Abstract
Jojoba (Simmondsia chinensis) is an economically important plant due to its high oil content in the seeds. Fipronil is an extensively used phenylpyrazole insecticide. The present investigation aimed to assess the possible ameliorative effect of jojoba oil on fipronil induced toxicity in rats. Animals orally received the insecticide dissolved in corn oil by stomach tube at 1/10th LD50 for 28 days. Fipronil induced hepatorenal toxic effects evidenced by elevated serum ALT, AST, ALP, and LDH activities, and urea and creatinine levels, with histomorphological changes in the liver and kidney. Brain GABA was elevated with histopathological alterations in the brain tissue. Oxidative stress was demonstrated in liver, brain, and kidney as indicated by elevated MDA and NO levels with reduction in GSH level and activities of SOD and CAT. In addition, caspase-3 gene expression was enhanced, while Bcl2 gene expression was downregulated in the three organs. Increased DNA fragmentation was recorded in the liver and kidney. Cotreatment of jojoba oil with fipronil ameliorated the toxic effects of fipronil on various organs with improvement of the antioxidant status, the rate of apoptosis and the histopathological alterations. In conclusion, jojoba oil provided significant protection against fipronil induced hepatorenal- and neuro-toxicity, by its antioxidant and antiapoptic effects, making it a possible beneficial protective of natural origin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fipronil is a phenylpyrazole insecticide with a trifluoromethylsulfinyl moiety (Gupta and Anadon 2018). It has a broad-spectrum activity against many insects, and a greater efficacy compared to classical insecticides such as pyrethroids, organophosphates and carbamates (Narahashi et al. 2007). Thus, it is widely used in agriculture for seed coating and soil treatment, veterinary medicine specially on dogs and cats, and in public hygiene management to control many insects (Gupta and Anadon 2018).
Fipronil, or its metabolites, exert neurotoxic effects through suppression of the inhibitory effect of gamma aminobutyric acid (GABA) by targeting GABAA-regulated chloride channels. This inhibits chloride influx into nerve cells leading to hyperexcitation, paralysis, and death (Wang et al. 2016). It has a higher specificity for the GABAA receptor (β3 subunit) present in insects than GABAA receptors of mammals, thereby contributing to fipronil’s 500-fold selective toxicity to insects (Narahashi et al. 2010). Fipronil is rapidly metabolized with persistence of significant amounts of metabolites in the tissues, especially adipose tissue and brain, for about 1 week after administration (Woodward 2012).
Fipronil is moderately toxic to mammals, with oral LD50 values in rats and mice of 97 and 95 mg/kg body, respectively (EPA 1996). The public health concerns about fipronil have increased after reports documenting thyroid cancer in rats (Hurley et al. 1998). It has been reported that fipronil exerts toxic effects on vital organs like liver, kidney, and brain by suppressing mitochondrial respiratory chain and calcium homeostasis, oxidative and nitrosative stress, in addition to damage to nucleic acids and proteins (Gupta and Anadon 2018).
Oxidative stress results from shortage in the defense offered by antioxidants or increased generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Laskin et al. 2009; Lenaz and Strocchi 2009). The enzymatic and nonenzymatic antioxidants protect the cells from the damage caused by free radicals. GSH substrate and the antioxidant enzymes serve as redox biomarkers, because they are the first to indicate the antioxidant state through oxidation/reduction processes (Lenaz and Strocchi 2009).
Previous experiments have demonstrated that increased ROS generation plays a major role in fipronil-induced toxicity in animals (Badgujar et al. 2015; Mossa et al. 2015; Swelam et al. 2017; Kartheek and David 2018; Khalaf et al. 2019). Oxidative stress affects various biological processes and cell signaling pathways. Thus, significant changes in apoptosis, the cell cycle, and stimulation or inhibition of signal transduction is expected to cause toxicological injury (Jin et al. 2014; Chtourou et al. 2015).
Apoptosis is a form of physiological cell death characterized by cell shrinkage, blebbing of membranes, apoptotic bodies formation, and nuclear DNA fragmentation into oligonucleosomal subunits (Ray and Corcoran 2009; Wang et al. 2013). It is triggered through two major pathways; the extrinsic (death receptor dependent) and intrinsic (mitochondrial-dependent) pathways. In the intrinsic pathway, as a response to endogenous and exogenous factors, the mitochondrial outer membrane becomes permeable, followed by the release of cytochrome c and the activation of caspases, leading to cell death (Ray and Corcoran 2009).
Natural plant oils are now used in medicine worldwide. They are usually easily accessible, effective, and have low price. Many oils have ingredients with antioxidant and anti-inflammatory properties making them suitable for internal and topical therapeutic uses. Jojoba (Simmondsia chinensis) is a promising oil seed plant belonging to family Simmondsiaceae. It is a woody evergreen perennial shrub, reaching about 3 m in height. It grows in desert and semi-desert areas native to south-western USA and north-western Mexico (Phillips and Comus 2000).
As Jojoba has the advantage of being capable of growth on arid or semi-arid soils with warm and dry environment that lack adequate water content, its cultivation has been established in many countries worldwide including Egypt, Tunisia, Saudi Arabia, India, Mexico, Chile, Argentina, and Australia (Abdel-Mageed et al. 2014).
Jojoba oil (liquid wax esters) constitutes approximately 50% of the seed by weight (Miwa 1971). This oil has special composition when compared with other classic oils, as it is composed of long monounsaturated esters while other oils are mainly formed of triglycerides. This gives jojoba oil unique characteristics and properties which are important for chemical industry and production of pharmacological preparations (Sánchez et al. 2016).
Jojoba is expected to protect from oxidative damage induced by free radicals, due to its content of lipoxygenase inhibitors (Abdel-Mageed et al. 2014). These are capable of inhibiting leukotriene synthesis and subsequent ROS production (Haeggström and Funk 2011). Furthermore, the antioxidant property of jojoba oil based on nitric oxide and DPPH scavenging assays has been demonstrated (Manoharan et al. 2016). Besides, Badr et al. (2017) have shown that jojoba oil is rich in total phenolic and flavonoid contents which give jojoba oil good antioxidant capacities compared with ascorbic acid.
