Abstract
This study investigated the effect of Ulva fasciata and Sargassum lacerifolium seaweeds as heavy metal remediators for soil and on the growth of radish (Raphanus sativus L.). The soil was inoculated by dry biomass of each seaweed alone and by their mixture. Seaweeds inoculation increased the organic matter content, clay-size fraction, and nutrients in the soil. Seaweeds mixture treatment caused a significant reduction in the contents of Pb, Cu, Zn and Ni in the soil samples and reduced them to the tolerable limits (40.2, 49.3, 43.8 and 1.1 mg kg-1, respectively), while Cd, Cr, Fe, and Mn contents were closely decreased to the tolerable limits. Biosorption of soil heavy metals by seaweeds decreased the bioaccumulated concentrations of metals in radish plant roots and/or translocated to its shoots compared to control. For seaweeds mixture-treated soil, cultivated radish roots were able to phyto-extract Cd, Cu, Cr, and Ni from the soil (bioaccumulation factor values > 1) of 7.45, 1.18, 3.13, and 26.6, respectively. Seaweeds inoculation promoted the growth of cultivated radish and improved the germination percentage and the morphological and biochemical growth parameters compared to control plants. The achieved soil remediation by dried seaweeds might be due to their efficient metal biosorption capacity due to the existence of active functional groups on their cell wall surfaces. Increased growth observed in radish was as a result of nutrients and growth hormones (gibberellins, indole acetic acid, and cytokinins) present in dried seaweeds. This study shows the efficiency of seaweeds as eco-friendly bioremediators for controlling soil pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollutants in the soil are very hazardous due to their toxic effects and accumulation throughout the food chain (Zhuo et al. 2019). Hence, they seriously affect human health and ecosystem balance (Ayangbenro and Babalola 2017; Zhang et al. 2018). Therefore, it is an urgent requirement to apply an efficient, eco-friendly, and low-cost remediation method for heavy metals in soil (Sud et al. 2008).
The adsorption by biomass is an important technique for the removal of heavy metals from the environment. Marine macroalgae (seaweeds) are one of the most promising types of biomasses suitable for their use as biosorbents (Das et al. 2016; Nasab et al. 2017). The potential of seaweeds as biosorbents for removal of heavy metal has been discussed in many research works, showing that seaweeds offer continuous availability and reusability of sorbent in numerous cycles (Abd-Elhady 2015; Fatemeh Faraji et al. 2016; Pandya et al. 2017).
Seaweeds possess efficient metal biosorption capabilities due to the existence of active functional groups on the surface of their cell walls such as polysaccharides, proteins, amino, hydroxyl, carboxyl, and sulfate. These groups act as binding sites for metals (Kang et al. 2012). The capacity of seaweeds for biosorption of heavy metals depends on the number of functional groups on their cell surface, the sorbed ion coordination number, availability, and affinity of the binding groups for the metal ions as well as the chemical state of the formed complexes. According to the composition of the cell wall in different seaweeds, the metal biosorption capacity will differ (Mehta and Gaur 2005). The green seaweed Ulva sp. is particularly useful in this respect because of its wide distribution and relatively simple structure. Ulva sp. has a sheet-like thallus of two cells in thickness, resulting in a relatively high surface area of structurally uniform and physiologically active cells (Sarl and Tuzen 2007). Badescu et al. (2016) estimated the biosorption capacity of Ulva lactuca and discovered it was as much as 29.63 mg Zn (II)/g. The biosorption of different metals by seaweed biomasses has shown that brown algae exhibited a higher biosorption capacity. Metal biosorption has been attributed to the presence of alginates in their cell walls, which have a high affinity for divalent cations; besides sulfated polysaccharides are responsible for the uptake of trivalent cations (Murphy et al. 2008; Kang et al. 2012). Abd-Elhady and El-Zabalawy (2014) and Abd-Elhady (2015) reported the efficient use of the mixture of Ulva sp. and Gelidium sp. as dry biomass for remediation of contaminated soil with Fe, Zn, Mn, and Pb heavy metals. Also, the authors recommend using the dry biomass mixture of these seaweeds as phyto-remediators for soil which cultivated with plants that either the leaves and/or roots are eaten, such as radish and lettuce.
Seaweeds do not only remediate soils but also serve as biofertilizers and biostimulants for both soils and plants (Melo et al. 2020). The fertilizer obtained from seaweed is biodegradable, non-polluting, non-toxic, and non-hazardous to humans and other animals, including birds (Raghunandan et al. 2019). In addition, slow release of seaweeds in soils can enhance plant efficiency and minimize environmental pollution (Wang et al. 2017). Furthermore, seaweeds are an excellent source of micro- and macroelements required for plant nutrition (Patel et al. 2017). They help to enhance the biochemical constituents of carbohydrates, lipids, proteins, fibers, ash, phenol, and dietary fiber in plants. Soil fertilization by seaweeds improve seed germination, shoot and root elongation, water, and nutrient uptake, frost and saline resistance and resistance toward phytopathogenic organisms (Kasim et al. 2015; Nabti et al. 2017; Silva et al. 2019).
Raphanus sativus L. (radish), one of the essential feeding crops, belongs to the family Brassicaceae. It is grown all over the world for its fleshy, edible tuberous root, which can be eaten either raw or cooked. A wide variety of cultivars are available, producing taproots that range from 2 cm up to 1 m long and from red to pink, white, purple, or black. It is an ancient vegetable in both tropical and temperate regions (Poudel et al. 2018). World production of radish roots was estimated at 7 million tons per year, about 2% of the total world production of vegetables (Schippers 2004). It is essential in Southeast Asia and North Africa: the total area cultivated with radish was 18,190 ha and total production of 9000 tons in Egypt (FAO 2014). Radish is grown in Egypt during the winter season for local consumption and exportation. It is a good source of vitamin C and minerals like calcium, potassium, and phosphorus. It also had medicinal uses as it stimulates the appetite and digestion, having intestines tonic and laxative effect and indirectly stimulating the flow of bile (Schippers 2004).
Heavy metal accumulation in plants depends upon plant species and the efficacy of different plants in absorbing metals. This was assessed by either plant uptake or soil-to-plant transfer factors of the metals, i.e., bioaccumulation factor. The bioaccumulation capability of heavy metals in the underground organs acts as a defensive mechanism to protect the plant shoots against the harmful effects of toxic metal levels, which may affect photosynthetic processes (Bonanno et al. 2017). Cultivation of crops for human or livestock consumption on contaminated soil can lead to the uptake and accumulation of heavy metals in the edible parts of plants with a resulting danger to human and animal health (Shehata and Galal 2020).
Many studies discussed the removal of heavy metals by seaweeds from polluted water, but studies based on seaweeds for the removal of heavy metals from the contaminated soil are still few. According to the cited literature, the two species of U. fasciata and S. lacerifolium used in this study are employed for heavy metal remediation of polluted soils for the first time. In addition, they are common seaweeds in the Egyptian coasts. Hence, the present study aimed at evaluating the efficiency of the dry biomasses of two seaweeds: U. fasciata (green seaweed) and S. lacerifolium (brown seaweed) as remediators for contaminated soil. The study also assessed the effect of the applied seaweeds on the growth parameters, nutrients, pigments, and organic contents of the radish plants cultivated in heavy metal contaminated soil and determining its heavy metal bioaccumulation and translocation factors.
Materials and methods
Soil sampling and analyses
Three composite soil samples were collected in summer 2018 from the agricultural field at the industrial Fifteen of May city, Egypt (29°51`N and 31° 23′ E) as a profile of 0–50 cm below the soil surface. The sampled soil was brought to the laboratory in plastic bags directly after collection, spread over paper sheets, air dried, and passed through a 2-mm sieve to remove gravels and debris. Soil texture analysis was assessed by Bouyoucos hydrometer method, whereby the percentages of clay, silt, and sand were calculated. The content of organic matter was determined using the loss-on-ignition method at 450 °C (Allen et al. 1986). Determination of calcium carbonate was carried out using Bernard’s calcimeter of the type described by Betremieux (1948). Soil water extract (1:5 w/v) was prepared for determination of soil salinity by electrical conductivity (EC) using electric conductivity meter and soil pH by pH meter (914 pH/Conductometer-Metrohm AG).
