Abstract
The wastes from the macro-fungus Agaricus bisporus were used as an eco-friendly and low-cost adsorbent for the treatment of colored effluents containing the recalcitrant dyes, acid red 97 (AR97) and crystal violet (CV). The macro-fungal waste presented an amorphous structure, composed of particles with different sizes and shapes. Also, it presents typical functional chemical groups of proteins and carbohydrates with a point of zero charge of 4.6. The optimum conditions for the dosage were found to be as follows: 0.5 g L−1 with an initial pH at 2.0 for the AR97 and 8.0 for the CV. From the kinetic test, it was found that it took 210 min and an adsorption capacity of 165 mg g−1 for the AR97. Concerning the CV kinetics, it took 120 min to reach the equilibrium and it achieved an adsorption capacity of 165.9 mg g−1. The Elovich model was the most proper model for describing the experimental data, achieving an R2 ≥ 0.997 and MSE ≤ 36.98 (mg g−1)2. The isotherm curves were best represented by the Langmuir model, predicting maximum adsorption capacity of 372.69 and 228.74 mg g−1 for the AR97 and CV, respectively. The process was spontaneous and favorable for both dyes. The ∆H0 values were 9.53 and 10.69 kJ mol−1 for AR97 and CV, respectively, indicating physical and endothermic adsorption. Overall, the wastes from Agaricus bisporus have the potential to adsorb cationic and anionic dyes, thus solving environmental problems related to water quality and residue disposal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, the industrial activities are more intensive, thereby the consumption of tons of dyes in different industrial processes has been growing. It is recognized that after each of these processes, a considerable number of effluents are generated, which need proper treatment before being disposed into the environment. The key problem is due to high toxicity, which affects the aquatic biome and other living beings (Gupta et al. 2013). Among the employed dyes, the crystal violet (CV) is known to be carcinogenic and mutagenic. However, it is still highly used in diverse sections of the textile industry, biological coloration, and dermatological agent. At lower concentrations, it can penetrate the skin, causing irritation and problems in the digestive system; in extreme cases, it can lead to breathing difficulties, kidney failure, and permanent blindness (Sarma et al. 2016). Another highly used dye in the textile and leather industries is the acid red 97 (AR97). Living organisms cannot degrade it, so in small concentrations, it can already damage animals and humans (Bankole et al. 2018). In this sense, the removal of these dyes from water resources is of great importance (Rigueto et al. 2020).
The adsorption is an efficient and easy operation method and is economical when employing low-cost adsorbents (Dotto and McKay 2020). Several studies utilizing different biomass based on macro-fungi for the removal of dyes have been reported. These fruiting bodies present great advantages: high availability, low cost, good texture, and physical and chemical resistance, showing good properties for the development of adsorbents (Maurya et al. 2006). Fu and Viraraghavan (2002) used the fungal biomass of Aspergillus niger for the removal of several dyes. Bayramoğlu and Yakup Arıca (2007) utilized the Trametes versicolor inactive biomass for the removal of direct blue 1 and direct red 128. Akar et al. (2009) have used a mixture of A. bisporus and Theileria orientalis for adsorption of reactive blue 49. Fomes fomentarius and Phellinus igniarius were used for the removal of rhodamine B and methylene blue (Maurya et al. 2006). Last, Georgin et al. (2019a) used the residues of the filamentous fungus Beauveria bassiana in the adsorption of the acid red 97 dye.
The Agaricus bisporus macro-fungus, commonly known as champignon mushroom, is an eatable and traded Basidiomycota. The principal components of the fungal cell wall are polysaccharides, chitin being a characteristic component of basidiomycetes (Vetter 2007). The production of these mushrooms generates high quantities of residues. The commercialized part is the upper rounded structure, which is above the ground, whereas the roots and stalk are discarded. Until the present data, no studies were observed that utilize the wastes from Agaricus bisporus macro-fungi as adsorbent. This work is aimed at studying the possibility of using the wastes from the macro-fungus Agaricus bisporus as a low-cost and eco-friendly adsorbent for the treatment of ideal colored effluents containing AR97 and CV. The fungal biomass wastes were prepared and characterized by different techniques. The kinetics, equilibrium, and thermodynamics of the adsorption operation were analyzed.
