Abstract
The research was designed to examine oxidative stress of the liver of turtle Mauremys reevesii caused by cadmium (Cd). Turtles were injected intraperitoneally with cadmium at the concentration of 7.5, 15, and 30 mg/kg, and 5 turtles were taken from each group after exposure for 1 week (1 w), 2 weeks (2 w), and 3 weeks (3 w). The activities of SOD and CAT as well as the contents of GSH and MDA in liver tissues were detected by using a kit. The results showed that the difference between the control group and the Cd-treated group was statistically significant with the increase of Cd concentration and the prolongation of exposure time, which suggested that Cd caused oxidative stress on the liver of turtles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a worldwide freshwater aquatic pollutant (Mehinto et al. 2014; Novelli et al. 2000). Cd excretion by the body is very slow, so its biological half-life is relatively long, up to 15–20 years (Joseph. 2009). Therefore, Cd is able to be plentiful through the food web (Rose et al. 2015). While the turtle is in the upper layer of the food chain, Cd is likely to be enriched higher in the turtle. However, there are only a few studies on Cd poisoning of turtle (Dayna et al. 2016; Huo et al. 2017a, b, 2018, 2020a, b; Yu et al. 2013).
The mechanism of Cd poisoning is not very clear, and several interpretations are presented (Varoni et al. 2017). The studies of toxicological Cd indicate that it can cause an increase in reactive oxygen species (ROS), which is able to induce multiple structural and functional damages, such as cellular lipid peroxidation, protein destruction, and DNA mutation (Cuypers et al. 2010; Huo et al. 2017a, b, 2018, 2020a, b; Wu et al. 2015). One of the products of lipid is malondialdehyde (MDA), which is able to bind to the amino acids of protein and trigger the internal or mutual connection of protein, leading to cell damage (Li et al. 2012). Therefore, the content of MDA can reflect the attack degree of ROS on cells, and it can be referenced as one marker of membrane damage (Amin et al. 2018). There is an antioxidant system in the body, which includes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) (Jiao et al. 2017; Koim-Puchowska et al. 2020; Laurent et al. 2017; Serdar et al. 2018; Yuan et al. 2016). ROS can be cleared by the antioxidant system. SOD can convert ROS into hydrogen peroxides (H2O2), and GSH-Px or CAT is able to degrade H2O2 (Afonso et al. 2007). Cd regulates the activities of SOD, CAT, and GSH-Px of the antioxidant system (Yuan et al. 2016).
The purpose of this research was to investigate the effects of Cd on oxidative stress in liver of turtle Mauremys reevesii.
Materials and methods
Animals and treatments
After acclimation, healthy, similar-weight (130 ± 10 g) Mauremys reevesii were selected for experiment. Mauremys reevesii were randomly divided into one control group (5 individuals) and three experiment groups (45 individuals, 15 turtles in each group). The control group turtles were injected with 0.85% sodium chloride solution, and the experiment group turtles were injected with 7.5, 15, and 30-mg/kg cadmium chloride respectively for only once.
Sample collection
Five turtles were randomly sacrificed from each group after 1 week (1 w), 2 weeks (2 w), and 3 weeks (3 w) of Cd exposure. Samples of liver tissue were excised and weighed immediately. Add 9 times medium (0.85% saline solution) to liver tissue by weight (g):volume (ml) equal to the proportion of 1:9, homogenize, and centrifuge at 2500 r/min for 10 min at 4 °C. Then, the supernate was stored at – 80 °C for detecting the oxidation index.
Biochemical assays
Under the manufacturer’s protocols, the content of GSH and MDA and the activities of SOD and CAT in supernatant were detected with the detection kits. All the detections were performed by using a microplate reader.
Statistical analysis
Statistical analyses were performed with the SPSS 20.0 software package. The data was representative of mean values of five animals of each group, and the results were shown as means ± standard deviations (SD). The probability value less than 0.05 was statistically different. If the probability value was less than 0.01, the difference was statistically significant.
