidacloprid was detected was equal to the number detected using identification based on fragmentation (i.e. 13). However, using the calibration method, clothianidin was only detected in four samples and thiacloprid was not detected in any honey sample (Table 2).
Overall, only 27% of honeys contained quantifiable amounts of neonicotinoids and imidacloprid was the only neonicotinoid that could be quantified (n = 8) (Table 2). The concentration of imidacloprid ranged from 0.007 to 0.018 mg/kg. Clothianidin and thiacloprid could not be quantified in honey samples as concentrations were < LOQ. Method validation parameters are shown in Table S3. At all levels, inter day precision was lower than 25%.
Relationship with landscape
At least one neonicotinoid was detected in 70% of honey samples, of which 14% came from SNH, 43% came from agricultural habitats and another 43% came from urban habitats (Fig. 5a, Table 3). Of the two honey samples that contained all three neonicotinoids, one of these came from the agricultural habitat category and one came from the urban habitat category (Fig. 5a, Table 3).
Honeys from urban habitats had the highest number of samples where clothianidin and thiacloprid were detected. The highest number of honey samples where imidacloprid was detected came from hives located within the agricultural habitat category (Fig. 5b, Table 3). Clothianidin and thiacloprid were least frequently detected in honey samples from SNH, while imidacloprid was detected in two samples from SNH and two from urban habitats.
Clothianidin was more frequently detected in honeys from urban habitats compared to SNH (p = 0.02) (Fig. 5a, Table 4). Although clothianidin was more frequently detected in honey samples from agricultural habitats compared to SNH, this difference was not statistically significant. Imidacloprid was more frequently detected in honeys from agriculture habitats compared to both SNH and urban habitats (p = 0.005, Fig. 5a, Table 4). There was no statistically significant association between the detection of thiacloprid and habitat category (Table 4); however, the number of detected samples was highest in urban habitats (Fig. 5a). The detection of at least one neonicotinoid was significantly higher in samples from both agricultural and urban habitats compared to SNH (p = 0.02, Fig. 5b). Finally, no statistically significant difference was observed between habitats when at least two neonicotinoids were detected (Table 4).
There were significant differences in the area of nine different land covers surrounding the hive according to the presence or absence of one of the neonicotinoids (Table 5). In samples where clothianidin was detected, there was significantly less coastal wetlands in the surrounding landscape (Table 5). Conversely, in samples where clothianidin was detected, there was a greater area of artificial surfaces and artificial non-agricultural vegetated areas surrounding the hives. For samples where thiacloprid was detected, there were significantly lower areas of heterogeneous agricultural areas and by land principally occupied by agriculture surrounding hives. Finally, for samples in which imidacloprid was detected, there were more agricultural areas and areas of pastures surrounding the hive, and lower areas of artificial surfaces and arable land surrounding the hive (Table 5).
Six of the eight honeys where imidacloprid was quantified came from the agricultural habitats category, one came from urban habitats and one came from SNH. The highest concentration of imidacloprid quantified came from the agricultural habitat category. A moderate negative relationship was observed between arable land and the concentration of imidacloprid in honey; however, this relationship was not statistically significant (Table S4). A moderate positive relationship was observed between forestry and the concentration of imidacloprid; however, again, this relationship was not statistically significant (Table S4).
