Abstract
The adsorption of acid red 97 dye (RED 97) by the waste of the filamentous fungus Beauveria bassiana was analyzed. The adsorbent was obtained as a waste of a fermentative process, and characterized by scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FT–IR), X–ray powder diffractometry (XRD), and specific surface area (BET). After the characterization, adsorption tests were carried out to determine the ideal conditions of pH, adsorbent mass, and contact time for the process. Adsorption isotherms, thermodynamic studies, and the treatment of textile effluent were also investigated. The adsorbent characterization allowed the visualization of its amorphous structure, with irregular and heterogeneous particles. The pore diameter was 51.9 nm and the surface area was 0.247 m2 g−1. 1.2 g L−1 of the adsorbent and pH of 2.0 were the ideal conditions for RED 97 adsorption. The pseudo–second–order kinetic model was the most appropriate to represent the experimental data, being the equilibrium reached in about 110 min. The Langmuir model was the most suitable to represent the equilibrium data, with maximum adsorption capacity of 194.1 mg g−1 at 45 °C. The adsorption processes was thermodynamically spontaneous, favorable, and exothermic. In the treatment of a real textile effluent, 5 g L−1 of the spores was capable to decolorize 70% of the solution. Therefore, spore wastes of Beauveria bassiana were promising for RED 97 adsorption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Azo dyes are constantly and widely applied directly or indirectly by pharmaceutical, paper, printing, pulp, food, leather, and petroleum industries (Saratale et al. 2013). This class represents up to 70% of the total textile dyestuffs used in industry. Up to half the amount of the original dye used is lost into wastewaters during dyeing and dye fixation processes (Bumpus 1995). As consequence, high quantities of dyes are dumped into the environment. A significant increase in oxygen demand (dissolved chemical and biological) is caused by the improper discharge of dyes in water bodies (Solis et al. 2012). In addition, azo dyes are toxic to plants, human, and animals, causing grave environmental concerns owing to their characteristic brilliance color, carcinogenic potential, and recalcitrance (Lang et al. 2013). Therefore, to reduce the effect of these discharges, it is very necessary the treatment of dye effluents.
Only few of several physicochemical decolorization technologies have been found worthy by the textile waste managers (Da Silva and Faria 2003). In recent years, several physicochemical methods have been employed in the removal of dyes from textile effluents. These methods have been certified unsuitable and uneconomical due to the production of large amounts of toxic sludge, aromatic amines, toxic substances, and secondary waste products (Alhassani et al. 2007). These degradation intermediates viz: phenols, benzene, naphthalene, and aromatic amines are very toxic and recalcitrant. Also, Myslak and Bolt (1998) reported the carcinogenic and mutagenic nature of aromatic amines produced after biodegradation of azo dyes. Alternatively, fungal biomass can be used as adsorbent to uptake azo dyes from effluents. This practice has proven to be more eco–friendly, cheap, and sustainable. In addition, no undesirable intermediates are formed (Banat et al. 1996).
Due to their massive biomass production, great surface to cell ratio solid–liquid, and extensive hyphal development, a number of filamentous fungal species have been explored for azo dye biodegradation (Mishra and Malik 2013). However, as mentioned, biodegradation leads to undesirable intermediates. In this way, it was proposed the application of Beauveria bassiana filamentous fungi biomass wastes as dye adsorbent. This fungus is widely used in biological control in several countries, including products marketed in Brazil. The application of this material as adsorbent is interesting, since the spore residues can be obtained as wastes of fermentation, which in turn, produce hydrolytic enzymes. Beauveria bassiana was already used to uptake metals like Zn(II), Cu(II), Cd(II), Pb(II), Cr(VI), and Ni(II) from aqueous media (Hussein et al. 2011; Gola et al. 2016). Recently, Beauveria bassiana was also used to uptake Reactive remazol red, Yellow 3RS, Indanthrene blue, and Vat novatic gray dyes from aqueous solutions (Gola et al. 2018). It is important highlight that in this work, it was verified the potential of Beauveria bassiana wastes, after a fermentation that generated hydrolytic enzymes.
