Abstract
The microbial fuel cell coupled constructed wetland (CW-MFC) was used for treatment sewage and simultaneously generating electricity. The main aim of this study was to explore the optimal conditions for the treatment of hexavalent chromium (Cr (VI)) wastewater by the CW-MFC system. The performance of CW-MFC in removing Cr (VI) and chemical oxygen demands (COD) contained in wastewater and its electricity generation were studied. Electrode spacing, Cr (VI) and COD concentration, and hydraulic retention time (HRT) had certain effects on the performance of CW-MFC. For the electrode spacing of 10 cm, the highest power density of 458.2 mW/m3 could be obtained with the influent concentration of Cr (VI) (60 mg/L) and COD (500 mg/L). The highest Cr (VI) and COD removal rate were obtained with the HRT of 3 days. Compared with CW system, the electrical energy generated in CW-MFC was beneficial to improving the removal efficiency of COD and Cr (VI). Thus, the results confirmed that CW-MFC is a promising technology to remove Cr (VI) from wastewater and achieve bioelectricity production simultaneously.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The heavy metal chromium is widely utilized in the industrial process, including electroplating, leather tanning, aerospace, nuclear reactors, shipbuilding, steel fabrication, and pigment manufacturing; therefore a lot of chromium wastewater is produced inevitably (Cheng et al. 2019; Malaviya and Singh 2011). Chromium species are usually existed in the form of hexavalent chromium (Cr (VI)) and trivalent chromium (Cr (III)) in wastewater. A small amount of Cr (III) is an essential nutrient for humans that can help the body use sugar, protein, and fat, while Cr (VI) is extremely toxic toward all organisms due to its high solubility and migration in water (Gupta et al. 2001). Therefore, the World Health Organization (WHO) has regulated that the threshold value of Cr (VI) in industrial wastewater should be less than 0.1 mg/L due to the potential hazards of all Cr (VI) compounds. Cr (VI) can enter into the human body mainly through breathing, skin contacting, and drinking water, which can be kept for a long time in the ecosystem due to non-biodegradability and cause cancer even death (Espinoza-Sánchez et al. 2019). Thus, the US Environmental Protection Agency (USEPA) stipulates that the maximum pollution concentration of hexavalent chromium in domestic water should not exceed 0.05 mg/L. Hence, it is important to explore an effective treatment method for wastewater containing Cr (VI). Conventional treatment methods for chromium-contaminated wastewater include adsorption, precipitation, chemical reduction, membrane separation, ion-exchange, and biological (Barrera-Díaz et al. 2012; Sharma and Weng 2007). However, these technologies have some intrinsic shortcomings, such as excessive consumption of chemicals, high cost, and secondary pollutants.
Currently, microbial fuel cell (MFC) has shown a remarkable promotion on the efficient removal of Cr (VI) and electricity generation. Wang et al. (2008) were the first to use MFC to achieve the cathode reduction of Cr (VI) and simultaneous power generation. Cr (VI), as an electron acceptor of MFC cathodes, can recover metal resources, reduce operating costs, and dramatically improve environmental benefits (Li et al. 2018b; Li et al. 2008; Song et al. 2016). Nevertheless, MFC have the deficiencies of small treatment scale and difficult application in practice. The emergence of microbial fuel cell coupled constructed wetland (CW-MFC), a new technology by embedding MFC into constructed wetland (CW), has attracted the attention of researchers. The presence of redox potential gradient in the CW system can be well coupled with MFC (Yadav et al. 2012; Oodally et al. 2019). An improvement in the ability of water treatment and power generation performance on the microbial fuel cell can be obtained by CW-MFC system, but it is still in the infant stage of development. At present, most of the studies on CW-MFC system are in the laboratory scale for treating kinds of wastewater. Zhao et al. (2013) constructed two lab-scale CW-MFC systems, which operated in continuous flow mode and batch mode of for treating the swine wastewater containing high-concentration organic. Xu