Abstract
The aim of this study was to investigate the gaseous emissions (CH4, N2O, and NH3) and compost quality during the pig manure composting by adding spent mushroom substrate (SMS) as a bulking agent. The control treatment was also studied using corn stalk (CS) as a bulking agent. The experiment was conducted in a pilot scale composting reactor under aerobic condition with the initial C/N ratio of 20. Results showed that bulking agents significantly affected gaseous emissions and compost quality. Using SMS as a bulking agent improved composting efficiency by shortening the time for maturity. SMS increased germination index and humic acid of the final compost (by 13.44 and 41.94%, respectively) compared with CS. Furthermore, composting with SMS as a bulking agent could reduce nitrogen loss, NH3, and N2O emissions (by 13.57, 35.56, and 46.48%, respectively) compared with the control. SMS slightly increased CH4 emission about 1.1 times of the CS. However, a 33.95% decrease in the global warming potential of CH4 and N2O was obtained by adding SMS treatment. These results indicate that SMS is a favorable bulking agent for reducing gaseous emissions and increasing compost quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, livestock feedlots are increasing in size and in animal density with large amounts of solid manure produced on a relatively small land base. Without proper treatment, these wastes can cause a series of problems such as hygiene hazards, odor pollution, and ground and surface water pollution (Santos et al. 2016). Due to the high organic matter and nutrient content, livestock manure is a valuable resource for organic fertilizer. However, fresh manure is unsuitable for direct land application owing to the unstable organic matter (OM), weed seeds, and pathogens (Guo et al. 2012). Analysis in literature indicates that composting is an effective, sustainable, and economically feasible technology for the treatment of livestock manure prior to land application (Awasthi et al. 2016; Lim et al. 2016).
However, mismanagement of the composting process results in emissions of harmful gases (such as NH3, CH4, and N2O) which lead not only to the decline in value of the compost as a fertilizer, but also the malodor problem during composting (Lim et al. 2016; Santos et al. 2016). Due to the degradation of N-containing compounds, NH3 emission appeared especially in the thermophilic phase (Jiang et al. 2015). CH4 is formed by the deoxidization of CO2/H and acetic acid by methanogens under anaerobic conditions (Santos et al. 2016). The formation of N2O occurs during incomplete nitrification/denitrification processes that normally convert NH4+ into N2. During denitrification; N2O can be synthesized where there is a lack of O2 or a nitrate (or nitrite) accumulation (Nigussie et al. 2016; Reino et al. 2017). Besides, N2O can also be produced in the presence of O2 or low availability of degradable carbohydrates during nitrification (Nigussie et al. 2016; Reino et al. 2017). Therefore, N2O can be produced under both aerobic and anaerobic conditions. Moreover, CH4 and N2O are both significant greenhouse gases (GHGs) with the global warming potential over a 100-year time frame of 34 and 298 times, respectively, compared with CO2 (IPCC 2013).
C/N ratio is one of the major factors that can affect composting (Guo et al. 2012; Onwosi et al. 2017). Low C/N ratio can lead to a high NH3 emission, while high C/N ratio might make this process very slow due to the excessive amount of degradable OM (Bernal et al. 2009; Onwosi et al. 2017). The optimum C/N ratio for composting is agreed to be in the range of 15–30, especially 20–25 (Guo et al. 2012). The C/N ratio of pig manure is about 12–14 (Guo et al. 2012; Jiang et al. 2015). Bulking agent, which was found to have a relative high C/N ratio, could be added to balance the C/N ratio of pig manure during composting (Chowdhury et al. 2014). In addition, bulking agent plays an important role in the conditioning of the starting composting mixtures, which are usually used to create inter-particle voids, providing air space, regulating the moisture content of waste, and also providing carbon source for micro-organisms (Santos et al. 2016; Yang et al. 2013). Corn stalk is a common and economical bulking agent (Guo et al. 2012). Previous study has found that using corn stalk as a bulking agent not only improves the maturity of compost but also reduces gaseous emissions during composting and increases the amounts of reusable nutrients that are present in the compost (Yuan et al. 2016).