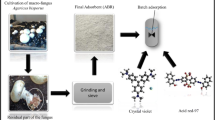
This study aimed to investigate the potential of Beauveria bassiana wastes in the removal of RED 97 dye from textile effluents. Concerning the adsorption of RED 97 dye, other materials were already investigated. Ghazi Mokri et al. (2015) investigated the application of walnut shells and found 73.33% of dye removal at 60 min of contact time, agitation rate of 140 rpm, and 12 g L−1 of adsorbent at 291 K. Balarak and Mostafapour (2016) evaluated the potential of canola stalks and found adsorption capacity of 19.8 mg g−1. Zulfiqar Ahmed et al. (2018) used α–Fe2O3 nanopowder and found a decolorization of 76.39% using 0.9 g L−1 of adsorbent during 35 min. Here, the potential of Beauveria bassiana wastes, a co–product of fermentation, was evaluated. Fungus wastes were characterized using scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FT–IR), X–ray powder diffractometry (XRD), and specific surface area (BET). Adsorption isotherm studies and adsorption kinetics were performed to understand the process. Thermodynamic parameters were also calculated. Finally, the adsorbent was applied in the treatment of a simulated effluent, aiming to verify the efficiency of the material in real process conditions.
Material and methods
Production of Beauveria bassiana spores by solid–state fermentation
Spore residues were obtained from laboratory studies that used the fermentation broth and discarded the biomass, with the objective to produce hydrolytic enzymes (endocellulase, exocellulase, chitinase, and β–1.3–glucanase).
Beauveria bassiana strains (IBCB 66) were provided by the Biological Institute (São Paulo–Brazil). For the pre–inoculum, the culture grown on PDA medium in 15 mL Falcon tubes was transferred to a 50-mL Falcon tube with 10 mL of a PD (potato, dextrose) and micronutrient solution containing (NH4)2SO4, (2 g L–1), FeSO4·7H2O (1 g L−1), MnSO4·H2O (1 g L−1), Mg SO4·H2O (0.5 g L−1), and K2HPO4 (1 g L−1) (Souza et al. 2015) and maintained for 24 h at 28 °C. Afterwards, the solution was transferred to an Erlenmeyer flask containing 90 mL of the solution described previously (PDA and micronutrient solution) and remained at 28 °C and 120 rpm for 72 h.
For spore production, rice was used as substrate with addition of CaCO3 (0–2% w/w) and micronutrient solution (0–1% w/w). The process was conducted in closed cylindrical glass flasks, for 10 days at 25 °C. Initial moisture varied from 40 to 60% and inoculum concentration from 5 to 10% (w/w).
After fermentation, distilled water (proportion 1:5) was used to obtain the fermentation broth. The extraction was performed using glass flasks and shaking at 150 rpm and 28 °C for 60 min. Then, the broth was separated from the solid mass by gauze filtration followed by centrifugation (Eppendorf, 5804R) for 5 min at 20 °C and 4000 rpm. The sediment formed after centrifugation, containing the spores, was dried in an oven for 24 h at 100 °C and macerated to obtain the powdered adsorbent.
Fungus characterization
B. bassiana wastes were characterized by FT–IR, XRD, BET, and SEM. The Fourier transform infrared spectroscopy (FT–IR) analysis (Prestige, 21210045, Japan) was performed using KBr pellets, in the range from 4500 to 500 cm−1 (Silverstein et al. 2007), aiming to identify the main groups on the spore surface and verify the possible modifications after the adsorption process. X–ray diffraction (XRD) (Rigaku, Miniflex 300, Japan) (Brindley and Brown 1980) was used to verify the crystalline/amorphous structure of the spores. Scanning electron microscopy (SEM) images with different magnifications and accelerating voltage of 10 kV (Jeol, JSM–6610LV, Japan) (Goldstein et al. 1992) were used to verify the morphology of the spores. The specific surface area (BET) was calculated from the N2 adsorption data using the Brunauer–Emmett–Teller equation (Micrometrics, ASAP, 2020) (Thommes et al. 2015).
Batch adsorption assays
The azo dye acid red 97 (RED 97) was used as target molecule. Its structure and characteristics are shown in Fig. 1. RED 97 concentrations in liquid phase were determined by spectrophotometry at 497 nm (Biospectro, Brazil), using a calibration curve previously obtained from successive dilutions of a stock solution (1.0 g L−1). All adsorption experiments were performed on a thermostated agitator (Marconi, MA 093, Brazil) at 200 rpm.
