Abstract
The acceptance of combined pre-composting and vermicomposting systems is increasing because of the advantage in rapidly stabilizing organic wastes and reducing emission of greenhouse gasses (GHG). However, GHG emission during the pre-composting phase is often neglected when evaluating the system. This study aimed to quantify GHG emission from a combined pre-composting and vermicomposting system and to investigate the effects of earthworms on GHG emission. A combined system using Eisenia fetida was employed to stabilize maize stover and cow dung (mixing ratio 60:40). The inoculating densities were 60 (T1), 120 (T2), and 180 (T3) earthworms per kilogram of substrate. A traditional composting system without earthworms was set as a control (T0). The results indicated that earthworms increased CO2 while decreased CH4 and N2O emissions compared to the control. Higher emission of CO2 suggested that the earthworms promoted the degradation of the substrates. Lower emission of CH4 and N2O showed the advantage of the combined system because CH4 and N2O possess extremely higher global warming potential than that of CO2. T2 is recommended for stabilizing maize stover and cow dung when making a tradeoff between stabilization rate and reduction of GHG. The percentages of GHG emission during pre-composting relative to total GHG emission in T1, T2, and T3 were 34%, 35%, and 30%, respectively. GHG emission is non-negligible when using a combined system, especially the emission of GHG during the pre-composting phase cannot be ignored.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Composting is an efficient approach for stabilizing agricultural organic wastes. The obtained compost can be used as a natural fertilizer for improving soil structure (Zhu-Barker et al. 2017). Thermophilic composting and vermicomposting are widely used for bioconversion of agricultural wastes into natural fertilizers. Thermophilic composting or traditional composting is a high-temperature process (> 45 °C) (Nigussie et al. 2017), and complex microbes get involved in it. The emission of greenhouse gasses (GHG) such as carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O) during composting is a major concern when it comes to the utilization of this biological method to stabilize organic wastes (Christensen et al. 2009). The production of CO2 during the composting process is due to the degradation of organic carbon materials, and it is a major component of GHG. CH4 is generated from anoxic or anaerobic microsites (Chan et al. 2011; Zhu-Barker et al. 2017). Incomplete ammonia oxidation and incomplete denitrification are responsible for the emission of N2O (Zhu-Barker et al. 2017). At the same time, large amounts of nitrogen are lost during traditional composting because high temperature increases ammonia volatilization (Pagans et al. 2006). Compared to traditional composting, vermicomposting is a mesophilic process (< 30 °C). The decomposition and stabilization of the organic wastes are the combined action of earthworms and associated microorganisms in the process of vermicomposting (Nigussie et al. 2017; Mupambwa and Mnkeni 2018). In comparison with traditional composting, vermicomposting is more acceptable for the reduced greenhouse gas emissions and faster stabilization rates. In particular, vermicomposting emits less CH4 under aerobic conditions created by the burrowing action of the earthworms (Girotto and Cossu 2019). However, the mesophilic condition during vermicomposting cannot kill all pathogens. Weeds inside the wastes cannot be eradicated totally. Thereby, a combined system of thermophilic composting and vermicomposting has been proposed to produce high agronomic value and pathogen-free end products (Nigussie et al. 2017). In fact, nearly all the current vermicomposting systems are carried out as combined systems. In such combined systems, the thermophilic composting, also called pre-composting, plays a role of pre-treatment and is generally introduced prior to the mesophilic process (Lim et al. 2016). This pre-composting of organic wastes involving thermal stabilization usually lasts for 2–3 weeks (Lim et al. 2016) and fulfills the following functions: firstly, efficient pre-composting avoids exposure of the earthworms to high temperature (Chattopadhyay 2012); secondly, the pathogens borne by the organic waste and weed seeds in the substrates can be eradicated after this pre-treatment phase; thirdly, volatile gasses which are toxic to earthworms can be released by the pre-treatment; lastly, pre-treatment enhances initial microbial degradation and softening of the wastes (Lim et al. 2016; Nigussie et al. 2017). In most researches pertinent to vermicomposting processes, pre-composting is a common practice to render the organic waste suitable for the following vermicomposting step (Lim et al. 2016). Currently, a short duration of thermophilic composting is frequently used to pre-treat the waste before vermicomposting at the later stage. Although pre-composting is a common practice in combined systems, the emission of GHG during the pre-composting phase is often neglected when evaluating the advantage of a combined pre-composting and vermicomposting system in reducing GHG.