Discussion
Clothianidin, imidacloprid and thiacloprid were detected in Irish honey samples, with 70% of samples containing at least one of these three compounds, and almost half (48%) containing residues of more than one compound. This is consistent with Mitchell et al. (2017a) who detected neonicotinoids in 79% of European honey samples, with 45% of honey samples having multiple neonicotinoid contamination. Although thiacloprid was used on a number of crops in Ireland and is available in a wide variety of products (for amateur and professional use), it was the least frequently detected neonicotinoid in our samples, possibly due to its shorter half-life in soil. The more persistent imidacloprid was the most frequently detected neonicotinoid in our study, but is the active ingredient of only six plant protection products (PPPs) approved for professional use in Ireland (Department of Agriculture Food and the Marine in Ireland 2018). The frequency of imidacloprid in honey samples may not be limited to agricultural PPP use, but may also arise due to its presence in a range of commercial products, including products for maintaining turf for sport and recreation, in plant nurseries, in domestic gardens and by local authorities in public parks. For example, a recent study reported that 70% of ornamental plants sold in the UK as “bee friendly” contained neonicotinoids (Lentola et al. 2017), and as not all garden plants sold in Ireland are home grown, exposure may be underestimated if only agricultural PPP use is considered (Central Statistics Office 2017). Additionally, neonicotinoid exposure can arise not only as a result of the number of products on the market; volume of sales must also be considered. Clothianidin was the active ingredient of only one PPP approved for professional use in Ireland; however, four times more of this product was applied between 2011 and 2014 than imidacloprid and thiacloprid (Table S1). Given the widespread availability of neonicotinoids in many PPPs, and their persistence in soils and plant material, it is not surprising that honey that originates from the nectar of plants foraged by bees contains these pesticides.
The concentration at which neonicotinoids were found in Irish honey was only quantifiable for imidacloprid. Although low, the average concentration of imidacloprid found here was higher than concentrations which have been shown to induce negative effects on honey bees. For example, Brandt et al. (2016) showed that oral exposure of clothianidin, imidacloprid and thiacloprid affect the immunocompetence of honey bees, reducing haemocyte density, encapsulation response and the antimicrobial activity of honey bees. Imidacloprid has also been shown to cause negative effects on the behaviour of bumble bees (Moffat et al. 2016), and one honey sample contained concentrations of imidacloprid exceeding the dosage concentration shown to have negative effects. Although 70% of samples tested positive for at least one neonicotinoid, concentrations were below the admissible limits for human consumption according to current EU regulations (i.e. MRLs). Mitchell et al. (2017) found that thiacloprid dominated the European honey samples in terms of concentration. The concentration of thiacloprid found here was lower than the LOQ and thus cannot be compared with the European honey; however, the concentration in both studies is lower than the European MRL.
This is the first study to show that honey from urban areas can be more contaminated than honey from rural areas for some chemical residues. We found a difference in neonicotinoid residues depending on the landscape in which honey bees were foraging. For logistical reasons, our urban samples were all from the east of Ireland, which is more densely populated, while our SNH samples were mostly from the west, where agriculture tends to be less intensive (Sullivan et al. 2017). Thus, landscape context is potentially confounded with geographical location. Nevertheless, previous studies have also reported differences: Lambert et al. (2013) found that chemical residues in beehive matrices (bees, beebread and pollen) from apiaries in rural-cultivated landscapes were more contaminated with chemical residues than urban landscapes and islands, but the differences were not significant. Subsequently, David et al. (2016) reported, based on analysis of pollen in nests, that pesticide exposure of bumble bee colonies in rural areas was higher than in urban areas. Our findings suggest that there was a difference in the chemical residues detected in honeys from different geographical locations, but that the difference depended on the chemical residue being analysed. Imidacloprid was more likely to be detected in honeys from agricultural habitats including pastures, but not arable land. Clothianidin was more likely to be detected in honeys originating from urban areas, or landscapes containing a high proportion of artificial surfaces, as well as artificial non-agricultural vegetated areas. Even though clothianidin is used in high quantities in Ireland, most of its use is on arable crops (winter wheat, winter barley, spring oats and winter oats). It is therefore unexpected that it was detected in honeys originating from both agricultural and urban areas. The highest number of samples where thiacloprid was detected came from urban habitats, and with more heterogeneous agricultural areas and land principally occupied by agriculture surrounding the hive, it was less likely that thiacloprid would be detected in the honey. In 2013, thiacloprid was the active ingredient of five PPPs approved for amateur use (Department of Agriculture Food and the Marine in Ireland 2013) and sold in most garden centres. Thus, the frequency of detection of this compound in honey samples from urban land covers may be due to the broad range of products available on the market.