Results
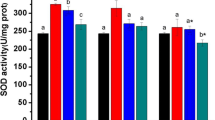
Correlations between SOD activities and Cd treatments in liver
From Fig. 1, SOD activities in liver of turtles exposed to 7.5 mg/kg gradually increased with the extension of exposure time, and the activities of SOD for 2 weeks and 3 weeks after Cd treatment were markedly increased (P < 0.01). But SOD activities in the liver of turtles exposed to 15 and 30 mg/kg declined with prolongation of exposure time, even SOD activities in the 30 mg/kg group after 3 weeks of Cd exposure were significantly lower than that of the control turtles (P < 0.01).
The activities of SOD in the liver gradually increased as the dose increases for 1 week. In comparison with the control group, the activities of SOD in the 7.5 mg/kg group had no statistical difference, but SOD activities in the 15 and 30 mg/kg groups were markedly increased (P < 0.01). SOD activities in the 7.5, 15, and 30 mg/kg groups after 2 weeks of Cd treatment were significantly increased (P < 0.01), but the activities of SOD in liver gradually decreased in a dose-dependent mode for 2 weeks. The activities of SOD in the liver increased first and then decreased in a dose-dependent mode after the turtles being exposed to Cd for 3 weeks. Compared with the control group, the activities of SOD in the 7.5 mg/kg group were markedly increased (P < 0.01), while the activities of SOD in the 15 mg/kg group had no statistical difference, but the activities of SOD in the 30 mg/kg group were markedly reduced (P < 0.01).
Overall, the activities of SOD of the liver of turtles Mauremys reevesii increased except that those of the 30 mg/kg group decreased after 3 weeks of Cd treatment. In brief, low-dose Cd could induce the increase of SOD activities in the turtle liver; however, high dose of Cd-induced SOD activities in the turtle liver increased first then decreased. The results showed that the changes of SOD activities were dose-dependent and time-dependent.
Correlations between CAT activities and Cd treatments in the liver
As shown in Fig. 2, all of the CAT activities in the liver of turtles Mauremys reevesii decreased. The activities of CAT in liver of turtles exposed to 7.5 and 30 mg/kg gradually decreased with prolongation of exposure time. However, the downward trend of the CAT activities in the liver of turtles exposed to 15-mg/kg CAT is not typical. The activities of CAT in the liver decreased for 1 week. The declining degree of CAT activities in the 15 mg/kg group was the most obvious. Compared with the control group, the activities of CAT in the 7.5 mg/kg group had no statistical difference, but the activities of CAT in the 15 and 30 mg/kg groups were markedly decreased (P < 0.01). CAT activities in liver decreased for 2 weeks. The declining degree of CAT activities in the 15 mg/kg group was the most obvious. The activities of CAT in the 7.5, 15, and 30 mg/kg groups were significantly decreased (P < 0.01). The activities of CAT in the liver decreased dose-dependently after being exposed to Cd for 3 weeks. Compared with the control group, the activities of CAT in the 7.5, 15, and 30 mg/kg group were markedly reduced (P < 0.01).
In short, CAT activities decreased obviously with the increase of dose and time. The outcomes indicated that changes of the activities of CAT were dose-dependent and time-dependent.
Correlations between the content of GSH and Cd treatments in the liver
GSH content in the liver of turtles exposed to 7.5 mg/kg declined slightly (1 W) and then significantly increased (2 W) followed by a little decrease (3 W) with prolongation of exposure time (Fig. 3). GSH content in the liver of turtles exposed to 15 and 30 mg/kg increased first and then gradually decreased with the extension of exposure time. The content of GSH in the liver decreased first (7.5 mg/kg) then increased (15 and 30 mg/kg) as the dose increases for 1 week. In comparison with the control group, GSH content in the 7.5 mg/kg group had no statistical difference, but the contents of GSH in the 15 and 30 mg/kg groups were markedly increased (P < 0.01). GSH content in the liver increased first (7.5 and 15 mg/kg) then decreased (30 mg/kg) for 2 weeks. The content of GSH in the 7.5 mg/kg group was significantly increased (P < 0.01); the 15 and 30 mg/kg groups had no statistical difference with that in the control group. GSH content in the liver increased first (7.5 mg/kg) then decreased (15 and 30 mg/kg) after being exposed to Cd for 3 weeks. Compared with the control group, GSH content in the 7.5 and 15 mg/kg groups had no statistical difference, but the content of GSH in the 30 mg/kg group was markedly reduced (P < 0.01).