The soils were digested using the acid digestion method adopted by Wade et al. (1993) for the determination of N, P, and K mineral nutrients as well as the heavy metal (Cd, Pb, Cu, Cr, Fe, Zn, Ni, and Mn) contents in the collected samples. One gram of soil sample was digested in 20 ml of a tri-acid mixture of HNO3/H2SO4/HClO4 (5:1:1, v/v/v) for 8 h at 80 °C. The digestion was continued until the solution became clear; then, the transparent digests were filtered using a 0.45-μm pore size cellulose nitrate membrane filter paper (Millipore) and diluted up to 50 ml with distilled water before it was stored refrigerated for analysis. The total soluble nitrogen (N) was assessed by the Kjeldahl method; phosphorus (P) was assessed by the molybdenum blue method using a spectrophotometer (U-3900/3900H/ Hitachi-VWR), while potassium (K) was assessed using Flame Photometer (Sherwood Scientific M420). Concentrations of total Cd, Pb, Cu, Cr, Fe, Zn, Ni, and Mn in soil samples were determined using a Perkin-Elmer 3100 Atomic Absorption Spectrophotometer. All of these analyses were according to Allen (1989).
Seaweeds sampling
Two seaweed species were collected from different localities during summer 2018. S. lacerifolium seaweed (Phaeophyta) was collected from Hurghada beach (Red Sea), while the other seaweed species was U. fasciata (Chlorophyta), collected from Ras Al Bar beach (Mediterranean Sea). The gathered seaweeds were brought immediately to the Phycology Laboratory in plastic bags containing seawater to minimize vaporization. The taxonomical identification of the seaweed samples was carried out according to Aleem (1993) and confirmed by using Algae Base https://www.algaebase.org/ website (Guiry and Guiry 2019). The two seaweeds were identified to be S. lacerifolium (Turner) C. Agardh and U. fasciata Delile. The seaweeds were cleaned thoroughly using tap water for removing sand, epiphytes, and debris. A portion was preserved in 5% formalin in seawater for the taxonomical identification, and the remaining portion was dried in the air on a retentive paper at room temperature (25–30 °C). The dried samples were milled with an electrical grinder to obtain a fine powder form. Finally, seaweed powdered samples were kept until use in a clean sealed glass vials in the refrigerator.
The taxonomic position of these seaweeds was confirmed as following: U. fasciata (Delile); Kingdom, Plantae; Phylum: Chlorophyta; Class: Ulvophyceae; Order: Ulvales; Family: Ulvaceae; Genus: Ulva. and S. lacerifolium (Turner) C. Agardh; Kingdom: Chromista; Phylum: Ochrophyta; Class: Phaeophyceae; Order: Fucales; Family: Sargassaceae; Genus: Sargassum.
Seaweeds inoculation and plant growth
A pot experiment was carried out at the experimental farm of Botany Department, Faculty of Science, Tanta University, Egypt, during winter 2018. Soil samples (500 g) were spread in 12 plastic pots of 15 cm in diameter. Pots were prepared in a complete randomized design, and each treatment was replicated three times. Pots were sown with 10 g dry weight of each seaweed species, separately. Another treatment was sown with 10 g dry weight of the mixture of both seaweeds (in equal amounts). The dried seaweeds were inoculated in the wetted soil 2 weeks before cultivating the plant seeds. All pots were placed under 12-h photoperiod at 30 °C, and they were maintained wet by regular spraying with a constant volume of distilled water. Radish (R. sativus) seeds (15 seeds) were sown in each pot of the seaweed-inoculated soil for 45 days in a growth chamber with a light intensity of 45 μEm−2 s−1 under 12 h light/dark cycle. The germination percentage of the radish seedlings was recorded after 1 week of cultivation. The other growth criteria were recorded after 45 days of cultivation which include root lengths, shoot lengths, as well as the fresh weight of the plants’ roots and shoots. The dry weights of roots and shoots were also evaluated after drying at 40 °C for 3 days until constant weight. Leaf area was recorded using Ushikata x-plan 360d Planimeter (Featonby-Smith and Van Staden 1983).
Plant analyses
Three composite samples of the radish roots and shoots were prepared from each treatment and grounded into a powder using a metal grinder for nutrient analysis (Galal 2016). From each composite powder, 0.5–1 g was digested by a mixed-acid digestion method. The concentration of N, P, and K nutrients and the heavy metals (Cd, Pb, Cu, Cr, Fe, Zn, Ni, and Mn) in plant samples were determined as previously mentioned for soil analysis (Allen et al. 1986). In addition, calcium (Ca) and magnesium (Mg) were determined by titration against 0.01 N versenate solution using meroxide and eriochrome black T as indicators and ammonium hydroxide and ammonium chloride as buffers. Sodium was also determined using a flame photometer (Sherwood Scientific M420) (Allen et al. 1986).
Photosynthetic pigments content of the plant
Photosynthetic pigments in the radish leaves were estimated according to the method of Metzner et al. (1965). The pigments in a known weight of fresh leaves were extracted with 85% cold acetone and allowed to extract overnight in the refrigerator. Each extract was prepared in 3 replicates. The absorbance of the pigment extracts was measured at 663, 644, and 452 nm by using a spectrophotometer for chlorophyll a, chlorophyll b, and carotenoids, respectively. To estimate the pigment constituents (mg/g dry weight), the following equations were applied:
Where E is the absorbance at each specified Wavelength (nm).
The total soluble protein content of the plant
Total soluble protein was measured quantitatively in the borate buffer extract of the tested plant, according to Lowry et al. (1951). A sample of 1 ml of borate extract was mixed with 1 ml of 0.2% sodium carbonate in 4% sodium hydroxide and 0.5% copper sulfate in 1% sodium tartrates. The mixture was allowed to stand at room temperature for at least 10 min before the addition of 0.1 ml phenol-folin reagent. After 30 min, the absorbance was measured using a spectrophotometer at 700 nm. The protein content was calculated as mg/g dry weight using a calibration curve of bovine serum albumin as a standard protein.
The total carbohydrate content of the plant
Total carbohydrate content was quantitatively determined by the method of phenol-sulfuric acid described by Dubios et al. (1956). One ml of 5% phenol was added to 0.5 ml of the plant borate buffer extract, and then, 5 ml of conc. H2SO4 solution was added directly on the surface of the tube. The sample was allowed to stand at room temperature for 30 min. The absorption was measured against the blank at 490 nm. The carbohydrate concentration (mg/g dry weight) was estimated after the preparation of a calibration curve using glucose as a standard.
Seaweed analyses
Element analysis
The concentration of nutrients (N, P, K, Ca, Na, and Mg) was determined as previously mentioned in the plant analyses section (2.4).
Exopolysaccharides (EPS) content of the tested seaweeds
The exopolysaccharides (EPS) content of the two tested seaweed species were isolated from the culture medium, according to Sudo et al. (1995). To precipitate proteins from the algal culture, trichloroacetic acid was added to a final concentration of 4%, and the algal culture was stirred for 2 h. Cells and precipitated proteins were removed by centrifugation at 6000 rpm for 30 min at 4 °C. The clear supernatant, which contains EPS, was collected. The EPS was then precipitated by adding 2 V of absolute cold ethanol to a known volume of the clear culture supernatant under continuous stirring, and the solution was allowed to stand overnight at 4 °C. The crude EPS was then obtained by centrifugation at 6000 rpm for 15 min at 4 °C, and the white material was collected. The crude EPS was then further purified by dialysis against cool distilled H2O for 48 h and changed twice daily. The dialyzed samples were then recovered by drying at 37 °C to get a fibrous white powder which was weighed.