Insecticides are just one group of PPPs that can be detected in honey. Glyphosate is the active ingredient in over 100 products approved for use in Ireland (Department of Agriculture Food and the Marine in Ireland 2018) and has been detected in honey from all over the world, including Switzerland (Zoller et al. 2018), Hawaii (Berg et al. 2018) and other US states (Rubio et al. 2014). It is not known if this chemical compound is present in Irish honey, nor whether it varies according to the landscapes in which hives are located. Given the frequency of application and quantity of PPPs used in Ireland and their detection in honey from multiple countries around the world, it is highly likely that there are other contaminants in Irish honey; and this deserves further investigation.
Conclusion
This research for the first time has identified the presence of clothianidin, imidacloprid and thiacloprid in Irish honey from a range of hive sites across a range of land use types. The concentrations detected were below the maximum residue level authorised for human consumption and are not thought to risk human health. However, the presence of these neonicotinoids in honey may have negative effects on honey bees, and their presence in nectar may have negative effects on other nectar-feeding insects. To our knowledge, this is the first study to show potential exposure of honey bees to pesticides in urban areas and the first study to find differences in the occurrence of neonicotinoids in honey from urban and rural areas. The authors recommend that these results should be confirmed by further studies due to the size of the sample dataset (n = 30) and the inability to quantify the residues of two of the three targeted analytes. The higher proportion and concentration of neonicotinoids in honeys from both agricultural and urban habitats, compared with semi-natural or other land covers, suggests that highly modified environments are more harmful for bees in terms of exposure to neonicotinoids. Given global trends of agricultural intensification and urbanisation, these findings are of concern. Although previous results show that urban areas have a positive relationship with honey quality in terms of phenolic content (Kavanagh et al. 2019), this may be offset by pesticide exposure. Given that these compounds have been shown to have adverse effects on honey bees, wild bees and other organisms, their detection in honey is of concern, and potential contamination routes should be explored further.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Al-Waili N et al (2012) Antibiotic, pesticide, and microbial contaminants of honey: human health hazards. Sci World J 2012:930849
Bargańska Ż, Ślebioda M, Namieśnik J (2013) Pesticide residues levels in honey from apiaries located of Northern Poland. Food Control 31:196–201
Basley K, Goulson D (2018) Neonicotinoids thiamethoxam and clothianidin adversely affect the colonisation of invertebrate populations in aquatic microcosms. Environ Sci Pollut Res 25:9593–9599
Beekman M, Ratnieks FLW (2000) Long-range foraging by the honey-bee, Apis mellifera L. Funct Ecol 14:490–496
Berg CJ et al (2018) Glyphosate residue concentrations in honey attributed through geospatial analysis to proximity of large-scale agriculture and transfer off-site by bees. PLoS One 13:e0198876
Botías C, David A, Horwood J, Abdul-Sada A, Nicholls E, Hill E, Goulson D (2015) Neonicotinoid residues in wildflowers, a potential route of chronic exposure for bees. Environ Sci Technol 49:12731–12740
Brandt A, Gorenflo A, Siede R, Meixner M, Büchler R (2016) The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.). J Insect Physiol 86:40–47
Campillo N, Viñas P, Férez-Melgarejo G, Hernández-Córdoba M (2013) Liquid chromatography with diode array detection and tandem mass spectrometry for the determination of neonicotinoid insecticides in honey samples using dispersive liquid-liquid microextraction. J Agric Food Chem 61:4799–4805
Casado J, Brigden K, Santillo D, Johnston P (2019) Screening of pesticides and veterinary drugs in small streams in the European Union by liquid chromatography high resolution mass spectrometry. Sci Total Environ 670:1204–1225
Central Statistics Office, (2017). Trade Statistics February 2018, Available at: https://www.cso.ie/en/media/csoie/releasespublications/documents/externaltrade/2017/trade_feb2017.pdf. Accessed 1 Sep 2018.