Materials and methods
Preparation of the Agaricus bisporus macro-fungal wastes
The Agaricus bisporus macro-fungal wastes, composed of roots and stalks (Fig. 1), were collected at local producers in the southern Brazil. Initially, the material was washed to remove impurities and dirt, mostly constituted by the substrate used in culture growth. Second, the samples were dried at 50 °C for 48 h. After this step, around 60% of the mass was lost, considering that it is predominantly composed of water. Last, the material was grounded and sieved (60 mesh), therefore obtaining a gray power with a particle diameter inferior to 150 μm. The material was stored for further utilization and labeled ABR (Agaricus bisporus residue).
Characterization of the ABR adsorbent
ABR was characterized by scanning electron microscopy (SEM) (Duarte et al. 2019), X-ray powder diffraction (XRD) (Oliveira et al. 2019), Fourier transform infrared spectroscopy (FT-IR) (Rojas et al. 2019), and point of zero charge (pHpzc) (Postai et al. 2016).
Adsorption assays using the ABR
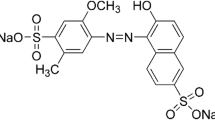
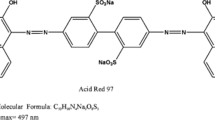
For the adsorption experiments, the AR97 (empirical formula C32H20N4Na2O8S2, Mw 698.6 g mol−1, CAS number 10169-02-05) and CV (empirical formula C25H30N3Cl, Mw 407.9 g mol−1, CAS number 548-62-9) dyes were utilized, which were acquired from Sigma-Aldrich (Germany). At first, stock solutions of the dyes were prepared by dissolving 1.00 g onto 1000 mL of distilled water. The experimental solutions were prepared by diluting the stock solution until reaching the desired concentration. All tests described below were performed separately for each dye under identical experimental conditions. The dye concentrations were determined using a UV-Vis spectrophotometer from Shimadzu (model UV1700, Japan) at the wavelengths of 497 and 590 nm for the AR97 and CV, respectively.
The influence of pH was tested for the range of 2 to 10. The pH values were adjusted using NaOH or HCl solutions (0.1 mol L−1). The dosage of 0.75 g L−1 was added to 50 mL of the dye with an initial concentration of 100 mg L−1 and stirred for 120 min at 298 K. The ABR dosage effect was evaluated for the 0.25, 0.5, 0.75, 1.0, and 1.25 g L−1, at 298 K at the optimum pH. For this, the adsorbent was put in contact with 50 mL of dye solution with the initial concentration of 100 mg L−1. The solutions were agitated for 2 h at 150 rpm.
The kinetic tests were conducted using the optimum dosage and pH conditions. The kinetic curves were evaluated for the different initial concentrations (50, 100, and 200 mg L−1). The samples were collected at various times (from 0 to 240 min). The equilibrium data were obtained for four different temperatures (298, 308, 318, and 328 K) with different initial concentrations (0, 25, 50, 100, 200, 300, 400, and 500 mg L−1).
After all the experiments, the samples were centrifuged (Cenrtibio, 80-2B, Brazil) at 4000 rpm for 20 min, to ensure the separation between the phases. All tests were made in triplicate. The percentage of dye removal (R, %), adsorption capacity at any time (qt, mg g−1), and equilibrium (qe, mg g−1) were used to evaluate the adsorption operation.
Kinetics, equilibrium, and thermodynamics
The kinetic curves were evaluated by the pseudo-first-order (Lagergren 1898), pseudo-second-order (Ho and Mckay 1999), general order (Liu and Shen 2008), and Elovich models (Elovich and Larinov 1962) (supplementary material (S.1)). For the adsorption isotherms, the models of Langmuir (1918) and Freundlich (1906) were tested (supplementary material (S.2)). The estimation of the thermodynamic parameters was done according to Lima et al. (2018) (supplementary material (S.3)). The parameter estimation and fit quality evaluation were made using scripts on MATLAB 2017. The fit quality evaluation was performed through the coefficient of determination (R2), adjusted determination coefficient (R2adj), average relative error (ARE), and minimum squared error (MSE) (supplementary material (S.4)).