Plant growth hormones of the tested seaweeds
Spectrophotometric techniques were used to determine the amounts of indole acetic acid (IAA), gibberellic acid (GA3), and cytokinin according to the method of Ünyayar et al. (1996). One gram of each seaweed sample was taken and combined with 60 ml of ethanol: chloroform: 2 N ammonium hydroxide (12:5:3 v/v/v). Each combined extract (60 ml) was kept in a bottle at − 20 °C in deep freeze for further analysis. The combined extract was treated with 25 ml of distilled water. The chloroform phase was discarded. The water-methanol phase was evaporated. The water phase was adjusted to the exact pH value of 2.5 or 7 or 11 with 1 N HCl or 1 N NaOH, respectively, and 15 ml ethyl acetate was added at each of three steps. This procedure provided the isolation of free-form of IAA, GA3, and cytokinin from the extraction solvent. After an incubation period of 1 h at 70 °C, evaporation of ethyl acetate was performed at 45 °C using a rote-evaporator system (B. chi Instruments). The spectrophotometric assay was done using 222 and 280 nm wavelengths for IAA, 254 nm for GA3, and 269 nm for cytokinin. The amounts of IAA, GA3, and cytokinin in the samples were expressed as standard synthetic IAA, GA3, and zeatin equivalent.
Statistical methodology
After testing the data for normality, the difference in soil and plant parameters with and without algal treatments was assessed by one-way ANOVA. Significant differences between means among control and treatments were identified using Duncan’s multiple range test. The effects of soil algal treatments and different plant organs on different available nutrients (N, P, K, Na, Ca, and Mg), heavy metals, and growth parameters were assessed using repeated measurement ANOVAs (ANOVA-2). The difference between means of nutrients ((N, P, K, Na, Ca, and Mg), hormones, and polysaccharide contents in both algae was tested by using unpaired t test. All values were expressed as a mean of three replicates ± standard deviation (SD). The statistical analyses were performed using SPSS (V. 23.0) software SPSS (2006). The bioaccumulation factor (BF), measuring the plant’s ability to accumulate a specific metal in relation to its concentration in the soil, was calculated as follows: BF = Croot/Csoil, where Croot and Csoil represent the heavy metal concentrations in the root and soil. The translocation factor (TF), indicate the relative translocation of heavy metal from root to shoot of the plant, was calculated as TF = Cshoot/Croot, where Cshoot and Croot denote the heavy metal concentrations in the plant shoot and root (Galal 2016).
Results
Soil characteristics
Soil characteristics show that treatment with seaweeds had a positive effect on its physical and chemical characters (Table 1). The percentage of clay and organic matter (OM) was significantly increased in seaweed-treated soil (according to the F value). The percentage of clay was significantly increased from 32.5% in the control soil to 36.4% and 36.4% in U. fasciata and S. lacerifolium treated soil, respectively, and reached its maximum percentage (37.4%) in seaweeds mixture-treated soil.
Nevertheless, the studied soil had a reasonable percentage of OM (7.56%) and was slightly alkaline (pH = 8.1); the soil pH decreased when it was remediated by U. fasciata (pH = 7.2) and seaweeds mixture (pH = 7.7), while an increased alkalinity value (pH = 8.5) is recorded for S. lacerifolium treated soil (Table 1).
Generally, all nutrients exhibited a significant increase in the soil treated with seaweeds compared to control soil. Nitrogen concentrations increase from 217.5 mg kg−1 in control to 220.7, 218.1, and 225.1 mg kg−1, while phosphorus concentrations increase from 178.9 mg kg−1 in control to 180.2, 179.3, and 180.9 mg kg−1 in U. fasciata, S. lacerifolium, and seaweeds mixture-treated soils, respectively (Table 1).
At the same time, the concentration of all studied heavy metals was significantly reduced as a result of seaweed treatment of soil. The percentages of decrement were as follows: Cd: 84.5, 83.6, and 85.4%; Pb: 30.1, 25.4, and 37.8%; Cu: 22.5, 11.5, and 26.6%; Cr: 46.7, 51.0, and 53.5%; Fe: 39.3, 0.1, and 46.2%; Zn: 36.3, 16.1, and 44%; Ni: 46.2, 30.8, and 57.7%; and Mn: 53, 4.9, and 57.9% for U. fasciata, S. lacerifolium, and the seaweeds mixture-treated soil, respectively. The order of reduction of heavy metal concentrations was Cd > Mn > Ni > Cr > Fe > Zn > Pb > Cu in the treated soil. Also, the concentration of Pb, Cu, Zn, and Ni is reduced to the tolerable limits (Table 1).
Plant analyses
Growth parameters
As shown in Table 2, seaweed treatments in soil induce significant increase (P < 0.01) in all growth parameters compared to control plants. The most pronounced increase was detected in germination (%) and root fresh weight (41.9% and 82.5% respectively) in plants cultivated in the soil treated with the mixture of seaweeds. On the other hand, U. fasciata treated soil recorded the highest increase in root and shoot lengths and dry weight of shoot and leaf area of radish plants which were amounted to be 1.02-fold, 52.22%, 87.5%, and 80.39%, respectively, whereas S. lacerifolium treated soil caused the highest increase in the root dry weight (1.83-fold) and the shoot fresh weight (63.64%) compared to control plants.
Inorganic nutrients
The results listed in Table 3 show that, generally, all seaweed treatments caused significant (P < 0.001) increase in the content of the inorganic nutrients in the root and shoot of radish plants. The highest increase content was recorded in plants cultivated in the soil treated with seaweeds mixture compared to plants cultivated in the control soil and the soil treated with each seaweed alone as well.
In roots of radish, the seaweed mixture treatment recorded the highest increase in all studied nutrients followed by S. lacerifolium then U. fasciata except for K, seaweeds mixture followed by U. fasciata then S. lacerifolium compared to plants cultivated in the control soil. On the other hand, in shoots of radish, the highest increase in N and Na contents was recorded in the seaweed mixture treatment followed by U. fasciata and then S. lacerifolium. The other nutrients (P, K, Ca, and Mg) are also increased in plants cultivated in soil treated with seaweeds according to the following order: the seaweeds mixture followed by S. lacerifolium and then U. fasciata compared to their values estimated in the control plant shoots (Table 3).
Heavy metals
Generally, all treatments of soil with seaweeds caused a highly significant decrease (P < 0.001) in all tested heavy metal concentrations in radish roots and shoots compared to control. The highest decrease is recorded in plants cultivated in the soil treated with seaweeds mixture compared to plants cultivated in the control soil and soil treated with each seaweed alone (Table 3).
Compared to root of the radish plants harvested from the control, a reduction in the concentrations of Cd (91.4, 13.1, and 12.6%), Fe (91.3, 33.2, and 18.9%), Zn (76.1, 35.1, and 24.4%), and Mn (92.8, 21.1, and 20.7%) was recorded in roots of radish plants cultivated in soil treated with seaweeds mixture, S. lacerifolium and U. fasciata, respectively. On the other hand, a reduction in the concentrations of Pb (89.1, 32.2, and 35.1%), Cu (67.7, 27.2, and 27.1%), Cr (74.7, 34.1, and 32.3%), and Ni (55.27, 26.3, and 23.5%) is recorded in root of the radish plants cultivated in soil treated with seaweeds mixture followed by U. fasciata and then S. lacerifolium (Table 3). Based on the maximum reduction percentage recorded in the radish roots cultivated in soil treated with seaweeds mixture, the heavy metals were mitigated in the following order: Mn ˃ Cd ˃ Fe ˃ Pb ˃ Zn ˃ Cr ˃ Cu ˃ Ni.
Compared to shoot of the radish plant harvested from the control, the reduction concentrations of Cd (85.9, 41.3, and 36.3%), Pb (82.1, 40.7, and 21.4%), Cr (81.9, 49.4, and 45.2), Fe (84.8, 41.7, and 30.3), and Zn ( 92.5, 60.5, and 54.0%) were recorded in radish shoots cultivated in soil treated with seaweeds mixture, S. lacerifolium and U. fasciata, respectively. On the other hand, reduction concentrations of Cu (84.1, 53.1, and 50.9%), Ni (72.8, 18.6, and 6.0%), and Mn (90.9, 47.2, and 40.8%) are recorded in shoots of the radish plants cultivated in soil treated with seaweeds mixture followed by U. fasciata and then S. lacerifolium (Table 3). The order of reduction in heavy metal concentrations was Zn ˃ Mn ˃ Cd ˃ Fe ˃ Cu ˃ Pb ˃ Cr ˃ Ni for the radish shoots cultivated in seaweeds mixture-treated soil.