In China, with the country urbanizes, the standard of living and income level increases which leads to higher consumption of industrial crop such as mushrooms. Moreover, with the development of mushroom industry, large quantities of waste are being produced within concentrated areas. Spent mushroom substrate (SMS) is the remains of the substrate in which mushrooms are produced. Due to the high OM content, SMS has been widely used as energy feedstock and organic fertilizers in recent years (Paredes et al. 2009; Wu et al. 2013). However, the presence of phytotoxic compounds and active OM in SMS may cause hygiene hazard problems during agricultural utilization (Medina et al. 2012). SMS is thought to require a 1–2-year weathering cycle prior to being commercially soil-applied (Chefetz et al. 2000). Thus, using SMS as a bulking agent during composting cannot only condition the initial composting material but also enhance the stabilization degree of SMS. Moreover, previous studies reported that the fungi that existed in SMS could accelerate the degradation of polycyclic aromatic hydrocarbon (PHA) and immobilization of heavy metals (García-Delgado et al. 2013).
However, to our knowledge, the effect of adding SMS as a bulking agent on gas emission coupled with advanced maturity indicators such as humification indices has not yet been systematically studied. In order to address the research gaps, the objectives of this study were to (1) evaluate the effect of SMS as a bulking agent on gas emissions during composting process, including CH4, N2O, and NH3; (2) comprehensively evaluate and compare compost quality using maturity (including humus indices) and stability indices.
Materials and methods
Composting materials and experimental design
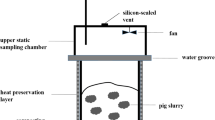
Pig manure was collected from Sujiatuo pig farm (Beijing, China). Corn stalk was taken from Shangzhuang Experimental Station and chopped to lengths of 2–3 cm. Spent mushroom substrate was provided by a mushroom farm in Beijing. After three harvesting cycles of mushroom, the SMS was air-dried and crumbed by hand to 2–3 cm prior to use. The physical and chemical properties of these raw materials and the initial compost mixture are all presented in Table 1.
Composting reactors were a series of cement rotting box with the dimension of 1.08 m × 0.8 m × 1.4 m (length × width × height, pilot scale). Detailed descriptions of these reactors are also available elsewhere (Jiang et al. 2015). Continuous aeration rate was set at 0.24 L kg−1DM min−1.
Two treatments with three replicates were carried out, namely, CS: pig manure + corn stalk; SMS: pig manure + spent mushroom substrate. The CS was a control treatment. Pig manure and bulking agent (corn stalk or spent mushroom substrate) were mixed with the C/N ratio of 20. Distilled water was added to adjust the moisture to approximately 60%. On the 14th day, water was added to adjust the moisture content to 60% in order to satisfy microbial needs (Hough et al. 2010).
Compost sampling and analytical methods
The treatments were operated for 28 days. Temperature was measured by a thermocouple sensor inserted into the middle of the composting materials and continuously recorded by a computer. Homogeneous samples of compost were taken after totally mixing during the turning on the 0th, 3rd, 7th, 14th, 21st, and 28th days. The solid samples were collected using multipoint sampling method (from more than 5 different locations). A part of samples was air-dried, milled with mixer mill (Retsch MM400, Germany), and passed a 0.15-mm mesh screen while the other was stored at 4 °C for analysis. Fresh samples were prepared for the measurement of moisture content (MC), seed germination index (GI), pH, and E4/E6. Air-dried samples were prepared for the determination of total organic carbon (TOC), total Kjeldahl nitrogen (TKN), extractible carbon (CEx), humic acid carbon (CHA), and fulvic acid carbon (CFA). All indices were replicated three times for each sample.
The moisture content was determined by drying the solid samples in an oven at 105 °C for about 8 h until a constant weight was achieved. The pH, GI, and E4/E6 were measured by adding deionized water to the samples at a solid to water ratio of 1:10 (w/v). Then, pH was measured by a pH probe (PHS-3C, China) and E4/E6 was determined by the colorimetric method. GI was determined using the method described by Yang et al. (2013). TOC and TKN of compost samples were measured according to Chinese national standard of organic fertilizer (NY 525-2012). CEx, CFA, and CHA were extracted and measured following the method described previously by Li et al. (2017).