Based on these mechanistic insights, we hypothesized that jojoba oil will represent a potential therapeutic option for protection against fipronil-induced toxic effects. Therefore, the current investigation endeavored to evaluate the protective effect of jojoba oil against oxidative effect of fipronil induced hepatotoxicity, nephrotoxicity, neurotoxicity, and apoptosis in rats.
Materials and methods
All methods and experiment were carried out in accordance with relevant guidelines and regulations.
Chemicals
Fipronil was obtained as a commercial formulation (Coach 20%SC) from Shoura Chemicals Co., Egypt. Jojoba oil was purchased from Pure Life Co., Egypt. The test kits used for biochemical analysis were purchased from Biodiagnostic and Spectrum Co., Egypt. All other chemicals used were of analytical grade.
Animals
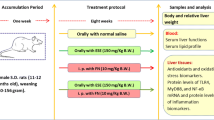
Sixty male Sprague Dawley rats, weighing between 170 and 180 g, were supplied with standard diet and water ad libitum and housed for 2 weeks to be acclimatized before starting the experimental study under standardized conditions (12 h light/dark period, temperature 23 ± 2 °C, and humidity 50%). The experiment was performed in strict accordance with the recommendations in Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) (Approval number: VUSC-011-2-19), Faculty of Veterinary Medicine, University of Sadat City, Egypt.
Experimental design
Animals were randomly allocated into 4 groups (15 rats/group). Animals in all groups were treated for consecutive 28 days as following: The 1st group served as a control, and received corn oil once daily by gavage. The 2nd group was given fipronil dissolved in corn oil daily by gavage (Fipronil group), at dose level of 9.7 mg/kg, equivalent to 1/10th LD50 (EPA 1996). The 3rd group fed on a basic diet mixed with 2.5% jojoba oil (Jojoba oil group) (Nassar et al. 2017). The 4th group received both fipronil and jojoba oil (Fipronil + Jojoba oil group) at the doses indicated for the 2nd and 3rd groups, respectively. Fipronil was given every morning, 2 h before supplying the animals with their daily feed.
At the end of experimental period (on day 29), blood samples were collected from the retro-orbital venous plexus, left to clot, and then centrifuged at 3500 rpm at 4 °C for 15 min, and the obtained sera were stored at – 20 °C for biochemical assessment of liver and kidney function markers. Under diethyl ether anesthesia, animals from all groups were euthanized by cervical dislocation and liver, kidney, and brain were instantly resected and washed in cold saline. Animal whole organs were store at – 80 °C to be used for oxidant/antioxidant, GABA, and DNA fragmentation studies. Other set of organs from animals in different groups was divided into two parts; the first part was stored at – 80 °C and used for the gene expression study, while the other part was fixed in 10% neutral buffered formalin solution for the histopathological examination.
Liver and kidney functions
For estimation of liver function, assay kits from Biodiagnostic, Egypt, were used for measuring serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) (Cat. No: AL1031(45), AS1061(45), and AP1020, respectively), while lactate dehydrogenase (LDH) was estimated using the assay kit of Spectrum, Egypt (Cat. No. 279002), following the manufacturer’s instructions. Kidney function was assessed by measuring serum urea and creatinine using test kits from Biodiagnostic, Egypt (Cat. No. UR2010 and Cr1251, respectively), according to the manufacturer’s protocols.
Assessment of gamma aminobutyric acid in brain tissue
Brain lysates in 75% aqueous HPLC grade methanol (10% w/v) was used for gamma aminobutyric acid (GABA) measurement (Arafa et al. 2010). The homogenate was spun at 4000 rpm for 10 min and the supernatant was dried using vacuum (70 Millipore) at room temperature and used for GABA estimation by high-performance liquid chromatography (HPLC) using the precolumn PITC derivatization technique, according to the method of Heinrikson and Meredith (1984).
Oxidant/antioxidant biomarkers
The oxidant/antioxidant biomarkers were investigated in liver, kidney, and brain of treated and control animals. Assay kits from Biodiagnostic, Egypt, were used to estimate malondialdehyde, MDA (Cat. No. MD2529); nitric oxide, NO (Cat. No. NO2533); reduced glutathione, GSH (Cat. No. GR2511); superoxide dismutase, SOD (Cat. No. SD2521); and catalase, CAT (Cat. No. CA2517) as indicated by the manufacturers.
Assessment of hepatorenal apoptosis by DNA fragmentation
DNA fragmentation was determined according to the method described by Perandones et al. (1993). Briefly, 10–20 mg were ground in 400-μl hypotonic lysis buffer, centrifuged at 3000×g for 15 min at 4 °C. The supernatant was divided into 2 parts; one used for the gel electrophoresis and the other was used with the pellet for quantification of fragmented DNA by the diphenylamine at 578 nm. DNA fragmentation percentage in each sample was expressed by the formula:
%DNA fragmentation = (O.D Supernatant/O.D Supernatant + O.D Pellet) × 100
Quantitative real-time polymerase chain reaction of caspase-3 and B cell lymphoma 2 genes
Total RNA in liver, kidney, and brain samples was extracted using QIAmp RNA mini kit (QIAGEN, Hilden, Germany) as indicated by the manufacturer. Total RNA purity and concentration were obtained using a nanodrop ND-1000 spectrophotometer. The isolated RNA was used for cDNA synthesis using reverse transcriptase (Fermentas, EU). Real-time PCR (qPCR) was performed in a total volume of 20 μl using a mixture of 1 μl cDNA, 0.5 mM of each primer (Table 1), iQ SYBR Green Premix (Bio-Rad 170-880, USA). PCR amplification and analysis were achieved using Bio–Rad iCycler thermal cycler and the MyiQ realtime PCR detection system. Each assay includes triplicate samples for each tested cDNAs and no-template negative control; the expression relative to control is calculated using the equation 2-ΔΔCT (Livak and Schmittgen 2001).
Histopathology
The liver, kidney, and brain samples fixed in neutral buffered formalin 10% solution were trimmed, washed, dehydrated in ascending grades of ethyl alcohol, cleared in methyl benzoate, and processed through the conventional paraffin embedding technique. Paraffin blocks were prepared, from which 3–5-μm thick sections were obtained using a manual Leica rotary microtome (LEICA RM 2135), then routinely stained by hematoxylin and eosin (H&E) stain according to Bancroft and Gamble (2008). Stained slides were microscopically analyzed using light microscopy. Histopathological photos were photographed using a digital Leica photomicroscope (LEICA DMLB Germany).