Protein and carbohydrate contents
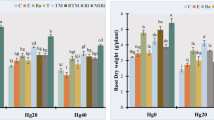
As shown in the results presented in Fig. 1, the roots and shoots of radish plants cultivated in the seaweed-treated soil were observed to contain significant amounts of carbohydrates compared to the control plants. The carbohydrate content in radish roots was increased by 40.8, 32.9, and 23.4% in plants cultivated in the soil treated with seaweeds mixture, U. fasciata and S. lacerifolium, respectively, compared to the carbohydrate content of control plant roots. Likewise, the carbohydrate content was increased by 16.2, 5.7, and 0.7% in the shoot of radish plants cultivated in the soil with U. fasciata, seaweeds mixture, and S. lacerifolium, respectively compared to the carbohydrate content in the shoots of control plants.
Carbohydrate and protein contents (mg/g dry wt.) in roots and shoots of radish (Raphanus sativus L.) plant with and without seaweed treatments. Falgal treatment = 165.2**, Fplant organ = 69.3ns, and Falgal treatment x plant organ = 198.9*, where *P < 0.05 and **P < 0.01. ns, not significant (i.e., P > 0.05)
In contrast, the protein content recorded significantly lower values than carbohydrates. The protein content was increased by 73.4% in the roots of plants cultivated in soil treated with the seaweeds mixture, followed by S. lacerifolium 55.5% and then U. fasciata 38.1%, compared to protein in the roots of the control plants. However, the protein content in the shoots of the treated plants was not substantially affected by the presence of seaweeds. The protein contents in the shoots were increased by 25.9, 10.8, and 7.1% in plants cultivated in soil treated with seaweeds mixture, U. fasciata and S. lacerifolium, respectively, compared to its content in the shoots of the control plants (Fig. 1).
Pigments content
As a general trend, seaweed treatment in soil induces an increase in the pigment contents (chlorophyll a, chlorophyll b, and carotenoids) of the radish leaves compared to the control plant leaves (Fig. 2). Chlorophyll a content was increased by 97.5, 72.9, and 53.2% in the plant leaves cultivated in soil treated with U. fasciata, seaweeds mixture, and S. lacerifolium, respectively. On the other hand, chlorophyll b and carotenoid concentrations are increased by 92.7, 56.4, and 44.5% and 2.14-, 1.80-, and 1.33-folds in the plant leaves cultivated in seaweeds mixture, U. fasciata and S. lacerifolium treated soil, respectively (Fig. 2).
Heavy metal bioaccumulation and translocation
The BF and TF of the radish plants for heavy metals are listed in Table 4. It was found that Cd had the highest BF values (71.5 and 67.2) followed by Ni (34.5 and 27.8) in plants cultivated in the soil treated with U. fasciata and S. lacerifolium, respectively. Seaweeds mixture treatment caused a significant decrease in all tested bioaccumulated heavy metals in the roots of radish (except for Ni of 26.6 as BF value) compared to control plants. The bioaccumulation of heavy metals in plants roots were of the following descending order: Ni > Cd > Cr > Cu > Pb > Zn > Mn > Fe.
While, the highest TF was found for Ni (1.4 and 1.56), followed by Cd (1.35 and 1.25) and then Cr (1.2 and 1.1) in plants cultivated in soil treated with the U. fasciata and S. lacerifolium, respectively, in addition, for plants grown in the soil treated with seaweeds mixture, Cd records the highest TF value (3.04), followed by Fe (1.52), and finally Cr (1.03) (Table 4).
Seaweed analyses
Data illustrated in Fig. 3 show that the concentrations of gibberellin hormone (92.16 and 88.62 mg/g dry) was higher than the concentration of indole acetic acid (IAA) (11.85 and 5.9 mg/g dry wt.) and cytokinin (4.8 and 3.0 mg/g dry wt.) for S. lacerifolium and U. fasciata, respectively. On the other hand, U. fasciata had a higher concentration of extracellular polysaccharides (149.9 mg/g dry wt.) than S. lacerifolium (80.7 mg/g dry wt.). For the inorganic nutrients, S. lacerifolium seaweed had higher concentrations of P (96.5 mg/100 g), Ca (67 mg/100gm), and Mg (43 mg/100 g), while U. fasciata recorded maximum concentrations of N (7.5 mg/100 g), K (46.7 mg/100gm), and Na (36.4 mg/100 g) (Fig. 4).
Discussion
Soil analyses
Soil remediation is necessary to abolish a threat to the environment and human beings from toxic metals. Several studies reported overexcited metal accumulation by plants, and thus, phyto-extraction of metals emerged as a feasible technology for remediation of metal-polluted soils (Garg and Kataria 2010). In the present study, the beneficial effect of seaweed treatments was markedly manifested on soil characteristics. Seaweed treatments slightly decrease the pH and the EC values (Table 1). On the other side, a significant increase in the soil organic matter content and clay percentage was noticed, especially in the treatment with the seaweeds mixture. These findings were in agreement with Gheda and Ahmed (2015), Nabti et al. (2017), and Silva et al. (2019) who reported that seaweed extracts slightly decreased the EC values in a manner not harmful to the plants because they include many bioactive organic molecules that could help plants to tolerate stress caused by elevated EC values. However, the recorded decrease in the pH values for U. fasciata and treatment with the mixture of seaweeds may be attributed to the increased solubility of the polysaccharides content of these treatments, which released more anions in the soil. This explanation could be related to the increased percentage of the aggregated clay particles of the soil for both treatments rather than that treated with S. lacerifolium (Table 1).
Concerning the organic matter percentage estimated in the soil samples, many studies reported the superior ability of seaweeds than chemical fertilizer to increase the organic matter in the soil and thus recover soil fertility and plant yield (Raghunandan et al. 2019) and improve the uptake of water and minerals in the upper soil layers (Abdel-Raouf et al. 2012). In addition, major polymeric carbon compounds of seaweeds, like carrageenans, laminarins, and ulvans as well as alginate, are used to fertilize the soil as being less readily degradable as organic matter by soil microbes (Thirumaran et al. 2009) and so to stimulate overall plant growth (Davari et al. 2012).
The addition of different seaweeds in adequate quantities improves the physical, chemical, and biological properties of the soil and expands the level of soil nutrients like N, P, and K and other minerals necessary for plant growth (Thirumaran et al. 2009). In this connection, the results of the present study (Table 1) show that the nutrient content (K, N, and P, respectively) was amended in the soil samples with different seaweed treatments, especially with seaweeds mixture. According to Abdel-Raouf et al. (2012), seaweeds, when used as biofertilizers, could mitigate the runoff of nitrogen and phosphorus after the application of livestock manure. Seaweeds content of K, N, P, micronutrients, humic acid, and polysaccharides (e.g., laminarin, alginates, and carrageenans), besides growth-promoting phyto-hormones, pointed them admirable biofertilizers for crop plants (Nabti et al. 2017). In addition, these polysaccharide molecules could act as a water-holding complex, and so affect water distribution within the soil, and its particles aggregate stability (Khan et al. 2009). This seaweed-induced soil microbial community might boost nutrient turnover in the soil, which eventually could benefit both the plant growth and soil health (Nabti et al. 2017).
Heavy metal removal
Many studies endorsed seaweeds (either fresh or dry biomass) ability for heavy metal biosorption since their macroscopic structure affords the production of biosorbent particles (Michalak et al. 2016; Ismail and Ismail 2017). As reported by many studies, non-living (dead) seaweed biomass might be more efficient than active (living) seaweeds for heavy metal removal by biosorption, mainly due to the increased surface area of the biomass biding sites. (Kang et al. 2012; Abd-Elhady 2015). (Kang et al. 2012). Commonly, physical treatments of seaweeds by boiling, drying, freezing, and grounding derived an enhanced metal ion biosorption capacity by releasing cell components that probably bind to the heavy metal ions (López Errasquín and Vázquez 2003). In this context, the results of this study revealed an efficient role of dried seaweeds as heavy metal biosorbents from polluted soils.