et al. (2018) reported a lab-scale CW-MFC with multiple bio-cathodes for increasing energy production and enhancing simultaneous N removal, owning to the reduction of energy losses on electrodes and the interaction between bioelectrical generation and nitrification and denitrification process. CW-MFC was also applied to remove the heavy metal of Zn (II) from wastewater via the electrochemical reduction reaction in cathode (Wang et al. 2020). In addition, a few researches have conducted CW-MFC system in pilot-scale. Villaseñor Camacho et al. (2017) constructed a pilot-scale horizontal subsurface flow CW-MFC, which could operate in high salinity (< 9.5 g/L) for contaminant removing and simultaneous electricity generation. Moreover, a pilot-scale system of CW-MFC was studied in treating domestic wastewater COD which was collected from the outlet of an anaerobic primary treatment, confirming the potential practical application of CW-MFC (Corbella et al. 2019). Whereas, the low-power output still limited the application of CW-MFC. To effectively utilize the electrical energy generated by CW-MFC system, a system biofilm electrode reactor (BER) coupled with CW-MFC was constructed and laid the foundation for further practical application of CW-MFC (Li et al. 2018a; Zhang et al. 2018). Therefore, the CW-MFC is rapidly developing and shows the potential of large-scale application, owing to its high-efficiency and low cost in removing organic pollutant, total nitrogen, heavy metal and other contaminants from wastewater, and simultaneous power generation. Furthermore, CW-MFC system is expected to be applied in removing chromium from water.
It is well known that both MFC and CW systems have been studied for removing the heavy metals of Cr (VI) from wastewater (Hadad et al. 2018; Sinha et al. 2017; Sultana et al. 2015; Srivastava et al. 2020). However, to our best knowledge, there are rarely about removing Cr (VI) in wastewater via the CW-MFC system. Hence, CW-MFC was proposed to remove Cr (VI) through bioelectrochemical reduction, adsorption on substrate, and the comprehensive effect of microorganisms in this work. In order to reduce the influencing factors and the complexity of the experiment, no plants were planted in the CW-MFC system in this study. The electric energy is conducive to the removal Cr (VI); hence, to balance the removal Cr (VI) and electricity generation, the effects of electrode spacing, influent concentration of COD, and Cr (VI) and hydraulic retention time (HRT) were investigated in this experiment. The performance of the unplanted CW-MFC system was explored in different conditions, revealing the bio-electricity production and the effects of removal Cr (VI) in CW-MFC.
Materials and methods
CW-MFC construction
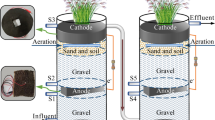
The up-flow CW-MFC reactors were constructed in the polyacrylic plastic cylinder with the internal diameter of 18 cm and the height of 52 cm to simulate unplanted CW-MFC (Fig. 1). There were six layers from the bottom to the top in CW-MFC system, which composed of a gravels layer (3 cm in thickness), a bottom layer (14 cm in thickness), an anode layer (8 cm in thickness), a middle layer, a cathode layer (8 cm in thickness), and a top layer. The role of the gravel layer (4–6 mm in diameter of gravel) was to improve the distribution of wastewater in the system. The bottom, middle, and top layers were filled with volcanic cinder (3–5 mm in diameter) as the main media material. The anode and cathode were made by granular activated carbon (GAC, 3–5 mm in diameter with a specific surface area of about 900 m2/g) and 12-mesh stainless steel mesh (4 cm in thickness and 16.9 cm in diameter). The stainless steel mesh as the electron collector was embedded into the activated carbon layer (Fang et al. 2013). The anode and cathode were connected with external 1000 Ω resistance to form a circuit by copper wires. In order to investigate the effects of electrode spacing on wastewater treatment efficiency and electricity generation, four CW-MFC systems were designed, and the distance between anode and cathode was varied among 5, 10, and 15 cm by adjusting the position of cathode layer. At the same time, another reactor with the electrode spacing of 10 cm was operated under an open circuit as control.