Semiquantitative histopathological evaluation of hepatic, renal, and brain tissues
The histopathological lesions in the examined tissues were evaluated in three rats from each group and five randomly selected sections were examined from each rat. Semiquantitative evaluation was performed in examined fields (n = 15) according to the percentage, degree, and extent of tissue damage and were scored according to Michael (2008) as follows: (−): normal appearance (absence of pathological lesion 0%), (+): mild (< 25% of sections), (++): moderate (25–50% of sections), (+++): severe (51–75% of sections), and (++++): very severe (> 75% of sections).
Statistical analysis
The obtained data are given as means ± S.E of the mean. Comparisons between different groups were carried out by one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test for post hoc analysis using SPSS software, version 17 (IBM, USA). The level of significance was set at p ≤ 0.05.
Results
Liver and kidney functions
The serum biochemical markers are shown in Table 2. Fipronil treatment for 28 days caused significant (p ≤ 0.05) elevation of serum ALT, AST, ALP, and LDH activities, and levels of urea and creatinine, compared to control values. Cotreatment of jojoba oil with fipronil significantly (p ≤ 0.05) ameliorated the hepato- and nephro-toxicity of fipronil, although the values of all parameters except creatinine were still significantly (p ≤ 0.05) higher than control values (Table 2).
Brain GABA finding
The concentration of GABA (nmol/ml) significantly (p ≤ 0.05) increased (7.5 ± 0.7) in fipronil group compared to control, while fipronil-jojoba group showed significant (p ≤ 0.05) improvement (4.5 ± 0.2), in comparison to the fipronil group. However, the values are still significantly (p ≤ 0.05) higher than control values (Table 3).
Oxidant/antioxidant biomarkers findings
Subacute intoxication with fipronil was associated with significant (p ≤ 0.05) elevations in MDA and NO levels in liver, kidney, and brain, compared to control (Table 4). In addition, the concentration of GSH and activities of SOD and CAT were significantly (p ≤ 0.05) reduced in these organs, compared to control. In fipronil-jojoba group, significant (p ≤ 0.05) recovery of all oxidant/ antioxidant biomarkers was noticed, although the values of most parameters showed significant (p ≤ 0.05) difference from control values (Table 4).
DNA fragmentation assay finding
As displayed in Fig. 1, fipronil significantly (p ≤ 0.05) enhanced the DNA fragmentation (61.7 ± 1.5, 55 ± 1.7) in liver and kidney, respectively. Interestingly, combined administration of fipronil with jojoba oil restored the normal control values.
DNA fragmentation % in different groups. a liver and kidney DNA fragmentation percentage. Values are means ± SE, n = 5. Bars carrying different letters (a, b, and c) are significantly different at p ≤ 0.05. b Electrophoretic mobility of fragmented DNA in different groups on 1% agarose gel. For liver tissue; lane 1: control, lane 2: Fipronil, lane 3: Jojoba oil, lane 4: Fipronil + Jojoba. For kidney tissue; lane 5: control, lane 6: Fipronil, lane 7: Jojoba oil, lane 8: Fipronil + Jojoba oil, M: DNA ladder
Caspase-3 and Bcl2 gene expression finding
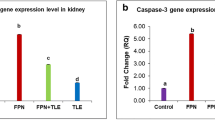
The relative expression of caspase-3 gene was significantly upregulated (p ≤ 0.05) 373-, 338-, and 93-fold in liver, kidney, and brain, respectively, in fipronil group compared to the control and other treated groups. However, cotreatment of fipronil and jojoba oil significantly (p ≤ 0.05) downregulated the gene expression, in comparison to the fipronil group (Fig. 2).
Quantitative RT-PCR of Casp-3 gene expression in different organs in various groups. a Evaluation of Casp-3 gene expression in different organs in fipronil group compared with control and other groups. Values are expressed as mean ± SE, n = 5. Bars carrying different letters (a, b, and c) are significantly different at p ≤ 0.05. b Cropped gel of electrophoretic mobility of quantitative RT-PCR products of Casp-3 and GAPDH (internal control) genes on 2% agarose gel. Lane 1: control, lane 2: Fipronil, lane 3: Jojoba oil, lane 4: Fipronil + Jojoba oil
The expression of B cell lymphoma 2 (Bcl2) gene was significantly (p ≤ 0.05) upregulated 44-, 2.5-, and 13-fold in liver, kidneys, and brain, respectively, in fipronil + jojopa group, compared to the fipronil group (Fig. 3).
Quantitative RT-PCR of Bcl2 gene expression in different organs in various groups. a Evaluation of Bcl2 gene expression in different organs in fipronil group compared with control and other groups. Values expressed as mean ± SE, n = 5. Bars carrying different letters (a, b, c, and d) are significantly different at p ≤ 0.05. b Cropped gel of electrophoretic mobility of quantitative RT-PCR products of Bcl2 and GAPDH (internal control) genes on 2% agarose gel. Lane 1: control, lane 2: Fipronil, lane 3: Jojoba oil, and lane 4: Fipronil + Jojoba oil
Histopathological findings
Liver
Liver sections of control rats showed normal histologic architecture of central vein and hepatic cords (Fig. 4a). Fipronil group showed congestion with inflammatory cells infiltration of central vein, ballooning degeneration of hepatocytes, and area of coagulative necrosis infiltrated by inflammatory cells (Fig. 4b). Animals treated with jojoba oil showed normal histologic architecture of liver tissue as control group (Fig. 4c). The liver of fipronil-jojoba oil group revealed normal central vein and hepatic cords, while some hepatic cells suffered from hydropic degeneration and mild inflammatory cells infiltration in hepatic sinusoids (Fig. 4d). Marked amelioration of the lesions in the fipronil-jojoba group was noticed on the semiquantitative analysis (Table 5).