Results and discussion
Features of ABR adsorbent
The SEM images with different magnifications were done for studying the surface morphology of the ABR, as presented in Fig. 2. It was verified that ABR is composed of particles with distinct irregular shapes and sizes, with a rugous surface. Similar characteristics have been reported by Akar et al. (2009) using the same fungi and by Grassi et al. (2019) for Diaporthe schini.
The XRD pattern for the ABR is exhibited in Fig. 3, variating the 2θ from 0 to 100°. In this case, the pattern presented a broad peak between 10 and 50°, without the presence of crystalline phases. These arrangements are composed of functional groups present on the fungal cell wall, containing amide, carboxyl, and phosphate (Li et al. 2018). These disorganized structures can facilitate the adsorption of dyes on the mushroom (Zazycki et al. 2018). The profile of this structure was equally found by Georgin et al. (2019a), in the fungus Beauveria bassiana.
The FT-IR spectrum for the ABR adsorbent is presented in Fig. 4. The broadband at 3420 cm−1 is related to the elongation of OH (Pavan et al. 2008). The band at 2932 cm−1 can be attributed to the CH bonds; after this, the intense bands at 1640 and 1465 cm−1 are related to the amide and ether group vibrations (Kumar and Ahmad 2011). The following band is 1015 cm−1, which is related to the C–OH and C–O– vibrations. The latter bands 710 and 498 cm−1 are associated with the C–H and C–N–C bonds, respectively (Román et al. 2013). From this spectrum, it is expected that ABR contains functional groups of natural chitin, including the hydroxyl, amide I (carbonyl vibration C–N), and amide II (NH deformation) groups. These groups on the ABR surface contain elements present in proteins and carbohydrates that can facilitate the adsorption of the dyes.
The point of zero charge (pHpzc) is the value where the charges at the surface of the materials tend to be zero or equivalent. In this case, the ABR presented a pHpzc of 4.6, according to Fig. 5. Therefore, the surface of the adsorbent is positively charged for pH values below 4.6. On the other hand, the surface is negatively charged for pH values above 4.6.
Evaluation of pH and adsorbent dosage
It is recognized that pH is one of the most relevant parameters in the adsorption process. It is a controller of the superficial charge, ionization degree, and the dissociation of functional groups (Bairagi et al. 2011; Nekouei et al. 2015). Figure 6 shows the adsorption capacity of AR97 and CV as a function of pH. In this figure, an inverse behavior of the dyes concerning the pH was obtained, mainly due to their contrary ionic nature. Concerning the AR97, the adsorption capacity decreased from 99.3 to 3.3 mg g−1 with an increase of pH. The optimum adsorption occurred at pH 2. This trend occurred because AR97 is an anionic dye, being negatively charged in the solution. In parallel, the ABR adsorbent is positively charged at pH values lower than 4.6 (Fig. 5). Hence, at a pH of 2, the interactions are facilitated. For the CV dye, the adsorption increased from 35.6 to 117.4 mg g−1 with the increase of the solution pH. The better pH for CV adsorption was 8. This behavior occurred because CV is a cationic molecule, being positively charged in solution. In parallel, the ABR adsorbent is deprotonated, leading to a notable attraction with the CV dye (Gupta et al. 2012). From these results, the ideal initial pH of the solution was selected as 2.0 for AR97 and 8.0 for CV.
The effect of ABR dosage in the adsorption of AR97 and CV (adsorption capacity and removal percentage) is presented in Fig. 7. For the AR97 (Fig. 7a), the ABR dosage increase led to an increase in removal percentage from 53.4 to 84.9% and a decrease in the adsorption capacity from 213.4 to 67.9 mg g−1. A similar trend was observed for the adsorption of CV (Fig. 7b), where the removal percentage increased from 66.5 to 88% and the adsorption capacity decreased from 266.0 to 70.4 mg g−1. The substantial increase in the removal percentage (R), for both dyes, at ABR dosage until 0.7 g L−1, is due to the greater number of vacant sites on the adsorbent surface, so the dye molecules are easily allocated. In contrast, the modest increase in removal percentage at higher ABR dosages is due to the lack of available sites (Pathania et al. 2016). Similar behavior has also been found by Mittal et al. (2010) when studying the adsorption of chrysodine. In this case, the ABR dosage of 0.5 g L−1 was adopted, because this dosage provides good values of both removal percentage and adsorption capacity.