The adsorbent dosage effect was verified with dosages of 0.5, 1.0, 1.5, 2.0, and 2.5 g L−1 at 25 °C and pH 2. The adsorbent was contacted with 50 mL of a 100 mg L−1 dye solution, and stirred for 2 h. The pH effect was verified using 2, 4, 6, 8, and 10, at 25 °C, initial dye concentration of 100 mg L–1, and contact time of 2 h. The adsorption kinetics were obtained using the best adsorbent dosage and pH (previous determined), varying the time in the intervals of 5, 10, 15, 20, 40, 60, 75, 90, 120, 150, and 180 min, at RED 97 concentrations of 50, 100, and 200 mg L−1. The isotherms were obtained at the same adsorbent dosage and pH that adsorption kinetics, at 25 °C, 35 °C, 45 °C, and 55 °C. To obtain different equilibrium concentrations (Ce, mg L−1), initial concentrations of RED 97 dye were of 25, 50, 100, 200, 300, 400, and 500 mg L−1. The equilibrium was defined when the measurement of the concentration in the liquid phase (Ce) showed a coefficient of variation of less than 5% in three consecutive measurements at intervals of 2 h (Piccin et al. 2017).
Data analysis
The dye removal percentage (R, %), adsorption capacity at any time (qt, mg g−1), and adsorption capacity at equilibrium (qe, mg g−1) were calculated as follows:
where C0, Ct, and Ce are the dye concentrations at t = 0, at any time and at equilibrium, respectively, W is the adsorbent mass (g), and V is the volume of dye solution (L).
Kinetic equilibrium and thermodynamics
Pseudo–first–order and pseudo–second–order models were used to represent the experimental data (Liu and Shen 2008). These models are respectively presented as follows:
where k1 (min−1) and k2 (g mg−1 min−1) are the rate constants of pseudo–first order and pseudo–second order, respectively; q1 and q2 are the theoretical values for the adsorption capacity (mg g−1).
Langmuir (Langmuir 1918), Freundlich (Freundlich 1906), and Redlich–Peterson (Redlich and Peterson 1959) models were used to represent the adsorption equilibrium as follows:
where qmax is the maximum adsorption capacity for Langmuir (mg g−1), KL is the Langmuir constant (L mg–1), KRP is the Redlich–Peterson constant (L mg−1), aRP is the affinity coefficient (L mg−1)β, β is the heterogeneity factor (dimensionless and 0 ≥ β ≤ 1), 1/nf is the heterogeneity factor (dimensionless), and Kf is the Freundlich constant (mg g−1) (L mg−1)−1/nf.
The adsorption thermodynamics was evaluated by standard Gibbs free energy change (ΔG0, kJ mol−1), enthalpy change (ΔH0, kJ mol−1), and entropy change (ΔS0, kJ mol−1 K−1). The equilibrium constant was estimated through the parameters of the best isotherm model (Lima et al. 2019).
where ke is the equilibrium constant (dimensionless), k is the constant parameter from the most suitable isotherm fit (L mg−1), MW is the molecular weight of the red 97 (mg mol−1), [red97]0 is the standard dye concentration (1 mol L−1), γ is the unitary activity coefficient (dimensionless), T is the temperature (K), and R is the universal gas constant (8.31 × 10–3 kJ mol−1 K−1).
The above parameters (isotherm, kinetic, and thermodynamics) were calculated by nonlinear regression, by the minimization of the least square function using the software Statistic 8.0 (Statsoft, EUA). The fit quality was evaluated by the determination coefficient (R2), adjusted determination coefficient (R2adj), and average relative error (ARE) (Dotto et al. 2013).
Treatment of simulated textile effluent
A synthetic solution was prepared based on the industrial textile effluents (Table 1) (Hessel et al. 2007). The simulated effluent was placed in contact with 5.0 g L−1 of spores, under stirring of 200 rpm during 1 h at ambient temperature. The effluent was scanned in the range of 400 to 800 nm in a spectrophotometer (Shimadzu, UV mini 1240, Japan), before and after the treatment. The color removal percentage was estimated through the ratio between the areas from the spectroscopic curves.
Results and discussion
Characteristics of Beauveria bassiana wastes
Figure 2 shows the SEM images of B. bassiana spores. Figure 2a reveals that the fungal biomass is a particulate material formed by irregular and deformed particles. The surface is irregular with cavities (Fig. 2b). In addition, smaller cavities were observed in the material internal structure (Fig. 2c).