Representative photomicrographs of liver sections (hematoxylin and eosin stain × 20. Scale bar 50 μm). a Control group showing normal histologic architecture of central vein and hepatic cords. b Fipronil group showing congestion with inflammatory cells infiltration of central vein (yellow arrow), ballooning degeneration of hepatocytes (black arrow), and area of coagulative necrosis infiltrated by inflammatory cells (circle). c Jojoba group showing normal histologic architecture of liver tissue as control group. d Fipronil + Jojoba group showing normal central vein and hepatic cords, some hepatic cells suffered from hydropic degeneration (black arrow) and mild inflammatory cells infiltration in hepatic sinusoids (yellow arrow)
Kidney
Kidneys of control (Fig. 5a) and jojoba oil (Fig. 5c) groups showed normal histologic architecture of glomeruli and renal tubules. Kidney of fipronil-treated group showed shrinkage of glomerular tuft with increased Bowman’s space, congestion of glomeruli and interstitial tissue, necrosis of glomerular cells and renal tubular epithelium with pyknotic nuclei, cloudy swelling of renal tubular epithelium with occlusion of their lumen, periglomerular edema, and in the interstitial tissue with inflammatory cells infiltration (Fig. 5b). The group that received both fipronil and jojoba oil revealed nearly normal glomeruli and renal tubules except for the presence of congestion of glomeruli and interstitial tissue and cloudy swelling of renal epithelium of scattered tubules (Fig. 5b). The semiquantitative analysis of renal lesions revealed marked amelioration of lesions in the fipronil-jojoba group (as displayed in Table 5).
Representative histopathological photomicrographs of rat kidney (hematoxylin and eosin stain × 20. Scale bar 50 μm). a Control group showing normal histologic architecture of glomeruli and renal tubules of kidney. b Fipronil group showing shrinkage of glomerular tuft with increased Bowman’s space (head arrow), congestion of glomeruli and interstitial tissue (blue arrow), necrosis of glomerular cells and renal tubular epithelium with pyknotic nuclei (red arrow), cloudy swelling of renal tubular epithelium with occlusion of their lumen (black arrow), periglomerular edema, and in the interstitial tissue (star) with inflammatory cells infiltration (yellow arrow). c Jojoba oil group showing normal histologic architecture of renal tissue as control group. d Fipronil + Jojoba oil group showing nearly normal glomeruli and renal tubules except for presence of congestion of glomeruli and interstitial tissue (blue arrow), cloudy swelling of renal epithelium of scattered tubules
Brain
Control group (Fig. 6a) and jojoba oil group (Fig. 6c) revealed normal histologic architecture of cerebrum. Fipronil group (Fig. 6b) showed vacuolation (spongiosis) of neuropil of frontal cerebrum, neuronal degeneration, peri-neuronal edema, satellitosis, and proliferation of microglia cells around dead neurons (beginning of neuronophagia). Administration of jojoba oil ameliorated the damage induced by fipronil and brain only revealed mild demyelination, peri-neuronal edema, neuronophagia, and peri-vascular cuffing (Fig. 6d). The semiquantitative analysis of brain lesions revealed marked amelioration of lesions in the fipronil-jojoba group (Table 5).
Representative histopathological photomicrographs of rat brain cerebrum (hematoxylin and eosin stain × 20. Scale bar 50 μm). a Control group showing normal histologic architecture of cerebrum. b Fipronil group showing vacuolation (spongiosis) of neuropil of frontal cerebrum (star), neuronal degeneration (black arrow), peri-neuronal edema (green arrow), satellitosis (yellow arrow), proliferation of microglia cells around dead neurons (beginning of neuronophagia) (blue arrow). c Jojoba oil group showing normal histologic architecture of cerebrum as control group. d Fipronil + Jojoba oil group showing mild spongiosis (star), peri-neuronal edema (green arrow), and peri-vascular cuffing (head arrow)
Discussion
In the current investigation, we have studied the possible protective effect of jojoba oil against the toxic effects induced by fipronil administered orally at 1/10th LD50 for 28 days on liver, kidney, and brain of rats.
Our data indicated significant elevations in the activates of ALT, AST, ALP, and LDH which are consistent with previous studies of Mossa et al. (2015), Kartheek and David (2018), Elgawish et al. (2019), and AlBasher et al. (2020). Elevated blood transaminases indicate liver dysfunction, where cellular degeneration causes leakage of intracellular ALT to the blood. ALT is a specific marker of hepatocellular degeneration which correlates well with the damage degree and consequent leakage out membranes. AST is less specific to the liver, as it is present also in heart (Whalan 2015). Elevated ALP activity may indicate liver disease, cholestasis, or osteoclastic activity (Whalan 2015). LDH is found nearly in almost all tissues making it a general marker for non-specific cytotoxicity and cell lysis (Bagchi et al. 1995).
In line with the changes in liver function biomarkers, the histomorphological examination of the liver tissue revealed congestion with inflammatory cell infiltration of central vein, ballooning degeneration of hepatocytes and area of coagulative necrosis infiltrated by inflammatory cells. Similar findings were previously reported by Mossa et al. (2015) and Abdel-Daim et al. (2019).
Herein, the nephrotoxic effect of fipronil was demonstrated by elevated serum urea and creatinine levels in addition to glomerular and tubular degeneration with interstitial congestion, edema, and leukocytic infiltration. Our results agree with the studies of Badgujar et al. (2015), Mossa et al. (2015), and Abdel-Daim et al. (2019). Urea is a waste product of protein metabolism produced by liver and freely filtered by glomeruli, while some urea is reabsorbed passively with water in the proximal tubules. Creatinine is a non-protein nitrogen produced from muscle creatine and phosphocreatine. It is a more specific marker of impaired glomerular filtration than urea, as it is filtered almost entirely by the glomeruli (Whalan 2015).
In this study, the neurotoxicity of fipronil was evidenced by increased brain GABA concentration accompanied with neuronal degeneration, peri-neuronal edema, neuronophagia, satellitosis, and proliferation of microglia cells around dead neurons. Similarly, Badgujar et al. (2015) and Khalaf et al. (2019) have shown histopathological alterations in the brain of fipronil-intoxicated animals. GABA is an important inhibitory neurotransmitter in the CNS (Schousboe and Waagepetersen 2007). The concentration of GABA depends upon the dynamic balance between its catabolism and new synthesis, mainly from l-glutamate, in the GABAergic neurons by the activity of glutamate decarboxylase (GAD). GABA is catabolized in astrocytes by GABA transaminase (GABA-T) which transforms GABA to succinic semialdehyde (SSA) (de Graaf et al. 2006). The accumulation of GABA in living organisms may result from elevated GAD activity or reduced GABA-T activity (de Graaf et al. 2006; Shimajiri et al. 2013). In addition, the rise in brain GABA level could be attributed to accumulation of GABA at the synaptic junctions (Gunasekara et al. 2007; Simon-Delso et al. 2015) due to targeting GABA receptor in the CNS (Wang et al. 2016).