Adsorption of AR97 and CV on ABR: kinetics, isotherms, and thermodynamics
The kinetic profiles for the adsorption of AR97 and CV onto the ABR are presented in Figs. 8 a and b, respectively. It can be noted in Fig. 8 that, for both dyes, the adsorption capacity increased with the contact time, attaining a constant value. Besides, for both dyes, the equilibrium time was little dependent on the initial concentration. For AR97, the equilibrium was achieved at 210 min for all initial concentrations, with a maximum adsorption capacity of 165.91 mg g−1 for 200 mg L−1. For the CV dye, the equilibrium time was reached at 125 min for all concentrations, reaching an adsorption capacity of 259.57 mg g−1 for the initial concentration of 200 mg L−1. Through a comparative evaluation between the dyes, it was observed that the adsorption occurred faster for CV than for AR97. This trend occurred because CV has little molecular volume and size in comparison with AR97, facilitating the mass transfer aspects. Similar tendencies were found in the adsorption of methyl orange using mesoporous carbon material (Mohammadi et al. 2011).
For describing the adsorption kinetics of both dyes onto the ABR, the pseudo-first-order, pseudo-second-order, general order, and Elovich models were adjusted. The parameters are presented in Tables 1 and 2 (AR97 and CV, respectively). The Elovich model was the most suitable for describing the kinetic behavior for both adsorbate/adsorbent systems. In the case of AR97, the statistical indicators were R2adj ≥ 0.975 and MSE ≤ 24.6 (mg g−1)2. For the CV, an R2adj ≥ 0.981 and MSE ≤ 37.0 (mg g−1)2 were found. According to Wu et al. (2009), this model is suitable for adsorption on heterogeneous surfaces. This behavior is following the system proposed in this work, where the adsorbent (ABR) has adsorption sites of different natures and energies. The parameter “b” is the initial adsorption rate because (dqt/dt) tends to “b” when qt tends to 0. In this work, for all initial concentrations, the parameter “b” was higher for CV than for AR97. This trend is an indication that at the initial stages, the CV adsorption is faster than the AR97 adsorption. This behavior can be easily observed comparing Fig. 8 a and b. This behavior is also associated with the little molecular volume and size of the CV dye.
The equilibrium data for the adsorption of AR97 and CV are presented in Fig. 9a, b, respectively. According to Giles et al. (1974), the isotherms can be classified as L2 type. For both cases, the temperature increase caused an increase in the adsorption capacity, indicating endothermic adsorption. However, the inclination of the curves differs according to the system, being more favorable for the AR97, where the adsorption capacity achieved 362.09 mg g−1 at 328 K. For the CV, adsorption capacity reached 202 mg g−1 at 328 K. The temperature effect on the adsorption capacity can be explained by the increase in the dispersion of the dye molecules through the outer boundary layer and the internal pores of the adsorbent particles, as a result of the solution viscosity reduction (Güzel et al. 2015). Similar behavior has been observed by Akar et al. (2009) in the adsorption of acid red 44 on the biomass of the fungus Agaricus bisporus. Grassi et al. (2019) also found that the adsorption capacity of CV onto Diaporthe schini fungi increased with the temperature. Streit et al. (2019), studying the adsorption of allura red and CV on activated carbon, identified a similar trend concerning the temperature. According to these authors, this behavior is also associated with the desolvation of the dye molecules.
The estimated parameters for the equilibrium models are presented in Tables 3 and 4. From the statistical indicators, it was found that the Langmuir model is the most suitable for describing the adsorption isotherms for both adsorbate/adsorbent systems. For the AR97, the Langmuir model presented an R2adj ≥ 0.941 and an MSE ≤ 523.45 (mg g−1)2. Following the same criteria, for the CV, the Langmuir model achieved an R2adj ≥ 0.984 and an MSE ≤ 48.83 (mg g−1)2. The model parameters (qL and KL) agree to the experimental data, increasing with the temperature. This trend shows that the adsorption capacity and also the affinity adsorbate/adsorbent have increased with the temperature. Furthermore, the parameters qL and KL were higher for the AR97 dye. This behavior shows that the adsorption capacity for this dye was higher, and also, its affinity for ABR was higher. This trend can be attributed to the more ramified structure of the AR97 dye, which, in turn, leads to a more significant probability of interacting with the ABR surface.