Inoculation and synthetic wastewater
Activated sludge was collected from the secondary sedimentation tank of the Haibo River Wastewater Treatment Plant (Qingdao, China), and it was used as original inoculum of the CW-MFC system. Each reactor system was inoculated with the sludge for 2 months prior to the experimental startup. During the cultivation of microorganisms, glucose (500 mg/L) was added to the solution as a carbon source for anaerobic sludge. After inoculation, the synthetic wastewater containing glucose was continuously pumped into the reactors by the peristaltic pump (BT100-1 L, Baoding Longer Precision Pump Co., Ltd., China) from the bottom and then discharged from the top of reactors, and the synthetic wastewater containing Cr (VI) was supplied to the reactors during the experiment period. The main composition of the synthetic wastewater is shown in Table 1. It was stored in a 25 L tank and renewed every 2 days. Besides, the COD and Cr (VI) concentrations varied by glucose and dichromate concentration, respectively. The pH value of the synthetic wastewater was 6.5–7.1. After the experimental study of the electrode spacing, the later experiments operated in the electrode spacing of 10 cm reactor and the control reactor.
Analytics and calculations
In the experiment period, samples were taken from water inlet and outlet. The concentrations of COD and soluble Cr (VI) content in wastewater samples were measured. The soluble COD was measured using dichromate method. The COD removal rate (δCOD) was calculated by Eq. (1):
where CODin and CODout represent the influent COD concentration (mg/L) and the effluent COD concentration (mg/L), respectively.
Soluble Cr (VI) content was analyzed by 1,5-diphenylcarbazide spectrophotometric method. The Cr (VI) removal rate (δCr(VI)) was calculated by Eq. (2):
where Cr (VI)in and Cr (VI)out represent the influent Cr (VI) concentration (mg/L) and the effluent Cr (VI) concentration (mg/L), respectively.
The cell voltage (U) across the 1000 Ω external resistance was automatically collected every 5 min by a data acquisition device (USB DAQ-680i, Suzhou Aumanyu Intelligent Technology Co. Ltd., China). The power density was calculated by the anode volumetric power density (P, W/m3) as:
where U is the voltage (V), R is the external resistance (Ω), and A is the anode effective working volume (m3).
The external resistance was varied from 10 to 100,000 Ω for investigating the power density and polarization curve. The power density curve and polarization curve can be applied to evaluate the electricity generation performance. The internal resistance (Rint) of the CW-MFCs was calculated by the linear region of polarization curve.
Scanning electron microscope
The scanning electron microscope (SEM, JSM-6700F, JEOL, Japan) was used to observe the morphologies of the electrode biofilm patterns taken from anodes and cathode of the CW-MFC. The samples were collected from anode and biocathode as the CW-MFC system operating stably and processed as the following steps. The samples were fixed in 2.5% glutaric dialdehyde (pH = 6.8) for 2 h, and then taken out and rinsed three times (15 min each time) in 0.1 mol/L phosphate buffered solution (PBS, pH = 7.0). Thereafter, the samples were dehydrated with gradient ethanol solutions (25, 50, 75, 95, and 100%) each for 15 min and 1:1 mixture of 100% ethanol and isopentyl acetate for 30 min. Finally, the samples were immersed in pure isopentyl acetate for 2 h and dried by a freeze drier.
Results and discussion
Effects of electrode spacing on wastewater treatment and electricity generation
The electrode spacing is one of the key factors in wastewater treatment of CW-MFC system. The influences on the removal rate of Cr (VI) and COD by changing the distance between the bottom of cathode and top of anode were investigated when the initial concentration of Cr (VI) and COD were 40 mg/L and 500 mg/L, respectively (Table 2). The Cr (VI) removal rates were 90.2%, 93.4%, and 92.4%, respectively at the electrode spacing of 5 cm, 10 cm, and 15 cm. Besides, the lowest removal rate of 83.1% was observed in the control reactor, indicating that the microorganisms acting as biocatalysts could use protons and electrons from the anode for Cr (VI) reduction in the cathode (Tandukar et al. 2009; Wu et al. 2015). The COD removal rates were 84.9%, 88.3%, 86.9%, and 83.8% at the spacing of 5 cm, 10 cm, 15 cm, and the control group, respectively. The overall COD removal rate was relatively high, owing to the easily degradable characteristic of glucose organic matter. The removal rate of the control group was slightly lower than other groups. This might be due to electricity generation could promote organics degradation (Doherty et al. 2015).