Analysis of the soil samples before and after applying different seaweed treatments showed a significant reduction in the assessed heavy metal content, especially when using the mixture of seaweeds. Besides, the concentration of Pb, Cu, Zn, and Ni metals is apparently reduced to the tolerable limits (Table 1). This is might be due to the ability of these seaweeds to retain heavy metals in soil by the formation of stable compounds with the cationic-ion contaminants or by immobilization of these heavy metals as oxide, hydroxide, or phosphate (Chen 2012; Abd-Elhady and El-Zabalawy 2014). The adsorption of such metals by these chemical groups may employ several mechanisms such as adsorption, electrostatic attraction, ion-exchange complexation, covalent binding, and microprecipitation (Raize et al. 2004). The inherent functional groups (e.g., carboxyl, hydroxyl, sulfate, phosphate, and amine groups) present in the cell walls of brown and green seaweeds play a key role in the metal binding by biosorption (Gupta and Rastogi 2008). Furthermore, the presence of protein and polysaccharide molecules with wealthy content of such active reacted groups could also act as binding sites for heavy metals by an ion-exchange mechanism between these heavy metals and the basic light ions (such as Ca2+ and Mg2+ and Na+ and K+) (Ahmady-Asbchin et al. 2009), thus rendering these ions unavailable in the soil solution when analyzed. Furthermore, the presence of protein and polysaccharide molecules of actively reacted groups such as amino, hydroxyl, carboxyl, and sulfate groups could act as binding sites for such metals and thus mitigating its absorption by the plant roots (Abirami et al. 2013; Abd-Elhady 2015). The capture of the metals by these chemical groups includes several mechanisms such as adsorption, electrostatic attraction, ion exchange, complexation, covalent binding, and microprecipitation (Raize et al. 2004). However, metal uptake is triggered mainly by their availability, either in the external (soil associated) or in the internal (plant-associated) boundaries. Besides, a portion of these heavy metal ions may be leached down with irrigating water to the lower layers of the soil. This could explain the recorded decrement in the content of metals in the seaweed-treated soils. In the same connection, many studies recommended seaweeds as bioremediating agents for polluted soils. Brown seaweed species Kappaphycus alvarezii and Eucheuma denticulatum were efficiently utilized for Cd2+ biosorption (Kang et al. 2012). A mixture of brown, red, and green algae (Padina tetrastromatica, Gracilaria edulis, and U. reticulate) was applied to remove Cr pollution by biosorption. As revealed from the analysis of these seaweed materials, Cr was adsorbed by functional groups of protein and polysaccharide at the seaweeds surface (Abirami et al. 2013).
Plants analyses
It has been reported that soil pH affects not only metal bioavailability but also the metal uptake by the plant roots (Galal and Shehata 2015a). Metal solubility in soils is predominantly controlled by pH and the oxidation state of the system (Galal and Shehata 2015b). The solubility of heavy metals is greater within the pH range of normal agricultural soil. Low pH was found to be optimal for metal availability but was adverse to the plants since the solubility of heavy metal cations, e.g., Cd, Cu, Ni, Pb, and Zn, increased with decreasing pH (Galal and Shehata 2015a). Neutral and high soil pH could stabilize toxic metals in soil, resulting in reduced leaching effects (Badr et al. 2012).
Some metals such as Cd, Cr, and Ni are very toxic even in minute amounts (Ahmed and Slima 2018). In the nutrient medium of the plant, elevated heavy metal concentration has a damaging effect on the roots, which reduces the uptake of most other nutrients (Eltaher et al. 2019). In the present study, Pb, Cu, Zn, and Ni elements were found in higher concentrations in the radish plant roots than their shoots; this was in a match with many studies and recordings that trace metals are mostly reserved in the underground organs (Bonanno 2013). Moreover, in this study, the pH was slightly above neutral for U. fasciata and seaweeds mixture-treated soil or even increased in the soil treated with S. lacerifolium, implying an increased mobility of metal ions and better availability of them for plant uptake (Ahmed and Slima 2018; Slima and Ahmed 2020). In this study, the nutrients N, P, and K contents in radish plants (shoot and root) are found to be increased as the concentration of the studied heavy metals decreases (Table 3).
The increase in germination percentage, especially with seaweed mixture treatment (Table 2), might be attributed to the presence of growth regulatory substances such as auxins (indole acetic acid), cytokinins, and gibberellins in the supplemented seaweed biomasses which exhibited phyto-active properties (Osman et al. 2010; Arioli et al. 2015; Silva et al. 2019). Hormones or hormone-like compounds have been isolated from different seaweeds, green seaweed, e.g., Ulva lactuca (El-Naggar et al. 2005), and brown species, e.g., Ascophyllum, Fucus, Sargassum, and Laminaria (Breure 2014; Silva et al. 2019), and may modulate inborn phyto-hormone biosynthesis pathways in the treated plants (Wally et al. 2012; Divya et al. 2015a, b). It has been reported that gibberellic acid (GA3) could stimulate seed germination of different plants by inducing the hydrolytic enzymes around the endosperm and activating the amylase genes in the aleurone layer cells (Sun and Gubler 2004). Since green, brown, and red algae are very rich in gibberellic acid (Jennings 1968), they could boost a faster seed germination process. It has been reported that S. latifolium or U. lactuca and the mixture of their extracts provoked the oxidative damaging on Triticum aestivum L. plants under drought stress by either directly activating the antioxidative enzyme system as well as by providing phyto-hormones and micro-nutrients supply for wheat plants growth (Kasim et al. 2015). Seaweed extracts could antagonize environmental stresses due to the presence of cytokinins and gibberellic and abscisic acids, which represented a recovery route to support plant growth under stress conditions (Kasim et al. 2015; El Shoubaky and Salem 2016). In addition to the presence of soluble carbohydrates, proteins, fiber, fat, several mineral nutrients and bioactive molecules of antioxidant and osmoprotectant properties which are essential parts of the seaweeds composition, enhance plant growth (Hernández-Herrera et al. 2014; Ismail and El-Shafay 2015; Ismail 2017). According to Erulan et al. (2009); Patel et al. (2017), and Melo et al. (2020), these organic compounds enclosed in seaweeds are cheap, abundant, and eco-friendly for sustainable farming, besides being valuable to maintaining P soil fertility. The same explanation was also applied to the recorded high increase in the radish plant morphological criteria of the root and shoot as well as their leaf area measurements, which indicated a better tolerance of the plants to the stress of the heavy metal in the soil compared to control plants.
Many studies have reported a wide range of beneficial effects due to seaweeds application on plants, either as a liquid extract, foliar spray, small pieces, or powder soil fertilizer (Thirumaran et al. 2009; Nabti et al. 2017; Silva et al. 2019). Seaweeds and their compounds also stimulate early seed germination, general plant growth, and its resistance to biotic stress conditions (Safinaz and Ragaa 2013; Silva et al. 2019). Similarly, the overall increase in radish plant growth might result from a higher chlorophyll content, a better photosynthetic capacity, and a larger leaf area (Akila and Jeyadoss 2010) which might enhance crop yield and quality as well. Furthermore, organic compounds in seaweeds can chelate and/or correct marginal shortages of some minerals since seaweeds normally contain essential amounts of trace elements such as Mg, Zn, Mn, Fe, Cu, Co, Ni, Mo, and B (Khan et al. 2009; Craigie 2011; Nabti et al. 2017) which are essential for plant growth.
These organic and inorganic composites of the studied seaweed species promotionally affected the mineral content (K, N, Ca, P, Mg, and Na) of the cultivated radish plant roots and shoots. However, the obtained results showed a significant stimulation effect for the same previous parameters in radish plants cultivated in soils pretreated with seaweeds mixture, implying the feasibility of the mixture treatment over the individual ones, probably due to the mixing of both seaweed components. Similar previous studies conformed with our results. Hernández-Herrera et al. (2014) found that K content in the seaweed extracts positively enhanced photosynthesis, meristematic growth, and water status in the treated plants. Also, P content enabled root proliferation and increased the root/shoot ratio. The Ca present in seaweed extracts assisted cell elongation, cell stability, and enzyme activation in the treated plants (Zheng et al. 2016), while the growth rate of the plants was linked to N supply (Zheng et al. 2016). Sridhar and Rengasamy (2002) recommended using the mixture of Sargassum wightii (brown seaweed) with U. lactuca (green seaweed) to increase the growth of peanut (Arachis hypogaea) plants. Also, Mathur et al. (2015) reported the enhancement in seed germination, general growth, and biochemical structures of legume plant (Glycine max) when treated with a mixture of liquid fertilizer of S. wightii, U. lactuca, and Enteromorpha intestinalis seaweeds. Silva et al. (2019) stated that rice and lettuce plants are grown at different concentrations of Ascophyllum nodosum and S. muticum seaweeds liquid extracts absorbed almost half amount of P, K, Ca, and Mg nutrients compared with the nutrients from the control soil (without seaweeds).