Take into account the literature and the results found in this work; a comparison was considered for the adsorption capacity, presented in Table 5 (AR97). Several adsorbents used for the removal of AR97 and CV were analyzed (Table 5). For both dyes, the ABR adsorbent reached the third-best adsorption capacity, being 372.69 and 228.74 mg g−1, respectively. These results demonstrate that in addition to the low cost of obtaining the material, since it is a residue and requires practically not even a reagent for its preparation, the ABR demonstrates the considerable potential for removing textile dyes, making it a promising adsorbent material.
The computed thermodynamic parameters for both systems are presented in Table 6. The estimate has to use the Langmuir constant (KL) for the determination of the equilibrium constant (Ke). For both systems, it was found that the adsorption was a spontaneous process since ΔG0AR97 and ΔG0CV were negative. Both systems were endothermic with ΔH0AR97 = 9.53 and ΔH0CV = 10.69 kJ mol−1 (Gupta et al. 1997). The magnitude of ΔH0 indicates that the ion-dipole, dipole-dipole, or hydrogen bonds can be involved in the adsorption of CV and AR97 on ABR (Chang and Thoman 2014). Minor rearrangements occurred at the surface of the adsorbent for both systems, considering the positive ΔS0 values.
Conclusion
The macro-fungal (Agaricus bisporus) wastes were evaluated as an adsorbent in the removal of the acid red 97 and crystal violet dyes from ideal colored effluents. It was obtained that the Agaricus bisporus wastes presented an amorphous structure composed of irregular particles, formed by chemical elements present in proteins and carbohydrates. The adsorbent presented a pHpzc of 4.6. The adsorption was favorable at acidic pH (2) for AR97 and basic pH (8) for CV with the optimum ABR dosage of 0.5 g L−1. The adsorption equilibrium has been achieved at 210 and 125 min, for the AR97 and CV. The Elovich model is the proper model to describe the kinetic data for both systems. For the isothermal studies, the Langmuir model showed strong affinity of the dyes with the ABR, obtaining a maximum capacity of 372.69 mg g−1 for AR97 and 228.74 mg g−1 for CV. The adsorption for both systems was spontaneous, favorable, and endothermic. All these results show that the wastes from the cultivation of Agaricus bisporus have great potential in the treatment of ideal colored effluents.
References
Akar ST, Gorgulu A, Kaynak Z, Anilan B, Akar T (2009) Biosorption of reactive blue 49 dye under batch and continuous mode using a mixed biosorbent of macro-fungus Agaricus bisporus and Thuja orientalis cones. Chem Eng J 148:26–34
Bairagi H, Khan MMR, Ray L, Guha AK (2011) Adsorption profile of lead on Aspergillus versicolor: a mechanistic probing. J Hazard Mater 186:756–764
Bankole PO, Adekunle AA, Govindwar SP (2018) Enhanced decolorization and biodegradation of acid red 88 dye by newly isolated fungus, Achaetomium strumarium. J Environ Chem Eng 6:1589–1600
Bayramoğlu G, Yakup Arıca M (2007) Biosorption of benzidine based textile dyes “direct blue 1 and direct red 128” using native and heat-treated biomass of Trametes versicolor. J Hazard Mater 143:135–143
Bellatin L, Herrera O, Navarro A, Sun-kou R, Llanos B (2014) Biosorption study of acid red 18, basic blue 99 and basic yellow 57, present in hair dyes, with residues of green tea leaves. Rev Soc Chem Peru 80
Chang R, Thoman JW (2014) Physical chemistry for the chemical sciences. University Science Books
Demarchi CA, Campos M, Rodrigues CA (2013) Adsorption of textile dye reactive red 120 by the chitosan-Fe(III)-crosslinked: batch and fixed-bed studies. J Environ Chem Eng 1:1350–1358