There is a large body of literature concerning the emission of GHG during vermicomposting, and the reports are contradictory. Some of the studies showed that vermicomposting systems increased the emission of GHG (Nigussie et al. 2017), whereas others indicated that the emission of GHG was decreased by the vermicomposting systems (Chan et al. 2011; Wang et al. 2014; Nigussie et al. 2016). Moreover, most of previous studies about the emission of GHG from vermicomposting systems tended to focus on the emission from the mesophilic stage, and the emission from the thermophilic stage (pre-treatment stage) is often neglected (Nigussie et al. 2017). However, Hobson et al. (2005) reported that pre-treatment removed considerable amount of carbon and nitrogen prior to vermicomposting, which caused significantly reduced emission of CH4 and N2O during the second phase (vermicomposting phase) compared to the emission from other studies. According to Hobson et al. (2005), considerable amount of GHG was emitted during pre-treatment, but limited information is available about the amount of GHG emission in the thermophilic pre-composting phase when using combined pre-composting and vermicomposting systems to stabilize organic wastes. The emission of GHG during the pre-composting phase should be included when evaluating emission of GHG from a combined system. The aims of this study were (1) to quantify the emission of GHG during pre-composting and vermicomposting phases in a combined system and (2) to investigate the effect of earthworm Eisenia fetida on the emission of GHG during stabilizing maize stover and cow dung.
Materials and methods
Materials
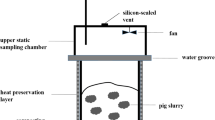
Maize stover and cow dung were obtained from a farm near Changchun, Jilin Province, China. The variety of the maize is Nianjiu, which is widely cultivated in Northeast China. Total organic carbon contents of the maize stover and cow dung were 479.32 and 432.35 g kg−1 dry matter (DM), respectively. Total nitrogen contents of the maize stover and cow dung were 5.75 and 8.06 g kg−1 DM, respectively. Maize stover and cow dung were naturally air-dried. Maize stover was cut into the length less than 5 mm, and dry cow dung was broken into small lumps for the experiment. The earthworm species is E. fetida, which was purchased from a local market. Adult earthworms were used in this vermicomposting system. The average weight of the earthworm is around 0.30 g. The earthworms were cultured in a mixture of maize stover and cow dung for 20 days in the laboratory. The container used in the experiment was made of polypropylene box with 40-cm length × 30-cm width × 17-cm height (Fig. 1a). These containers were drilled on the bottom and the walls. The holes were sealed and the lids of the containers were screwed tightly when gas sample collection was carried out (Fig. 1b).
Experimental design
The mixing ratio of maize stover to cow dung was 60:40 based on dry weight, and this mixing ratio was obtained from the preliminary study. One kilogram of the well-mixed substrates was placed into each container. The mixed substrates were pre-composted for 15 days (Lim et al. 2016), and this phase was designated as pre-composting. After the pre-composting phase, adult earthworms, E. fetida, were inoculated. The earthworm density was set as 0 (which served as the control), 60, 120, and 180 earthworms per kilogram substrate. Corresponding groups were designated as T0, T1, T2, and T3, respectively. This phase, designated as phase II, lasted for 45 days. During phase II, T0 continued the traditional composting while other groups entered the vermicomposting phase. The moisture content of the substrates was maintained around 55–65% during pre-composting and phase II by periodic sprinkling of adequate quantity of sterilized water. Each treatment was done in triplicate. The substrates in all containers were turned thoroughly every 3 days during the pre-composting phase, and then turning was not performed during phase II. Sampling was taken at 15-day intervals. The temperature inside the substrate was monitored daily during pre-composting and then was recorded at each sampling date.
Earthworm biomass
All earthworms were carefully taken out from the substrate at each sampling date. The earthworms were cleaned with distilled water to remove the substrate. Filter paper was used to absorb the water attached on their body. Total biomass of earthworms in each treatment was recorded respectively.
Gas sampling
The lids of the containers were screwed tightly and the holes were sealed during gas sampling (Fig. 1b). Gas samples were taken using an air-tight syringe at four time points: 0, 10, 20, and 30 min, respectively. The samples were injected into pre-evacuated aluminum foil gas-collecting bags. The gas samples were analyzed using gas chromatography (Shimazu, GC-2010PLUS). The concentration of CO2 was determined using a thermal conductivity detector. N2O and CH4 concentrations were measured using an electron capture detector and a flame ionization detector, respectively. Gas fluxes (Gf) in g kg−1 DM day−1 for CO2 and mg kg−1 DM day−1 for CH4 and N2O were calculated according to Czepiel et al. (1996) and Nigussie et al. (2016):
where ΔC is the change in the concentration of gas (ppm) at time interval Δt (hour), V is the headspace volume (L), A is the surface area of the container (m2), M is the molecular weight of the gas, Vs is the volume occupied by 1 mol of gas at standard temperature and pressure, P is the barometric pressure (bar), P0 is the standard pressure, T is the temperature inside the container (K), and W is the initial dry weight of the substrate (kg).