Heavy metals bioaccumulation and translocation
The plant’s ability to accumulate metals from soils can be estimated using the BF, while its ability to translocate metals from the roots to the shoots is measured using the TF. Both BF and TF can be used to estimate a plant’s potential for phyto-remediation purposes. According to Zhao et al. (2007), TF > 1 indicates a very efficient ability to transport nutrients from roots to shoots, likely due to an efficient metal transport system. It is worth noting that radish plants showed BFs values (for Cd, Pb, Cu, Cr, and Ni) as well as TFs values (for Cd, Cr, and Fe) more than unity; therefore, it is a suitable plant for phyto-extraction of these metals from the soil. According to Eltaher et al. (2019), plants exhibiting TF and particularly BF values > 1 are improper for phyto-extraction. Furthermore, the TF values < 1 estimated for Cu, Zn, Ni, and Mn agreed with several studies (e.g., Eid et al. 2012; Galal and Shehata 2015a; Galal et al. 2018; Slima and Ahmed 2020) according to which heavy metal concentrations in the underground organs are generally higher than the above-ground organs. In addition, BF values < 1 reflect the ability of seaweeds to chelate and stabilize these metals in the soil. But for TF values > 1 reflect the ability of the root to translocate these metals to its shoot and the sequestration of such metals in tissues or cellular barriers of the plants (e.g., central vacuoles). Translocation of the excess metals into old leaves (sooner before they fell) could be tolerance machinery by these plants (Weis and Weis 2004). In this connection, both BF and TF values were significantly lower than unity for the most studied metals, indicating its beneficial role for heavy metal decontamination from the soil. In a recent study, Abd-Elhady (2015) endorsed using a mixture of green (Ulva sp.) and red algae (Gelidium sp.) dry biomass for lettuce plant cultivation in contaminated soil to ameliorate the existed heavy metal content. The same study recommended avoiding eating leaves or roots of such plants which have been grown in the heavy metal contaminated soil. In the same direction, radish plants have been endorsed with a phyto-extraction ability of different heavy metals from soils including Cd, Cr, Cu, Ni, Zn (Marchiol et al. 2004), Pb (Kapourchal et al. 2009), and As (Gutierrez et al. 2010). Recently research trends are mainly focusing on the enhanced technologies for heavy metal phyto-remediation by using, for example, field crops, microbes/plants genetically engineered technologies, and agromining (Li et al. 2019). In the present study, applying different seaweed groups as treatments along with radish plants in the polluted soil not only decreased the concentration of the heavy metals in the soil but also transported lower concentrations of these metals to the organs of the plant. This may in turn, upon repeated practicing, deliver a dynamic in situ technique for removing heavy metals from polluted soils as well as mitigating its existence in the edible plants.
Conclusion
This study presented a promising approach by using U. fasciata and S. lacerifolium seaweeds (as dry matter) to remediate heavy metal polluted soil and improved the growth of cultivated radish plants, especially when applying a mixture of both seaweeds. For soil chemical properties, seaweeds inoculation caused a slight increment in the inorganic nutrients (N, P, and K) and decreased the alkaline soil pH toward neutral soil conditions (pH = 7.2). It is worth noting that the application of the seaweeds mixture reduced the content of Pb, Cu, Zn, and Ni metals in soil and reached them to the safe limits. At the same time, the soil content of Cd, Cr, Fe, and Mn metals was conveyed closely to the tolerable limits. Furthermore, these treatments decreased the concentration of heavy metals bioaccumulated in root and/or translocated to its shoot compared to control, thus boosting the plant tolerance to the heavy metal stress. In the case of seaweeds mixture treatment, radish roots showed phyto-extraction ability for Cd, Cu, Cr, and Ni from the soil. These findings implied that biosorption of heavy metals by the studied seaweeds relief the stress on the radish plants and enhance their growth as they can play a dual role as heavy metal bioremediators of soil and as plant biofertilizers. Seaweeds richness content of hormones, organic matter, and minerals strongly potentiate these functions. Further studies are still needed using different seaweed concentrations to determine the safe concentration which can be used for soil treatment and to detect the critical toxic concentration of seaweeds to be avoided to get healthy plants for human consumption is appreciable to a certain extent in the current climate of pandemics.
Data availability
Please contact the authors for data requests.
References
Abd-Elhady ESE (2015) Evaluation of algae dry biomass as a biochemical soil remediation for polluted soil. Inter J Environ 4:309–314
Abd-Elhady ESE, El-Zabalawy KM (2014) Remediation of a soil contaminated with heavy metals using some seaweeds. J Soil Sci and Agric Eng, Mansoura Univ. 5(12):1623–1633
Abdel-Raouf N, Al-Homaidan AA, Ibrahem IBM (2012) Agricultural importance of algae. Afr J Biotechnol 11:11648–11658
Abirami S, Srisudha S, Gunasekaran P (2013) Comparative study of chromium biosorption using brown, red and green macroalgae. Int J Biol Pharm Res 4:115–129
Ahmady-Asbchin S, Andres Y, Gérente C, Le Cloirec P (2009) Natural seaweed waste as sorbent for heavy metal removal from solution. Environ Technol 30:755–762
Ahmed DA, Slima DF (2018) Heavy metal accumulation by Corchorus olitorius L. irrigated with wastewater. Environ Sci Pollut Res 25:14996–15005
Akila N, Jeyadoss X (2010) The potential of seaweed liquid fertilizer on the growth and antioxidant enhancement of Helianthus annuus L. Orient J Chem 26:1353–1360
Aleem AA (1993) In: Aleem AA (ed) The marine algae of Alexandria, Egypt. Faculty of science. University of Alexandria, Alexandria
Allen SE (1989) Chemical analysis of ecological materials. Blackwell Scientific Publications, London
Allen SE, Grimshaw HM, Parkinson JA, Quarmby C, Roberts JD (1986) In: Moore PD, Chapman SB (eds) Methods in plant ecology, 2nd edn. Blackwell, Oxford, pp 411–466
Arioli T, Mattner SW, Winberg PC (2015) Applications of seaweed extracts in Australian agriculture: past, present and future. J Appl Phycol 27:2007–2015
Ayangbenro AS, Babalola OO (2017) A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Res Public Health 14:94–109
Badescu IS, Bulgariu D, Bulgariu L (2016) Alternative utilization of algal biomass (Ulva sp.) loaded with Zn (II) ions for improving of soil quality. J Appl Phycol 29:1069–1079
Badr N, Fawzy M, Al-Qahtani KM (2012) Phytoremediation: an ecological solution to heavy metal-polluted soil and evaluation of plant removal ability. World Appl Sci J 16(9):1292–1301
Betremieux R (1948) Trait6 de chimie vegetale. Publi6 sous la direction de Brunel, Id. p. 342 (in Espiau, Pet Larguier, M. 1967)
Bonanno G (2013) Comparative performance of trace element bioaccumulation and biomonitoring in the plant species Typha domingensis, Phragmites australis and Arundo donax. Ecotoxicol Environ Saf 97:124–130
Bonanno GJ, Borg A, DiMartino V (2017) Levels of heavy metals in wetland and marine vascular plants and their biomonitoring potential: a comparative assessment. Sci Total Environ 576:796–806
Breure MS (2014) Exploring the potential for using seaweed (Ulva lactuca) as organic fertilizer. MSc Thesis Plant Production Systems. PPS-80436, Wageningen University. Netherlands
Chen, JP (2012) Decontamination of heavy metals: processes, mechanisms and applications. CRC Press/Taylor and Francis Group.