Djelad A, Mokhtar A, Khelifa A, Bengueddach A, Sassi M (2019) Alginate-whey an effective and green adsorbent for crystal violet removal: kinetic, thermodynamic and mechanism studies. Int J Biol Macromol 139:944–954
Dotto GL, McKay G (2020) Current scenario and challenges in adsorption for water treatment. J Environ Chem Eng 8:103988
Duarte AL, DaBoit K, Oliveira MLS, Teixeira EC, Schneider IL, Silva LFO (2019) Hazardous elements and amorphous nanoparticles in historical estuary coal mining area. Geosci Front 10:927–939
El-Bindary AA, Diab MA, Hussien MA, El-Sonbati AZ, Eessa AM (2014) Adsorption of acid red 57 from aqueous solutions onto polyacrylonitrile/activated carbon composite. Spectrochim Acta Part A: Mol Biomol Spect 124:70–77
Elovich SY, Larinov OG (1962) Theory of adsorption from solutions of non-electrolytes on solid (I) equation adsorption from solutions and the analysis of its simplest form, (II) verification of the equation of adsorption isotherm from solutions. Zvestiya Akademii Nauk SSSR 2:209–216
Franco DSP, Georgin J, Drumm FC, Netto MS, Allasia D, Oliveira MLS, Dotto GL (2020) Araticum (Annona crassiflora) seed powder (ASP) for the treatment of colored effluents by biosorption. Environ Sci Pollut Res 27:11184–11194
Freundlich H (1906) Ubber die adsorption in lusungen. J Am Chem Soc 61:2–28
Fu Y, Viraraghavan T (2002) Dye biosorption sites in Aspergillus niger. Bioresour Technol 82:139–145
Georgin J, Alves E, Drumm F, Tonato D, Grassi P, Piccin JS, Oliveira MLS, Dotto GL, Mazutti M (2019b) Application of Beauveria bassiana spore waste as adsorbent to uptake acid red 97 dye from aqueous medium. Environ Sci Pollut Res 26:36967–36977
Georgin J, Drumm FC, Grassi P, Franco D, Allasia D, Dotto GL (2018a) Potential of Araucaria Angustifolia bark as an adsorbent to remove gentian violet dye from aqueous effluents. Water Sci Technol 78:1693–1703
Georgin J, Franco DSP, Grassi P, Tonato D, Piccilli DGA, Meili L, Dotto GL (2019a) Potential of Cedrella fissilis bark as an adsorbent for the removal of red 97 dye from aqueous effluents. Environ Sci Pollut Res 26:19207–19219
Georgin J, Marques BS, Peres EC, Allasia D, Dotto GL (2018b) Biosorption of cationic dyes by Pará chestnut husk (Bertholletia excelsa). Water Sci Technol 77:1612–1621
Ghazali A, Shirani M, Semnani A, Zare-Shahabadi V, Nekoeinia M (2018) Optimization of crystal violet adsorption onto. Date palm leaves as a potent biosorbent from aqueous solutions using response surface methodology and ant colony. J Environ Chem Eng 6:3942–3950
Giles CH, Smith D, Huitson A (1974) A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J Colloid Interface Sci 47:755–765
Gopi S, Pius A, Thomas S (2016) Enhanced adsorption of crystal violet by synthesized and characterized chitin nanowhiskers from shrimp shell. J water Process Eng 14:1–8
Graba Z, Hamoudi S, Bekka D, Bezzi N, Boukherroub R (2015) Influence of adsorption parameters of basic red dye 46 by the rough and treated Algerian natural phosphates. J Ind Eng Chem 25:229–238
Grassi P, Reis C, Drumm FC, Georgin J, Tonato D, Escudero LB, Dotto GL (2019) Biosorption of crystal violet dye using inactive biomass of the fungus Diaporthe schini. Water Sci Technol 79:709–717
Gupta VK, Pathania D, Sharma S, Agarwal S, Singh P (2013) Remediation and recovery of methyl orange from aqueous solution onto acrylic acid grafted Ficus carica fiber: isotherms, kinetics, and thermodynamics. J Mol Liq 177:325–334
Gupta VK, Ali I, Saleh TA, Siddiqui MN, Agarwal S (2012) Chromium removal from water by activated carbon developed from waste rubber tires. Environ Sci Pollut Res 20:1261–1268