To elucidate the biochemical pathways underlying fipronil-induced hepatorenal- and neuro-toxicity, oxidant/antioxidant status was studied in organs of intoxicated rats. Our results revealed that fipronil induced oxidative injury in the liver, kidney, and brain evidenced by elevated MDA and NO concentrations with reduction of the GSH level and the activities of enzymatic antioxidants SOD and CAT. Our findings are in accordance with previous investigations demonstrating oxidative damage by fipronil in intoxicated rats (Mossa et al. 2015; Swelam et al. 2017; Kartheek and David 2018; Khalaf et al. 2019; AlBasher et al. 2020), mice (Badgujar et al. 2015), and Japanese Quails (Khalil et al. 2019).
The elevation of the lipid peroxidation marker malonaldehyde (MDA) in liver, kidney, and brain indicates damage of cellular membranes by ROS associated with inability of the cellular enzymatic and non-enzymatic antioxidants to detoxicate the ROS in these organs (Nordberg and Arnér 2001). The increase in nitric oxide (NO) level may be attributed to activation of inducible nitric oxide synthase. NO may further combine with superoxide anion to form peroxynitrite, a noxious nitrogen species (Pacher et al. 2007). Thus, NO may contribute to hepatorenal and brain damage by inducing nitrosative stress response in the fipronil-treated rats.
Glutathione (GSH), the major intracellular antioxidant, is very critical for detoxication and clearance of poisons, acting as scavenger of free radicals, and as substrate for the antioxidant enzymes GPx and GST, in addition to its role in regenerating vitamins E and C to their bioactive form (Meister and Anderson 1983; Deyashi and Chakraborty 2016). Herein, the depletion of GSH may reflect failure of hepatic, renal, and brain antioxidant systems to comate the oxidizing effect of ROS in fipronil-treated animals.
The diminished catalase (CAT) activity could be attributed to utilization of the enzyme in the detoxification of H2O2 produced by oxidative stress. If H2O2 level is elevated, it may generate hydroxyl radical through Fenton reaction, and thus further exaggerates the oxidative stress (Ratliff et al. 2016). The reduction of SOD activity reflects consumption of that enzyme in detoxication of the superoxide radical. The decrease in antioxidant enzyme activities may be attributed to direct action of ROS on these enzymes, substrate depletion, and/or reduction of transcription and translation processes (Yonar and Sakin 2011). Herein, the oxidative damage of liver, kidney, and brain of intoxicated rats is correlated with the observed histopathological alterations in these organs.
In the present study, our data indicated an apoptotic effect of fipronil in liver, kidney, and brain of intoxicated rats evidenced by increased DNA fragmentation, enhanced caspase-3 expression, and downregulation of Bcl2 gene expression. Consistent with our results, Park et al. (2016) reported that treatment of SH-SY5Y cells with fipronil induced apoptosis via activation of caspase-9 and -3, leading to nuclear condensation. Abdel-Daim et al. (2019) reported that fipronil induced overexpression of the proapoptotic Bax gene, while it downregulated the expression of the antiapoptotic Bcl2 gene in liver and kidney of rats. Additionally, Khalaf et al. (2019) reported that the insecticide upregulated the expression of brain caspase-3 and Bax genes.
Of note are the findings of Wang et al. (2013). They have shown that fipronil-induced apoptosis in Spodoptera frugiperda (Sf9) cell line is mediated, at least in part, by the increased •OH radical, depleted GSH, and ATPase. Apoptosis is responsible for the cytotoxicity of various poisons causing nuclear DNA fragmentation (Elmore 2007). It has two pathways; the intrinsic one is initiated by intracellular signals such as DNA damage and mediated by disruption of mitochondrial membrane potential releasing death factors into cytosol, and regulation of Bcl2 family proteins. On the other hand, the extrinsic pathway is mediated by death receptors. In both pathways, activation of caspases causes cell death by degrading key nuclear and structural proteins (Ray and Corcoran 2009).
Critical to preventing the toxic effects induced by insecticides like fipronil is seeking effective protectives of natural origin. Jojoba (Simmondsia chinensis) is an economically important plant due to its high oil contents in seeds (50%), ability to grow in desert with inadequate water (Abdel-Mageed et al. 2014), and using its meal as animal and fish feed (Bouali et al. 2008; Khalil et al. 2009).
In the current study, jojoba oil, interestingly, ameliorated the toxic effects produced by fipronil in rats as evidenced by improved liver and kidney function biomarkers, and diminished lipid peroxidation measured by MDA and NO levels in liver, kidney, and brain. It also enhanced the antioxidant status by improving cellular GSH level, and CAT and SOD activities. Moreover, it ameliorated the histomorphological alterations induced by fipronil in liver, kidney, and brain. Of note, jojoba oil demonstrated marked anti-apoptic effect shown by reduced DNA fragmentation and caspase-3 expression, and overexpression of Bcl2 gene.
Previous studies confirm the protective effect of jojoba oil against the toxic insults induced by various toxicants. Moawad et al. (2018) reported that jojoba oil ameliorated the toxic effects of diethyl nitrosamine (DEN) in rats as indicated by reduction of the serum transaminases activities, liver MDA content, and the histopathological alterations. Sobhy et al. (2016) have demonstrated hepatoprotective and anti-apoptic effects of jojoba oil in rats intoxicated with aflatoxins. Also, Sobhy et al. (2015) and Nassar et al. (2017) indicated the hepatoprotective effect of jojoba oil in rats poisoned with cadmium and CCl4, respectively. Moreover, jojoba ethanolic extract was shown to have a similar protective effect against fumonisin hepatotoxicity (Reda et al. 2009; Abdel-Wahhab et al. 2016) and nephrotoxicity (Reda et al. 2009) in rats.