The method of gas sample collection was according to that of Jiang et al. (2015). The composting reactors were covered by a box, and samples were taken 10 min after covering. Gas samples were collected with the air sampler and analyzed four times per day for the first 2 weeks and two times per day for the remaining days. The CH4 and N2O were analyzed using the gas chromatograph (3420A, Beifen, China), and they were examined by a flame and ionization detector and an electron capture detector, respectively. The NH3 was captured in washing bottle with boric acid and titrated against H2SO4. The O2 was measured by an O2 detector (BIOGAS-5000, Geotech, UK).
Statistical analysis
The mean value and standard deviation of three replicates of each treatment were reported. The differences between treatments were compared by a t test analysis. Statistical Analysis System 8.2 for Windows was used for the variance analysis.
Results and discussion
Temperature and oxygen
The changes in ambient and compost temperatures are shown in Fig. 1a. The composting materials went through three typical degradation phases: mesophilic, thermophilic, and curing. The temperature of CS and SMS treatments increased to over 50 °C (thermophilic phase) both on the 3rd day and remained above this level for 20 and 11 days, respectively. Such quick increases indicate fast biodegradation of the materials due to the presence of easily degradable forms of organic matter in the mixtures (Lim et al. 2016). The thermophilic phase of the two treatments lasted more than 3 days, both met the sanitation standards (Yang et al. 2013). However, the temperature in SMS treatment was lower than that in CS treatment during composting. Temperature variations during composting are the result of the thermal balance between the heat generated by the microorganisms and the heat loss through convection, conduction, evaporation, and radiation (Santos et al. 2016). Thus, the energy generated by microorganisms and its effect on the temperature during composting depend on the thermal properties of the bulking agent and its proportion (Pandey et al. 2016; Santos et al. 2016). Moreover, the thermophilic phase was shorter in SMS treatment than CS treatment (p < 0.0001), which may be due to the higher hydrolysable characteristic of SMS after the growth of mushroom (Zhu et al. 2012) and the more extracellular enzymes secreted by the fungi in SMS (Chatterjee et al. 2017; García-Delgado et al. 2013). Thus, using SMS as a bulking agent improved composting efficiency by shortening the time for maturity.
Due to oxygen consumption by aerobic microorganisms for biodegrading organic matters,oxygen contents of the two treatments decreased rapidly when composting started (Fig. 1b). Following this sharp decrease, a slowly fluctuating increasing trend of oxygen concentrations was observed. The oxygen content in SMS treatments returned to ambient level with the exhausting of organic matter after 21 days, while oxygen content in CS treatments became close to ambient level at the end of composting, indicating the slower OM degradation in CS treatment. Previous study showed that cellulose and lignin in the substrate decreased by 50 and 30% after mushroom growth, respectively, while the contents of crude protein and fat were doubled (Zhu et al. 2012). In contrast, the higher amount of recalcitrant compounds (data not shown), such as cellulose and lignin, in CS treatment might explain the prolonged oxygen consumption (Yamada and Kawase 2006).
Compost quality
Compost quality is a primary concern for soil application. According to previous research, compost quality could be evaluated by pH, EC, GI, humification, and some other stability and/or maturity indices (Bernal et al. 2009).
The optimum moisture content for composting varies with the waste to be composted, but generally the mixture should be at 50–60% (Gajalakshmi and Abbasi 2008; Onwosi et al. 2017). During composting, large quantity of water can evaporate in order to control excessive temperature, and as water content diminishes the rate of decomposition decreases, then rewetting should be required in order to maintain the optimum moisture content for the microbial activity (Bernal et al. 2009). As shown in Table 2, the moisture contents in the two treatments were both dropped below 50%; then, water was added to adjust the moisture content to 60% (Hough et al. 2010).
A pH at the range of 6.7–9.0 can support a better microbial activity during composting (Miller 1992). The pH of the composts in CS and SMS treatments was 7.01–7.99 and 7.52–8.26, respectively (Table 2), which indicated good microbial activity in both treatments. The pH decreased at the beginning of the composting due to the rapid degradation of readily available OM and accumulation of organic acids (Pandey et al. 2016), while later on, gradually increased pH might be ascribed to the degradation of proteinaceous materials and the accumulation ammonium (Zhang and Sun 2016). Then, with the ammonia volatilization and humification process after thermophilic phase pH decreased. The pH values in SMS treatments are slightly higher than those found in CS treatments during all composting phases, possibly due to a greater OM stabilization and humification degree in the SMS than CS (Bernal et al. 2009; Paredes et al. 2009).