Gupta VK, Rastogi A, Dwivedi MK, Mohan D (1997) Process development for the removal of zinc and cadmium from wastewater using slag-A blast furnace waste material. Sep Sci Technol 32:2883–2912
Güzel F, Sayğılı H, Akkaya Sayğılı G, Koyuncu F (2015) New low-cost nanoporous carbonaceous adsorbent developed from carob (Ceratonia siliqua) processing industry waste for the adsorption of anionic textile dye: characterization, equilibrium and kinetic modeling. J Mol Liq 206:244–255
Heibati B, Rodriguez-Couto S, Al-Ghouti MA, Asif M, Tyagi I, Agarwal S, Gupta VK (2015) Kinetics and thermodynamics of enhanced adsorption of the dye AR 18 using activated carbons prepared from walnut and poplar woods. J Mol Liq 208:99–105
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Khodam F, Rezvani Z, Amani-Ghadim AR (2015) Enhanced adsorption of acid red 14 by co-assembled LDH/MWCNTs nanohybrid: optimization, kinetic and isotherm. J Ind Eng Chem 21:1286–1294
Kumar R, Ahmad R (2011) Biosorption of hazardous crystal violet dye from aqueous solution onto treated ginger waste (TGW). Desalination 265:112–118
Lagergren S (1898) Zur Theorie der Sogenannten Adsorption Gelöster Stoffe. Kung Svenska Vetenskap 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica, and platinum. J Amer Chem Soc 40:1361–1403
Li X, Zhang D, Sheng F, Qing H (2018) Adsorption characteristics of copper, zin and mercury by four kinds of immobilized fungi residues. Ecotoxicol Environ Saf 147:357–366
Liang YD, He YJ, Wang TT, Lei LH (2019) Adsorptive removal of gentian violet from aqueous solution using CoFe2O4/activated carbon magnetic composite. J Water Proc Eng 27:77–88
Lima EC, Hosseini-Bandegharaei A, Moreno-Piraján JC, Anastopoulos I (2018) A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J Mol Liq 273:425–434
Liu Y, Shen L (2008) A general rate law equation for biosorption. Biochem Eng J 38:390–394
Maurya NS, Mittal AK, Cornel P, Rother E (2006) Biosorption of dyes using dead macro fungi: effect of dye structure, ionic strength, and pH. Bioresour Technol 97:512–521
Mittal A, Mittal J, Malviya A, Gupta VK (2010) Removal and recovery of Chrysoidine Y from aqueous solutions by waste materials. J Colloid Interface Sci 344:497–507
Miyah Y, Lahrichi A, Idrissi M, Boujraf S, Taouda H, Zerrouq F (2017) Assessment of adsorption kinetics for removal potential of crystal violet dye from aqueous solutions using Moroccan pyrophyllite. J Assoc Arab Univ Basic Appl Sci 23:20–28
Mohammadi N, Khani H, Gupta VK, Amereh E, Agarwal S (2011) Adsorption process of methyl orange dye onto mesoporous carbon material-kinetic and thermodynamic studies. J Colloid Interface Sci 362:457–462
Nekouei F, Nekouei S, Tyagi I, Gupta VK (2015) Kinetic, thermodynamic and isotherm studies for acid blue 129 removal from liquids using copper oxide nanoparticle-modified activated carbon as a novel adsorbent. J Mol Liq 201:124–133
Oliveira M, Izquierdo M, Querol X, Lieberman RN, Saikia BK, Silva LFO (2019) Nanoparticles from construction wastes: a problem to health and the environment. J Clean Prod 219:236–243
Pal A, Pan S, Saha S (2013) Synergistically improved adsorption of anionic surfactant and crystal violet on chitosan hydrogel beads. Chem Eng J 217:426–434
Pathania D, Katwal R, Kaur H (2016) Enhanced photocatalytic activity of electrochemically synthesized aluminum oxide nanoparticles. Int J Miner Metall Mater 23:358–371
Pavan FA, Lima EC, Dias SLP, Mazzocato AC (2008) Methylene blue biosorption from aqueous solutions by yellow passion fruit waste. J Hazard Mater 150:703–712