The cumulative emissions (CEt(ab)) were calculated according to Nigussie et al. (2016):
where (CEt(ab)) is the cumulative emission between the measurement day a (ta) and day b (tb), and Gta and Gtb are gas fluxes at the two measurement dates.
Total cumulative emission was calculated as the sum of cumulative emissions on each day according to Nigussie et al. (2016):
The emissions of CH4 and N2O were converted into CO2 equivalents (CO2-eq) using global warming potentials of 25 and 298 for CH4 and N2O, respectively (Solomon et al. 2007). The sum of the three gasses was the total emission of GHG.
Chemical parameter analysis
pH values of the substrate were determined using a digital pH meter. The collected samples taken at each date were suspended in deionized water at a ratio of 1:10 (w/v). The solution was agitated mechanically for 20 min and filtered. The filtrate was used to determine pH values.
Total carbon was measured using the potassium dichromate and concentrated sulfuric acid oxidation method (Nelson and Sommers 1996). Total nitrogen was measured according to the semi-micro Kjeldahl method (Jackson 1973). Lost carbon and nitrogen were calculated according to Nigussie et al. (2016).
where Wi and Wf are total dry weight at the beginning and end of the experiment and Ci and Cf are carbon or nitrogen concentration at the beginning and the end of the experiment.
Statistical analyses
The statistical analyses were carried out using SPSS 16.0. The homogeneity of variances was tested using Levene’s test. One-way ANOVA was used to test the effect of sampling date on the total biomass of earthworms and the effect of earthworm density on the carbon and nitrogen changes in the substrate and on the cumulative emission of gasses. A two-way ANOVA was used to determine the effect of sampling date and earthworm density on the temperature, pH, and fluxes of gasses. Post hoc comparisons of the means were performed by using a Student–Newman–Keuls (SNK) test at α = 0.05. All values were given as mean ± SD.
Results and discussion
Earthworm biomass
Total biomass of the earthworms fluctuated in each treatment followed by a decrease at the end of the experiment (Fig. 2), which was consistent with reports by Marhan et al. (2007). The shortage of available nutrients in the substrate in the later stage may be one of the reasons for the decrease of earthworm biomass (Castillo et al. 2013). Moreover, the ambient condition during vermicomposting was not so favorable for earthworm growth due to the formation of toxic intermediate metabolites during the degradation of substrates (Marhan et al. 2007; Suthar et al. 2017), which may also be one reason for the decline of total biomass of the earthworms. In T2 and T3, total biomass of earthworms showed significant difference between different sampling dates (p < 0.05), while in T1, no significant difference was observed between different sampling dates. This was due to relatively lower earthworm density and less intense competition for food in T1.
Changes in temperature and pH of the substrate
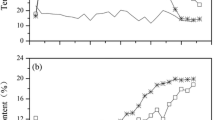
Temperature profiles are shown in Fig. 3a. In the first 2 days, the temperature decreased drastically from 35 to around 24 °C and fluctuated within a narrow range until the end of the experiment. The highest temperature was lower than 45 °C in the present study, which was not consistent with that in traditional composting systems. Generally, the temperature in traditional composting systems reaches at least 45 °C (Nigussie et al. 2017). However, it has been reported that low temperature is typical in small-scale composting such as home composting (Lim et al. 2016). The substrate in the present system was not conducive to the heat buildup, and the heat produced in the substrate quickly dissipated to the surrounding. As a result, the temperature was lower than that in traditional composting systems.
The changes in pH are shown in Fig. 3b. It was obvious that the pH change was greatly influenced by the number of earthworms applied. For all treatments, pH values increased from 7.25 to 7.85 during the first 15 days (pre-composting phase), and then decreased rapidly until day 30. From day 30 to day 45, pH of T0 continued to drop to almost the initial value (pH 7.25), while pH for other three treatments exhibited an increase. From day 45 to day 60, except T1, all other three treatments showed an increase in pH. The pH value for T3, in particular, increased to around 8.10. The increase of pH during the pre-composting phase may be due to the decomposition of nitrogen organic matter and the formation of ammonium ions. The decline of pH may be due to the mineralization of organic matter, the production and fixation of CO2, and the formation of carboxylic, phenolic, and humic acids. Carboxylic, phenolic, and humic acids and ammonium ions exert opposite effect on the pH of the substrate. Carboxylic, phenolic, and humic acids reduce the pH value; however, ammonium ions increase the pH value of the substrate (Sharma and Garg 2019). The increase or decrease of pH in the substrate during the composting process is decided by the joint effect of the above basic and acidic compounds.
