Abstract
Rice ( Oryza sativa L.) is one of the main staple food crops which is inherently low in micronutrients, especially iron (Fe), and can lead to severe Fe deficiency in populations having higher consumption of rice. Soils polluted with nickel (Ni) can cause toxicity to rice and decreased Fe uptake by rice plants. We investigated the potential role of biochar (BC) and gravel sludge (GS), alone and in combination, for in situ immobilization of Ni in an industrially Ni-contaminated soil at original and sulfur-amended altered soil pH. Our further aim was to increase Fe bioavailability to rice plants by the exogenous application of ferrous sulfate to the Ni-immobilized soil. Application of the mixture of both amendments reduced grain Ni concentration, phytate, Phytate/Fe, Phyt/Zn molar ratios, and soil DTPA-extractable Ni. In addition, the amendment mixture increased 70 % Fe and 229 % ferritin concentrations in rice grains grown in the soil at original pH. The Fe and ferritin concentrations in S-treated soil was increased up to 113 and 383 % relative to control respectively. This enhanced Fe concentration and corresponding ferritin in rice grains can be attributed to Ni/Fe antagonism where Ni has been immobilized by GS and BC mixture. This proposed technique can be used to enhance growth, yield, and Fe biofortification in rice by reducing soil pH while in parallel in situ immobilizing Ni in polluted soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal toxicity in agricultural soils is a serious concern for quality food production on these soils (Adrees et al. 2015a; Ali et al. 2015; Rizwan et al. 2012, 2016a). Among heavy metals, contamination of soil with nickel (Ni) has become a serious global issue, leading to hazardous health effects as they enter the food chain and cause reduction in agricultural yield (Guo and Marschner 1995; Salt et al. 1995; Khaliq et al. 2016). Nickel is released into the atmosphere from both natural and anthropogenic sources (Rajkumar and Freitas 2008). Nickel is considered an essential micronutrient for proper functioning of plants, and its high concentration in soil caused toxic effects on plants grown in these soils (Roitto et al. 2005; Gajewska et al. 2009). Excess Ni affects a number of biochemical and physiological processes in plants resulting in an inhibition of plant growth and yield (Bertrand et al. 2001; Gajewska et al. 2006; Khaliq et al. 2016).
The conventional technologies for cleaning up contaminated soils like soil washing strategies, electrokinetic extraction, solidification, and soil replacement have their own limitations (Mulligan et al. 2001; Sruthy and Jayalekshmi 2014). Recently, the use of a variety of organic and inorganic amendments has attained a considerable attention for remediation of heavy metal-contaminated soils (Usman et al. 2013; Almaroai et al. 2014; Adrees et al. 2015b; Rehman et al. 2015; Rizwan et al. 2016b). Recently, a carbon-rich economically viable soil amendment like biochar (BC), produced by pyrolyzing biowaste materials under limited oxygen supply, has attained significant interest to improve soil physical, chemical, and biological properties due to its high organic carbon content, surface area, cation exchange capacity, etc. (Almaroai et al. 2014; Rizwan et al. 2016c). Biochar is also widely used as a soil amendment to immobilize toxic metals and hence reduced their toxicity to plants when applied in contaminated soils (Houben et al. 2013a; Houben et al. 2013b; Almaroai et al. 2014; Rizwan et al. 2016c, 2016d). However, little information is available on the role of BC in reducing Ni toxicity in plants (Rizwan et al. 2016c). Furthermore, it has been reported that gravel sludge (GS) application improved plant growth under metal stress (Iqbal et al. 2012).
Iron (Fe) acts as cofactor for many enzymes which is involved in a number of physiological processes both in plants and animals (e.g., respiration, photosynthesis, and oxygen transport) that are impaired under Fe deficiency. Iron is not deficient in mineral soil, but due to high pH and calcareousness, it is merely available to plants because at high pH, Fe+2 ions (absorbed by plants) converted into less soluble Fe+3 oxides and hydroxides and disappear from the soil solution (Marschner 1995). Nickel and Fe have an antagonistic effect in which high concentration of Ni in the soil reduces endogenous Fe concentration and reduces its bioavailability to crops (Chen et al. 2009; Ghasemi et al. 2009; Nishida et al. 2012). Interestingly, different authors have also confirmed that high concentration of Fe+2 suppresses Ni+2 absorption and translocation in plants (Cataldo et al. 1978; Marschner 1995).
Rice is the most widely consumed staple food crop and a primary food source for 50 % of the world’s population (Wang et al. 2013a). Around 3 billion people, mostly in Asia, depend on rice for 35–59 % of their caloric intake, and the dietary contribution of rice is substantially higher in many developing countries (Meng et al. 2005). Rice is poor in Fe contents which are about 7.5–24.5 mg kg−1 that are insufficient to meet daily Fe requirements of people whose staple food is rice (Welch and Graham 2004). Decreased concentration of Fe and other mineral nutrients has been observed under Ni stress in many plant species, including rice (Rubio et al. 1994; Rahman et al. 2005).
There are several promoting and inhibitory factors which are involved in the Fe bioavailability to rice plants. Ferritin is a long-term stable iron storage protein in grains and is known to be a major source of iron (Zielińska-Dawidziak 2015). Ferritin-Fe has been shown to have a bioavailability similar to that of ferrous sulfate (Theil and Briat 2004; Lonnerdal 2007). Some anti-nutrients like phytate, polyphenolic compounds, and tannic acids act as Fe inhibitory factors, by forming insoluble complexes which limit the Fe bioavailability to humans (Bouis and Welch 2010).
Bioavailability of trace elements especially Fe to crop plants can be increased by lowering soil pH by the application of elemental sulfur in alkaline soils (Qureshi et al. 2004; Olaniran et al. 2013). It is reported that oxidizing each mole of S produces two moles of hydrogen ions (H+) in the soil and reduces soil pH that leads to dissolution of nutrients in the root zone (Modaihsh et al. 1989; Kaplan and Orman, 1998; Iqbal et al. 2012). Different studies have been reported on micronutrient biofortification in plants under normal soils (Hussain et al. 2013). However, to the best of our knowledge, no such study is reported on Fe biofortification under pH-manipulated Ni-contaminated soil. Therefore, a novel approach was used for Fe biofortification by the remediation of Ni under normal and pH-manipulated Ni-contaminated soil with the aim of increasing Fe bioavailability and nutrition value by decreasing Ni content in rice grain.