Figure 3 shows the N2 adsorption–desorption isotherms of the biomass waste. According to the International Union of Pure and Applied Chemistry (IUPAC), the sample presented a type IV isotherm, exhibiting characteristics predominantly of mesoporous materials. In addition, the material had a pore diameter of 51.96 nm, a surface area of 0.247 m2 g−1, and a total pore volume of 0.001062 cm3 g−1. These values are low compared with traditional adsorbents, but are in the adequate range in relation to biomasses (Escudero et al. 2019).
The FT–IR vibrational spectrum of B. bassiana (Fig. 4) showed an intense band at 3319.4 cm−1 due to the stretching of the OH group. At 1757.1 cm−1, it was verified the stretching of N=N group. Other intense bands were found at 2946.9 cm−1 and 2835.3 cm−1, related to the stretching of C–H groups. These groups can be potential receptor sites for the dye molecules, depending of the experimental condition.
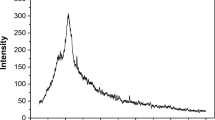
The XRD pattern of the fungal biomass residues is shown in Fig. 5. The fungal biomass residue was identified by a 2θ phase analysis, ranging from 10° to 100°. This pattern showed a broad band pronounced at 2θ in the range of 10° to 50°, with no crystalline phase, indicating that this material is composed of an amorphous structure. This factor may be related to the presence of the amide, carboxyl, and phosphate functional groups in the composition of the fungal cell wall (Li et al. 2018). The amorphous region has a disorganized structure which facilitates the dye adsorption (Zazycki et al. 2018). The profile of this adsorbent structure was also found by Georgin et al. (2018), which utilized Pará chestnut husk (Bertholletia excelsa) (PCH) as an adsorbent in the removal of crystal violet (CV) and methylene blue dyes (MB) from aqueous solutions.
Effects of fungal dosage and pH
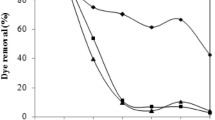
The effects of fungal biomass dosage on the RED 97 removal percentage and adsorption capacity are depicted in Fig. 6a, b, respectively. The removal percentage increased from 37 to 75% when the adsorbent dosage increased (Fig. 6a). This occurred because the amount of adsorption sites to absorb RED 97 was increased. However, the increase in fungal biomass dosage led to a strong decrease in adsorption capacity (Fig. 6b). This occurred because, even with more adsorption sites, the available adsorption sites per unit of mass decreased due to aggregation. So, it is required the selection of an adequate biomass dosage to find simultaneously good values of removal percentage and adsorption capacity. In this work, the intersection of the curves in Fig. 6a, b was selected. This point is a biomass dosage of 1.2 g L−1. Under these conditions, the adsorbent presented adsorption capacity of 44.83 mg g−1, associated with removal percentages of 67.25%. The solution discoloration after the adsorption process with fungal biomass was also observed by Przystaś et al. (2015).
The pH effect on RED 97 adsorption was studied at 2, 4, 6, 8, and 10 with initial dye concentration of 100 mg L−1, adsorbent dosage of 1.2 g L−1, and temperature of 25 °C, during 1 h of agitation. The results are presented in Fig. 6c. It was found that the pH increase from 2 to 6 led to a strong decrease in adsorption capacity from 48 to 8 mg g−1. A new increase in pH from 6 to 10 presented no effect. The adsorption capacity was strongly favored by the pH decrease, with the maximum values obtained at pH 2. The maximum adsorption of RED 97 dye in acidic pH can be attributed to the electrostatic interactions between the positively charged cells of the fungal biomass and the negatively charged anions of the dye (Bankole et al. 2018). Similar trend was found by Bankole et al. (2018) in the adsorption of anionic dyes with the fungus Achaetomium strumarium.
Adsorption kinetics
Adsorption kinetic curves were obtained with initial dye concentrations of 50, 100, and 200 mg L−1, adsorbent dosage of 1.2 g L−1, 25 °C, and pH 2. The profiles are presented in Fig. 7. In the RED 97 adsorption by B. bassiana spores, there was a gradual increase in the adsorption capacity. These profiles indicated that the dye molecules progressively occupied the adsorption sites. For dye concentration of 50 mg L−1, the equilibrium was reached up to 60 min. However, with the increase of dye concentration to 100 and 200 mg L−1, the equilibrium was reached at 75 min and 110 min, indicating a longer contact time. The adsorption capacity increased with the initial dye concentration reaching 73.66 mg g−1 at 200 mg L−1. Another point to be highlighted is that the adsorption of RED 97 on B. bassiana spores was a fast process, being interesting for real applications.