From Table 2, the GI decreased slowly during the early phase. Previous studies attributed this drop to the production of low molecular weight short chain volatile fatty acids (mainly acetic acid) and ammonia (Guo et al. 2012). The GI increased with the decomposition of these toxic materials and the stabilization of the compost. After 14 days, GI reached more than 80% in both of the two treatments, indicating phytotoxic-free and mature of compost (Zhang and Sun 2016). Compared to CS treatments, SMS treatments could increase the final GI by 13.44%. Similar to the lower temperature in SMS treatments, this also implied the high degree of maturity. The statistical analysis showed that the bulking agent had a significant influence on GI (p = 0.0096).
The humified fraction of OM is the most important one responsible for organic fertility functions as it is the fraction most resistant to microbial degradation (Bernal et al. 2009). As shown in Table 2, the contents of humic substances (CEx, including HA and FA) decreased from 92.41 to 37.56 g kg−1 and 101.61 to 48.39 g kg−1 in the CS and SMS treatments, respectively. Contents of CEx declined possibly due to the dramatic decrease of FA (from 80.78 to 11.14 g kg−1 and 87.88 to 10.89 g kg−1 in the CS and SMS treatments, respectively, Table 2). The noticeable reduction in FA could be explained by the feature of FA containing easily degradable compounds which were firstly exposed to microbial attack (Sellami et al. 2008). The degradation of the readily available organic substances, including the FA, provided energy to the microorganism (Zhou et al. 2014). The bio-oxidation of these compounds led to the production of substances with more stable structures like HA in mature composts. Hue and Liu (1995) proposed CFA ≤ 12.5 g kg−1 for the maturity of different origin composts. Compost in these two treatments both attained this maturity standard at the end of the composting.
On the contrary, HA contents increased from 11.63 to 26.42 g kg−1 and 13.73 to 37.50 g kg−1 in the CS and SMS treatments, respectively. Similar results were found by Awasthi et al. (2016) and Zhou et al. (2014). The increase of HA may be ascribed to the high content of readily available OM decomposed, leading to a higher rate of HA generation than decomposition. Alternatively, parts of the HA may be transformed from other forms of humic substances, such as FA (Li et al. 2017; Zhou et al. 2014). SMS treatments could significantly increase the final HA by 41.94% compared to CS treatments (p = 0.0057). The higher contents of HA in SMS treatments indicated a higher level of humification in SMS.
The HA-to-FA (HA/FA) ratio is widely used to reflect the stability and maturity of the final compost (Li et al. 2017). From Table 2, the HA/FA ratio increased with the higher change in the SMS treatments (from 0.16 to 3.44) than the CS treatments (from 0.14 to 2.37). The increase in the HA/FA ratio, also known as the “degree of polymerization,” reflected the formation of complex molecules (HA) from more simple molecules (FA) and a diminution in the non-humic components of other organic acid fraction which are the most easily degraded by microorganisms (Sellami et al. 2008). During the humification process, SMS provided rich substrates for aromatization and oxidation with more fiber-structure components (Wu et al. 2013), leading to a higher HA/FA ratio in SMS treatment than CS treatment. Indeed, for CS and SMS treatments, the ratios of CHA/CFA were both higher than 1.6, which would indicate an extended degree of maturity achieved by organic matter in both mixtures (Sellami et al. 2008). Their evolution during composting reveals the humification process of the OM, but a limit value cannot be fixed for expressing compost maturity.