Studies have shown that microorganisms acting as biocatalyst could oxidize carbon sources to promote electricity generation. The surface morphologies of virgin activated carbon and biofilm of electrodes can be observed from the SEM images (Fig. 2). The virgin activated carbon had a protuberance and rough surface, which created a beneficial living condition for bacterial growth. Comparing with the virgin activated carbon, biofilm was formed on the surface of the anodes and biocathode electrode. Hence, there was a stable operation of the CW-MFC system during the experiment.
To explore the effects of electrode spacing on the bioenergy output, electrical performance was conducted. The highest power density of 441.4 mW/m3 was obtained at the electrode spacing of 10 cm, and the maximum power densities were 422.7 mW/m3 and 272.8 mW/m3 for the electrode spacing of 5 cm and 15 cm, respectively (Fig. 3). The electrode spacing had a certain impact on electricity generation performance of CW-MFCs. The voltage also increased initially, and then decreased with the increasing of electrode spacing. Meanwhile the internal resistance increased with increasing electrode spacing, the corresponding internal resistances were 144 Ω (5 cm), 150 Ω (10 cm), and 261 Ω (15 cm). Although the internal resistance was minimum with the electrode spacing of 5 cm, it would cause oxygen diffusion to the anode area and damage the anaerobic condition (Fang et al. 2017; Logan et al. 2006). Too small electrode spacing caused that the partial electrons were directly used by microorganisms in the anode without passing through the external circuit, resulting in lower power output (Xie et al. 2018). For the electrode spacing of 15 cm, the voltage value and power density were reduced due to the increase of internal resistance. Considering the performance of wastewater treatment and electricity generation, the electrode spacing of 10 cm was the suitable spacing and applied in the subsequent experiment.
Effects of Cr (VI) concentrations on wastewater treatment and electricity generation
Cr (VI) removal using the biocathode CW-MFC was tested at different initial Cr (VI) concentrations (Fig. 4a). For the influent Cr (VI) concentration increased from 20 to 100 mg/L, the Cr (VI) removal showed a decrease trend. More than 96% Cr (VI) was removed at an influent Cr (VI) concentration of 20 mg/L. For the influent Cr (VI) concentration increased to 60 mg/L, the Cr (VI) removal could still be up to 90%. After further increasing the Cr (VI) concentration to 80 mg/L, the Cr (VI) removal rate had a slightly decrease. However, with increasing the concentration of Cr (VI) to 100 mg/L, the Cr (VI) removal rate decreased to 74.3%. The COD removal trend increased firstly and then decreased, and the high concentration of Cr (VI) was not conducive to the removal of COD (Fig. 4b). The microorganisms present in CW-MFC have a certain tolerance to Cr (VI), but high concentrations of Cr (VI) were toxic to microorganisms (Cervantes et al. 2001). Additionally, both the Cr (VI) and COD removal in control group of CW-MFC were significantly lower than that of closed circuit group of CW-MFC. It indicated that the closed circuit of CW-MFC could promote the removal of Cr (VI) and COD.
The polarization curve and power density curve showed electricity generation performance under different Cr (VI) concentrations in Fig. 5. The voltage and power density firstly increased and then decreased with the increase of Cr (VI) concentration. When the concentration of Cr (VI) was 60 mg/L, the highest voltage and maximum power density were 588.2 mV and 458.2 mW/m3, respectively. The presence of Cr (VI) had a positive impact on the power generation. Cr (VI) could obtain electron which produced by oxidizing organic matter and ammonia nitrogen to promote the electricity generation (Wang et al. 2020). Although many microorganisms that could reduce Cr (VI) to the trivalent form have a certain tolerant to Cr (VI), a high concentration of Cr (VI) is still toxic to microorganisms. Therefore, excessive concentration of Cr (VI) could inhibit the activity of bacteria due to its high toxicity, resulting in a decrease in power density (Vaiopoulou and Gikas 2012). Considering the performance of the removal rate and electricity generation, the Cr (VI) concentration of 60 mg/L was the suitable concentration and applied in the subsequent experiment.