In order to obtain more detailed information concerning to the kinetics, pseudo−first order (PFO) and pseudo−second order (PSO) models were used. The parameters of these models are presented in Table 2. PSO model had a satisfactory fit to the experimental data, as shown by the statistical parameters of Table 2 and the solid lines of Fig. 7. Moreover, the equilibrium adsorption capacity values predicted by the model were similar to the experimental values. For this reason, it can be stated that the best model to represent the adsorption kinetics of RED 97 dye by Beauveria bassiana spores was PSO. The parameters of PSO model demonstrated that the adsorption capacity (q2) increased with the initial dye concentration. However, the rate of adsorption (k2) decreased, demonstrating that the equilibrium was reached with a major contact time. This is due to the fact that, although there is a higher driving force with increasing concentration, the solute (dye) has to reach more adsorption sites inside the adsorbent particle. Thus, it is evident that the diffusive mechanism of the dye within the particle is the limiting step of the adsorption process. Similar results were observed for the adsorption of Purple NR5 Dye by Aristeus antennatus shells (Benchekor et al. 2018).
Equilibrium studies
Figure 8 shows the adsorption isotherms of RED 97 dye in B. bassiana spores obtained in four different temperatures. All the equilibrium curves were of L1 type (Chen et al. 2013), where the adsorption capacity is progressively increased. It was not observed a plateau for the equilibrium concentrations studied, indicating that all sites were not occupied, under the studied conditions. In addition, it can be verified that the adsorption was favored by the temperature increase, and the best results were obtained at 328 K. This fact is related to the higher solubility of RED 97 at higher temperatures and decrease of solution viscosity, which facilitates penetration of dye to adsorption sites within the particle. The adsorption capacity was favored at 328 K.
Freundlich, Langmuir, and Redlich–Peterson isotherm models were fitted to the experimental data in order to obtain more detailed information about the equilibrium. The results are presented in Table 3. All models presented determination coefficient (R2) and adjusted determination coefficient (R2adj) above 90%. However, Langmuir and Redlich–Peterson models presented lower values of ARE, being more adequate than the Freundlich model. It was observed that the β value for the Redlich–Peterson model was close to 1. In this case, the Redlich–Peterson model is simplified to the Langmuir model. For this reason, Langmuir model was considered the most adequate to represent the RED 97 dye adsorption isotherms on B. bassiana spores. In Table 3, it was observed that the parameter qm increased with the temperature, showing that the adsorption capacity was major at higher temperatures. However, at 308 K, it was observed the major value of kL, demonstrating that at this temperature, the adsorption is more effective at low equilibrium concentrations.
In order to verify the efficiency of Beauveria bassiana wastes as an adsorbent, a comparison with other materials was performed. The results are shown in Table 4. Among the 17 adsorbents used for the removal of RED 97 dye (Table 4), the adsorbent produced from Beauveria bassiana spores presented the fourth maximum adsorption capacity, indicating that this fungal biomass waste is a promising adsorbent, which can be used to remove the dye from aqueous medium. Moreover, this adsorbent has other advantages like low cost, since it is derived from residues; simple and fast to be prepared, since it only needs a process of centrifugation, drying, and milling; efficient, since the removal percentage was approximately 67.25%; and relatively fast kinetics, since more than 80% of the saturation was reached in 30 min.
Thermodynamics
The standard values of Gibbs free energy (ΔG0), enthalpy (ΔH0), and entropy changes (ΔS0) were estimated in order to obtain thermodynamic information about the adsorption process. At first, ke parameters were estimated from the Langmuir parameters (Table 5). The spontaneity of the adsorption process of RED 97 dye in fungal residues was confirmed by the negative values of ΔG0. The increase in temperature provided more negative values of ΔG0, indicating that the affinity between the dye and the adsorbent was higher at 328 K. The positive values of ΔH0 indicated that the adsorption process was endothermic and due to physical sorption, probably due to Van Der Walls interactions. Positive values of ΔS0 demonstrated that the solid/liquid interface disorder increased during the adsorption process.