In brief, low-dose Cd had complicated effect on the content of GSH in the turtle liver; however, high dose of Cd-induced GSH content in the turtle liver increased first then decreased with prolongation of exposure time and increase of dose.
Correlations between the content of MDA and Cd treatments in the liver
In comparison with the control group, the content of MDA in each exposure group and each exposure time increased (Fig. 4). The content of MDA in the liver of turtles exposed to 7.5 and 30 mg/kg increased first and then decreased with prolongation of exposure time. MDA content in the liver of turtles exposed to 15 mg/kg had no clear trend. In comparison with the control group, MDA content in the 7.5 mg/kg group had no statistical difference, but the contents of MDA in the 15 and 30 mg/kg group were markedly increased (P < 0.01) for 1 week. The content of MDA in the 7.5, 15, and 30 mg/kg groups increased significantly (P < 0.01) for 2 weeks. In comparison with the control group, MDA content in the 7.5 mg/kg group increased significantly (P < 0.01), but the 15 and 30 mg/kg groups had no statistical difference.
In conclusion, Cd could induce the increase of MDA content in the turtle liver.
Discussion
Incomplete reduction of oxygen at electron donor sites on several mitochondrial and extra-mitochondrial enzymes produces ROS in cells (Brand, 2016; Mailloux, 2018; Wong et al., 2019). ROS is involved in many cellular functions and regulatory networks (Sies 2017; Mailloux 2018).
Excessive production of ROS causes cells to enter the oxidative stress state, followed by cell necrosis or apoptosis (Wu et al. 2014; Gilgun-Sherki et al. 2002). MDA is one of the widely recognized biomarkers of oxidative stress and cellular lipid peroxidation (Lawal et al. 2011; Zhou et al. 2017).
ROS produced by heavy metal poisoning can be cleared by the cellular defense enzyme system, such as SOD, CAT, and GSH (Sevcikova et al. 2011). The proximal ROS produced by the mitochondria is superoxide anion (·O2−) and H2O2 (Mailloux, 2018). O2− results from single electron reduction of molecular oxygen, then·O2− is dismutated to H2O2 and O2 spontaneously or catalyzed into H2O2 and O2 by manganese superoxide dismutase (Mn-SOD) (mitochondrial matrix) and copper–zinc superoxide dismutase (Cu/Zn-SOD) (cytosol and mitochondrial intermembrane space) (Okoye et al., 2019). H2O2 is also produced by paired electron reduction of oxygen directly (Andreyev et al., 2015; Brand, 2016). H2O2 is a secondary messenger to coordinate oxidative metabolism with changes in cell physiology. The level of H2O2 is regulated through its production and degradation (Mailloux, 2018). CAT is able to catalyze H2O2 into H2O and O2 (Fomazier et al., 2002). GSH plays an important role in neutralizing ROS and directly countering Cd toxicity (Varoni et al. 2017).
Cd cannot stimulate the body to produce ROS directly, but it can indirectly induce the body to produce excessive ROS and destroy the antioxidant defense system (Cuypers et al. 2010). Cd increases the levels of lipid peroxidation and decreases total antioxidant capacity in hepatic tissue (Moradkhani et al. 2020). Cd can induce the body to produce O2−, H2O2, and hydroxyl radicals (·OH) by altering the expression of ROS-related genes (Patra et al. 2011). When there are no enough GSH and CAT enzymes, excessive ·OH is produced by Fenton’s reaction (Abdeen et al. 2019). ·OH has the highest rate of reactivity compared with other ROS, so it is the most harmful radical (Sies 2017). It results from the breakdown of the unsaturated fatty acid content (Avery 2011). ·OH can cause lipid peroxidation and production of MDA by diffusing or reacting with distant molecules such as cell membranes (Avery 2011).