Usually, the E4/E6 decreases with the increasing of molecular weight and degree of HA condensation but it is not related to the molecular quantity of HA (Yuan et al. 2016). In the beginning of composting, large values of E4/E6 ratio (Table 2) have been connected with the presence of smaller size organic molecules or more aliphatic structures and usually with a higher content of functional groups (Li et al. 2017). With the increase in composting time, E4/E6 ratio decreased significantly indicating that the easily degradable organic matters were mineralized and phenolic compounds were oxidized and bound to methoxyl groups and/or aliphatic side chains in humic substances (Sellami et al. 2008). At the end of composting, a sufficient level of stability and maturity was achieved by a lower E4/E6 ratio. Moreover, SMS treatments showed smaller E4/E6 as compared to CS treatments, indicating that HA in SMS had the larger molecular weight and the higher humification degree than HA in CS treatments.
In short, temperature data combined with other maturity and stability properties clearly indicates that SMS-amended compost has higher humic substances and maturity degree.
Gaseous emissions
Methane emission
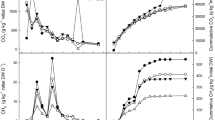
The CH4 emissions of both treatments mainly occurred during thermophilic phase of the composting (Fig. 2a). In the thermophilic phase, microorganisms could degrade organics rapidly, and then partially, anaerobic conditions occurred in composting piles and leading to CH4 production in this period (Awasthi et al. 2016; Chowdhury et al. 2014). Subsequently, with the turning activities breaking the big particles, destroying the anaerobic regions, and with the biodegradation of OMs, CH4 emissions in the two treatments experienced a gradual decline (Santos et al. 2016) whereas oxygen contents increased simultaneously (Fig. 1b). These results were similar to the study by Yang et al. (2013) during kitchen waste composting and Luo et al. (2014) during pig manure composting. CH4 emission pattern in SMS treatments was similar with that in CS treatments. Statistical analysis of CH4 emission showed no significant difference between the two treatments (p = 0.4849). However, from Fig. 2b, the cumulative CH4 emission in SMS treatments (1509 g CH4-C·t−1DM) was about 1.1 times of the CS treatments (1370 g CH4-C·t−1DM). The inconsistency should be explained by the characteristics of the materials that SMS contains more easily decomposable materials after mushroom cultivation (Zhu et al. 2012). Similar result was found by Nigussie et al. (2016) during composting of vegetable waste that a high proportion of easily decomposable materials increase CH4 production.
Nitrous oxide emission
The dominant emissions of N2O for the two treatments occurred in the first 2 weeks and peaked at the beginning of composting (Fig. 3a), which was consistent with previous study by Awasthi et al. (2016). This finding clearly indicated the presence of anaerobic pockets during the beginning of the composting and may be the result of composting feedstock settlement reducing the oxygen availability (Santos et al. 2016). According to previous studies, there are two possible mechanisms for this phenomenon: nitrification or denitrification could occur in raw materials before the start of composting (Nigussie et al. 2016); the initial temperature and oxygen concentration are favorable to nitrifies on the surface of composting piles (Hao et al. 2004). In this study, the N2O emissions at the beginning could be created by the nitrification of ammonia (Fig. 4a). However, N2O emission in CS treatments was also detected during the maturation phase. This can be attributed to the nitrification of NH4+-N, which might accumulate in the thermophilic phase, and thus nitrifying bacteria activity increased during the curing phase (Yuan et al. 2016). Statistical analysis shows that there was a significant difference in N2O emissions between the two treatments (p = 0.0024). Compared to the CS treatments, SMS treatments reduced N2O emission by 46.48% during composting (Fig. 3b), possibly due to the blockage of gaseous permeation by the CS (Fig. 1b).
Ammonia emission
The emissions of NH3 mainly occurred in the thermophilic phase for the two treatments (Fig. 4a); this could be attributed to the biodegradation of organic nitrogen to inorganic nitrogen such as NH4+ which could volatilize in form of NH3 under high temperature and pH (pH = 7–9 in this study) (Pagans et al. 2006; Santos et al. 2016). The decomposition of organic matter was also accompanied by a rise in pH of the composting pile (Table 2), which in turn accelerated the volatilization of NH3. The NH3 emission profile from our study was generally consistent with several previous studies (Awasthi et al. 2016; Pagans et al. 2006; Yuan et al. 2016). The NH3 emission of CS and SMS treatments reached their peak values at day 4 (876 g NH3-N·t−1DM) and day 5 (571 g NH3-N·t−1DM), which was attributed to the degradation of partially decomposed materials after turning (Jiang et al. 2015). During the curing phase (after day 14), the NH3 emissions of the two treatments decreased gradually with the decrease of easily degradable materials and composting temperature (Yang et al. 2013). Statistical analysis shows that there was a significant difference in NH3 emissions between the two treatments (p = 0.0150). At the end of the compost, the cumulative NH3 (Fig. 4b) losses from the CS treatments were 13.57% higher than those from the SMS treatments, which was caused by higher humus content in SMS treatment fixed more nitrogen in the compost (Table 2).