Changes in carbon and nitrogen mass
Retained carbon contents at the end of the experiment in T2, T3, and T4 were 42.54%, 39.96%, and 40.35% of the initial carbon, while it was 63.04% in T0 (Table 1). The percentage of lost carbon was equivalent to the result from Shah et al. (2012), who reported that 67% of the initial carbon of the original substrate was lost during composting. More carbon was lost in the combined system than that in the traditional composting system (control group), which indicated that more organic carbon was degraded in the combined system. This was in line with the results from Nigussie et al. (2016), in which vermicomposting increased total carbon loss. In the present study, most of the carbon was lost as CO2. In T1, T2, and T3, 34.09, 37.26, and 43.61 g carbon kg−1 substrate were lost as CO2, and 32.29 g carbon kg−1 substrate was lost as CO2 in T0. However, 2.97, 1.73, and 1.41 mg carbon kg−1 substrate were lost as CH4 in T1, T2, and T3, and it was 4.09 mg carbon kg−1 substrate in T0. The carbon lost as CH4 was much lower than that lost as CO2. This was consistent with the finding by Nigussie et al. (2016), who reported that most carbon was lost as CO2 during composting and vermicomposting.
Total nitrogen loss was higher in the traditional composting system (T0) (Table 2). Retained nitrogen in T1, T2, and T3 was 89.84%, 91.65%, and 87.42% of initial nitrogen, respectively. However, it was only 79.68% in T0. More nitrogen was retained compared to the findings by Shah et al. (2012), who reported that 46% of the initial nitrogen of the original substrate was lost during composting. This may be due to the differences in the substrates, composting systems, and composting periods. Eklind and Kirchmann (2000) reported that nitrogen loss was 43–62% after 590 days of composting of organic household waste. Results about nitrogen loss from the present study were comparable with those results from Yang et al. (2019), who reported that nitrogen losses were 13–16% in the composting of dairy manure and rice straw. In the present study, most of nitrogen was lost as NH3. In T1, T2, and T3, 20.33, 27.89, and 35.40 mg NH3-N kg−1substrate were lost, respectively. While in T0, the loss amounted to 13.01 mg NH3 kg−1 substrate. Different from the situation of NH3, nitrogen loss as N2O was significantly higher (p < 0.05) in the traditional composting system than in the combined systems of T1, T2, and T3 (Table 2). The decomposition of nitrogen organic matter produces \( {NH}_4^{+} \) in composting or vermicomposting systems. NH3 volatilization is common during the composting or vermicomposting process. Under aerobic condition, NH3 is oxidized to nitrous acid by ammonia-oxidizing bacteria and further converted into nitric acid by nitrite-oxidizing bacteria. Nitrous acid and nitric acid can be transformed into N2 by denitrifying bacteria under anaerobic condition. N2O is an intermediate product in transformation of nitrous acid/nitric acid into N2. The ammonia-oxidizing process is a rate-limiting step of the nitrification process (Lv et al. 2019). This may be one of the reasons for the lower N2O than NH3 in the present study.
CO2 emission from the degradation process
As shown in Fig. 4a, the emission of CO2 was the highest during the initial stage of the experiment. On day 0, the flux of CO2 emission was around 5.00 g kg−1 DM day−1. By the end of the experiment, it decreased to 1.35 to 2.34 g kg−1 DM day−1. The sampling time and earthworm density significantly affected CO2 emission (p < 0.01). The lower CO2 emission during phase II suggested that the substrates tended to be stable as composting proceeded. This was corroborated by the temperature profiles. The rapid evolution of CO2 in the initial stage can be attributed to the decomposition of the easily biodegradable substances (Fernández-Gómez et al. 2010). The cumulative emission of CO2 in T1, T2, and T3 was higher than that in the control (Fig. 5a). This is supported by the retained amount of carbon in the corresponding groups. During the pre-composting phase, the cumulative emissions of CO2 was around 43.00 g CO2 kg−1 substrate. The cumulative emissions of CO2 during the mesophilic vermicomposting phase in T1, T2, and T3 were 82.27, 88.86, and 112.15 g CO2 kg−1 substrate, respectively. In T0, the cumulative emission of CO2 was 75.68 g CO2 kg−1 substrate from day 15 to day 60. There were significant differences in cumulative emission of CO2 in four earthworm density treatments (p < 0.01). The presence of the earthworms promoted the emission of CO2 in this study. This was in line with some other studies. Lubbers et al. (2013) reported that earthworms increased emission of CO2 during a relatively short experimental period, and the earthworm-induced CO2 emission decreased with experimental duration. The movement of earthworms inside the substrate increased the oxygen penetration, which promoted the aerobic degradation of organic matter (Ba et al. 2020). This is one of the reasons for the increased emission of CO2 in treatments with earthworms compared to the control. The substrate composition affects the emission of CO2. Wang et al. (2014) reported that CO2 emission was not altered during vermicomposting of duck manure, while the addition of reed straw increased CO2 emission by 20%. The percentages of CO2 emission during the pre-composting phase relative to the total CO2 emission in T0, T1, T2, and T3 were 36%, 34%, 35%, and 30%, respectively (Fig. 6a). That is to say, at least one-third of total CO2 was emitted during the pre-composting phase using a combined pre-composting and vermicomposting system. The emission of CO2 during pre-composting should be included when the emission of CO2 from a combined system is estimated.