Treatment of simulated effluent
Fungal residues were tested for the treatment of a simulated textile effluent containing different dyes and inorganic compounds (Table 1). In these tests, 5 g L−1 of adsorbent was added to the effluent. The solution was stirred at 200 rpm for 2 h at 25 °C. The visible spectra of the effluent before and after treatment are shown in Fig. 9. In quantitative terms, B. bassiana spores removed 70% of the color. Considering that the concentration of RED 97 dye in textile effluents is in the range of mg L−1 (Hessel et al. 2007; Ghazi Mokri et al. 2015; Balarak and Mostafapour 2016; Zulfiqar Ahmed et al. 2018), it can be concluded that Beauveria bassiana spore wastes are an efficient material to treat colored effluents containing RED 97 dye.
Conclusion
Considering the development of eco–friendly processes, the search for applications to the fermentation co–products is an alternative. In this sense, the wastes of the fungus Beauveria bassiana, after the fermentation for the production of hydrolytic enzymes, were used as an adsorbent for the first time. It was analyzed the use of wastes from Beauveria bassiana spores as an adsorbent in the removal of RED 97 dye. The results showed that under acid pH, at the minimum time of 90 min, using 1.2 g L−1 of adsorbent, the biomass waste was able to remove more than 65% of the color present in the solution. The adsorption kinetic was relatively fast and the curves were better represented by the PSO model. The equilibrium was better represented by the Langmuir model, with the maximum adsorption capacity of 194.1 mg g−1 at 318 K. The adsorption process was spontaneous, favorable, and endothermic. Thus, it can be stated that the use of Beauveria bassiana spore wastes is a promising alternative in the removal of the RED 97 anionic dye. Residues of Beauveria bassiana proved to be an efficient adsorbent in the treatment of effluents containing several dyes and inorganic compounds, providing 70% of color removal.
References
Alhassani HA, Rauf MA, Ashraf SS (2007) Efficient microbial degradation of Toluidine Blue dye by Brevibacillus sp. Dyes Pigments 75:395–400
Balarak D, Mostafapour FK (2016) Adsorption behavior of acid red 97 dye on canola stalks. J Sci Eng Res 3:148–154
Banat IN, Nigam P, Singh D, Marchant R (1996) Microbial decolorization of textile dye–containing effluents: a review. Bioresour Technol 58:217–227
Bankole PO, Adekunle AA, Govindwar SP (2018) Enhanced decolorization and biodegradation of acid red 88 dye by newly isolated fungus, Achaetomium strumarium. J Environ Chem Eng 6:1589–1600
Bellatin L, Herrera O, Navarro A, Sun-Kou R, Llanos B (2014) Estudio de la biosorción de Rojo ácido 18, Azul básico 99 y Amarillo básico 57, presentes en los tintes de cabellos, con residuos de hojas de té verde. Rev Soc Quím Perú 80:9–23
Benchekor H, Iddou A, Hentit H, Aziz A, Piccin JS (2018) Multilayer adsorption of purple NR5 industrial dye by Aristeus antennautus shell in aqueous solution. Key Eng Mater 762:109–114
Brindley GW, Brown G (1980) Crystal structures of clay minerals and their X–ray identification. Mineralogical Society, London. https://doi.org/10.1180/mono-5
Bumpus JA (1995) Microbial degradation of azo dyes. In: Singh VP (ed) Biotransformation: microbial degradation of health–risk compounds. Elsevier Science, Amsterdam, pp 157–175
Chen JJ, Ahmad AL, Ooi BS (2013) Poly(N–isopropylacrylamide–co–acrylic acid) hydrogels for copper ion adsorption: equilibrium isotherms, kinetic and thermodynamic studies. J Environ Chem Eng 1(2013):339–348
Da Silva CG, Faria JL (2003) Photochemical and photocatalytic degradation of an azo dye in aqueous solution by UV irradiation. J Photochem Photobiol A Chem 155:133–143
Demarchi CA, Campos M, Rodrigues CA (2013) Adsorption of textile dye reactive red 120 by the chitosan–Fe(III)–crosslinked: batch and fixed–bed studies. J Environ Chem Eng 1:1350–1358
Dotto GL, Costa JAV, Pinto LAA (2013) Kinetic studies on the biosorption of phenol by nanoparticles from Spirulina sp. LEB 18. J Environ Chem Eng 1:1137–1143