Cd can combine with SOD sulfhydryl groups to form the Cu–Cd–SOD complex by instead of Zn in the SOD, thus causing the decrease or even disappearance of the SOD activities (Wu et al. 2014). The activities of SOD reduce obviously in the mice liver of the damage group (Gong et al. 2017; Pang et al. 2010). The activities of SOD decline in rats’ liver (Wang et al. 2018; Yu et al. 2006). SOD activities in the liver of Bufo gargarizans are declined (Jia et al. 2004). SOD activities in the liver of Orechromis niloticus decrease (Wang et al. 2016). The activities of SOD in the hepatopancreas of crucian carp under the Cd alone–exposed group are lower than that in the control group (Zhuo et al. 2016). SOD activities in the liver of cock decline (Wang et al. 2007B). The activities of SOD in grass carp’s liver tissues decrease first then increase and decrease once again when Cd2+ presents low-concentration pollution, while the activity of SOD is inhibited obviously all along when Cd2+ presents high-concentration pollution (Wang et al. 2007A). Our results of the 30 mg/kg group in 3 weeks (Fig. 1) were in accordance with these reports, which may be explained that the turtle had a strong tolerance to Cd exposure. There are also reports that SOD activities change in the opposite direction. SOD activities in the liver of the frog Rana nigromaculata are increased (Wang et al. 2006). Our results in the early days (Fig. 1) were in accordance with this report.
The activities of CAT reduce obviously in the mice liver of the Cd group (Gong et al. 2017). CAT activities in the liver of Bufo gargarizans are declined (Jia et al. 2004). CAT activities in the liver of Orechromis niloticus decrease (Wang et al. 2016). The activities of CAT in the hepatopancreas of crucian carp under the Cd-exposed group are lower than that in the control group (Zhuo et al. 2016). Our outcomes indicated a reduction of the activities of CAT in the turtle liver after Cd exposure (Fig. 2).
The content of GSH in rats’ liver increases obviously (Yu et al. 2006). GSH contents are induced significantly in the liver of the frog Rana nigromaculata with an increase of exposure time (Wang et al. 2006). Our study also showed an increase of the content of GSH in the turtle liver after Cd treatment (Fig. 3). However, it is not in agreement with some study that animals exposed to Cd decrease the content of GSH. GSH content decreases in the liver of Bufo gargarizans (Jia et al. 2004) and Orechromis niloticus (Wang et al. 2016). The difference may come from dosage, exposure duration, the way of administration, species, and age of the experimental animals.
The content of MDA in the liver of mice increases with the prolonged time (Gong et al. 2017; Pang et al. 2010). MDA content in rats’ liver increases (Wang et al. 2018). MDA content in the liver of the frog Rana nigromaculata increases rapidly with the prolonged time (Wang et al. 2006). The MDA content in the Bufo gargarizans liver increases (Jia et al. 2004). MDA content in the hepatopancreas of crucian carp under the Cd-exposed group increases (Zhuo et al. 2016). MDA content in the liver of cock increases (Wang et al. 2007B). Our study indicated that the content of MDA increased in the turtle liver (Fig. 4).
Conclusions
Low-dose Cd could induce the increase of SOD activities in the turtle liver; however, high dose of Cd-induced SOD activities in the turtle liver increased first then decreased. CAT activities decreased with the increase of dose and time. Low-dose Cd increased the content of GSH in the turtle liver; however, high dose of Cd-induced GSH content in the turtle liver increased first then decreased with prolongation of exposure time. Cd could induce the increase of MDA content in the turtle liver. The results of our experiments indicated that Cd is able to induce oxidative stress and damage in turtles Mauremys reevesii. But turtles seemed to have some tolerance to Cd exposure.
