Abstract
The interactions between Cd and Zn in their effects on plants are inconsistent and difficult to predict. A hydroponic experiment was conducted to investigate the effects of Cd and Zn and their interactions on root morphology and metal translocation in two populations of Hylotelephium spectabile (Boreau) H. Ohba (Crassulaceae, HB1 and HB2). Both populations showed relative tolerance to high levels of Cd and Zn, except that the leaf biomass of HB1 significantly decreased by 44.6% with 5-mg/L Cd plus 10-mg/L Zn. Root growth was inhibited in both populations by addition of 20-mg/L Zn under Cd stress, while 10-mg/L Zn showed little impact on the root growth inhibition of HB2. Roots with diameter 0.1–0.4 mm contributed most of the total root length (RL) and root surface area (RSA) of H. spectabile. In both populations, these root parameters showed greater suppression with the combined stress of Cd plus Zn than under Cd or Zn single stress, except by adding 10-mg/L Zn under Cd stress. Moreover, HB2 maintained relatively higher RL and RSA than HB1 under the different treatments, which implied that HB2 might possess a more effective mechanism than HB1 for coping in response to Cd and Zn stress. The addition of Zn not only affected the absorption of Cd but also significantly affected the distribution of Cd in different tissues of H. spectabile. A low level of Zn led to increased Cd in the stem of HB2, but an increase in Cd in the leaf and root of HB1. Addition of 10-mg/L Zn led to a significant increase by 188% and 170% in Cd accumulation in aboveground part of HB2 under 2- and 5-mg/L Cd stress, whereas the addition of Zn had little effect on Cd accumulation in HB1. Thus, strong positive interactions of Cd and Zn occurred in HB2, which showed great potential for application in phytoremediation of soil contaminated with both Cd and Zn, warranting further investigation under field condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is one of the most toxic trace metals in the environment. Cd inhibits plant growth, reduces chlorophyll content, raises levels of reactive oxygen species in cells, and disrupts nutrient absorption (Choppala et al. 2014; Tran and Popova 2013). Cd is easily accumulated in organisms via the food chain and poses a threat to human health due to its high mobility and non-degradability (Sarwar et al. 2014). Zinc (Zn), an element in the same metal family (group IIB) as Cd, is an essential micronutrient for plants and participates in many metabolic processes, such as chlorophyll synthesis, photosynthesis, and protein metabolism (Aravind and Prasad 2004; Emamverdian et al. 2015; Hajiboland and Amirazad 2010; Hänsch and Mendel 2009). However, excessive Zn inhibits the key steps of photosynthesis and can lead to microelement deficiency such as Fe, Mg, and Mn in plant (Qiu et al. 2011).
There has been much research on the effects of Cd or Zn stress on plants; however, heavy metal contamination in the environment rarely occurs as one metal alone. Cadmium naturally exists in Zn ores and is often released accompanying Zn with mining and smelting these ores (Green et al. 2017). Cd possesses similar electron configurations and ionic radii as Zn, resulting in similar geochemical and environmental characteristics between the two elements (Li et al. 2009). Therefore, Cd might act as a competitive ion for Zn absorption by plant roots and interfere with the normal functions of Zn in metabolism (Hassan et al. 2005; Sarwar et al. 2015). The interactions of Zn and Cd on plant growth and metal translocation may be either synergistic or antagonistic, depending on the plant species, environmental conditions, or the Zn/Cd ratios in the medium (Sarwar et al. 2015; Wang et al. 2017).
Phytoremediation, that is, using plants to extract and accumulate heavy metals from contaminated soil, has been proposed as an environmentally friendly and cost-effective technique for soil remediation (Guo et al. 2017). The root system is the primary interface for ion exchange between the plant and the soil; it is the most sensitive to heavy metal stress in the environmental medium and directly influences the metal translocation of phytoaccumulators (Li et al. 2009). Plants might change their root morphology to cope with unfavorable growth conditions under Cd stress, and studies of root morphology might provide more valuable parameters than a focus simply on quantitative root parameters (Aravind and Prasad 2003; Wei and Zhou 2006). Hyperaccumulating and non-hyperaccumulating ecotypes of Sedum alfredii Hance exhibited significant differences in root morphology and metal translocation in response to Zn and Cd interactions (Li et al. 2009).
Hylotelephium spectabile (Boreau) H. Ohba (Crassulaceae), a perennial ornamental plant, was proved to be a Cd accumulator with considerable Cd phytoextraction capacity (Guo et al. 2017). Previous studies on the mechanism of Cd tolerance and accumulation of H. spectabile focused mainly on the subcellular distribution of Cd and the antioxidant system, while little information is available about the effects of Cd/Zn interactions on root morphology and metal translocation (Guo et al. 2018; Yang et al. 2018).
The main aim of this study was to determine the root morphology and Cd/Zn translocation of two populations of H. spectabile in response to Cd and/or Zn treatments and their interactions. We aimed to provide a more comprehensive understanding of the morphological characteristics associated with variations in Cd accumulation among different populations of H. spectabile.