El-bindary AA, Diab MA, Hussien MA, El-Sonbati AZ, Eessa AM (2014) Adsorption of Acid Red 57 from aqueous solutions onto polyacrylonitrile/activated carbon composite. Spectrochim Acta – Part A: Molec Biomolec Spectroscopy 124:70–77
Errais E, Duplay J, Elhabiri M, Khodja M, Ocampo R, Baltenweck-Guyot R, Darragi F (2012) Anionic RR120 dye adsorption onto raw clay: surface properties and adsorption mechanism. Colloids Surfaces A: Physicochem Eng Aspects 403:69–78
Escudero LB, Quintas PY, Wuilloud RG, Dotto GL (2019) Recent advances on elemental biosorption. Environ Chem Lett 17:409–427
Freundlich H (1906) Over the adsorption in solution. Z Phys Chem 57:358–471
Fu F, Xiong Y, Xie B, Chen R (2007) Adsorption of Acid Red 73 on copper dithiocarbamate precipitate–type solid wastes. Chemosphere 66:1–7
Georgin J, Silva Marques B, Peres EC, Allasia D, Dotto GL (2018) Biosorption of cationic dyes by Pará chestnut husk (Bertholletia excelsa). Water Sci Technol 77:1612–1621
Ghazi Mokri HS, Modirshahla N, Behnajady MA, Vahid B (2015) Adsorption of C.I. Acid Red 97 dye from aqueous solution onto walnut shell: kinetics, thermodynamics parameters, isotherms. Int J Environ Sci Technol 12:1401–1408
Gola D, Dey P, Bhattacharya A, Mishra A, Malik A, Namburath M, Ahammad SZ (2016) Multiple heavy metal removal using an entomopathogenic fungi Beauveria bassiana. Bioresour Technol 218:388–396
Gola D, Malik A, Namburath M, Ahammad SZ (2018) Removal of industrial dyes and heavy metals by Beauveria bassiana: FTIR, SEM, TEM and AFM investigations with Pb(II). Environ Sci Pollut Res 25:20486–20496
Goldstein JI, Newbury DE, Echil P, Joy DC, Romig AD Jr, Lyman CE, Fiori C, Lifshin E (1992) Scanning electron microscopy and X–ray microanalysis. Plenum Press, New York. https://doi.org/10.1007/978-1-4613-0491-3
Graba Z, Hamoudi S, Bekka D, Bezzi N, Boukherroub R (2015) Influence of adsorption parameters of basic red dye 46 by the rough and treated Algerian natural phosphates. J Ind Eng Chem 25:229–238
Heibati B, Couto SR, Al-Ghouti MA, Asif M, Tyagi I, Agarwal S, Gupta VK (2015) Kinetics and thermodynamics of enhanced adsorption of the dye AR 18 using activated carbons prepared from walnut and poplar woods. J Mol Liq 208:99–105
Hessel C, Allegre C, Maisseu M, Charbit F, Moulin P (2007) Guidelines and legislation for dye house effluents. J Environ Manag 83:171–180
Hussein KA, Hassan SHA, Joo JH (2011) Potential capacity of Beauveria bassiana and Metarhizium anisopliae in the biosorption of Cd2+ and Pb2+. J Gen Appl Microbiol 57:347–355
Khodam F, Rezvani Z, Amani-Ghadim AR (2015) Enhanced adsorption of Acid Red 14 by co–assembled LDH / MWCNTs nanohybrid: optimization, kinetic and isotherm. J Ind Eng Chem 21:1286–1294
Lang W, Sirisansaneeyakul S, Ngiwsara L, Mendes S, Martins LO, Okuyama M, Kimura A (2013) Characterization of a new oxygen insensitive azo reductase from Brevibacillus laterosporus TISTR1911: toward dye decolorization using a packed bed metal affinity reactor. Bioresour Technol 150:298–306
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Li X, Zhang D, Sheng F, Qing H (2018) Adsorption characteristics of copper (II), zinc (II) and mercury (II) by four kinds of immobilized fungi residues. Ecotoxicol Environ Saf 147:357–366
Lima EC, Hosseini-Bandegharaei A, Moreno-Piraján JC, Anastopoulos I (2019) A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong Use of Equilibrium Constant in the Van’t Hoof Equation for Calculation of Thermodynamic Parameters of Adsorption. J Mol Liq 273:425–434
Liu Y, Shen L (2008) A general rate law equation for biosorption. Biochem Eng J 38:390–394
Mishra A, Malik A (2013) Recent advances in microbial metal bioaccumulation. Crit Rev Environ Sci Technol 43:1162–1222
Myslak ZW, Bolt HM (1998) Occupational exposure to azo dyes risk of bladder cancer. Zbl Arbeitsmed 38:310–321
Piccin JS, Cadaval TRS, Pinto LAA, Dotto GL (2017) Adsorption isotherms in liquid phase: experimental, modeling, and interpretations. Chapter 2. In: Bonilla–Petriciolet A, Mendoza–Castillo DI, Reynel–Ávila E. (ed) Adsorption processes for water treatment and purification. Springer International Publishing, Cham, pp 31–52.