Central Statistics Office, (2016). Environmental indicators Ireland - land use [Internet]. 2016. Available at: https://www.cso.ie/en/releasesandpublications/ep/peii/eii2016/lu/ [Accessed December 21, 2020].
Copernicus, (2018). CLC 2018. Available at: https://land.copernicus.eu/pan-european/corine-land-cover/clc2018 [Accessed April 26, 2020].
Copernicus, (2020). Corine land cover nomenclature guidelines. Available at: https://land.copernicus.eu/user-corner/technical-library/corine-land-cover-nomenclature-guidelines/html/index-clc-111.html [Accessed December 21 2020].
David A, Botías C, Abdul-Sada A, Nicholls E, Rotheray EL, Hill EM et al (2016) Widespread contamination of wildflower and bee-collected pollen with complex mixtures of neonicotinoids and fungicides commonly applied to crops. Environ Int 88:169–78
Department of Agriculture Food and the Marine, (2014). Pesticide usage in Ireland soft fruit crops survey report, Available at: http://www.pcs.agriculture.gov.ie/media/pesticides/content/sud/pesticidestatistics/PesticideUsage2014SoftFruitCropsSurveyReport100317.pdf. Accessed 1 Sep 2018.
Department of Agriculture Food and the Marine, (2011). Pesticide usage in Ireland vegetable crops survey report. Available at: http://www.pcs.agriculture.gov.ie/media/pesticides/content/sud/pesticidestatistics/PesticdeUsage-2011VegetableSurveyReport.pdf. Accessed 1 Sep 2018.
Department of Agriculture Food and the Marine in Ireland (2013). Plant protection products book 2013, Available at: http://www.pcs.agriculture.gov.ie/media/pesticides/content/products/book2013/Chapter01-ProductRegister.pdf. Accessed 1 Sep 2018.
Department of Agriculture Food and the Marine in Ireland (2018). Plant protection products book 2018. Available at: http://www.pcs.agriculture.gov.ie/media/pesticides/content/plantprotectionproducts/pppbook2018/PlantProtectionProducts2018FullBook230218.pdf. Accessed 1 Sep 2018.
EFSA (European Food Safety Authority) (2018) Conclusion on the peer review of the pesticide risk assessment for bees for the active substance clothianidin considering the uses as seed treatments and granules. EFSA J 16:5177
Environmental Protection Agency (2021) Common Data - CORINE Landcover 2012. Available at: https://gis.epa.ie/GetData/Download. Accessed 1 Jun 2015
Fernández-Bayo JD, Nogales R, Romero E (2009) Effect of vermicomposts from wastes of the wine and alcohol industries in the persistence and distribution of imidacloprid and diuron on agricultural soils. J Agric Food Chem 57:5435–5442
Gibbons D, Morrissey C, Mineau P (2015) A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environ Sci Pollut Res 22:103–118
Goulson D (2013) An overview of the environmental risks posed by neonicotinoid insecticides. J Appl Ecol 50:977–987
Guilleminot SH et al (2019) A national wide survey of neonicotinoid insecticides in agricultural land with implications for agricultural -and with implications for agri-environment schemes. J Appl Ecol 56:1502–1514
Henry M, Beguin M, Requier F, Rollin O, Odoux JF, Aupinel P, Aptel J, Tchamitchian S, Decourtye A (2012) A common pesticide decreases foraging success and survival in honey bees. Science 336:348–350