Materials and methods
Experimental soil
Nickel-contaminated soil was collected from the top 0–15 cm of soil from the industrial area near Lahore, Pakistan, for the use in this experiment. The soil was continuously affected from the last 25 years by receiving the effluents from the nickel plating industry. Prior to use in experiment, soil was air-dried and passed through a 2-mm particle size. Soil texture was clay loam (clay 43 %; silt 41 %; sand 16 %) as measured by hydrometer method (Gee and Bauder 1986). Soil pH was measured by pH meter (JENCO pH meter, 671 P model). The electrical conductivity of the saturated soil paste extract was measured by EC meter. Soil organic matter was determined by Nelson and Sommers (1982). The amount of calcium carbonate (CaCO3) was determined by acid dissolution (Allison and Moodie 1965). Iron, Ni, and Zn in the soil were measured by atomic absorption spectrophotometer (PerkinElmer, AAnalyst 100, Waltham, USA) after extraction with 0.005 MDTPA (Lindsay and Norvell 1978). Phosphorus (Watanabe and Olsen 1965), nitrogen (Bremner and Mulvaney 1982), and extractable potassium (Flame Photometer at 767 nm wavelength) were measured according to the standard procedures. Both physical and chemical properties of experimental soil are given in Table 1.
Soil additives
Immobilizing agents
Both BC and GS were used for immobilizing Ni in soil. Biochar used in this study was prepared from rice straw feed stock at Government College University Faisalabad, Pakistan. This BC was prepared in a closed reactor by pyrolyzing the rice straw feedstock with the end temperature 350 °C. The BC was air-dried, ground in a blender, and sieved to <1 mm size prior to use in this experiment. Representative properties of BC were pH 7.65, EC 1.79 dS m−1, organic C 54.03 %, C/N ratio 5:1, Fe 312.2 mg kg−1, Cu 143.3 mg kg−1, Mn 142.6 mg kg−1, and Zn 169.5 mg kg−1. Gravel sludge is a product of gravel industry having 30–70 g kg−1 Fe2O3, 400–650 g kg−1 SiO2, 100–140 g kg−1 Al2O3, 40–60 g kg−1 MgO, and 50–120 g kg−1 CaO (Friesl et al. 2006; Friesl-Hanl et al. 2009; Iqbal et al. 2012). For immobilization of Ni, BC and GS were applied at 1 % (w/w) in experimental soil when used in a single treatment and 0.5 % each when applied in combination.
Solubilizing agents
Elemental sulfur (S) was used for slow and steady acidification of soil and related Fe solubilization in this experiment. Acidity in soil is produced by microbial oxidation of S under aerobic conditions to sulfuric acid (Iqbal et al. 2012):
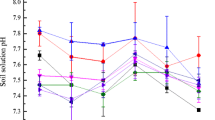
To calculate the amount of S to lower the pH of experimental soil, a preliminary incubation experiment was conducted in which different amounts (1.5, 2, 2.5, 3 g kg−1 soil) of elemental S were added to the experimental soil. After the addition of S, experimental soil was incubated for 17 weeks at 55 % water holding capacity. After the completion of incubation experiment, the soil samples were suspended in deionized water at a soil/solution ratio of 1:2.5 and were measured for pH. These pH data were then used to calculate the amount of S to achieve the pH 6.5 in experimental soil (Table 2).
Iron source in experimental soil
Ferrous sulfate was used as Fe source and was applied at 10 kg ha−1. To this end, ferrous sulfate heptahydrate (purity ≥99 %; Sigma-Aldrich Co. St Louis, MO, USA) was homogeneously mixed in the soil with the help of a spatula (Table 2).
Pot experiment
Both agents (BC and GS) were mixed with soil to obtain ten treatments (Table 2). Powdery soil amendments were homogeneously mixed in soil with the help of a spatula and were moistened to 65 % WHC. The soil was incubated for 10 days in a plastic container (25 °C) in the dark and was air-dried. Each treatment was performed in triplicate. For each treatment, 10 kg air-dried soil was filled in drained plastic pots and was placed on plastic trays to avoid water losses during experiment via evaporation. The pots were transferred to an outdoor greenhouse and arranged in a randomized manner. Recommended doses of nitrogen (60 mg kg−1), phosphorus (45 mg kg−1), and potassium (30 mg kg−1) were uniformly applied in soil by using urea, single super phosphate, and potassium sulfate as source. Rice seeds of variety “Basmati 385” were obtained from Ayub Agriculture Research Institute (AARI), Faisalabad. This rice variety is mainly cultivated in the country and has strong resistance against pests and diseases. The pot experiment was conducted in a botanical garden where the temperature was in the range 18–25 °C and humidity was 70 % at the time of sowing and 30–35 °C and 85 % humidity at the time of harvesting. Uniform and healthy seeds were transferred into gunny bags and soaked in water for 24 h. Seeds were grown in small trays. Three 25-day-old rice seedlings were transplanted into plastic pots and plants were grown for 16 weeks from mid-July to mid-November 2014. All pots were carefully watered to maintain the moisture during the experiment.
Plant parameters and analysis
Physiological parameter
After 6 weeks of transplanting rice seedlings, physiological parameters like photosynthesis rate (A), transpiration rate (E), stomatal conductance (g s ), and substomatal conductance rate (C i ) were measured during the time period of 10 to 12 a.m. by using IRGA (Li-Cor 6400 XT).
Biological parameter
The rice crop was harvested carefully when 90 % of grain became golden yellow. Plant root, shoot, and grain weights were measured at harvest. Shoot and root dry weight was recorded after sun-drying, and then harvested plants were oven-dried at 70 °C till constant weight.