Effects of COD concentrations on wastewater treatment and electricity generation
To investigate the effects of organic loading amount on wastewater treatment and electricity generation, the performance of CW-MFC with COD concentration range from 0 to 1000 mg/L was recorded. The Cr (VI) removal rate of control group was significantly lower than that of 10 cm group. The Cr (VI) removal rate of 10 cm group increased from 74.2% to maximum (90.7%) with COD increased from 100 to 500 mg/L (Fig. 6a). Then Cr (VI) removal rate decreased with continuously increasing the COD concentration. Cr (VI) removal reduced to 84.0% at a COD concentration of 700 mg/L, which was only slightly decreased compared with the highest removal rate (90.7%). The Cr (VI) contained in wastewater can be used as the electron acceptor of the cathode. In the closed circuit CW-MFC, electrons produced by oxidizing organics and ammonia nitrogen can accelerate electron transfer between electrodes and affect the Cr (VI) removal. For the high COD concentration, too much organic matter was accumulated in the system, which was not conducive to the transfer of electrons (He et al. 2015). Moreover, the corresponding effluent COD concentrations were measured, and the removal rates were shown in Fig. 6b. The removal rate of COD in 10 cm group and control group both tended to decrease with the increase of COD concentration, but the COD removal rate of 10 cm group showed a smaller decline compared with that of the control group, which benefits from the synergy of CW and MFC system. Obviously, a closed circuit system could enhance the capability of degradation of pollutants (Srivastava et al. 2015; Fang et al. 2013).
Organic loading amount in CW-MFC was closely related to the power generation capacity of system. The voltage of the CW-MFC increased from 508.6 to 588.2 mV with influent COD increased from 100 to 500 mg/L. But the voltage decreased to 569.9 mV as the COD increased to 700 mg/L (Fig.7). Besides, there was a sluggish decrement of the internal resistance with COD concentration increased from 100 to 500 mg/L (178 Ω at 100 mg/L to 147 Ω at 500 mg/L). Nevertheless, an obvious increasing trend was observed in terms of the internal resistance with the influent COD concentration increased to 700 mg/L (162 Ω). As shown in the power density curve, the power density progressively increased from 299.1 to 458.2 mW/m3 as the COD concentration increased from 100 to 500 mg/L. However, it did not cause a corresponding power density increment that the COD further increased to 700 mg/L (423.5 mW/m3 at COD 700 mg/L). When the concentration of COD was less than 500 mg/L, with the increase of organic matter, the microorganisms could oxidize more organic and produce a large amount of electrons, which contributed to generate higher electrical energy (Xu et al. 2019). Nevertheless, further increased of COD to 700 mg/L, too much organic matter may lead to the accumulation of organic acids in bacterial cells, thereby inhibiting the activity of microorganisms and reducing the generation of electricity (Wei et al. 2012).
To evaluate the correlation between COD concentration and voltage, the dynamic response of the system to different influent COD concentrations is shown in Fig. 8. The voltage production was firstly linearly increased from 508.6 to 588.2 mV with the COD increased from 100 to 500 mg/L, which R2 was calculated to be 0.9626. And then linearly decreased from 588.2 to 569.9 mV with COD increased from 500 to 700 mg/L (R2 = 0.9123). The maximum voltage was obtained as the COD concentration was 500 mg/L. In comparison with the linear slope in the range of 100 ~ 500 mg/L COD concentration, the linear at 500 to 700 mg/L had a flatter slope. This may be that COD can reach saturated for exoelectrogens in CW-MFC, a further increase of COD concentration would not conducive to electricity production (Zhang et al. 2015).
Effects of HRT on wastewater treatment and electricity generation
To research the effects of HRT on the performance of CW-MFC, the concentration of Cr (VI) and COD was kept at 60 mg/L and 500 mg/L, respectively. According to Table 3, Cr (VI) removal increased from 81.0 to 93.0% with HRT increased from 1 to 3 days. The decrease of Cr (VI) concentration was attributed to multiple mechanisms of treatment including adsorption on substrate and bioremediation, electrochemical reduction, and precipitation. When the HRT was longer, adsorption was almost saturated; bioremediation and electrochemical played a major role in removal Cr (VI). The removal efficiency of Cr (VI) was closely related to the power generation performance of the system. Hence the Cr (VI) removal rate decreased to 89.6% when the HRT was extended to 4 days. Therefore the Cr (VI) removal capacity was enhanced with an appropriate increase in HRT. The COD removal rate was firstly increased to maximum and then decreased with the elongation of the HRT. When the HRT was 3 days, the highest COD removal rate was obtained. It showed that proper prolongation of HRT was beneficial to the removal of pollutants in wastewater, but too long HRT would affect the removal rate of pollutants.