Hladik ML, Corsi SR, Kolpin DW, Baldwin AK, Blackwell BR, Cavallin JE (2018) Year-round presence of neonicotinoid insecticides in tributaries to the Great Lakes, USA. Environ Pollut 235:1022–1029
Hou J, Xie W, Hong D, Zhang W, Li F, Qian Y, Han C (2019) Simultaneous determination of ten neonicotinoid insecticides and two metabolites in honey and Royal-jelly by solid−phase extraction and liquid chromatography−tandem mass spectrometry. Food Chem 270:204–213
Jeschke P et al (2010) Overview of the status and global strategy for neonicotinoids. J Agric Food Chem 59:2897–2908
Kavanagh S, Gunnoo J, Marques Passos T, Stout JC, White B (2019) Physicochemical properties and phenolic content of honey from different floral origins and from rural versus urban landscapes. Food Chem 272:66–75
Krupke CH et al (2012) Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS One 7:e29268
Lambert O et al (2013) Widespread occurrence of chemical residues in beehive matrices from apiaries located in different landscapes of Western France. PLoS One 8:e67007
Lentola A, David A, Abdul-Sada A, Tapparo A, Goulson D, Hill EM (2017) Ornamental plants on sale to the public are a significant source of pesticide residues with implications for the health of pollinating. Environ Pollut 228:297–304
Lundin O et al (2015) Neonicotinoid insecticides and their impacts on bees: a systematic review of research approaches and identification of knowledge gaps. PLoS One 10:e0136928
Mitchell EAD, Mulhauser B, Mulot M, Mutabazi A, Glauser G, Aebi A (2017a) A worldwide survey of neonicotinoids in honey. Science 358:109–111
Mitchell EAD, Mulhauser B, Mulot M, Mutabazi A, Glauser G, Aebi A (2017b) Supplementary materials for a worldwide survey of neonicotinoids in honey. Science 358:109–111
Moffat C, Buckland ST, Samson AJ, McArthur R, Chamosa Pino V, Bollan KA, Huang JTJ, Connolly CN (2016) Neonicotinoids target distinct nicotinic acetylcholine receptors and neurons, leading to differential risks to bumblebees. Sci Rep 6:24764
Mullin CA et al (2010) High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS One 5:e9754
Nauen R, Ebbinghaus-Kintscher U, Elbert A, Jeschke P, Tietjen K (2001) Acetylcholine receptors as sites for developing neonicotinoid insecticides. In: Ishaaya I (ed) Biochemical sites of insecticide action and resistance. Springer, New York, pp 77–105
Paradis D, Bérail G, Bonmatin JM, Belzunces LP (2014) Sensitive analytical methods for 22 relevant insecticides of 3 chemical families in honey by GC-MS/MS and LC-MS/MS. Anal Bioanal Chem 406:621–633
Pisa LW, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Downs CA, Goulson D, Kreutzweiser DP, Krupke C, Liess M, McField M, Morrissey CA, Noome DA, Settele J, Simon-Delso N, Stark JD, van der Sluijs JP, van Dyck H, Wiemers M (2015) Effects of neonicotinoids and fipronil on non-target invertebrates. Environ Sci Pollut Res 22:68–102
Raghunandan KS, Basavarajappa S (2013) Analysis of multifloral honey of the giant honeybee, Apis Dorsata F., for pesticide residues in Southern Karnataka, India. Eur J Zool Res 2:22–28
Rexrode, M. et al., 2003. EFED risk assessment for the seed treatment of clothianidin 600FS on corn and canola, Washington.