Chemical analysis
Grain samples of rice were dried at 60 °C in a hot air oven, ground, and digested in a diacid mixture (HNO3/HClO4 9:4) (Jackson 1973). Iron, Zn, and Ni in rice grain digest were measured on AAS (PerkinElmer, Analyst 100, Waltham, USA). All the chemicals and reagents used in this study were obtained from Sigma-Aldrich (St. Louis, MO, USA) to ensure the quality and purity of the chemicals.
Biochemical analysis
For the determination of phytate in rice grains, 60 mg finely ground rice was extracted with 10 mL of 0.2 N HCl at room temperature after shaking the mixture continuously for 2 h. Phytate in the extract was determined by an indirect method (Haug and Lantzsch 1983) at 519 nm with a spectrophotometer (Shimadzu, UV-1201, Kyoto, Japan). Ferritin quantification from rice grains was carried out through the method described by Barcelo et al. (1997) and Laulhere et al. (1988) with slight modifications described by Lukac et al. (2009). The final concentration of ferritin was measured by enzyme-linked immunosorbent assay using antibody-coated microtiter wells (catalog number BC-1025).
Physico-chemical analysis
The paddy was cleaned to remove dust trash, stone, and foreign matter; de-hulled; and milled by passing through “Stake sheller” (polisher) to obtain different fractions of rice. A portion of rice fractions was also ground by passing through “UDY Cyclone Mill” to get rice flour. Samples were analyzed for ash, fiber, protein, and fat by the methods of AOAC (2003).
Statistical analysis
The pot experiment was conducted in a completely randomized design. Treatment effects on plant physiological, biological, biochemical, and physiochemical parameters were evaluated using two-way analysis of variance (ANOVA). Least significant differences (at p = 0.05) were used for comparison between treatment (Steel et al. 1997). Statistical analyses were performed using Statistix 9® for Windows (Analytical Software, Tallahassee, USA). The regression/correlation analysis was calculated by Microsoft Excel 2012 professional edition.
Results
Dry matter yield
Table 3 shows the dry matter yield in the treatments of unaltered pH soil and the pH-amended soil. Application of BC and GS alone or their mixture significantly increased the shoot dry weight per pot when compared to control treatment. The maximum increase in shoot dry weight (91 %) was observed in T10 relative to control. Similarly, a significant increase in root biomass was observed in T10 compared to T1 (control). Incorporation of GS and BC in pH-amended soil (T10) significantly increased root dry weight when compared to control treatment (T1). Grain yield per pot on unaltered soil pH and the pH-amended soil was generally higher in the treatments where GS or BC alone or their mixture was added as immobilizing amendments. Interestingly, a significantly highest grain yield [pH 6.5 (27.2 g pot−1)] was observed in the treatment receiving GS, BC, and S together (T10) which was 56 % higher when compared to control (T1).
Photosynthetic parameters of rice plant
Table 3 presents the photosynthetic rate (A) of rice leaves under different treatments. Application of Fe only (T2) did not significantly increase A relative to control treatment. However, Fe along with immobilizing amendments (GS or BC or their combination) and elemental S showed a significant increase in A relative to T1. The maximum increase (184 %) in A was observed in T10 (S + Fe + BC + GS) application relative to control. Transpiration rate (E) values ranged from 2.7 to 5.8 μmol H2O m−2 s−1 with the maximum value found in T10 (S + Fe + BC + GS). Maximum increase (115 %) in E was observed in T10 treatment relative to control. The values of stomatal conductance (gs) in rice leaves were ranged from 235 to 479.7 μmol m−2 s−1. Application of S with GS or BC or their combination (T8, T9, and T10) in the presence of Fe showed statistically similar values of gs but had significantly higher values relative to control (T1). Similarly, the maximum significant decrease in substomatal CO2 conductance (Ci) in the leaves of rice plants was found in T10 (29 %) and T8 (28 %) relative to the non-amended control (T1).
Grain Fe, Zn, and Ni concentration
In our experiment, the application of Fe significantly improved Fe concentration in rice grains (Table 4) when compared to control where no Fe was added. The concentration of Fe was in the range from to 27.9 to 59.6 mg kg−1 dry weight (DW) of rice grains in our results. Interestingly, incorporation of elemental S (altered soil pH) significantly increased grain Fe concentrations (in most of the treatments) when compared to T1. Maximum increase of 70 % (T5) and 113 % (T10) in Fe concentration in the grains was observed when compared to control (T1).
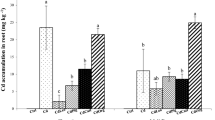
Zinc concentration in rice grain ranged from 18.5 mg kg−1 (original soil pH) to 27.4 mg kg−1 (altered soil pH). Generally, the Fe concentrations in the grains were found higher in the soil at an original pH (8) as compared to the likewise treatments in the soil with altered pH (6.5). Maximum increase (39 %) in grain Zn concentration was observed with T9 application relative to T1. The concentration of Ni ranged from 10.2 to 17.3 mg kg−1 in the rice grains (Table 4). Application of immobilizing agents (GS and BC) and S affected the Ni concentration in grains.
Total and DTPA-extractable Ni concentrations in the soil
The effect of solubilizing and immobilizing agents on the DTPA and total Ni concentration after crop harvest in the soil is presented in Table 4. Data showed a positive effect of these amendments on the amount of chemically available Ni in the soil. Highest concentration of DTPA-extractable Ni was observed in T6 (4.36 mg kg−1 soil) (altered soil pH) where a significant lowest DTPA-extractable Ni was observed in T5 (2.12 mg kg−1 soil) which was 44 % lower when compared to control (T1). The significant increase in the total Ni concentration in soil was observed in T5 when compared to control. In contrast, lowest total Ni concentration was observed in the treatment T6 (71.1 mg kg−1 soil) which was 5.1 % lower when compared to T1 [control (74.9 mg kg−1 soil)]. Both total and DTPA-extractable Ni concentrations were higher in the soil at original pH (8) as compared to the likewise treatments in the soil with altered pH (6.5).