CH4 emission from the degradation process
In most cases, the emission of CH4 was higher in T0 compared to T1, T2, and T3 (p < 0.05) (Fig. 4b). T0 was not inoculated with earthworms. The highest flux in T0 appeared on day 60 with the value of 0.30 mg kg−1 DM day−1. It was twice the CH4 flux from T1, three times the flux from T2, and nine times the flux from T3. It was reported that CH4 production can be inhibited to some extent under vermicomposting. The lower emission of CH4 in the initial stage was in agreement with other studies (Andersen et al. 2010; Zhu-Barker et al. 2017). CH4 was formed under anoxic conditions, and \( {NO}_3^{-} \) has an inhibitory effect on methanogenesis (Zhu-Barker et al. 2017). CH4 emission started when oxygen and \( {NO}_3^{-} \) inside the substrates were depleted. The cumulative emissions of CH4 during the pre-composting phase were less than 0.50 mg kg−1 substrate in T0, T1, T2, and T3 (Fig. 5b). From day 15 to day 60, the cumulative emissions of CH4 in T0, T1, T2, and T3 were 5.00, 3.46, 2.04, and 1.70 mg kg−1 substrate, respectively. This indicated that the emission of CH4 was significantly decreased by the presence of earthworms (p < 0.01). The emission of CH4 is dependent on oxygen level. Methanogens are strictly anaerobic organisms and are found in anoxic environments (Welte and Deppenmeier 2014). High aeration condition can decrease CH4 emission. Thus, forced aerated composting is often adopted because of the advantage of high processing rate and good control of process variables (Tsutsui et al. 2013). Earthworms improve air circulation inside the compost pile through continuous turning of the substrates like mechanical aeration, thereby maintaining aerobic conditions (Nigussie et al. 2016; Lee et al. 2018). The percentages of cumulative emission of CH4 during pre-composting relative to the total emission of CH4 was 9%, 13%, 9%, and 11% in T0, T1, T2, and T3, respectively (Fig. 6b). The emission of CH4 was a little higher in the later stage, though the absolute amount was quite low. The emission on day 60 was 0.30, 0.16, 0.11, and 0.03 mg kg−1day−1 DM in T0, T1, T2, and T3, respectively. The values were below 0.04 mg kg−1day−1 DM on day 0 in all treatments. The amount of CH4 emitted was much lower compared to that of CO2, which was analogous to the results obtained by Nigussie et al. (2016), who reported that most carbon was lost as CO2 during composting and vermicomposting processes. The production of methane accounted for only 0.5 – 1% of total carbon loss in the study conducted by Nigussie et al. (2016). In the present study, the production of methane accounted for 3 – 8% in treatments with earthworms and 11% in the control group.
N2O emission from the degradation process
N2O flux from each group is shown in Fig. 4c. In T1, the flux of N2O became stable starting from day 45, and the value was 0.27 mg kg−1 DM day−1. In T2 and T3, the peak values of N2O flux appeared on day 15. In the control group (T0), the flux of N2O showed an increasing trend. It was 0.92 mg kg−1 DM day−1 by the end of the experiment in T0. This demonstrated that the flux of N2O was significantly affected by the earthworm (p < 0.05). From day 30 to day 60, the application of earthworms caused decrease of N2O emission. This was consistent with the results by Wang et al. (2014), who reported that N2O flux was decreased by the earthworms during vermicomposting of duck manure. Cumulative emission of N2O in T0, T1, T2, and T3 during the pre-composting phase was around 0.30 mg kg−1 substrate (Fig. 5c). Cumulative emissions of N2O from day 15 to day 60 in T0, T1, T2, and T3 were 18.61, 8.64, 1.35, and 0.70 mg kg−1 substrate, respectively. This indicated that earthworms decreased the emission of N2O. The decreasing extent was related to the earthworm density (p < 0.01, r = − 0.923). This was different from some reports. Hu et al. (2013) and Tsutsui et al. (2013) found that high N2O emission was triggered immediately when the anoxic condition was switched to oxic condition due to the inactivation of N2O reductase by oxygen. The emission of N2O from the ingested substrates was increased compared to the group without earthworms (Chapuis-Lardy et al. 2010), which was due to the positive influence on the relative abundance of denitrifiers by earthworms through soil ingestion and subsequent egestion (Chapuis-Lardy et al. 2010). Nigussie et al. (2016) and Wu et al. (2015) reported that organic nitrogen transformations such as mineralization, volatilization, nitrification, and denitrification during vermicomposting were affected by earthworms and their interaction with associated microbes, although earthworms hardly produced any N2O themselves. In addition to that, the gut of earthworms was an optimal environment for N2O-producing microflora, and the microflora was stimulated by the passage of earthworm gut (Wu et al. 2015). It was reported that the low redox potentials, organic carbon