Postai DL, Demarchi CA, Zanatta F, Melo DCC, Rodrigues CA (2016) Adsorption of rhodamine B and methylene blue dyes using the waste of seeds of Aleurites Moluccana, a low-cost adsorbent. Alexandria Eng J 55:1713–1723
Rigueto CVT, Piccin JS, Dettmer A, Rosseto M, Dotto GL, Schimitz APO, Perondi D, Freitas TSM, Loss RA, Geraldi CAQ (2020) Water hyacinth (Eichhornia crassipes) roots, an amazon natural waste, as an alternative biosorbent to uptake a reactive textile dye from aqueous solutions. Ecol Eng 150:105817
Rojas JC, Sánchez NE, Schneider I, Oliveira MLS, Teixeira EC, Silva LFO (2019) Exposure to nanometric pollutants in primary schools: environmental implications. Urban Clim 27:412–419
Román S, Valente Nabais JM, Ledesma B, González JF, Laginhas C, Titirici MM (2013) Production of low-cost adsorbents with tunable surface chemistry by the conjunction of hydrothermal carbonization and activation processes. Microporous Mesoporous Mater 165:127–133
Sarma GK, Sen Gupta S, Bhattacharyya KG (2016) Adsorption of crystal violet on raw and acid-treated montmorillonite, K10 in aqueous suspension. J Environ Manag 171:1–10
Shakoor S, Nasar A (2018) Adsorptive decontamination of synthetic wastewater containing crystal violet dye by employing Terminalia arjuna sawdust waste. Ground Sustain Dev 7:30–38
Sharma G, Kumar A, Naushad M, García-Peñas A, Al-Muhtaseb AH, Ghfar AA, Stadler FJ (2018) Fabrication and characterization of gum arabic-cl-poly (acrylamide) nano hydrogel for effective adsorption of crystal violet dye. Carbohydr Polym 202:444–453
Sonai GG, de Souza SMAGU, de Oliveira D, de Souza AAU (2016) The application of textile sludge adsorbents for the removal of reactive red 2 dye. J Environ Manag 168:149–156
Srikantan C, Suraishkumar GK, Srivastava S (2018) Effect of light on the kinetics and equilibrium of the textile dye (reactive red 120) adsorption by Helianthus annuus hairy roots. Bioresour Technol 257:84–91
Streit AFM, Côrtes LN, Druzian SP, Godinho M, Collazzo GC, Perondi D, Dotto GL (2019) Development of high quality activated carbon from biological sludge and its application for dyes removal from aqueous solutions. Sci Total Environ 660:277–287
Tahir N, Bhatti HN, Iqbal M, Noreen S (2017) Biopolymers composites with peanut hull waste biomass and application for crystal violet adsorption. Int J Biol Macromol 94:210–220
Thue PS, Sophia AC, Lima EC, Wamba AGN, de Alencar WS, dos Reis GS, Dias SLP (2018) Synthesis and characterization of a novel organic-inorganic hybrid clay adsorbent for the removal of acid red 1 and acid green 25 from aqueous solutions. J Clean Prod 171:30–44
Vetter J (2007) Chitin content of cultivated mushrooms Agaricus bisporus, Pleurotus ostreatus, and Lentinula edodes. Food Chem 102:6–9
Wu FC, Tseng RL, Juang RS (2009) Characteristics of the Elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems. Chem Eng J 150:366–373
Xu D, Gu C, Chen X (2013) Adsorption and removal of acid red 3R from aqueous solution using flocculent humic acid isolated from lignite. Procedia Environ Sci 18:127–134
Zazycki MA, Godinho M, Perondi D, Foletto EL, Collazzo GC, Dotto GL (2018) New biochar from pecan nutshells as an alternative adsorbent for removing reactive red 141 from aqueous solutions. J Clean Prod 171:57–65
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial Responsibility: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Drumm, F.C., Franco, D.S.P., Georgin, J. et al. Macro-fungal (Agaricus bisporus) wastes as an adsorbent in the removal of the acid red 97 and crystal violet dyes from ideal colored effluents. Environ Sci Pollut Res 28, 405–415 (2021). https://doi.org/10.1007/s11356-020-10521-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10521-9