Craigie JS (2011) Seaweed extract stimuli in plant science and agriculture. J Appl Phycol 23(3):371–393
Das C, Naseera K, Ram A, Ram MM, Ramaiah N (2016) Bioremediation of tannery waste water by a salt tolerant strain of Chlorella vulgaris. J Appl Phycol 29:235–243
Davari M, Sharma SN, Mirzakhani M (2012) Residual influence of organic material, crop residues, and biofertilizers on performance of succeeding mung bean in an organic rice-based cropping system. J Recycl Organic Waste Agricult 1:1–14
Divya K, Roja MN, Padal SB (2015a) Effect of seaweed liquid fertilizer of Sargassum wightii on germination, growth and productivity of brinjal. Int J Adv Res Sci Eng Technol 2:868–871
Divya K, Roja MN, Padal SB (2015b) Influence of seaweed liquid fertilizer of Ulva lactuca on the seed germination, growth, productivity of Abelmoschus esculentus (L). Int J of Pharmacol Res 5:344–346
Dubios M, Gilles KA, Hamelton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Eid EM, Shaltout KH, El-Sheikh MA, Asaeda T (2012) Seasonal courses of nutrients and heavy metals in water, sediment and above- and below-ground Typha domingensis biomass in Lake Burullus (Egypt): perspective for phytoremediation. Flora 207:783–794
El Shoubaky GA, Salem EA (2016) Effect of abiotic stress on endogenous phytohormones profile in some seaweeds. IJPPR 8:124–113
El-Naggar AH, Osman MEH, El-Sheekh MM, Gheda SF (2005) Influence of the aqueous extracts of Ulva lactuca and Chlorella kessleri on growth and yield of Vicia faba. Archiv fur̈ Hydrobiologie 116:213–229
Eltaher GT, Ahmed DA, El-Beheiry M, Sharaf El-Din A (2019) Biomass estimation and heavy metal accumulation by Pluchea dioscoridis (L.) DC. in the Middle Nile Delta, (Egypt): perspectives for phytoremediation. S Afr J Bot 127:153–166
Erulan V, Soundarapandian P, Thirumaran G, Ananthan G (2009) Studies on the effect of Sargassum polycystum (C. Agardh, 1824) extract on the growth and biochemical composition of Cajanus cajan (L.) Mill sp. Am Eurasian J Agric Environ Sci 6(4):392–399
FAO (2014) Stat. Database Internet. http://faostat.fao.org/default.aspx. Accessed Jan 2019
Fatemeh Faraji G, Sina D, Alireza R, Abdolhamid E, Mohammad JM, Mozhgan K, Sara GN, Farshid S (2016) Data on Fe (II) biosorption onto algae obtained from the Persian Gulf in Bushehr Port. Data Brief 9:823–827
Featonby-Smith BC, Van Staden J (1983) The effect of seaweed concentrate and fertilizer on the growth of Beta vulgaris. Z Pflanzenphysiol 112:155–162
Galal TM (2016) Impact of environmental pollution on the growth and production of Egyptian clover. Int J Plant Prod 10(3):303–316
Galal TM, Shehata HS (2015a) Bioaccumulation and translocation of heavy metals by Plantago major L. grown in contaminated soils under the effect of traffic pollution. Ecol Indic 48:244–251
Galal TM, Shehata HS (2015b) Impact of nutrients and heavy metals capture by weeds on the growth and production of rice (Oryza sativa L.) irrigated with different water sources. Ecol Indic 54:108–115
Galal TM, Khalafalla AA, Elawa OE, Hassan LM (2018) Human health risks from consuming cabbage (Brassica oleracea L. var. capitata) grown on wastewater irrigated soil. Int J Phytoremediation 20(10):1007–1016
Garg G, Kataria SK (2010) Phytoremediation potential of raphanus sativus (L.), brassica juncea (L.) and triticum aestivum (L.) for copper contaminated soil, E-Proceedings of International Society of System Sciences, Univertity of Queensland, Brisbane (Australia)
Gheda SF, Ahmed DA (2015) Improved soil characteristics and wheat germination as influenced by inoculation of Nostoc kihlmani and Anabaena cylindrica Rend Fis Ac. Lincei 26:121–131
Guiry MD, Guiry GM (2019) AlgaeBase world-wide electronic publication. National University of Ireland, Galway. http://www.algaebase.org. Accessed Mar 2019
Gupta VK, Rastogi A (2008) Sorption and desorption studies of chromium (VI) from nonviable cyano bacterium nostoc muscorum Biomass. J Hazard Mater 154(1–3):347–54
Gutierrez J, Hong CO, Lee BH, Kim PJ (2010) Effect of steel-making slag as a soil amendment on arsenic uptake by radish (Raphanus sativa L.) in an upland soil. Biol Fertil Soils 46:617–623
Hernández-Herrera RM, Santacruz-Ruvalcaba F, Ruiz-López MA, Norrie J, Hernández-Carmona G (2014) Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J Appl Phycol 26(1):619–628
Ismail GA (2017) Biochemical composition of some Egyptian seaweeds with potent nutritive and antioxidant properties. Food Sci Technol Campinas 37(2):294–302
Ismail MM, El-Shafay SM (2015) Variation in taxonomical position and biofertilizing efficiency of some seaweed on germination of Vigna unguiculata (L). IJESE 6:47–57
Ismail GA, Ismail MM (2017) Variation in oxidative stress indices of two green seaweeds growing under different heavy metal stresses. Environ Monit Assess 189:68–73
Jennings RC (1968) Gibberellin antagonism by material from a brown alga. New Phytol 68:683–688
Kang OL, Nazaruddin R, Musa A (2012) Cadmium (II) biosorption onto seaweed (Kappaphycus alvarezii and Eucheuma denticulatum) waste biomass: equilibrium and mechanism studies. Middle-East J Sci Res 11:867–872
Kapourchal SA, Kapourchal SA, Pazira E, Homaee M (2009) Assessing radish (Raphanus sativus L.) potential for phytoremediation of lead-polluted soils resulting from air pollution. Plant Soil Environ 55:202–206
Kasim WA, Hamada EAM, Shams El-Din NG, Eskander SK (2015) Influence of seaweed extracts on the growth, some metabolic activities and yield of wheat grown under drought stress. Int J Agri Agri R 7:173–189
Khan W, Rayirath UP, Subramanian S, Jithesh MN, Rayorath P, Hodges DM, Critchley AT, Craigie JS, Norrie J, Prithiviraj B (2009) Seaweed extracts as biostimulants of plant growth and development. J Plant Growth Regul 28:386–399
Li C, Ji X, Luo X (2019) Phytoremediation of heavy metal pollution: a bibliometric and scientometric analysis from 1989 to 2018. Int J Environ Res Public Health 16:4755
López Errasquín E, Vázquez C (2003) Tolerance and uptake of heavy metals by Trichoderma atroviride isolated from sludge. Chemosphere. 50(1):137–143
Lowry OH, Rosen BJ, Fan AC, Randel RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:225–275
Marchiol L, Assolari S, Sacco P, Zerbi G (2004) Phytoextraction of heavy metals by canola (Brassica napus) and radish (Raphanus sativus) grown on multicontaminated soil. Environ Pollut 132(1):21–27. https://doi.org/10.1016/j.envpol.2004.04.001
Mathur C, Rai S, Sase N, Krish S, Jayasri MA (2015) Enteromorpha intestinalis derived seaweed liquid fertilizers as prospective biostimulant for Glycine max. Braz Arch Biol Technol 58:813–820
Mehta SK, Gaur JP (2005) Use of algae for removing heavy metal ions from wastewater: progress and prospects. Crit Rev Biotechnol 25(3):113–152
Melo PC, Abreu K, Bahcevandziev G, Araujo PL (2020) Biostimulant effect of marine macroalgae bioextract on pepper grown in greenhouse. Appl Sci 10:4052. https://doi.org/10.3390/app10114052
Metzner H, Rauand H, Senger H (1965) Unter suchungen zur synchronisier barteit einzelner pigmentan angel mutanten von chlorella. Planta 65:186–194
Michalak I, Chojnacka K, Marycz K, Basinska K (2016) Bioaccumulation of microelements in seaweeds by scanning electron microscopy with an energy dispersive x-ray analytical system and inductively coupled plasma-optical emission spectrometer. Res Rev J Bot Sci 5:50–55