Proximate composition of rice grains
Proximate composition was significantly (p < 0.05) reduced in rice cultivar under control conditions when compared to most of the treatments (Fig. 1). Significantly highest proximate composition of rice grains was observed in T10 treatment (S + Fe + BC + GS) when compared to control (T1). The ranges of percent age values of crude fat ranged from 0.9 to 1.53; crude protein from 4.8 to 7.5; crude fiber from 0.27 to 0.54; and ash content from 0.36 to 0.7 in our experiment. At original soil pH, application of immobilizing agents (BC and GS) with Fe (T5) in soil showed 44, 41, 59, and 66 % significantly higher crude fat, protein, fiber, and ash percentages in rice grains, respectively, when compared to the control treatment (T1). The application of S along with BC, GS, and Fe (T10) in pH-altered soil proved to be significantly effective in increasing 72, 56, 101, and 93 % crude fat, protein, fiber, and ash percentages, respectively, relative to the non-amended control (T1).
Proximate composition analysis of rice grains grown with various treatments (T1 control; T2 iron; T3 iron + biochar; T4 iron + gravel sludge; T5 iron + gravel sludge + biochar and the next five treatments were in the same order with elemental sulfur) under nickel-contaminated soil. Soil pH was manipulated from 8 to 6.5 with elemental sulfur prior to sowing of rice crop. Values are means with bars sharing the same letters which are statistically similar to each other at p ≤ 0.05
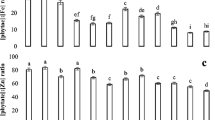
Phytate contents and molar ratios of phytate to Fe and Zn in rice grains
The phytate contents in rice grain ranged from 6.2 to 8.3 mg g−1 DW in our results (Fig. 2). Incorporation of BC, GS, and Fe (T5) significantly decreased the phytate contents (10 %) in rice grains relative to control (T1) where no amendments were added. This effect was more pronounced (34 % decreased) in the treatment (T10) where elemental S along with BC, GS, and Fe was incorporated (altered soil pH). BC along with Fe (T3) in the soil with original pH significantly reduced phytate contents up to 7 % when compared to control, but this effect was significantly more prominent (23 % decreased) in the treatment where S and Fe were added (T8).
Phytate concentration in rice grains and molar ratios of phytate to Fe and Zn grown with various treatments (T1 control; T2 iron; T3 iron + biochar; T4 iron + gravel sludge; T5 iron + gravel sludge + biochar and the next five treatments were in the same order with elemental sulfur) under nickel-contaminated soil. Soil pH was manipulated from 8 to 6.5 with elemental sulfur prior to sowing of rice crop. Values are means with bars sharing the same letters which are statistically similar to each other at p ≤ 0.05
Figure 2 presents the molar ratios of phytate to iron and zinc in rice grains. Phyt/Fe molar ratios ranged from 8.8 to 25.2 with the minimum value found in T10 (S + Fe + BC + GS). Application of Fe significantly (p < 0.05) reduced Phyt/Fe molar ratios, but the reduction was variable among different treatments. There was a 47 % decrease in Phyt/Fe molar ratio in T5 treatment (Fe + BC + GS) relative to the control (T1). Interestingly, this decrease was significantly more pronounced (65 %) in the treatment where elemental S along with BC, GS, and Fe was incorporated (T10). The values of the Phyt/Zn molar ratio in our experiment were in the range from 24 to 42.1 in the results. Treatment receiving only Fe (T2) was statistically similar to the control where neither Fe nor amendments were added (T1). Treatments receiving elemental S (altered soil pH) showed significantly lower Phyt/Zn molar ratios (Fig 2) when compared to the likewise treatments where no sulfur was added (originals soil pH).
Concentration of ferritin in rice grains
Ferritin concentration in rice grains is shown in Fig. 2. In our results, the concentration of ferritin in rice grain ranged from 0.03 to 0.15 μg g−1 DW. Application of Fe only (T2) in soil without any immobilizing amendments significantly increased the ferritin concentration in rice grains when compared to control (T1) where neither amendments nor S was added. The maximum increase (383 %) relative to control treatment was observed where Fe, GS, BC, and S together were applied (T10). Ferritin concentration was found lower in the soil at original pH (8) as compared to the likewise treatments in the soil with altered pH (6.5).
Correlation between Fe concentration and ferritin concentration in rice grains
There was a close positive correlation between Fe concentration and ferritin protein concentrations in the rice grains (r = 0.97) (Fig. 4).
Discussion
The results of the present study showed that BC alone and its mixture with GS (original soil pH) increased growth and yield parameters of rice (Table 3). This effect was more pronounced with the application of elemental S in the growth medium. Such increased impact of BC on dry matter production of rice is in accordance with the results of previous studies (Kimetu et al. 2008). Compared to control, the highest grain yield was recorded in the S + Fe + GS + BC treatment in the altered soil pH (Table 3). Wang et al. (2012) found similar results when BC applied with N fertilizer increased wheat and rice grain biomass and yield. Similarly, an increase in the biomass of different plant species by the application of GS has also been previously reported in different studies (Friesl et al. 2006; Friesl-Hanl et al. 2009; Iqbal et al. 2012). The GS application showed a positive effect on plant yield on a site with initial inhibited plant growth. Thus, it is clear that enhanced plant growth and grain yield in rice crop can be attributed to the positive effect of BC. An increase in the yield parameters of rice can also be attributed to the immobilization of Ni in the soil (Table 4) which led to a decrease in the Ni uptake by plants, hence alleviating Ni toxicity to plants (Herath et al. 2015).
In the present study, the significant increase in A (184 %) and E (115 %) was observed with the application of S + Fe + GS + BC as compared to control (Table 3). Ippolito et al. (2011) reported that application of BC increased the moisture contents by about 3–7 % which enhanced the rate of photosynthesis. The maximum increase in E (115 % that of control) and gs was also observed with the application of combined amendments (Table 3). Borrell et al. (2000) reported reduction in E in the plants under stress conditions. It has been reported that Ni toxicity decreased gas exchange characteristics in many plant species (Ouzounidou et al. 2006; Khaliq et al. 2016). Similarly, a decrease in stomatal conductance and hence a decline in photosynthesis due to Ni stress in poplar (Populus nigra) were reported by Velikova et al. (2010). An increase in stomatal conductance was found by the application of BC as compared to control (Solaiman et al. 2010). It showed that BC combined with GS in the presence of S has given the stability to rice plants under Ni stress.