Data availability
Not applicable.
References
Abdeen A, Abou-Zaid OA, Abdel-Maksoud HA, Aboubakr M, Abdelkader A, Abdelnaby A, Abo-Ahmed AI, El-Mleeh A, Mostafa O, Abdel-Daim M, Aleya L (2019) Cadmium overload modulates piroxicam-regulated oxidative damage and apoptotic pathways. Environ Sci Pollut Res 26(24):25167–25177
Afonso V, Champy R, Mitrovic D, Collin P, Lomri A (2007) Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine 74:324–329
Amin MM, Rafiei N, Poursafa P, Ebrahimpour K, Mozafarian N, Shoshtari-Yeganeh B, Hashemi M, Kelishadi R (2018) Association of benzene exposure with insulin resistance, SOD, and MDA as markers of oxidative stress in children and adolescents. Environ Sci Pollut Res 25:34046–34052
Andreyev AY, Kushnareva YE, Murphy AN, Starkov, A.A (2015) Mitochondrial ROS metabolism: 10 years later. Biochem Mosc, 80, 517-531.
Avery SV (2011) Molecular targets of oxidative stress. Biochem J 434:201–210
Brand MD (2016) Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic Biol Med 100:14–31
Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, Opdenakker K, Nair AR, Munters E, Artois TJ, Nawrot T, Vangronsveld J, Smeets K (2010) Cadmium stress: an oxidative challenge. Biometals 23:927–940
Dayna L, Smith MJ, Cooper JM, Kosiara GA, Lamberti (2016) Body burdens of heavy metals in Lake Michigan wetland turtles. Environ Monit Assess 188:128
Fomazier RF, Ferreira RR, Viteria AP, Molina SMG, Lea PJ, Azevedo RA (2002) Effects of cadmium on antioxidant enzyme activities in sugar cane. Biol Plant 45(1):91–97
Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D (2002) Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev 54:271–284
Gong P, Ma FL, He RR, Zhao TL, Chang XN, Chen FX (2017) Protective effect of theacrine on cadmium-induced mice hepatic damage. J Shaanxi Univ Sci Technol 35(1):134–138
Huo JF, Dong AG, Wang YH, Lee SQ, Ma CG, Wang L (2017a) Cadmium induces histopathological injuries and ultrastructural changes in the liver of freshwater turtle (Chinemys reevesii). Chemosphere 186:459–465
Huo JF, Dong AG, Yan JJ, Wang L, Ma CG, Lee SQ (2017b) Cadmium toxicokinetics in the freshwater turtle, Chinemys reevesii. Chemosphere 182:392–398
Huo JF, Dong AG, Niu XJ, Dong AL, Lee SQ, Ma CG, Wang L (2018) Effects of cadmium on oxidative stress activities in plasma of freshwater turtle Chinemys reevesii. Environ Sci Pollut Res 25:8027–8034
Huo JF, Dong AG, Yan JJ, Dong AL (2020a) Effects of cadmium on the activities of ALT and AST as well as the content of TP in plasma of freshwater turtle Mauremys reevesii. Environ Sci Pollut Res 27:18025–18028
Huo JF, Dong AG, Yan JJ, Dong AL (2020b) Effects of cadmium on the gene transcription of the liver in the freshwater turtle (Chinemys reevesii). Environ Sci Pollut Res 27:8431–8438
Jia XY, Dong AH, Ma XM (2004) Effect of Cd2+ on lipid peroxidation in liver and kidney of Bufo gargarizans. Chin J Appl Environ Biol 10(1):92–94
Jiao XY, Yang K, An Y, Teng XJ, Teng XH (2017) Alleviation of lead-induced oxidative stress and immune damage by selenium in chicken bursa of Fabricius. Environ Sci Pollut Res 24:7555–7564
Joseph P (2009) Mechanisms of cadmium carcinogenesis. Toxicol Appl Pharmacol 238:272–279