Importantly, the antioxidant property of jojoba oil based on nitric oxide and DPPH scavenging assays has been illustrated (Manoharan et al. 2016). The protective effect of jojoba oil could be attributed to the high antioxidant content which enables it to scavenge ROS and comate the consequent oxidative damage. Kara (2018) has attributed the antioxidant activity of the ethanolic extract of jojoba leaves and seeds to the high total phenolic substances. Badr et al. (2017) have shown that jojoba oil demonstrated good antioxidant capacities compared with ascorbic acid due to its high total phenolic compounds (12.5 mg gallic acid/gram) and total flavonoid content (5.92 mg catechol/gram). In fact, the antioxidant effect of jojoba seed extract may be due to the presence of compounds such as quercetin, quercetin 3-glucoside, catechin, caffeic acid, and gallic acid (Belhadj et al. 2018). Jojoba oil was reported to contain a natural antioxidant demonstrated to be a hydroxytoluene allylic derivative (Kampf et al. 1986). Kono et al. (1981) demonstrated that tocopherol α, γ, and δ isomers are found in jojoba oil in different amounts, depending on the oil origin, with the γ-isomer being the most abundant. Besides, the anti-apoptic effect of jojoba oil could be due to the presence of polyphenols such as kaempferol, rutin, and apigenin (Belhadj et al. 2018). Interestingly, jojoba plant was reported to protect from free radicals due to presence of lipoxygenase inhibitors (Abdel-Mageed et al. 2014) which suppress leukotriene synthesis and consequent inflammation (Haeggström and Funk 2011).
Conclusion
Fipronil induced hepatorenal and neurotoxic effects in rats associated with apoptosis and histopathological alterations. Oxidative stress and apoptosis seem to play a pivotal role in mediating fipronil-induced toxic injury. Of note, cotreatment of jojoba oil with fipronil significantly ameliorated these toxic effects as evidenced by improved liver and kidney functions, oxidant/antioxidant status, brain GABA, and apoptosis markers (DNA fragmentation, and casp-3 and Bcl2 gene expression). These observations suggested that jojoba oil has promising antioxidant and anti-apoptic properties. However, additional investigations are warranted to further explore the biochemical pathways underlying jojoba oil protective effects.
Data availability
Not applicable.
References
Abdel-Daim MM, Dessouki AA, Abdel-Rahman HG, Eltaysh R, Alkahtani S (2019) Hepatorenal protective effects of taurine and N-acetylcysteine against fipronil-induced injuries: the antioxidant status and apoptotic markers expression in rats. Sci Total Environ 650:2063–2073. https://doi.org/10.1016/j.scitotenv.2018.09.313
Abdel-Mageed WM, Bayoumi SALH, Salama AAR, Salem-Bekhit MM, Abd-Alrahman SH, Sayed HM (2014) Antioxidant lipoxygenase inhibitors from the leaf extracts of Simmondsia chinensis. Asian Pac J Trop Med 7:S521–S526. https://doi.org/10.1016/S1995-7645(14)60284-4
Abdel-Wahhab MA, Joubert O, El-Nekeety AA, Sharaf HA, Abu-Salem FM, Rihn BH (2016) Dietary incorporation of jojoba extract eliminates oxidative damage in livers of rats fed fumonisin contaminated diet. Hepatoma Res 2:78–86. https://doi.org/10.4103/2394-5079.168078
AlBasher G, Abdel-Daim MM, Almeer R, Ibrahim KA, Hamza RZ, Bungau S, Aleya L (2020) Synergistic antioxidant effects of resveratrol and curcumin against fipronil-triggered oxidative damage in male albino rats. Environ Sci Pollut Res Int 27:6505–6514. https://doi.org/10.1007/s11356-019-07344-8
Arafa NM, Salem SM, Farid OA (2010) Influence of Echinacea extracts pre- or postnatal supplementation on immune and oxidative status of growing rabbits. Ital J Anim Sci 9:e63
Badgujar PC, Pawar NN, Chandratre GA, Telang AG, Sharma AK (2015) Fipronil induced oxidative stress in kidney and brain of mice: protective effect of vitamin E and vitamin C. Pestic Biochem Physiol 118:10–18. https://doi.org/10.1016/j.pestbp.2014.10.013
Badr AN, Shehata MG, Abdel-Razek AG (2017) Antioxidant activities and potential impacts to reduce aflatoxins utilizing jojoba and jatropha oils and extracts. Int J Pharmacol 13:1103–1114
Bagchi D, Bagchi M, Hassoun EA, Stohs SJ (1995) In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicol 104:129–140. https://doi.org/10.1016/0300-483x(95)03156-a
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques. 6th edn. New York, pp 440-450.
Belhadj S, Hentati O, Hamdaoui G, Fakhreddine K, Maillard E, Dal S, Sigrist S (2018) Beneficial effect of jojoba seed extracts on hyperglycemia-induced oxidative stress in RINm5f Beta cells. Nutrients 10:384. https://doi.org/10.3390/nu10030384
Bouali A, Bellirou A, Boukhatem N, Hamal A, Bouammali B (2008) Enzymatic detoxification of jojoba meal and effect of the resulting meal on food intake in rats. Nat Prod Res 22:638–647. https://doi.org/10.1080/14786410701614341
Chtourou Y, Aouey B, Kebieche M, Fetoui H (2015) Protective role of naringin against cisplatin induced oxidative stress, inflammatory response and apoptosis in rat striatum via suppressing ROS-mediated NFkappaB and P53 signaling pathways. Chem Biol Interact 239:76–86
de Graaf RA, Patel AB, Rothman DL, Behar KL (2006) Acute regulation of steady-state GABA levels following GABA-transaminase inhibition in rat cerebral cortex. Neurochem Int 48:508–514. https://doi.org/10.1016/j.neuint.2005.12.024
Deyashi M, Chakraborty SB (2016) Pesticide induced oxidative stress and the role of antioxidant defense system in animal body. Harvest 2:1–14
Elgawish RA, Abdelrazek HMA, Ismail SAA, Loutfy NM, Soliman MTA (2019) Hepatoprotective activity of Uncaria tomentosa extract against sub-chronic exposure to fipronil in male rats. Environ Sci Pollut Res Int 26:199–207. https://doi.org/10.1007/s11356-018-3615-5
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516. https://doi.org/10.1080/01926230701320337
Environmental Protection Agency (1996) New pesticide fact sheet. PB96-181516. epa 737-F-96-005. U.S. EPA Office of Prevention, Pesticides and Toxic Substances. May 1996.