When compared to control, a significant increase in Fe concentration in rice grains in receiving immobilizing agents (GS and BC) alone or in combination in both soils (original soil pH and altered soil pH) can be attributed to reduced Ni and Zn concentration in rice grains (Table 4). Several authors have reported that Ni and Fe show an antagonistic effect where high Ni reduced Fe concentration and/or bioavailability (Chen et al. 2009; Ghasemi et al. 2009; Nishida et al. 2012). It was reported that it is the Ni/Fe ratio, rather than the absolute concentration in plant tissues and organs, that is most tightly associated with reduced Fe bioavailability/use under high Ni conditions. Interestingly, Fe on Ni antagonism has also been proved in some studies where reduced Ni in soil increased the Fe concentration in plants (Bingham et al. 1986; Yang et al. 1996; Wood 2013).
In the present study, the significant increase in Fe concentration in rice grains can be associated with lower concentration of grain Ni in these treatments. This Ni-induced increase in grain Fe and Zn concentration implicates a Ni-induced disruption of cell homeostasis likely due to low bioavailability of Ni in soil solution. This low bioavailability of Ni in soil (Table 4) led to an increased uptake of Fe and Zn by rice plant. Many studies found that application of immobilizing agents like BC and GS reduced bioavailable Ni concentration (Friesl-Hanl et al. 2009; Namgay et al. 2010; Khanmohammadi et al. 2015). Biochar has larger surface areas and offers sorption sites that immobilize heavy metals by combined processes of chemisorption, complexation, ion exchange, and surface interaction (Cao et al. 2009; Park et al. 2011; Usman et al. 2013; Ahmad et al. 2014). Numerous studies have found that GS decreases soil available heavy metals and thus reduces uptake by plants (Lothenbach et al. 1998; Friesl et al. 2006; Friesl-Hanl et al. 2009; Iqbal et al. 2012). Mechanisms like increased metal adsorption due to increased soil pH (Iqbal et al. 2012), enhanced soil buffering capacity (Lothenbach et al. 1998), chemisorptions (Gray et al. 2006), and offering large surface area for metal binding provided by calcite, calcium carbonate, and clay minerals present in GS (Lothenbach et al. 1998; Krebs et al. 1999) have been suggested to explain the decreased metal solubility in soil after its application.
Phytate are complexes that are formed by relative binding of divalent mineral cations with phytic acid which is an acidic compound (Lott et al. 1995). In rice, the accumulation of phytate is mostly (about 80 %) in the embryo and in the aleurone layer of mature seeds (Ogawa et al. 1977). In present study, the phytate range was from 6.2 to 8.3 mg g−1 DW of rice grains with the lowest significant values found in T7, T8, T9, and T10 in the soil with altered pH. This significant lowest phytate concentration can be attributed to higher grain Zn concentrations in corresponding treatments (Table 4). According to literature, phytic acid (PA) is a major phosphorus storage compound of most seeds and cereal like rice (Zhou and Erdman 1995). Previous studies have shown that the concentration of PA in seeds is mostly dependent on uptake of phosphorus from root and its translocation from leaves to seeds (Michael et al. 1980; Raboy and Dickinson 1993). Since uptake of phosphorus via root and later its accumulation in seeds is greatly affected by Zn supply to roots (Loneragan et al. 1982; Rengel and Graham 1995), thus in our case it can be suggested that higher Zn accumulation in rice grains has affected PA contents in rice grains.
The molar ratio of Phy/mineral concentration is an estimate of phytate inhibitory effect on mineral absorption. The molar ratio of Phy/Zn and Phy/Fe is the most widely used to evaluate Fe and Zn bioavailability for human and animal absorption (Cook et al. 1991; Hambidge et al. 2010). Our results proved that application of BC and GS, alone or in combination (both in soil with original pH and altered soil pH), was able to decrease Phy/Fe ratio when compared to control. In the case of Phy/Zn molar ratio, lowest significant values were found in T8, T9, and T10. This decreased Phy/Fe ratio can be attributed to the higher content of Fe in rice grains which was due to the antagonistic behavior of Fe with Ni (Bingham et al. 1986; Yang et al. 1996; Wood 2013).
The ferritin concentration increased in rice grains by the application of BC and GS in original soil and soil with altered pH (Fig. 3). This increase in ferritin concentration can be attributed to low bioavailability of Ni in soil solution due to immobilization of Ni by GS and BC. This decreased Ni bioavailability in soil solution has led to enhanced uptake of Fe by rice plant and later its accumulation in grains. Our findings are in line with Panda et al. (2014) who found that application of Fe supplementation in the growing medium increased Fe accumulation, which in turn increased aconitase activity, and ferritin accumulation in the seedlings of two rice cultivars.
Ferritin concentration (μg g−1) in rice grains grown with various treatments (T1 control; T2 iron; T3 iron + biochar; T4 iron + gravel sludge; T5 iron + gravel sludge + biochar and the next five treatments were in the same order with elemental sulfur) under nickel-contaminated soil. Soil pH was manipulated from 8 to 6.5 with elemental sulfur prior to sowing of rice crop. Values are means with bars sharing the same letters which are statistically similar to each other at p ≤ 0.05
Therefore, not only increased Fe storage in grains but also enhanced Fe uptake from soil and hence its enhanced translocation within the rice plant body are thought to be the requirements for further Fe biofortification in rice grains. Interestingly, we found a strong correlation (R 2 = 0.97) between Fe concentration and ferritin concentration in rice grains (Fig. 4). Prom-u-thai et al. (2009) also reported a positive correlation between ferritin and Fe concentration in rice grains.
Ferritin is the only protein known to concentrate Fe to the level required by the cells, to store it in a soluble and biologically available form, to release it when required, and to protect the cells against the toxic effects of excess Fe (Theil and Briat 2004). Also, the molecular mechanism involved in the Fe uptake which is stored in ferritin is an important source for humans to use and avoid Fe deficiency (Thiel et al. 2012). Our results strengthen the suggestion that increasing the bioavailability of Fe in soil parallel to the immobilization of Ni with BC and GS can be an effective solution for improving Fe nutrition in the populations who are exposed to the risk of Fe deficiency.