Koim-Puchowska B, Drozdz-Afelt JM, Lamparski R, Menka A, Kaminski P (2020) Antioxidant defence barrier of great tit Parus major nestlings in response to trace elements. Environ Sci Pollut Res 27:20321–20334
Laurent H, Anne-Leila M, Olivier V (2017) Oxidative stress biomarkers in the Mediterranean pond turtle (Mauremys leprosa) reveal contrasted aquatic environments in Southern France. Chemosphere 183:332–338
Lawal AO, Lawal AF, Olongodudu A, Adeniran OY, Omonkhua A, Obi F (2011) Antioxidant effects of heated garlic juice on cadmium-induced liver damage in rats as compared to ascorbic acid. J Toxicol Sci 36(5):549–557
Li Y, Li M, Shi J, Yang X, Wang Z (2012) Hepatic antioxidative responses to PCDPSs and estimated short-term biotoxicity in freshwater fish. Aquat Toxicol 120-121:90–98
Mailloux RJ (2018) Mitochondrial antioxidants and the maintenance of cellular hydrogen peroxide levels. Oxidative Med Cell Longev 2018:1–10
Mehinto AC, Prucha MS, Colli-Dula RC, Kroll KJ, Lavelle CM, Barber DS, Vulpe CD, Denslow ND (2014) Gene networks and toxicity pathways induced by acute cadmium exposure in adult largemouth bass (Micropterus salmoides). Aquat Toxicol 152:186–194
Moradkhani S, Rezaei-Dehghanzadeh T, Nili-Ahmadabadi A (2020) Rosa persica hydroalcoholic extract improves cadmium-hepatotoxicity by modulating oxidative damage and tumor necrosis factor-alpha status. Environ Sci Pollut Res 27:31259–31268
Novelli F, Novelli E, Manzano MA, Lopes AM, Cataneo AC, Barbosa LL, Ribas BO (2000) Effect of alpha-tocopherol on superoxide radical and toxicity of cadmium exposure. Int J Environ Health Res 10:125–134
Okoye CN, MacDonald-Jay N, Kamunde C (2019) Effects of bioenergetics, temperature and cadmium on liver mitochondria reactive oxygen species production and consumption. Aquat Toxicol 214:105264
Pang YQ, Zhou M, Zheng ZM, Wei JQ (2010) Protective effect of tomato juice on liver and kidney damage in mice with cadmium poisoning. Chin J Public Health 26(12):1552–1553
Patra RC, Rautray AK, Swarup D (2011) Oxidative stress in lead and cadmium toxicity and its amelioration. Vet Med Int, 1-9
Rose M, Fernandes A, Mortimer D, Baskaran C (2015) Contamination of fish in UK fresh water systems: risk assessment for human consumption. Chemosphere 122:183–189
Serdar O, Yildirim NC, Tatar S, Yildirim N, Ogedey A (2018) Antioxidant biomarkers in Gammarus pulex to evaluate the efficiency of electrocoagulation process in landfill leachate treatment. Environ Sci Pollut Res 25:12538–12544
Sevcikova M, Modra H, Slaninova A, Svobodova Z (2011) Metals as a cause of oxidative stress in fish: a review. Vet Med-Czech 56(11):537–546
Sies H (2017) Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol 11:613–619
Varoni MV, Pasciu V, Gadau SD, Baralla E, Serra E, Palomba D, Demontis MP (2017) Possible antioxidant effect of Lycium barbarum polysaccharides on hepatic cadmium-induced oxidative stress in rats. Environ Sci Pollut Res 24(3):2946–2955
Wang GY, Hu XM, Zhou QX, Sun XQ (2007a) SOD effect of cadmium chloride on acute toxicity of grass carp. J Northeastern Univ (Natural Science) 28(12):1758–1761
Wang HY, Qin M, Wang KY (2018) The relationship between hepatotoxicity and nephrotoxicity induced by cadmium and zinc homeostasis. J Zunyi Med Univ 41(5):556–561
Wang JT, Li JL, Xu SW (2007b) Effect of oxidative stress on apoptosis induced by subchronic cadmium poisoning in liver and kidney. Chin J Vet Sci 27(3):366–368