Gunasekara AS, Truong T, Goh KS, Spurlock F, Tjeerdema RS (2007) Environmental fate and toxicology of fipronil. J Pest Sci 32:189–199
Gupta RC, Anadon A (2018) Fipronil. In: Gupta RC (ed) Veterinary Toxicology, Basic and Clinical Principles, 3rd edn. Academic press, London, pp 533–538
Haeggström JZ, Funk CD (2011) Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem Rev 111(10):5866–9588. https://doi.org/10.1021/cr200246d
Heinrikson RL, Meredith SC (1984) Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal Biochem 136:65–74. https://doi.org/10.1016/0003-2697(84)90307-5
Hurley PM, Hill RN, Whiting RL (1998) Mode of carcinogenic action of pesticides inducing thyroid follicular cell tumors in rodents. Environ Health Perspect 106:437–445. https://doi.org/10.1289/ehp.98106437
Jin ML, Park SY, Kim YH, Oh JI, Lee SJ, Park G (2014) The neuroprotective effects of cordycepin inhibit glutamate-induced oxidative and ER stress-associated apoptosis in hippocampal HT22 cells. Neurotoxicol 41:102–111. https://doi.org/10.1016/j.neuro.2014.01.005
Kampf A, Grinberg S, Galun A (1986) Oxidative stability of jojoba wax. J Am Oil Chem Soc 63:246–248. https://doi.org/10.1007/BF02546148
Kara Y (2018) Phenolic contents and antioxidant activity of jojoba (Simmondsia chinensis (Link). Schindler. Int J Sec Metabolite 4:142-147.
Kartheek RM, David M (2018) Assessment of fipronil toxicity on wistar rats: a hepatotoxic perspective. Toxicol Rep 5:448–456. https://doi.org/10.1016/j.toxrep.2018.02.019
Khalaf AA, Galal MK, Ibrahim MA, Abd Allah AA, Afify MM, Refaat R (2019) The Terminalia laxiflora modulates the neurotoxicity induced by fipronil in male albino rats. Biosci Rep 39:BSR20181363. https://doi.org/10.1042/BSR20181363
Khalil FF, Farrag FH, Mehrim AI (2009) Evaluation of using jojoba meal (Simmondsia chinensis) supplemented with methionine and Biogen instead of fish meal in the diet of mono-sex nile tilapia (Oreochromis niloticus). Egyptian J Nutrition and Feeds 12:141–156
Khalil SR, Mohammed WA, Zaglool AW, Elhady WM, Farag MR, El Sayed SAM (2019) Inflammatory and oxidative injury is induced in cardiac and pulmonary tissue following fipronil exposure in Japanese quail: mRNA expression of the genes encoding interleukin 6, nuclear factor kappa B, and tumor necrosis factor-alpha. Environ Pollut 251:564–572. https://doi.org/10.1016/j.envpol.2019.05.012
Kono Y, Tomita K, Katsura H Ohta S (1981) Antioxidant in jojoba crude oil. In: Puebla M (ed) Proceedings of the Fourth International Conference on Jojoba, Hermosillo, pp 239-256.
Laskin JD, Heck DE, Laskin DL (2009) Nitric oxide pathways in toxic responses. In: Ballantyne B, Marrs TC, Syversen T (eds) General and applied toxicology. John Wily and Sons Inc., USA, pp 425–438
Lenaz G, Strocchi P (2009) Reactive oxygen species in the induction of toxicity. In: Ballantyne B, Marrs TC, Syversen T (eds) General and Applied Toxicology. John Wily and Sons Inc., USA, pp 367–410
Livak KJ, Schmittgen HD (2001) Analysis of relative gene expression data using realtime quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Manoharan S, Vishnupriya V, Gayathri R (2016) Phytochemical analysis and in vitro antioxidant activity of jojoba oil. J Pharm Sci Res 8:512–516
Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52:711–760. https://doi.org/10.1146/annurev.bi.52.070183.003431
Michael JD (2008) The toxicologist’s pocket handbook, 2nd ed, Informa Healthcare, New York, NY, USA.