Materials and methods
Plant preparation
H. spectabile is usually propagated by cuttings rather than seeds. Two populations (HB1 and HB2) of H. spectabile were obtained from the Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, Beijing, China. These two populations displayed remarkable differences in the plant morphologies and Cd absorption in aboveground part (Fig. S1). Moreover, these two populations also exhibited some differences in Cd accumulation capabilities and detoxification mechanisms in our previous research (Guo et al. 2018). The third-generation tillers were grown on the non-polluted medium to ensure that they are free of heavy metal pollution. The obtained tillers were initially pre-cultured on the artificial substrate for 15 days, and then cuttings with uniform morphology were selected for hydroponic experiments.
Experimental design and plant growth
The tillers were transplanted into 1-L pots containing 400-mL Hoagland’s solution (half strength) with the following composition: 2.5-mmol/L Ca(NO3)2, 2.5-mmol/L KCl, 5-μmol/L KH2PO4, 0.5-mmol/L MgSO4, 25-μmol/L H3BO3, 2.25-μmol/L MnSO4, 1.9-μmol/L ZnSO4, 0.15-μmol/L CuSO4, 0.05-mmol/L (NH4)6Mo7O24, and 5-μmol/L Fe-EDTA acid (EDTA). The treatments were control (CK), 2-mg/L Cd (Cd2), 5-mg/L Cd (Cd5), 10-mg/L Zn (Zn10), 20-mg/L Zn (Zn20), 2-mg/L Cd + 10-mg/L Zn (Cd2 + Zn10), 2-mg/L Cd + 20-mg/L Zn (Cd2 + Zn20), 5-mg/L Cd + 10-mg/L Zn (Cd5 + Zn10), and 5-mg/L Cd + 20-mg/L Zn (Cd5 + Zn20), with four tillers per pot and four replicate pots for each treatment. Cd and Zn were supplied as CdCl2·2.5H2O and ZnSO4·7H2O (guaranteed reagents) at the desired concentrations. The pH of the nutrient solution was adjusted to pH 6.0 ± 0.1 with 0.1-mol/L NaOH. Plants were cultured under greenhouse condition at temperatures of 30/20 °C (day/night) with a photoperiod of 16 h, approximately 300-mE/m2/s light intensity and 60% average relative humidity. The tillers were grown in hydroponic culture for 16 days with continuously aeration, and the nutrient solution was renewed every 3 days. Hydroponic culture was conducted in a greenhouse located at the Center for Environmental Remediation, Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, Beijing, China.
Plant morphology analysis
The harvested plants were first washed with tap water and then with 10-mmol/L EDTA for 5 min to remove metal ions from the root surface, and finally rinsed three times with ultrapure water.
The cleaned plants were separated into leaves, stem, and roots. The root segments were first placed on a root scan apparatus (Epson Perfection V700) in a transparent plastic tray filled with ultrapure water, and a white plastic plate served as image background. The image was recorded at a resolution of 400 dpi and images were saved as TIFF (tagged image file format). The WinRHIZO™ 2000 software was used to recognize the root digital images and analyze root morphological parameters. For a better understanding of the root morphological characteristics, the root diameter (d) of each sample was defined as 10 classes, which were 0–0.1 mm, 0.1 < d < 0.2 mm, 0.2 < d < 0.3 mm, 0.3 < d < 0.4 mm, 0.4 < d < 0.5 mm, 0.5 < d < 0.6 mm, 0.6 < d < 0.7 mm, 0.7 < d < 0.8 mm, 0.8 < d < 0.9 mm, and d > 0.9 mm. Root length (RL), root surface area (RSA), root volume (RV), average root diameter (ARD), and numbers of root tips (RT) were determined for each diameter class. For each replicate, the roots of the four plants were analyzed.
After root morphology analysis, the leaves, stem, and roots were oven dried at 65 °C to a constant weight for determination of biomass and Cd concentration. The dry weight of each tissue was recorded separately using an electronic balance (0.1-mg accuracy).
Elemental analysis
The oven-dried samples were separately ground in a mill, and then digested with conc. HNO3 and HClO4 (5:1, v/v). Cd and Zn concentration was determined by using inductively coupled plasma mass spectrometry (ICP-MS, Elan DRC-e, Perkin Elmer, USA). Quality control was addressed by routinely analyzing plant standard reference materials for bush twigs and leaves (GBW07603: GSV-2), including blanks in digestion batches. The recovery of standard for each element ranged between 95 and 105%.
Calculation of growth potential and Cd translocation
The tolerance index (TI) was used to evaluate the growth response of H. spectabile under different Cd and Zn stresses. Transfer factors (TF) were used to evaluate the efficiency of H. spectabile in translocating the accumulated metal from roots to shoots. The TF from stem to leaf (named TF1) was defined as the ratio of metal concentration in plant leaf to that in stem, and the TF from root to stem (named TF2) was defined as the ratio of metal concentration in plant stem to that in root. The calculation equations were:
where Wm represents the dry biomass of H. spectabile under different Cd and Zn stress treatments and Wck represents the dry biomass of H. spectabile under the control treatment.
where Cleaf (mg/kg), Cstem (mg/kg), and Croot (mg/kg) represent the metal concentration in the dried leaf, stem, and root of H. spectabile, respectively.