Carbon and nitrogen balances and the global warming potential of CH4 and N2O
Table 3 presents the carbon and nitrogen balances and the global warming potential of CH4 and N2O at the end of the composting. After composting, more than 44% of the initial TOC content had been lost. The major pathway for the loss of initial TOC was in the form of CO2 (43.43–48.22%), whereas 0.38–0.43% of initial TOC was lost due to CH4 emission under partially anaerobic conditions in composting piles (Santos et al. 2016). This result was similar to that of Yang et al. (2013) in kitchen waste composting and Yuan et al. (2016) in sewage sludge composting.
The TN losses ranged from 29.84 to 34.32% of initial TN in raw materials (Table 3). Most nitrogen was lost in the form of NH3, while 1.24–2.30% of nitrogen was lost in the form of N2O. These results were similar to the previous study by Jiang et al. (2015) in pig manure composting and Nigussie et al. (2016) in food waste vermicomposting. Composting with SMS as a bulking agent could significantly reduce nitrogen loss, NH3, and N2O emissions (by 13.57, 35.56, and 46.48%, respectively) compared with the CS.
While CO2, CH4, and N2O are all important GHGs, the contribution of CO2 to greenhouse effect should be excluded during composting as it derives from microbial respiration (IPCC 2013). Therefore, only CH4 and N2O were taken into consideration when calculated the total GHG emissions in this study. The global warming potential of CH4 and N2O ranged from 124.44 to 196.66 kgCO2-eq·t−1DM, with the SMS treatments having the lower GHG emissions. The lower GHG budget from SMS-amended composting may be explained by the lower N2O production. Moreover, in terms of environmental benefits, using SMS as a bulking agent could significantly reduce the total GHG emission by 33.95% (about 70.96 kg CO2-eq·t−1DM) than that of using CS as a bulking agent. In summary, composting with SMS as a bulking agent is considered as a sustainable and cleaner process as it could significantly reduce the global warming potential of CH4 and N2O.
Conclusion
Bulking agents could significantly affect gaseous emissions and compost quality. Using SMS as a bulking agent improved composting efficiency by shortening the time for maturity. Moreover, adding SMS increased germination index and the humic acid of the final compost (by 13.44 and 41.94%, respectively) compared with CS treatment. Furthermore, composting with SMS as a bulking agent could reduce nitrogen loss, NH3, and N2O emissions (by 13.57, 35.56, and 46.48%, respectively). Although using SMS as a bulking agent could slightly increase CH4 emission, it reduced the global warming potential of CH4 and N2O by 33.95% compared with the CS treatment.