To explore the effects of HRT on electricity generation performance in CW-MFC, voltage value was monitored with an external resistor of 1000 Ω; power density curve and polarization curve were plotted (Fig. 9). When the HRT was increased from 1 to 2 days, the voltage increased from 550.8 to 588.2 mV and the internal resistance decreased from 197 to 147 Ω. However, further increasing the HRT to 4 days resulted in the decrease of the voltage to 545.6 mV and the internal resistance increased to 169 Ω. Meanwhile, it was evident that the power density was also affected by the HRT; the maximum power density was 458.2 mW/m3 at the HRT of 2 days. As the HRT was too short to ensure a sufficient contact time between the wastewater and the microorganisms in the MFC-CW, the organic matter supplying to the microorganisms in electrode was affected. Appropriate prolongation of HRT was beneficial to the full utilization of glucose by microorganisms for their own growth and reproduction. The enhancement of the electrochemical activity of the bacteria promoted the oxidation of organic matter and ammonia nitrogen to produce more electrons and boosted the mass transfer efficiency of the electrodes, resulting in lower internal resistance and higher power output (Liu et al. 2016). Yet, the power density of the system decreased from 458.2 (HRT = 2 days) to 372.5 mW/m3 (HRT = 4 days). The possible explanation was that excessive consumption of glucose may occur in the bottom layer at longer HRT, causing the shortage of the electron donor in the anode (Fang et al. 2017). Therefore, a suitable HRT would be promising to enhance both the performance of wastewater treatment and electricity generation in CW-MFC.
Conclusions
Factors such as electrode spacing, COD concentration, Cr (VI) concentration, and HRT can affect the capacity of wastewater treatment and electricity generation. Although the minimum internal resistance was obtained with the small electrode spacing, too small electrode spacing could decrease electricity generation. The Cr (VI) removal rate was 93.4% at the Cr (VI) concentration of 40 mg/L with the electrode spacing of 10 cm, which was higher than those of the electrode spacing of 5 cm and 15 cm. It should be noted that the suitable concentration of Cr (VI) and COD could enhance the electricity generation, and the maximum power density was 458.2 mW/m3 at 60 mg/L of Cr (VI) and 500 mg/L of COD, which was obtained 90.7% and 92.5% removal rate of Cr (VI) and COD, respectively. In addition, the results showed that a suitable HRT could enhance both the wastewater treatment and electricity generation in CW-MFC. Consequently, the high power density, high Cr (VI) removal confirmed that CW-MFC technology could be successfully used to treat Cr (VI) contained in wastewater and produce electricity simultaneously.