Przystaś W, Zabłocka-Godlewska E, Grabińska-Sota E (2015) Efficacy of fungal decolorization of a mixture of dyes belonging to different classes. Braz J Microbiol 46:415–424
Qu B, Zhou J, Xiang X, Zheng C, Zhao H, Zhou X (2008) Adsorption behavior of azo dye C. I. acid red 14 in aqueous solution on surface soils. J Environ Sci 20:704–709
Redlich O, Peterson DL (1959) A useful adsorption isotherm. J Phys Chem 63:1024–1026
Saratale RG, Gandhi SS, Purankar MV, Kurade MB, Govindwar SP, Oh SE, Saratale GD (2013) Decolorization and detoxification of sulfonated azo dye C.I. Remazol Red and textile effluent by isolated Lysinibacillus sp. RGS. J Biosci Bioeng 115:658–667
Silverstein RM, Webster FX, Kiemle DJ (2007) Spectrometric identification of organic compounds. Wiley, New York
Solis M, Solis A, Perez HI, Mnjarrez N (2012) Microbial decolouration of azo dyes: a review. Process Biochem 47:1723–1748
Sonai GG, Souza SMAGU, Oliveira D, Souza AAU (2016) The application of textile sludge adsorbents for the removal of Reactive Red 2 dye. J Environ Manag 168:149–156
Souza ARC, Baldoni DB, Lima J, Porto V, Marcuz C, Ferraz RC, Kuhn RC, Jacques RJS, Guedes JVC, Mazutti MA (2015) Bioherbicide production by Diaporthe sp. isolated from the Brazilian Pampa biome. Biocat Agric Biotec 4:575–578
Srikantan C, Suraishkumar GK, Srivastava S (2018) Effect of light on the kinetics and equilibrium of the textile dye (Reactive Red 120) adsorption by Helianthus annuus hairy roots. Bioresour Technol 257:84–91
Thommes M, Kaneko K, Neimark AV, Oliver JP, Rodriguez-Reinoso F, Rouquerol J, Sing KSW (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87:1051–1069
Thue PS, Sophia AC, Lima EC, Wamba AG, de Alencar WS, dos Reis GS, Dias SLP (2018) Synthesis and characterization of a novel organic–inorganic hybrid clay adsorbent for the removal of acid red 1 and acid green 25 from aqueous solutions. J Clean Prod 171:30–44
Wang L, Li J (2013) Adsorption of C.I. Reactive Red 228 dye from aqueous solution by modified cellulose from flax shive: kinetics, equilibrium, and thermodynamics. Ind Crop Prod 42:153–158
Wang L, Chen Z, Wen H, Cai Z, He C, Wang Z, Yan W (2018a) Microwave assisted modification of activated carbons by organic acid ammoniums activation for enhanced adsorption of acid red 18. Powder Technol 323:230–237
Wang L, Yan W, He C, Wen H, Cai Z, Wang Z, Chen Z, Liu W (2018b) Microwave–assisted preparation of nitrogen–doped biochars by ammonium acetate activation for adsorption of acid red 18. Appl Surf Sci 433:222–231
Xu D, Gu C, Chen X (2013) Adsorption and removal of acid red 3R from aqueous solution using flocculent humic acid isolated from lignite. Procedia Environ Sci 18:127–134
Zazycki MA, Godinho M, Perondi D, Foletto EL, Collazzo GC, Dotto GL (2018) New biochar from pecan nutshells as an alternative adsorbent for removing reactive red 141 from aqueous solutions. J Clean Prod 171:57–65
Zulfiqar Ahmed MN, Chandrasekhar KB, Nagabhushana BM (2018) Adsorption of Acid Red 97 by α–Fe2O3 nanopowder: isotherm and kinetic studies. Int J Sci Eng Manage 3:34–40
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Georgin, J., Alves, E., Drumm, F. et al. Application of Beauveria bassiana spore waste as adsorbent to uptake acid red 97 dye from aqueous medium. Environ Sci Pollut Res 26, 36967–36977 (2019). https://doi.org/10.1007/s11356-019-06792-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06792-6