richness, and nearly neutral pH of the earthworm gut could lead to the selective stimulation of ingested microorganisms capable of anaerobiosis (Depkat-Jakob et al. 2013). Under this circumstance, the ingested fermentative, methanogenic, and nitrate-reducing bacteria can be activated during gut passage (Depkat-Jakob et al. 2013). The result about N2O emission was partially consistent with the report from Chen et al. (2014), who reported more N2O emission in earthworm-worked dry soil than that without earthworms. Under this circumstance, N2O was thought to be a byproduct of the nitrification process. This means that it was released during hydroxylamine oxidation to nitrite by ammonia-oxidizing microorganisms. However, in wet soil, earthworms reduced N2O emission. The presence of earthworms could favor the reduction of N2O to N2. Also, earthworms altered soil microenvironment to slow N2O diffusion, and therefore, N2O was reduced to N2 before it was diffused into the air (Chen et al. 2014). Although there is no soil used in the present study, the substrates of maize stover and cow dung were inhabited by a diversity of microorganisms. The attached microorganisms acted like the microbiota in the soil and interacted with earthworms to affect the emission of N2O. The mechanism of N2O emission is quite complex. Though many studies found that denitrification or nitrifier denitrification was a main pathway for N2O production during the composting process, N2O production through nitrification also played an important role (Tsutsui et al. 2013). It needs further study to identify the origin of N2O in a combined pre-composting and vermicomposting system. The dominant microorganism species should be taken into account as an important factor in the future for clarifying the mechanism of the emission of N2O in addition to the physiochemical conditions.
The percentages of cumulative emissions of N2O during pre-composting relative to the total emission of N2O were 1%, 3%, 18%, and 29% in T0, T1, T2, and T3, respectively (Fig. 6c). The lower percentage in the control group was because of its higher total emission of N2O. Nearly 30% of N2O was emitted during pre-composting in T3. In the present study, small-size earthworm E. fetida decreased the emission of N2O. This was different from the report by Depkat-Jakob et al. (2013), who found that small-size earthworm was capable of emitting N2O. E. fetida is a typical small-size earthworm, and it is often used in vermicomposting systems. The effect of earthworms on the emission of N2O in the present study was similar to that obtained by Wang et al. (2014). Luth et al. (2011) attributed the discrepancy to the available organic nitrogen in the system. They suggested that there was probably a threshold of available organic nitrogen that determined whether earthworms would decrease or increase the emission of N2O. This needs to be further investigated in the combined pre-composting and vermicomposting system with maize stover and cow dung.
Total GHG emission from the degradation process
CH4 and N2O were converted into CO2 equivalents using conversion coefficient suggested by Solomon et al. (2007), and total emission of GHG was obtained (Fig. 5d). During the pre-composting phase, GHG emissions were around 43.00 g CO2-eq kg−1 substrate. During phase II, GHG emissions in T0, T1, T2, and T3 were 81.40, 84.95, 89.33, and 112.41 g CO2-eq kg−1 substrate, respectively. The percentages of GHG emission during pre-composting relative to total GHG emission in T0, T1, T2, and T3 were 34%, 34%, 35%, and 30%, respectively (Fig. 6d). This indicated that during the pre-composting phase, around one-third of GHG was emitted when using a combined pre-composting and vermicomposting system. Taking up a significant portion of total GHG emission, the GHG emitted during the pre-composting phase should be considered one of the noteworthy factors when evaluating a combined pre-composting and vermicomposting system. The total emissions of GHG in T0, T1, T2, and T3 were 124.22, 127.77, 137.65, and 160.73 g CO2-eq kg−1 substrate, respectively. It indicated that the presence of earthworms increased the emission of GHG and the emission amount increased with earthworm density. The emission of GHG in T3 was significantly higher than that in other three groups (p < 0.01). The combined system had an advantage though its total GHG emission was higher than that in the traditional composting system. This was due to the faster decomposition and stabilization in the combined system which was indicated by the higher emission of CO2. More importantly, the emission of N2O (p < 0.01) and CH4 (p < 0.01) was significantly decreased by the combined system, and the global warming potential of N2O and CH4 is extremely higher than that of CO2. T2 is recommended for stabilizing maize stover and cow dung when the tradeoff between the effectiveness of stabilization and the reduction of GHG is considered.