References
Adrees M, Ali S, Rizwan M, Ibrahim M, Abbas F, Farid M, Rehman MZ, Irshad MK, Bharwana SA (2015a) The effect of excess copper on growth and physiology of important food crops: a review. Environ Sci Pollut Res 22:8148–8162
Adrees M, Ali S, Rizwan M, Rehman MZ, Ibrahim M, Abbas F, Farid M, Qayyum MK, Irshad MK (2015b) Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: a review. Ecotoxicol Environ Saf 119:186–197
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33
Ali S, Bharwana SA, Rizwan M, Farid M, Kanwal S, Ali Q, Ibrahim M, Gill RA, Khan MD (2015) Fulvic acid mediates chromium (Cr) tolerance in wheat (Triticum aestivum L.) through lowering of Cr uptake and improved antioxidant defense system. Environ Sci Pollut Res 22:10601–10609
Allison LE, Moodie CD (1965) Carbonate. In: Black CA (ed) Methods soil analysis part 2: chemical and microbiological properties. Am Soc Agron Madison USA, p 1379–1396
Almaroai YA, Usman ARA, Mahtab A, Deok HM, Ju-Sik C, Young KJ, Choong J, Sang SL, Ok YS (2014) "Effects of biochar, cow bone, and eggshell on Pb availability to maize in contaminated soil irrigated with saline water. Environ Earth Sci 71:1289–1296
AOAC (2003) Official methods of analysis of the association of official’s analytical chemists, 17th edn. Association of official analytical chemists, Arlington, Virginia. Application on mycorrhizal root colonisation, growth and nutrition of wheat. Aust J Soil Res 48:546–554
Barcelo F, Miralles F, Arean CO (1997) Purification and characterization of ferritin from alfalfa seeds. J Inorg Biochem 66:23–27
Bertrand J, Mars A, Boyle C, Bove F, Yeargin-Allsopp M, Decoufle P (2001) Prevalence of autism in a United States population: the Brick Township, New Jersey, investigation. Pediatrics 108:1155–1161
Bingham FT, Pereyea FJ, Jarrell WM (1986) Metal toxicity to agricultural crops. Met Ions Biol Syst 20:119–156
Borrell AK, Hammer GL, Douglas ACL (2000) Does maintaining green leaf area in sorghum improve yield under drought? I. Leaf growth and senescence. Crop Sci J 40:1026–1037
Bouis HE, Welch RM (2010) Biofortification: a sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop Sci 50:20–32
Bremner JM, Mulvaney CS (1982) Nitrogen total. p. 595–624. In A. L. Page (ed.), Methods of soil analysis. Agron. No. 9, part 2: chemical and microbiological properties, 2nd ed., Am Soc Agron Madison WI USA
Cao XD, Ma LN, Gao B, Harris W (2009) Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ Sci Technol 43:3285–3291
Cataldo DA, Garland TR, Wildung RE (1978) Nickel in plants. I. Uptake kinetics using intact soybean seedling. Plant Physiol 62:563
Chen Z, Watanabe TT, Shinano T, Exawa J, Washaki K, Kimura T, Osaki M, Xhu Y (2009) Element interconnections in Lotus japonicas: a systematic study of the effects of element additions on different natural variants. Soil Sci Plant Nutr 55:91–101
Cook JD, Dassenko SA, Lynch SR (1991) Assessment of the role of nonheme-iron availability in iron balance. Am J Clin Nutr 54:717–722
Friesl W, Friedl J, Platzer K, Horak O, Gerzabek MH (2006) Remediation of contaminated agricultural soils near a former Pb/Zn smelter in Austria: batch, pot and field experiments. Environ Pollut 144:40–50
Friesl-Hanl W, Platzer K, Horak O, Gerzabek MH (2009) Immobilising of Cd, Pb, and Zn contaminated arable soils close to a former Pb/Zn smelter: a field study in Austria over 5 years. Environ Geochem Health 31:581–594
Gajewska E, Sklodowska M, Slaba M, Mazur J (2006) Effect of nickel on antioxidative enzyme activities, proline and chlorophyll content in wheat shoots. Biol Plant 50:653–659
Gajewska E, Wielanek M, Bergier K, Skłodowska M (2009) Nickel-induced depression of nitrogen assimilation in wheat roots. Acta Physiol Plant 31:1291–1300
Gee GW, Bauder JW (1986) Particle-size analysis. In: Klute A (ed) Methods soil anal. Part 1: physical and mineralogical methods. Agron Monogr 9. Soil Sci Soc Am. Madison, USA, p 383–409
Ghasemi R, Ghaderian SM, Kramer U (2009) Interference of nickel with copper and iron homeostasis contributes to metal toxicity symptoms in the nickel hyperaccumulator plant Alyssum inflatum. New Phytol 184:566–580
Gray CW, Dunham SJ, Dennis PG, Zhao FJ, McGrath SP (2006) Field evaluation of in situ remediation of a heavy metal contaminated soil using lime and red-mud. Environ Pollut 142:530–539
Guo Y, Marschner H (1995) Uptake, distribution, and binding of cadmium and nickel in different plant species. J Plant Nutr 18:2691–2706
Hambidge KM, Miller LV, Westcott JE, Sheng X, Krebs NF (2010) Zinc bioavailability and homeostasis. Am J Clin Nutr 91:1478–1483
Haug W, Lantzsch H (1983) Sensitive method for the rapid determination of phytate in cereals and cereal products. J Sci Food Agric 34:1423–1426
Herath I, Kumarathilaka P, Navaratne A, Rajakaruna N, Vithanage M (2015) Immobilization and phytotoxicity reduction of heavy metals in serpentine soil using biochar. J Soils Sediments 15:126–138
Houben D, Evrard L, Sonnet P (2013a) Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere 92:1450–1457
Houben D, Evrard L, Sonnet P (2013b) Beneficial effects of biochar application to contaminated soils on the bioavailability of Cd, Pb and Zn and the biomass production of rapeseed (Brassica napus L.). Biomass Bioenergy 57:196–204