Rubio F, Guo E, Kamp L (2014) Environmental & analytical toxicology survey of glyphosate residues in honey, corn and soy products. J Environ Anal Toxicol 5:249
Silva V, Mol HGJ, Zomer P, Tienstra M, Ritsema CJ, Geissen V (2019) Pesticide residues in European agricultural soils – a hidden reality unfolded. Sci Total Environ 653:1532–1545
van der Sluijs JP, Simon-Delso N, Goulson D, Maxim L, Bonmatin JM, Belzunces LP (2013) Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr Opin Environ Sustain 5:293–305
Sohn L, Brodie RJ, Couldwell G, Demmons E, Sturve J (2018) Exposure to a nicotinoid pesticide reduces defensive behaviors in a non-target organism, the rusty crayfish Orconectes rusticus. Ecotoxicology 27:900–907
Sullivan CA, Finn JA, Ó hÚallacháin D, Green S, Matin S, Meredith D, Clifford B, Moran J (2017) The development of a national typology for high nature value farmland in Ireland based on farm-scale characteristics. Land Use Policy 67:401–414
Tanner G, Czerwenka C (2011) LC-MS/MS analysis of neonicotinoid insecticides in honey: methodology and residue findings in Austrian honeys. J Agric Food Chem 59:12271–12277
The European Commission, (2018). Neonicotinoids. Available at: https://ec.europa.eu/food/plant/pesticides/approval_active_substances/approval_renewal/neonicotinoids_en [Accessed September 3, 2018].
Tosi S, Nieh JC (2017) A common neonicotinoid pesticide, thiamethoxam, alters honey bee activity, motor functions, and movement to light. Sci Rep 7:15132
Williamson SM, Willis SJ, Wright GA (2014) Exposure to neonicotinoids influences the motor function of adult worker honeybees. Ecotoxicology 23:1409–1418
Williamson SM, Wright GA (2013) Exposure to multiple cholinergic pesticides impairs olfactory learning and memory in honeybees. J Exp Biol 216:1799–1807
Wood TJ, Kaplan I, Zhang Y, Szendrei Z (2019) Honeybee dietary neonicotinoid exposure is associated with pollen collection from agricultural weeds. Proc R Soc B 286:20190989
Wood TJ, Goulson D (2017) The environmental risks of neonicotinoid pesticides: a review of the evidence post 2013. Environ Sci Pollut Res 24:17285–17325
Wyckhuys KAG, Aebi A, Bijleveld van Lexmond MFIJ, Bojaca CR, Bonmatin JM, Furlan L, Guerrero JA, Mai TV, Pham HV, Sanchez-Bayo F, Ikenaka Y (2020) Resolving the twin human and environmental health hazards of a plant-based diet. Environ Int 144:106081
Xie W, Han C, Qian Y, Ding H, Chen X, Xi J (2011) Determination of neonicotinoid pesticides residues in agricultural samples by solid-phase extraction combined with liquid chromatography–tandem mass spectrometry. J Chromatogr A 1218:4426–4433
Zimmermann J, Stout JC (2016) Underestimating neonicotinoid exposure: how extent and magnitude may be affected by land-use change. Sci Pollut Res 23:7050–7054
Zimmermann J, González A, Jones MB, O’Brien P, Stout JC, Green S (2016) Assessing land-use history for reporting on cropland dynamics - a comparison between the Land-Parcel Identification System and traditional inter-annual approaches. Land Use Policy 52:30–40
Zoller O, Rhyn P, Rupp H, Zarn JA, Geiser C (2018) Glyphosate residues in Swiss market foods: monitoring and risk evaluation. Food Addit Contaminants: Part B 11:83–91
Acknowledgements
The beekeepers of Ireland and Paula Meleady (NICB) are gratefully acknowledged.
Funding
Financial support was provided by the Irish Research Council, Ireland (Postgraduate Scholarship, S. Kavanagh) and DCU Career Start Fellowship (B. White).
Author information
Authors and Affiliations
Contributions
SK carried out all separations and sample analysis. SK also carried out the statistical analysis. MH contributed to experimental analysis and interpretation of the results. SK, BW, JCS and MH contributed to the text of the article. SK, BW, JCS and MH read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 204 kb)
Rights and permissions
About this article
Cite this article
Kavanagh, S., Henry, M., Stout, J.C. et al. Neonicotinoid residues in honey from urban and rural environments. Environ Sci Pollut Res 28, 28179–28190 (2021). https://doi.org/10.1007/s11356-021-12564-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12564-y