Wang MZ, Jia XY, Dong AH, He X, Chu JJ (2006) Effects of cadmium on oxidative stress and antioxidative ability in the frog Rana nigromaculata under a long-term exposure. Acta Ecol Sin 26(6):1718–1724
Wang XJ, Hu GC, Zhang LJ, Li JZ, Guo S, Liu Y, Cui K (2016) Influence of cadmium on antioxidant defense system injuvenile of Orechromis niloticus. Mar Environ Sci 35(5):647–651,657
Wong H, Benoit B, Brand MD (2019) Mitochondrial and cytosolic sources of hydrogen peroxide in resting C2C12 myoblasts. Free Radic Biol Med 130:140–150
Wu H, Li YJ, Lang XP, Wang L (2015) Bioaccumulation, morphological changes, and induction of metallothionein gene expression in the digestive system of the freshwater crab Sinopotamon henanense after exposure to cadmium. Environ Sci Pollut Res 22:11585–11594
Wu H, Xuan RJ, Li YJ, Zhang XM, Jing WX, Wang L (2014) Biochemical, histological and ultrastructural alterations of the alimentary system in the freshwater crab Sinopotamon henanense subchronically exposed to cadmium. Ecotoxicology 23:65–75
Yu DJ, Xu ZF, Wang Y, He AN, Xu B, Yang JH (2006) Experimental study on the effect of alpha-lipoic acid and taurine on sub-chronic toxicity of cadmium. Ind Health Occup Dis 32(1):32–36
Yu S, Halbrook RS, Sparling DW (2013) Correlation between heavy metals and turtle abundance in ponds near the Paducah Gaseous Diffusion Plant, Kentucky, USA. Arch Environ Contam Toxicol 65:555–566
Yuan HX, Qin FJ, Guo WQ, Gu HJ, Shao AH (2016) Oxidative stress and spermatogenesis suppression in the testis of cadmium-treated Bombyx mori larvae. Environ Sci Pollut Res 23:5763–5770
Zhou YY, Jing WX, Dahms H-U, Hwang J-S, Wang L (2017) Oxidative damage, ultrastructural alterations and gene expressions of hemocytes in the freshwater crab Sinopotamon henanense exposed to cadmium. Ecotoxicol Environ Saf 138:130–138
Zhuo LL, Tang S, Wang CC, Cui ZM, Li L, Yu XL, Liu YH (2016) Effects of Ca on Cd uptake and antioxidant enzymes activities of crucian carp under Cd stress. Ecol Environ Sci 25(6):1001–1005
Funding
The study was financed by the Natural Science Foundation of Shanxi Province (award number 201801D121229), Science and Technology Innovation Ability Cultivation Program of Shanxi University of Chinese Medicine (award number 2018PY-008), and Shanxi Key Laboratory of Chinese Medicine Encephalopathy (Shanxi University of Chinese Medicine) (award number CME-OP-2017006).
Author information
Authors and Affiliations
Contributions
Aiguo Dong and Junfeng Huo designed the study, performed the research, analyzed the data, and wrote the paper. Juanjuan Yan was a major contributor in performing the research. Ailing Dong was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This study was approved by Shanxi University of Chinese Medicine (permit number: SXZYYDXLL022).
Consent to publish
Not applicable.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Aiguo Dong and Junfeng Huo are Joint first co-authorship
Rights and permissions
About this article
Cite this article
Dong, A., Huo, J., Yan, J. et al. Oxidative stress in liver of turtle Mauremys reevesii caused by cadmium. Environ Sci Pollut Res 28, 6405–6410 (2021). https://doi.org/10.1007/s11356-020-11017-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11017-2