References
Barrera-Díaz CE, Lugo-Lugo V, Bilyeu B (2012) A review of chemical, electrochemical and biological methods for aqueous Cr (VI) reduction. J Hazard Mater 223-224:1–12
Cervantes C, Campos-García J, Devars S, Gutiérrez-Corona F, Loza-Tavera H, Torres-Guzmán JC, Moreno-Sánchez R (2001) Interactions of chromium with microorganisms and plants. FEMS Microbiol Rev 25:335–347
Cheng C, Hu Y, Shao S, Yu J, Zhou W, Cheng J, Chen Y, Chen S, Chen J, Zhang L (2019) Simultaneous Cr (VI) reduction and electricity generation in plant-sediment microbial fuel cells (P-SMFCs): synthesis of non-bonding Co3O4 nanowires onto cathodes. Environ Pollut 247:647–657
Corbella C, Hartl M, Fernandez-gatell M, Puigagut J (2019) MFC-based biosensor for domestic wastewater COD assessment in constructed wetlands. Sci Total Environ 660:218–226
Doherty L, Zhao Y, Zhao X, Wang W (2015) Nutrient and organics removal from swine slurry with simultaneous electricity generation in an alum sludge-based constructed wetland incorporating microbial fuel cell technology. Chem Eng J 266:74–81
Espinoza-Sánchez MA, Arévalo-Niño K, Quintero-Zapata I, Castro-González I, Almaguer-Cantú V (2019) Cr (VI) adsorption from aqueous solution by fungal bioremediation based using Rhizopus sp. J Environ Manag 251:109595
Fang Z, Song H, Cang N, Li X (2013) Performance of microbial fuel cell coupled constructed wetland system for decolorization of azo dye and bioelectricity generation. Bioresour Technol 144:165–171
Fang Z, Cheng S, Cao X, Wang H, Li X (2017) Effects of electrode gap and wastewater condition on the performance of microbial fuel cell coupled constructed wetland. Environ Technol 38:1051–1060
Gupta VK, Shrivastava AK, Jain N (2001) Biosorption of chromium (VI) from aqueous solutions by green algae Spirogyra species. Water Res 35:4079–4085
Hadad HR, Mufarrege MDLM, Di Luca GA, Maine MA (2018) Long-term study of Cr, Ni, Zn, and P distribution in Typha domingensis growing in a constructed wetland. Environ Sci Pollut R 25:18130–18137
He C, Mu Z, Yang H, Wang Y, Mu Y, Yu H (2015) Electron acceptors for energy generation in microbial fuel cells fed with wastewaters: a mini-review. Chemosphere 140:12–17
Li Z, Zhang X, Lei L (2008) Electricity production during the treatment of real electroplating wastewater containing Cr6+ using microbial fuel cell. Process Biochem 43:1352–1358
Li H, Song H, Yang X, Zhang S, Yang Y, Zhang L, Xu H, Wang Y (2018a) A continuous flow MFC-CW coupled with a biofilm electrode reactor to simultaneously attenuate sulfamethoxazole and its corresponding resistance genes. Sci Total Environ 637-638:295–305
Li M, Zhou S, Xu Y, Liu Z, Ma F, Zhi L, Zhou X (2018b) Simultaneous Cr (VI) reduction and bioelectricity generation in a dual chamber microbial fuel cell. Chem Eng J 334:1621–1629
Liu L, Chou T, Lee C, Lee D, Su A, Lai J (2016) Performance of freshwater sediment microbial fuel cells: consistency. Int J Hydrogen Energy 41:4504–4508
Logan BE, Hamelers B, Rozendal R, Schröder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K (2006) Microbial fuel cells: methodology and technology. Environ Sci Technol 40:5181–5192
Malaviya P, Singh A (2011) Physicochemical technologies for remediation of chromium-containing waters and wastewaters. Crit Rev Environ Sci Technol 41:1111–1172
Oodally A, Gulamhussein M, Randall DG (2019) Investigating the performance of constructed wetland microbial fuel cells using three indigenous south African wetland plants. J Water Process Eng 32:100930
Sharma YC, Weng CH (2007) Removal of chromium (VI) from water and wastewater by using riverbed sand: kinetic and equilibrium studies. J Hazard Mater 142:449–454
Sinha V, Manikandan NA, Pakshirajan K, Chaturvedi R (2017) Continuous removal of Cr (VI) from wastewater by phytoextraction using Tradescantia pallida plant based vertical subsurface flow constructed wetland system. Int Biodeterior Biodegradation 119:96–103
Song T, Jin Y, Bao J, Kang D, Xie J (2016) Graphene/biofilm composites for enhancement of hexavalent chromium reduction and electricity production in a biocathode microbial fuel cell. J Hazard Mater 317:73–80