Hussain S, Maqsood MA, Aziz T, Basra SMA (2013) Zinc bioavailability response curvature in wheat grains under incremental zinc applications. Arch Agron Soil Sci 59:1001–1016
Ippolito JA, Barbarick KA, Elliott HA (2011) Drinking water treatment residuals: a review of recent uses. J Environ Qual 40:1–12
Iqbal M, Puschenreiter M, Oburger E, Santner J, Wenzel WW (2012) Sulfur-aided phytoextraction of Cd and Zn by Salix smithiana combined with in situ metal immobilization by gravel sludge and red mud. Environ Pollut 170:222–231
Jackson ML (1973) Soil chemical analysis. Prentice-hall of India Pvt. Ltd., New Delhi
Kaplan M, Orman S (1998) Effect of elemental sulfur and sulfur containing waste in a calcareous soil in Turkey. J Plant Nutr 21:1655–1665
Khaliq A, Ali S, Hameed A, Farooq MA, Farid M, Shakoor MB, Mahmood K, Ishaque W, Rizwan M (2016) Silicon alleviates nickel toxicity in cotton seedlings through enhancing growth, photosynthesis and suppressing Ni uptake and oxidative stress. Arch Agron Soil Sci 62:633–647
Khanmohammadi Z, Afyuni M, Mosaddeghi MR (2015) Effect of pyrolysis temperature on chemical and physical properties of sewage sludge biochar. Waste Manag Res 33:275–283
Kimetu JM, Lehmann J, Ngoze S, Mugendi DN, Kinyangi JM, Riha S, Verchot L, Recha JW, Pell A (2008) Reversibility of soil productivity decline with organic matter of differing quality along a degradation gradient. Ecosystems 11:726–739
Krebs R, Gupta SK, Furrer G, Schulin R (1999) Gravel sludge as an immobilising agent in soils contaminated by heavy metals: a field study. Water Air Soil Pollut 115:465–479
Laulhere JP, Lescure AM, Briat JF (1988) Purification and characterization of ferritins from maize, pea, and soybean seeds distribution in various pea organs. J Biol Chem 263:10289–10294
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese and copper. Soil Sci Soc Am J 42:421–428
Loneragan JF, Grunes DL, Welch RM, Aduayi EA, Tengah A, Lazar VA, Cary EE (1982) Phosphorus accumulation and toxicity in leaves in relation to zinc supply. Soil Sci Soc Am J 46:345–352
Lonnerdal B (2007) The importance and bioavailability of phytoferritin- bound iron in cereals and legume foods. Int J Vitam Nutr Res 77:152–157
Lothenbach B, Krebs R, Furrer G, Gupta SK, Schulin R (1998) Immobilization of cadmium and zinc in soil by Al-montmorillonite and gravel sludge. Eur J Soil Sci 49:141–148
Lott JNA, Greenwood JS, Batten GD (1995) Mechanisms and regulation of mineral nutrient storage during seed development. In: Kigel J, Galili G (eds) Seed development and germination. Marcel Dekker, New York, pp. 215–235
Lukac RJ, Aluru MA, Reddy MB (2009) Quantification of ferritin from staple food crops. J Agric Food Chem 57:2155–2161
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press Inc., San Diego
Meng F, Wei Y, Yang X (2005) Iron content and bioavailability in rice. J Trace Elem Med Biol 18:333–338
Michael B, Zink E, Lantzsch HJ (1980) Effect of phosphate application on phytin-phosphorus and other phosphate fractions in developing wheat grains. Z Pflanzenernaehr Bodenkd 143:369–376
Modaihsh S, Al-mustafa WA, Metwally AE (1989) Effect of elemental sulfur on chemical changes and nutrient availability in calcareous soils. Plant Soil 116:95–101
Mulligan CN, Yong RN, Gibbs BF (2001) Surfactant-enhanced remediation of contaminated soil: a review. Eng Geol 60:371–380
Namgay T, Singh B, Singh BP (2010) Influence of biochar application to soil on the availability of As, Cd, Cu, Pb and Zn to maize (Zea mays L.). Aust J Soil Res 48:638–647
Nelson DW, Sommers LE (1982) Total carbon, organic carbon and organic matter. In: Klute A (ed) Methods of soil analysis, part 2: chemical and microbiological properties. Soil Science Society of America, Madison, pp. 570–571
Nishida S, Aisu A, Mizuno T (2012) Induction of IRT1 by the nickel-induced iron-deficient response in Arabidopsis. Plant Signal Behav 7:13–19
Ogawa M, Tanaka K, Kasai Z (1977) Note on the phytin containing particles isolated from rice scutellum. Cereal Chem 54:1029–1034
Olaniran AO, Balgobind A, Pillay B (2013) Bioavailability of heavy metals in soil: impact on microbial biodegradation of organic compounds and possible improvement strategies. Int J Mol Sci 14:10197–10228
Ouzounidou G, Moustakas M, Symeonidis L, Karataglis S (2006) Response of wheat seedlings to Ni stress: effects of supplemental calcium. Arch Environ Contam Toxicol 50:346–352
Panda BB, Sharma S, Mohapatra PK, Das A (2014) Iron nutrition vis-à-vis aconitase activity and ferritin accumulation in tropical indica rice cultivars differing in grain iron concentration. Am J Plant Sci 5:2829–2841
Park JH, Choppala GH, Bolan NS, Chung JW, Chuasavathi T (2011) Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 348:439–451
Prom-u-thai C, Glahn PR, Cheng Z, Fukai S, Rerkasem B, Huang L (2009) The bioavailability of iron fortified in whole grain parboiled rice. Food Chem 89:2565–2571
Qureshi S, Richards BK, Steenhuis TS, McBride MB, Baveye P, Dousset S (2004) Microbial acidification and pH effects on trace element release from sewage sludge. Environ Pollut 132:61–71
Raboy V, Dickinson DB (1993) Phytic acid levels in seeds of Glycine max and G. soja as influenced by phosphorus status. Crop Sci 33:1300–1305
Rahman H, Sabreen S, Alam S, Kawai S (2005) Effects of nickel on growth and composition of metal micronutrients in barley plants grown in nutrient solution. J Plant Nutr 28:393–404