Miwa TK (1971) Jojoba oil wax esters and derived fatty acids and alcohols: Gas chromatographic analyses. J Am Oil Chem Soc 48:259–264. https://doi.org/10.1007/BF02638458
Moawad M, Hegazy AE, Nasr GM, Sakr SA, Handa AK, Nasr MI (2018) Evaluation of the protective effect of jojoba natural products on hepatotoxicity of diethylnitrosamine in rats. Egypt J Genet Cytol 47:193–201
Mossa AH, Swelam ES, Mohafrash SMM (2015) Sub-chronic exposure to fipronil induced oxidative stress, biochemical and histopathological changes in the liver and kidney of male albino rats. Toxicol Rep 2:775–784. https://doi.org/10.1016/j.toxrep.2015.02.009
Narahashi T, Zhao X, Ikeda T (2010) Glutamate-activated chloride channels: unique fipronil targets present in insects but not in mammals. Pestic Biochem Physiol 97:149–152. https://doi.org/10.1016/j.pestbp.2009.07.008
Narahashi T, Zhao X, Ikeda T, Nagata K, Yeh JZ (2007) Differential actions of insecticides on target sites: basis for selective toxicity. Hum Exp Toxicol 26:361–366. https://doi.org/10.1177/0960327106078408
Nassar MH, Ibrahim IA, Sobhy HM, Saleh SY, Elbeltagy MA (2017) Effect of jojoba oil and extra virgin olive oil on genetic expressions and DNA damage in induced CCl4 toxicity in rats Egypt. J Chem Environ Health 3:93–109
Nordberg J, Arnér ES (2001) Reactive oxygen species, antioxidants and the mammalian thioredoxin system. Free Radic Biol Med 11:1287–1312. https://doi.org/10.1016/s0891-5849(01)00724-9
Pacher P, Beckman JS, Liaudet L (2007) Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87:315–424. https://doi.org/10.1152/physrev.00029.2006
Park JH, Park YS, Lee JB, Park KH, Paik MK, Jeong M, Koh HC (2016) Meloxicam inhibits fipronil-induced apoptosis via modulation of the oxidative stress and inflammatory response in SH-SY5Y cells. J Appl Toxicol 36:10–23. https://doi.org/10.1002/jat.3136 Epub 2015 Mar 13
Perandones CE, Illera AV, Peckham D, Stunz LL, Ashman RF (1993) Regulation of apoptosis in vitro in mature murine spleen T cells. J Immunol 151:3521–3529
Phillips SJ, Comus PW (2000) In: Phillips, S.J., Comus, P.W. (Eds.), A natural history of the Sonoran Desert. University of California Press, Berkeley and Los Angeles,CA, pp 256–257
Ratliff BB, Abdulmahdi W, Pawar R, Wolin MS (2016) Oxidant mechanisms in renal injury and disease. Antioxid Redox Signal 25:119–146. https://doi.org/10.1089/ars.2016.6665
Ray SD, Corcoran GB (2009) Cell Death and Apoptosis. In: Ballantyne B, Marrs TC, Syversen T (eds) General and Applied Toxicology. John Wily and Sons Inc., USA, pp 247–312
Reda M, Sharaf HA, Gaber EM, Embaby EM, El-Katan N, Abdel-Wahhab MA (2009) Evaluation of the protective effects of jojoba extract against fuminosin toxicity in rats. Egypt J Hosp Med 35:254–270
Sánchez M, Avhad MR, Marchetti JM, Martínez M, Aracil J (2016) Jojoba oil: a state of the art review and future prospects. Energy Convers Manag 129:293–304. https://doi.org/10.1016/j.enconman.2016.10.038
Schousboe A, Waagepetersen HS (2007) GABA: homeostatic and pharmacological aspects. Prog Brain Res 160:9–19. https://doi.org/10.1016/S0079-6123(06)60002-2
Shimajiri Y, Oonishi T, Ozaki K, Kainou K, Akama K (2013) Genetic manipulation of the γ-aminobutyric acid (GABA) shunt in rice: overexpression of truncated glutamate decarboxylase (GAD2) and knockdown of γ-aminobutyric acid transaminase (GABA-T) lead to sustained and high levels of GABA accumulation in rice kernels. Plant Biotechnol J 11:594–604. https://doi.org/10.1111/pbi.12050
Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C, Furlan L, Gibbons DW, Giorio C, Girolami V, Goulson D, Kreutzweiser DP, Krupke CH, Liess M, Long E, McField M, Mineau P, Mitchell EA, Morrissey CA, Noome DA, Pisa L, Settele J, Stark JD, Tapparo A, Van Dyck H, Van Praagh J, Van der Sluijs JP, Whitehorn PR, Wiemers M (2015) Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ Sci Pollut Res Int 22:5–34. https://doi.org/10.1007/s11356-014-3470-y
Sobhy HM, Abo Elmagd MK, El-kholy MA, Abo Elmagd EK, Laz ES (2016) Hepatoprotective effect of jojoba oil and Nigella sativa seeds in rats fed diet containing aflatoxin. Egypt J Chem Environ Health 2:21–37
Sobhy HM, Mansour KA, Zaki AA, Elkholy M (2015) Hepatoprotective effect of jojoba oil on DNA damage and antioxidant enzymes induced by cadmium in rats. Egypt J Chem Environ Health 1:94–112
Swelam ES, Abdallah IS, Mossa AH (2017) Ameliorating effect of zinc against oxidative stress and lipid peroxidation induced by fipronil in male rats. J Pharmacol Toxicol 12:24–32
Wang X, Martínez MA, Wu Q, Ares I, Martínez-Larrañaga MR, Anadón A, Yuan Z (2016) Fipronil insecticide toxicology: oxidative stress and metabolism. Crit Rev Toxicol 46:876–899. https://doi.org/10.1080/10408444.2016.1223014
Wang XQ, Li YG, Zhong S, Zhang H, Wang XY, Qi PP, Xu H (2013) Oxidative injury is involved in fipronil-induced G2/M phase arrest and apoptosis in Spodoptera frugiperda (Sf9) cell line. Pestic Biochem Physiol 105:122–130. https://doi.org/10.1016/j.pestbp.2012.12.008
Whalan JE (2015) Clinical chemistry. In: A Toxicologist’s Guide to Clinical Pathology in Animals. Springer International Publishing, Switzerland, pp 67–94
Woodward KN (2012) Veterinary pesticides. In: Marrs TC (ed) Mammalian toxicology of insecticides. RSC Publ, Cambridge, pp 348–426
Yonar ME, Sakin F (2011) Ameliorative effect of lycopene on antioxidant status in Cyprinus carpio during pyrethroid deltamethrin exposure. Pestic Biochem Physiol 99:226–231. https://doi.org/10.1016/j.pestbp.2010.12.008
Acknowledgments
We thank consult of Central Labs in both Faculty of Veterinary Medicine, University of Sadat City & of Faculty of Veterinary Medicine, Cairo University, as we had bench space to work.
Funding
The authors declare that there are no sources of funding to be acknowledged.
Author information
Authors and Affiliations
Contributions
Shimaa M. Abou-Zeid performed the experimental protocol and biochemical analysis of liver and kidney functions, and oxidant/antioxidant biomarkers. Huda O. AbuBakr participated in GABA estimation, apoptosis analysis and gene expression study. Enas A. Tahoun performed the histopathological examination. All authors have contributed to writing this article. All authors critically read and revised the manuscript, and approved its submission for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Ethics approval and consent to participate this study was approved by the Institutional Animal Care and Use Committee (IACUC) (Approval number: VUSC-011-2-19), Faculty of Veterinary Medicine, University of Sadat City, Egypt.
Consent for publication
Not applicable.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abou-Zeid, S.M., Tahoun, E.A. & AbuBakr, H.O. Ameliorative effects of jojoba oil on fipronil-induced hepatorenal- and neuro-toxicity: the antioxidant status and apoptotic markers expression in rats. Environ Sci Pollut Res 28, 25959–25971 (2021). https://doi.org/10.1007/s11356-020-12083-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-12083-2