Conclusions
GHG mainly emitted as CO2 from the combined pre-composting and vermicomposting system used in the present study. The GHG emission during the pre-composting phase accounted for around 30 – 35% of total emission from the combined system. Earthworms had an impact on the emission amount of GHG during the whole process. The emission of CO2 was increased by the presence of earthworms, whereas the emissions of CH4 and N2O were decreased by the earthworms. On the whole, the emission of GHG was increased by earthworms in the present study. T2 is recommended for stabilizing maize stover and cow dung at the present study context. Both of the emission amounts of GHG during pre-composting and vermicomposting phases cannot be neglected when evaluating a combined system.
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Andersen JK, Boldrin A, Samuelsson J, Christensen TH, Scheutz C (2010) Quantification of greenhouse gas emission from windrow composting of garden waste. J Environ Qual 39:713–724
Ba SD, Qu QB, Zhang KQ, Groot JCJ (2020) Meta-analysis of greenhouse gas and ammonia emissions from dairy manure composting. Biosyst Eng 193:126–137
Castillo JM, Romero E, Nogales R (2013) Dynamics of microbial communities related to biochemical parameters during vermicomposting and maturation of agroindustrial lignocellulose wastes. Bioresour Technol 146:345–354
Chan YC, Sinha RK, Wang W (2011) Emission of greenhouse gases from home aerobic composting, anaerobic digestion and vermicomposting of household wastes in Brisbane, Australia. Waste Manag Res 29:540–548
Chapuis-Lardy L, Brauman A, Bernard L, Pablo AL, Toucet J, Mano M, Weber L, Brunet D, Razafimbelo T, Chotte JL (2010) Effect of the endogeic earthworm Pontoscolex corethrurus on the microbial structure and activity related to CO2 and N2O fluxes from a tropical soil (Madagascar). Appl Soil Ecol 45:201–208
Chattopadhyay GN (2012) Use of vermicomposting biotechnology for recycling organic wastes in agriculture. Int J Recycl Org Waste Agric 1:8
Chen C, Whalen JK, Guo XB (2014) Earthworms reduce soil nitrous oxide emission during drying and rewetting cycles. Soil Biol Biochem 68:117–124
Christensen TH, Gentil E, Boldrin A, Larsen A, Weidema B, Hauschild M (2009) C banlance, carbon dioxide emissions and global warming potentials in LCA-modelling of waste management systems. Waste Manag Res 27(8):707–715
Czepiel P, Douglas E, Harriss R, Crill P (1996) Measurement of N2O from composted organic wastes. Environ Sci Technol 30:2519–2525
Depkat-Jakob PS, Brown GG, Tsai SM, Horn MA, Drake HL (2013) Emission of nitrous oxide and dinitrogen by diverse earthworm families from Brazil and resolution of associated denitrifying and nitrate-dissimilating taxa. FEMS Microbiol Ecol 83:375–391
Eklind Y, Kirchmann H (2000) Composting and storage of organic household waste with different litter amendments. II: nitrogen turnover and losses. Bioresour Technol 74:125–133
Fernández-Gómez MJ, Nogales R, Insam H, Romero E, Goberna M (2010) Continuous-feeding vermicomposting as a recycling management method to revalue tomato-fruit wastes from greenhouse crops. Waste Manag 30:2461–2468
Girotto F, Cossu R (2019) Role of animals in waste management with a focus on invertebrates’ biorefinery: an overview. Environ Dev 32:100454
Hobson AM, Frederickson J, Dise NB (2005) CH4 and N2O from mechanically turned windrow and vermicomposting systems following in-vessel pre-treatment. Waste Manag 25:345–352
Hu Z, Zhang J, Li S, Xie H (2013) Impact of carbon source on nitrous oxide emission from anoxic/oxic biological nitrogen removal process and identification of its emission sources. Environ Sci Pollut Res 20:1059–1069
Jackson ML (1973) Soil chemical analysis. Prentice Hall of India, New Delhi 1973:7–33
Lee LH, Wu TY, Shak KPY, Lim SL, Ng KY, Nguyen MN, Teoh WH (2018) Sustainable approach to biotransform industrial sludge into organic fertilizer via vermicomposting: a mini-review. J Chem Technol Biotechnol 93(4):925–935
Lim SL, Lee LH, Wu TY (2016) Sustainability of using composting and vermicomposting technologies for organic solid waste biotransformation: recent overview, greenhouse gases emissions and economic analysis. J Clean Prod 111:262–278
Lubbers IM, van Groenigen KJ, Fonte SJ, Six J, Brussaard L, van Groenigen JW (2013) Greenhouse-gas emissions from soils increased by earthworms. Nat Clim Chang 3:187–194
Luth RP, Germain P, Lecomte M, Landrain B, Li Y, Cluzeau D (2011) Earthworm effects on gaseous emissions during vermifiltration of pig fresh slurry. Bioresour Technol 102:3679–3686
Lv BY, Zhang D, ChenQ CYX (2019) Effects of earthworms on nitrogen transformation and the correspond genes (amoA and nirS) in vermicomposting of sewage sludge and rice straw. Bioresour Technol 287:121428
Marhan S, Langel R, Kandeler E, Scheu S (2007) Use of stable isotopes (13C) for studying the mobilisation of old soil organic carbon by endogeic earthworms (Lumbricidae). Eur J Soil Biol 43:S201–S208
Mupambwa HA, Mnkeni PNS (2018) Optimizing the vermicomposting of organic wastes amended with inorganic materials for production of nutrient-rich organic fertilizers: a review. Environ Sci Pollut Res 25(11):10577–10595
Nelson DW, Sommers LE (1996) Total carbon and organic carbon and organic matter, in: Page AL, Miller RH, Keeney DR (eds.), Method of Soil Analysis. ASA and SSSA, Madison 1996; pp. 539-579.