Murphy V, Hughes H, McLoughlin P (2008) Comparative study of chromium biosorption by red, green and brown seaweed biomass. Chemosphere 70(6):1128–1134
Nabti E, Jha B, Hartmann A (2017) Impact of seaweeds on agricultural crop production as biofertilizer. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-016-1202-1
Nasab SM, Naji A, Yousefzadi M (2017) Kinetic and equilibrium studies on biosorption of cadmium (II) from aqueous solution by Gracilaria corticata and agar extraction algal waste. J Appl Phycol:1–10
Osman MEH, Elsheekh MM, El-Naggar AH, Gheda SF (2010) Effect of two species of cyanobacteria as biofertilizers on some metabolic activities growh and yield of pea plant. Biol Fertil Soils 46:861–675
Pandya KY, Patel RV, Jasrai RT, Hatt NB (2017) Optimization of Cr and Cu biosorption by green marine algae Caulerpa racemose Var. Cylindracea and Ulva lactuca. Int J Adv Res 5(8):923–939
Patel RV, Pandya KY, Jasrai DRT, Brahmbhatt DN (2017) A review: scope of utilizing seaweeds as a biofertilizer in agriculture. Int J Adv Res 5(7):2046–2054
Poudel K, Karki S, Sah K, Lal M, Mandal J (2018) Evaluation of radish (Raphanus sativus L.) genotypes in Eastern mid-hills condition of Nepal. International Journal of Advanced Research (IJAR) 19:155–159
Raghunandan BL, Vyas RV, Patel HK, Jhala YK (2019) Perspectives of seaweed as organic fertilizer in agriculture. In: Panpatte DG, Jhala YK (eds) Soil Fertility Management for Sustainable Development. Springer Nature Singapore Pte Ltd. https://doi.org/10.1007/978-981-13-5904-0_13
Raize O, Argaman Y, Yannai S (2004) Mechanisms of biosorption of different heavy metals by brown marine macroalgae. Biotechnol Bioeng 87(4):451–458
Safinaz AF, Ragaa AH (2013) Effect of some red marine algae as biofertilizers on growth of maize (Zea mayz L.) plants. Int Food Res J 20(4):1629–1632
Sarl A, Tuzen M (2007) Biosorption of Pb (ll) and Cd (ll) from aqueous solution using green alga (Ulva lactuca) biomass" Department of chemistry, Gaziosmanpasa University, 60250 tokat, Turkey. Algae". Biotechnol Bioeng 41:819–825
Schippers RR (2004) Raphanus sativus L. In: Grubben GJH, Denton OA (eds) PROTA 2: Vegetables/Légumes. [CD-Rom]. PROTA, Wageningen
Shehata HS, Galal TM (2020) Trace metal concentration in planted cucumber (Cucumis sativus L.) from contaminated soils and its associated health risks. J Consum Prot Food S. https://doi.org/10.1007/s00003-020-01284-z
Silva LD, Bahcevandziev K, Pereira L (2019) Production of bio-fertilizer from Ascophyllum nodosum and Sargassum muticum (Phaeophyceae). J Ocean Limnol 37(3):918–927
Slima DF, Ahmed DA (2020) Trace metals accumulated in pea plant (Pisum sativum L.) as a result of irrigation with wastewater. J Soil Sci Plant Nutr. https://doi.org/10.1007/s42729-020-00341-8
SPSS (2006) SPSS base 15.0 Users guide SPSS inc. Chicago
Sridhar S, Rengasamy R (2002) Effect of seaweed liquid fertilizer obtained from Ulva lactuca on the biomass, pigments and protein content of Spirulina platensis. Sea Res Utili 24:145–149
Sud D, Mahajan G, Kaur PM (2008) Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions: a review. Bioresour Technol 99:6017–6027. https://doi.org/10.1016/j.biortech.2007.11.064
Sudo H, Burgess JG, Takemasa H, Nakamura N, Matsunaga T (1995) Sulphated exopolysaccharide production by the halophilic cyanobacterium Aphanocapsa halophytica. Curr Microbiol 30:219–222
Sun TP, Gubler F (2004) Molecular mechanism of gibberellin signaling in plants. Annu Rev Plant Physiol Plant Mol Biol 55:197–223
Thirumaran G, Arumugam M, Arumugam R, Anantharaman P (2009) Effect of seaweed liquid fertilizer on growth and pigment concentration of Abelmoschus esculentus (I) Medikus. American-Eurasian Journal of Agronomy (AEJA) 2(2):57–66
Ünyayar S, Topcuoglu SF, Ünyayar A (1996) A modified method for extraction and identification of indole-3- acetic acid (IAA), gibberellic acid (GA3), abscisic acid (ABA) and zeatin produced by Phanerochate chrysosporium ME 446. Bulg J Plant Physiol 22(3–4):105–110
Wade TL, Brooks JM, Kennicutt MC, McDonald TJ, Sericano JL, Jackson TL (1993) Trace metals and organic contaminants analytical techniques 5. In: Lauenstein GG, Cantillo AY (eds) Sampling and Analytical Methods of the National Status and Trend Program. National Benthic Surveillance and Mussel Watch Projects 1984–1992. NOAA Technical Memorandum NOS ORCA 71, Silver Spring, pp 121–139
Wally OS, Critchley AT, Hiltz D, Craigie JS, Han X, Zaharia LI, Abrams SR, Prithiviraj B (2012) Regulation of phytohormone biosynthesis and accumulation in Arabidopsis following treatment with commercial extract from the marine macroalga Ascophyllum nodosum. J Plant Growth Regul 32:324–339
Wang L, Li H, Zhao C, Li S, Kong L, Wu W, Kong W, Liu Y, Wei Y, Zhu JK, Zhang H (2017) The inhibition of protein translation mediated by AtGCN1 is essential for cold tolerance in Arabidopsis thaliana. Plant Cell Environ 40(1):56–68
Weis JS, Weis P (2004) Metal uptake, transport and release by wetland plants: implications for phytoremediation and restoration. Environ Int 30:685–700
WHO (1996) Health criteria other supporting information. In guidelines for drinking water quality, vol. 2 (2nd edn). Geneva, pp 31–388
Zhang P, Qin C, Hong X, Kang G, Qin M, Yang D, Pang B, Li Y, He J, Dick RP (2018) Risk assessment and source analysis of soil heavy metal pollution from lower reaches of Yellow River irrigation in China. Sci Total Environ 633:1136–1147
Zhao GQ, Ma BL, Ren CZ (2007) Growth, gas exchange, chlorophyll fluorescence and ion content of naked oat in response to salinity. Crop Sci 47:123–131
Zheng SY, He ML, Jiang J, Zou SM, Yang WN, Zhang Y, Deng J, Wang CH (2016) Effect of kelp waste extracts on the growth and lipid accumulation of microalgae. Bioresour Technol 201:80–88
Zhuo H, Fu S, Liu H, Song H, Ren L (2019) Soil heavy metal contamination and health risk assessment associated with development zones in Shandong, China. Environ Sci Pollut Res 26:30016–30028
Acknowledgments
Deep thanks from authors to the editor of the Environmental Science and Pollution Research Journal (Prof. Elena MAESTRI) for her patience and support. Thanks extend to reviewers who improve this manuscript to appear in this good form.
Author information
Authors and Affiliations
Contributions
Dalia Abd El-Azeem Ahmed: conception and design, acquisition, analysis, statistic analysis and interpretation of results. Also, drafting the article and revising it. She approved the final version to be submitted for publication.
Saly Farouk Gheda: design and interpretation of results. Also, drafting the article and revising it. She approved the final version to be submitted for publication.
Gehan Ahmed Ismail: design and interpretation of results. Also, drafting the article and revising it. She approved the final version to be submitted for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Responsible Editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmed, D.A.EA., Gheda, S.F. & Ismail, G.A. Efficacy of two seaweeds dry mass in bioremediation of heavy metal polluted soil and growth of radish (Raphanus sativus L.) plant. Environ Sci Pollut Res 28, 12831–12846 (2021). https://doi.org/10.1007/s11356-020-11289-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11289-8