References
Awasthi MK, Wang Q, Huang H, Li R, Shen F, Lahori AH, Wang P, Guo D, Guo Z, Jiang S, Zhang Z (2016) Effect of biochar amendment on greenhouse gas emission and bio-availability of heavy metals during sewage sludge co-composting. J Clean Prod 135:829–835. https://doi.org/10.1016/j.jclepro.2016.07.008
Bernal MP, Alburquerque JA, Moral R (2009) Composting of animal manures and chemical criteria for compost maturity assessment: a review. Bioresour Technol 100(22):5444–5453. https://doi.org/10.1016/j.biortech.2008.11.027
Chatterjee S, Sarma MK, Deb U, Steinhauser G, Walther C, Gupta DK (2017) Mushrooms: from nutrition to mycoremediation. Environ Sci Pollut Res 24(24):19480–19493. https://doi.org/10.1007/s11356-017-9826-3
Chefetz B, Heemst JDHV, Chen Y, Romaine CP, Chorover J, Rosario R, Gui MX, Hatcher PG (2000) Organic matter transformations during the weathering process of spent mushroom substrate. J Environ Qual 29(2):592–602. https://doi.org/10.2134/jeq2000.00472425002900020030x
Chowdhury MA, De NA, Jensen LS (2014) Potential of aeration flow rate and bio-char addition to reduce greenhouse gas and ammonia emissions during manure composting. Chemosphere 97:16–25. https://doi.org/10.1016/j.chemosphere.2013.10.030
Gajalakshmi S, Abbasi SA (2008) Solid waste management by composting: state of the art. Crit Rev Env Sci Technol 38(5):311–400. https://doi.org/10.1080/10643380701413633
García-Delgado C, Jiménez-Ayuso N, Frutos I, Gárate A, Eymar E (2013) Cadmium and lead bioavailability and their effects on polycyclic aromatic hydrocarbons biodegradation by spent mushroom substrate. Environ Sci Pollut Res 20(12):8690–8699. https://doi.org/10.1007/s11356-013-1829-0
Guo R, Li G, Jiang T, Schuchardt F, Chen T, Zhao Y, Shen Y (2012) Effect of aeration rate, C/N ratio and moisture content on the stability and maturity of compost. Bioresour Technol 112:171–178. https://doi.org/10.1016/j.biortech.2012.02.099
Hao X, Chang C, Larney FJ (2004) Carbon, nitrogen balances and greenhouse gas emission during cattle feedlot manure composting. J Environ Qual 33(1):37–44. https://doi.org/10.2134/jeq2004.3700
Hough RL, Crews C, White D, Driffield M, Campbell CD, Maltin C (2010) Degradation of yew, ragwort and rhododendron toxins during composting. Sci Total Environ 408(19):4128–4137. https://doi.org/10.1016/j.scitotenv.2010.05.024
Hue NV, Liu J (1995) Predicting compost stability. Compost Sci Util 3(2):8–15. https://doi.org/10.1080/1065657X.1995.10701777
IPCC (2013) Climate change 2013: the physical science basis. http://www.ipcc.ch/report/ar5/wg1/. Accessed 10 Sept 2016
Jiang T, Li G, Tang Q, Ma X, Wang G, Schuchardt F (2015) Effects of aeration method and aeration rate on greenhouse gas emissions during composting of pig feces in pilot scale. J Environ Sci 31:124–132. https://doi.org/10.1016/j.jes.2014.12.005
Li S, Li D, Li J, Li G, Zhang B (2017) Evaluation of humic substances during co-composting of sewage sludge and corn stalk under different aeration rates. Bioresour Technol 245(Pt A):1299–1302. https://doi.org/10.1016/j.biortech.2017.08.177
Lim SL, Lee LH, Wu TY (2016) Sustainability of using composting and vermicomposting technologies for organic solid waste biotransformation: recent overview, greenhouse gases emissions and economic analysis. J Clean Prod 111:262–278. https://doi.org/10.1016/j.jclepro.2015.08.083
Luo W, Yuan J, Luo Y, Li G, Nghiem LD, Price WE (2014) Effects of mixing and covering with mature compost on gaseous emissions during composting. Chemosphere 117:14–19. https://doi.org/10.1016/j.chemosphere.2014.05.043
Medina E, Paredes C, Bustamante MA, Moral R, Moreno-Caselles J (2012) Relationships between soil physico-chemical, chemical and biological properties in a soil amended with spent mushroom substrate. Geoderma 173–174:152–161
Miller FC (1992) Composting as a process based on the control of ecologically selective factors. In: Metting FB Jr (ed) Soil microbial ecology, applications in agricultural and environmental management. Marcel Dekker Inc., New York, pp 515–544