Nigussie A, Bruun S, de Neergaard A, Kuyper TW (2017) Earthworms change the quantity and composition of dissolved organic carbon and reduce greenhouse gas emissions during composting. Waste Manag 62:43–51
Nigussie A, Kuyper TW, Bruun S, Ade N (2016) Vermicomposting as a technology for reducing nitrogen losses and greenhouse gas emissions from small-scale composting. J Clean Prod 139:429–439
Pagans E, Barrena R, Font X, Sánchez A (2006) Ammonia emissions from the composting of different organic wastes, dependency on process temperature. Chemosphere 62:1534–1542
Shah GM, Oenema O, Lantinga EA (2012) Covered storage reduces losses and improves crop utilization of nitrogen from solid cattle manure. Nutr Cycl Agroecosyst 94(2-3):299–312
Sharma K, Garg VK (2019) Recycling of lignocellulosic waste as vermicompost using earthworm Eisenia fetida. Environ Sci Pollut Res 26:14024–14035
Solomon S, Qin D, Manning M, Alley RB, Berntsen T (2007) Technical summary. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds). Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA 2007; pp. 33
Suthar S, Pandey B, Gusain R, Gaur RZ, Kumar K (2017) Nutrient changes and biodynamics of Eisenia fetida during vermicomposting of water lettuce (Pistia sp.) biomass: a noxious weed of aquatic system. Environ Sci Pollut Res 24:199–207
Tsutsui H, Fujiwara T, Matsukawa K, Funamizu N (2013) Nitrous oxide emission mechanisms during intermittently aerated composting of cattle manure. Bioresour Technol 141:205–211
Wang JZ, Hu ZY, Xu XK, Jiang X, Zheng BH, Liu XN, Pan XB (2014) Emission of ammonia and greenhouse gases during combined pre-composting and vermicomposting of duck manure. Waste Manag 34:1546–1552
Welte C, Deppenmeier U (2014) Bioenergetics and anaerobic respiratory chains of aceticlastic methanogens. Biochim Biophys Acta 1837:1130–1147
Wu YP, Shaaban M, Zhao JS, Hao R, Hu RG (2015) Effect of the earthworm gut-stimulated denitrifiers on soil nitrous oxide emissions. Eur J Soil Biol 70:104–110
Yang X, Liu EK, Zhu XM, Wang HY, Liu HB, Liu X, Dong WY (2019) Impact of composting methods on nitrogen retention and losses during dairy manure composting. Int J Environ Res Public Health 16:3324
Zhu-Barker X, Bailey SK, Paw KT, Burger M, Horwath WR (2017) Greenhouse gas emissions from green waste composting window. Waste Manag 59:70–79
Acknowledgments
The authors express their gratitude to the anonymous reviewers for their valuable comments and suggestions.
Funding
This study was funded by the National Natural Science Foundation of China (31872178).
Author information
Authors and Affiliations
Contributions
LZ, methodology, investigation, interpretation, writing—original draft. TZ, data collection. ES, data collection. ZZ, data analysis. YZ, data analysis, writing—editing. YC, conceptualization, methodology, writing—review and editing, supervision, funding acquisition, project administration.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Ethics approval
The present study was carried out at the College of Biological and Agricultural Engineering, Jilin University, Changchun, China. The experimental protocol used in the study was approved by the Institutional Animal Care and Use Committee of Jilin University, Changchun, China.
Consent to participate
All authors agree to participate in this study.
Consent to publish
All authors allow the publication of this paper.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, L., Zhao, T., Shi, E. et al. The non-negligibility of greenhouse gas emission from a combined pre-composting and vermicomposting system with maize stover and cow dung. Environ Sci Pollut Res 28, 19412–19423 (2021). https://doi.org/10.1007/s11356-020-12172-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-12172-2