Rajkumar M, Freitas H (2008) Effects of inoculation of plant-growth promoting bacteria on Ni uptake by Indian mustard. Bioresour Technol 99:3491–3498
Rehman MZ, Rizwan M, Ghafoor A, Naeem A, Ali S, Sabir M, Qayyum MF (2015) Effect of inorganic amendments for in situ stabilization of cadmium in contaminated soil and its phyto-availability to wheat and rice under rotation. Environ Sci Pollut Res 22:16897–16906
Rengel Z, Graham RD (1995) Wheat genotypes differ in zinc efficiency when grown in the chelate-buffered nutrient solution. II. Nutrient uptake. Plant Soil 176:317–324
Rizwan M, Ali S, Adrees M, Rizvi H, Rehman MZ, Hannan F, Qayyum MF, Hafeez F, Ok YS (2016a) Cadmium stress in rice: toxic effects, tolerance mechanisms and management: a critical review. Environ Sci Pollut Res. doi:10.1007/s11356-016-6436-4
Rizwan M, Ali S, Qayyum MF, Ibrahim M, Rehman MZ, Abbas T, Ok YS (2016c) Mechanisms of biochar-mediated alleviation of toxicity of trace elements in plants: a critical review. Environ Sci Pollut Res 23:2230–2248
Rizwan M, Meunier JD, Davidian JC, Pokrovsky OS, Bovet N, Keller C (2016b) Silicon alleviates Cd stress of wheat seedlings (Triticum turgidum L. cv. Claudio) grown in hydroponics. Environ Sci Pollut Res 23:1414–1427
Rizwan M, Ali S, Abbas T, Rehman MZ, Hannan F, Keller C, Al-Wabel MI, Ok YS (2016d) Cadmium minimization in wheat: a critical review. Ecotoxicol Environ Saf 130:43–53
Rizwan M, Meunier JD, Hélène M, Keller C (2012) Effect of silicon on reducing cadmium toxicity in durum wheat (Triticum turgidum L. cv. Claudio W.) grown in a soil with aged contamination. J Hazard Mater 209-210:326–334
Roitto M, Rautio P, Julkunen-Tiitto R, Kukkola E, Huttunen S (2005) Changes in the concentrations of phenolics and photosynthates in Scots pine (Pinus sylvestris L.) seedlings exposed to nickel and copper. Environ Pollut 137:603–609
Rubio MI, Escrig I, Martínez-Cortina C, López-Benet F, Sanz A (1994) Cadmium and nickel accumulation in rice plants. Effects on mineral nutrition and possible interactions of abscisic and gibberellic acids. Plant Growth Regul 14:151–157
Salt DE, Blaylock M, Kumar NPBA, Dusenkov V, Ensley BD, Chet I, Raskin I (1995) Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Biotechnology 13:468–474
Solaiman ZM, Blackwell P, Abbott LK, Storer P (2010) Direct and residual effect of biochar application on mycorrhizal root colonisation, growth and nutrition of wheat. Aust J Soil Res 48:546–554
Sruthy OA, Jayalekshmi S (2014) Electrokinetic remediation of heavy metal contaminated soil. Int J Struct Civil Eng Res 3:103–111
Steel RGD, Torrie JH, Dickey DA (1997) Principles and procedures of statistics: a biometrical approach, 3rd edn. McGraw-Hill Publishing Co., New York
Theil EC, Briat JF (2004) Plant ferritin and non-heme iron nutrition in humans; harvest plus technical monograph 1; International Food Policy Research Institute and International Center for Tropical. Agriculture, Washington DC
Thiel J, Riewe D, Rutten T, Melzer M, Friedel S, Bollenbeck F, Weschke W, Weber H (2012) Differentiation of endosperm transfer cells of barley: a comprehensive analysis at the micro-scale. Plant J 71:639–655
Usman ARA, Alkredaa RS, Al-Wabel MI (2013) Heavy metal contamination in sediments and mangroves from the coast of RedSea: Avicennia marina as potential metal bioaccumulator. Ecotoxicol Environ Saf 97:263–270
Velikova V, Tsonev T, Loreto F, Centritto M (2010) Changes in photosynthesis, mesophyll conductance to CO2, and isoprenoid emissions in Populus nigra plants exposed to excess nickel. Environ Pollut 1–9
Wang J, Pan X, Liu Y, Zhang X, Xiong Z (2012) Effects of biochar amendment in two soils on greenhouse gas emissions and crop production. Plant Soil 360:287–298
Wang K, Gao F, Ji YX, Liu Y, Dan ZW, Yang PF, Zhu YG, Li SQ (2013a) ORFH79 impairs mitochondrial function via interaction with a subunit of electron transport chain complex III in Honglian cytoplasmic male sterile rice. New Phytol 198:408–418
Watanabe FS, Olsen SR (1965) Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts. Soil Sci Soc Am Proc 29:677–678
Welch RM, Graham RD (2004) Breeding for micronutrients in staple food crops from a human nutrition perspective. J Exp Bot 55:353–364
Wood BW (2013) Iron-induced nickel deficiency in pecan. Hortic Sci 48:1145–1153
Yang X, Baligar VC, Martens DC, Clark PB (1996) Plant tolerance to nickel toxicity. II Nickel effect on influx and transport of mineral nutrients in four plant species. J Plant Nutr 19:265–279
Zhou JR, Erdman JW Jr (1995) Phytic acid in health and disease. Crit Rev Food Sci Nutr 35:495–508
Zielińska-Dawidziak M (2015) Plant ferritin—a source of iron to prevent its deficiency. Nutrients 7:1184–1201
Acknowledgment
We are highly thankful to the Higher Education Commission (HEC), Pakistan, and Government College University, Faisalabad, Pakistan, for their financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Elena Maestri
Rights and permissions
About this article
Cite this article
Ramzani, P.M.A., Khan, WuD., Iqbal, M. et al. Effect of different amendments on rice (Oryza sativa L.) growth, yield, nutrient uptake and grain quality in Ni-contaminated soil. Environ Sci Pollut Res 23, 18585–18595 (2016). https://doi.org/10.1007/s11356-016-7038-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7038-x