Nigussie A, Neergaard AD, Bruun S, Kuyper TW (2016) Vermicomposting as a technology for reducing nitrogen losses and greenhouse gas emissions from small-scale composting. J Clean Prod 139:429–439. https://doi.org/10.1016/j.jclepro.2016.08.058
Onwosi CO, Igbokwe VC, Odimba JN, Eke IE, Nwankwoala MO, Iroh IN, Ezeogu LI (2017) Composting technology in waste stabilization: on the methods, challenges and future prospects. J Environ Manag 190:140–157. https://doi.org/10.1016/j.jenvman.2016.12.051
Pagans E, Barrena R, Font X, Sanchez A (2006) Ammonia emissions from the composting of different organic wastes. Dependency on process temperature. Chemosphere 62(9):1534–1542. https://doi.org/10.1016/j.chemosphere.2005.06.044
Pandey PK, Vaddella V, Cao W, Biswas S, Chiu C, Hunter S (2016) In-vessel composting system for converting food and green wastes into pathogen free soil amendment for sustainable agriculture. J Clean Prod 139:407–415. https://doi.org/10.1016/j.jclepro.2016.08.034
Paredes C, Medina E, Moral R, Pérezmurcia MD, Morenocaselles J, Bustamante MA, Cecilia JA (2009) Characterization of the different organic matter fractions of spent mushroom substrate. Commun Soil Sci Plan 40(1-6):150–161. https://doi.org/10.1080/00103620802625575
Reino C, Mcm VL, Carrera J, Pérez J (2017) Effect of temperature on N2O emissions from a highly enriched nitrifying granular sludge performing partial nitritation of a low-strength wastewater. Chemosphere 185:336–343. https://doi.org/10.1016/j.chemosphere.2017.07.017
Santos A, Bustamante MA, Tortosa G, Moral R, Bernal MP (2016) Gaseous emissions and process development during composting of pig slurry: the influence of the proportion of cotton gin waste. J Clean Prod 112:81–90. https://doi.org/10.1016/j.jclepro.2015.08.084
Sellami F, Hachicha S, Chtourou M, Medhioub K, Ammar E (2008) Maturity assessment of composted olive mill wastes using UV spectra and humification parameters. Bioresour Technol 99(15):6900–6907. https://doi.org/10.1016/j.biortech.2008.01.055
Wu S, Lan Y, Wu Z, Peng Y, Chen S, Huang Z, Xu L, Gelbič I, Guan X, Zhang L, Zou S (2013) Pretreatment of spent mushroom substrate for enhancing the conversion of fermentable sugar. Bioresour Technol 148:596–600. https://doi.org/10.1016/j.biortech.2013.08.122
Yamada Y, Kawase Y (2006) Aerobic composting of waste activated sludge: kinetic analysis for microbiological reaction and oxygen consumption. Waste Manag 26(1):49–61. https://doi.org/10.1016/j.wasman.2005.03.012
Yang F, Li G, Yang Q, Luo W (2013) Effect of bulking agents on maturity and gaseous emissions during kitchen waste composting. Chemosphere 93(7):1393–1399. https://doi.org/10.1016/j.chemosphere.2013.07.002
Yuan J, Chadwick D, Zhang D, Li G, Chen S, Luo W, Du L, He S, Peng S (2016) Effects of aeration rate on maturity and gaseous emissions during sewage sludge composting. Waste Manag 56:403–410. https://doi.org/10.1016/j.wasman.2016.07.017
Zhang L, Sun X (2016) Influence of bulking agents on physical, chemical, and microbiological properties during the two-stage composting of green waste. Waste Manag 48:115–126. https://doi.org/10.1016/j.wasman.2015.11.032
Zhou Y, Selvam A, Wong JW (2014) Evaluation of humic substances during co-composting of food waste, sawdust and Chinese medicinal herbal residues. Bioresour Technol 168:229–234. https://doi.org/10.1016/j.biortech.2014.05.070
Zhu H, Sun L, Zhang Y, Zhang X, Qiao J (2012) Conversion of spent mushroom substrate to biofertilizer using a stress-tolerant phosphate-solubilizing Pichia farinose FL7. Bioresour Technol 111:410–416. https://doi.org/10.1016/j.biortech.2012.02.042
Acknowledgments
This research was financially supported by the National Key R&D Program (2016YFD0800202) and Research Project in Cashmere Goat Industry of China (CARS-39-19).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Li, S., Li, D., Li, J. et al. Effect of spent mushroom substrate as a bulking agent on gaseous emissions and compost quality during pig manure composting. Environ Sci Pollut Res 25, 12398–12406 (2018). https://doi.org/10.1007/s11356-018-1450-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1450-3