Srivastava P, Yadav AK, Mishra BK (2015) The effects of microbial fuel cell integration into constructed wetland on the performance of constructed wetland. Bioresour Technol 195:223–230
Srivastava P, Abbassi R, Kumar Yadav A, Garaniya V, Kumar N, Khan SJ, Lewis T (2020) Enhanced chromium (VI) treatment in electroactive constructed wetlands: influence of conductive material. J Hazard Mater 387:121722
Sultana M, Chowdhury AKMM, Michailides MK, Akratos CS, Tekerlekopoulou AG, Vayenas DV (2015) Integrated Cr (VI) removal using constructed wetlands and composting. J Hazard Mater 281:106–113
Tandukar M, Huber SJ, Onodera T, Pavlostathis SG (2009) Biological chromium (VI) reduction in the cathode of a microbial fuel cell. Environ Sci Technol 43:8159–8165
Vaiopoulou E, Gikas P (2012) Effects of chromium on activated sludge and on the performance of wastewater treatment plants: a review. Water Res 46:549–570
Villaseñor Camacho J, Rodríguez Romero L, Fernández Marchante CM, Fernández Morales FJ, Rodrigo Rodrigo MA (2017) The salinity effects on the performance of a constructed wetland-microbial fuel cell. Ecol Eng 107:1–7
Wang G, Huang L, Zhang Y (2008) Cathodic reduction of hexavalent chromium [Cr (VI)] coupled with electricity generation in microbial fuel cells. Biotechnol Lett 30:1959–1966
Wang Q, Lv R, Rene ER, Qi X, Hao Q, Du Y, Zhao C, Xu F, Kong Q (2020) Characterization of microbial community and resistance gene (CzcA) shifts in up-flow constructed wetlands-microbial fuel cell treating Zn (II) contaminated wastewater. Bioresour Technol 302:122867
Wei L, Yuan Z, Cui M, Han H, Shen J (2012) Study on electricity-generation characteristic of two-chambered microbial fuel cell in continuous flow mode. Int J Hydrogen Energ 37:1067–1073
Wu X, Zhu X, Song T, Zhang L, Jia H, Wei P (2015) Effect of acclimatization on hexavalent chromium reduction in a biocathode microbial fuel cell. Bioresour Technol 180:185–191
Xie T, Jing Z, Hu J, Yuan P, Liu Y, Cao S (2018) Degradation of nitrobenzene-containing wastewater by a microbial-fuel-cell-coupled constructed wetland. Ecol Eng 112:65–71
Xu L, Zhao Y, Wang X, Yu W (2018) Applying multiple bio-cathodes in constructed wetland-microbial fuel cell for promoting energy production and bioelectrical derived nitrification-denitrification process. Chem Eng J 344:105–113
Xu F, Ouyang DL, Rene ER, Ng HY, Guo LL, Zhu YJ, Zhou LL, Yuan Q, Miao MS, Wang Q, Kong Q (2019) Electricity production enhancement in a constructed wetland-microbial fuel cell system for treating saline wastewater. Bioresour Technol 288:121462
Yadav AK, Dash P, Mohanty A, Abbassi R, Mishra BK (2012) Performance assessment of innovative constructed wetland-microbial fuel cell for electricity production and dye removal. Ecol Eng 47:126–131
Zhang X, He W, Ren L, Stager J, Evans PJ, Logan BE (2015) COD removal characteristics in air-cathode microbial fuel cells. Bioresour Technol 176:23–31
Zhang S, Song H, Yang X, Li H, Wang Y (2018) A system composed of a biofilm electrode reactor and a microbial fuel cell-constructed wetland exhibited efficient sulfamethoxazole removal but induced sul genes. Bioresour Technol 256:224–231
Zhao Y, Collum S, Phelan M, Goodbody T, Doherty L, Hu Y (2013) Preliminary investigation of constructed wetland incorporating microbial fuel cell: batch and continuous flow trials. Chem Eng J 229:364–370
Funding
This work was supported by the Program of China Scholarship Council (No. 20120370391) and the National Key Research and Development Program of China (NO. 2018YFC0408000, 2018YFC0408004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Alexandros Stefanakis
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mu, C., Wang, L. & Wang, L. Performance of lab-scale microbial fuel cell coupled with unplanted constructed wetland for hexavalent chromium removal and electricity production. Environ Sci Pollut Res 27, 25140–25148 (2020). https://doi.org/10.1007/s11356-020-08982-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08982-z