Statistical analysis
All experiment results in the tables and figures were analyzed using the SPSS 19.0 package. Data in the texts and tables were expressed as means with standard deviation, and error bars in the figures indicate standard deviations. The statistical significance of the differences between groups was evaluated by analysis of variance (ANOVA) and compared using the LSD test at P < 0.05, n = 4.
Results
Plant growth
The dry biomass of different H. spectabile tissues under various treatments of Cd, Zn, or Cd + Zn are presented in Table 1. Significant differences were recorded in the shoot and root biomasses between the two populations. Under various single stress conditions with Cd or Zn, both HB1 and HB2 populations grew normally without showing any toxic symptoms in the aboveground parts, expect that the leaf biomass of HB1 was significantly decreased by 44.6% under Cd5 + Zn10 treatment. The root growth of both HB1 and HB2 was significantly inhibited under 5-mg/L Cd or Cd + Zn combined treatments compared with the control, and the Cd + Zn combined stress showed stronger inhibition than single Cd stress. Moreover, the root biomass of HB2 was not decreased under the single stress of added Zn, while in HB1, the root biomass was significantly decreased by 60.0% under 20-mg/L Zn.
Root morphology
Both two populations of H. spectabile showed obvious changes in the root morphologies with different addition doses of Cd and Zn (Fig. S2). The results for RL, RSA, ARD, RV, and numbers RT under various treatments of Cd/Zn or Cd + Zn are presented in Table 2. The RL, RSA, and RV of both HB1 and HB2 showed a decreasing trend, while none of them reached the statistically significant trend. In addition, high level of Zn (20 mg/L) significantly decreased the RL and RSA of HB1 while showing little effect on those of HB2. The combined stress of Cd with 20-mg/L Zn exhibited a stronger inhibitory effect on the RL, RSA, and RV of both populations than Cd and Zn single stress, while no significant decrease was recorded under Cd stress with 10-mg/L Zn addition. The RL, RSA, and RV in HB1 and HB2 were lowest under 5-mg/L Cd + 20-mg/L Zn. For ARD, no significant differences were observed among the different treatments in either population.
Root diameter distribution
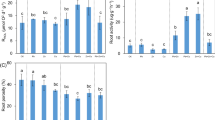
Ten root diameter classes were defined at intervals of 0.1 mm. The RL and RSA was predominantly found in the diameter classes 0.1–0.2 mm, 0.2–0.3 mm, and 0.3–0.4 mm for both populations under different Cd and Zn stress, which accounted for 70.1–85.3% of the total RL and for 63.3–83.0% of the total RSA (Fig. S3 and Fig. S4). The RL and RSA for root diameter class (0.1 mm–0.4 mm) are shown in Figs. 1 and 2. The RL of HB1 in the three diameter classes were on decline with increase of Cd or Zn stress, although the differences are not significant. In contrast to HB1, the RL in the diameter classes 0.1–0.2 mm and 0.2–0.3 mm of HB2 slightly increased under 2-mg/L Cd and significantly increased under 10-mg/L Zn. However, the negative effect of the combined stress of Cd + Zn on the RL between diameter classes 0.1–0.4 mm was greater than Cd/Zn single stress for both populations, except for Cd2 + Zn10 and Cd5 + Zn10 treatments. The roots in diameter classes 0.1–0.2 mm, 0.2–0.3 mm, and 0.3–0.4 mm contributed the most to the total RSA of the two populations (Fig. 2). The effect of different Cd, Zn, or Cd + Zn treatments on the RSA in these three diameter classes of HB1 and HB2 was also consistent with the results for RL.
Effect of different Cd and Zn additions to the nutrient solutions on the distribution of root length (RL) for different root diameters in two populations of H. spectabile. Bars represent standard deviation (SD) of four replicates. Different letters on the bars for the same population show significant differences between the treatments
Effect of different Cd and Zn additions to the nutrient solutions on the distribution of root surface area (RSA) for different root diameters in two populations of H. spectabile. Bars represent standard deviation (SD) of four replicates. Different letters on the bars for the same population show significant differences between the treatments
Cd and Zn uptake and translocation
The Cd concentrations in different tissues of both populations increased with increasing Cd stress and were in the order of root > stem > leaf (Fig. 3). The stem Cd concentration of HB1 was 3.85 and 3.39 times higher than that of HB2 under 2- and 5-mg/L Cd, respectively (P < 0.05). The combined stress of Cd + Zn decreased the leaf Cd concentration of HB1, except for Cd5 + Zn10 treatment, which significantly increased the leaf Cd concentration by 41.4%, as compared with 5-mg/L Cd treatment. Under 5-mg/L Cd stress, the stem Cd concentration of HB1 was significantly decreased by adding 10-mg/L Zn but significantly increased by addition of 20-mg/L Zn. In contrast to HB1, the stem Cd concentration of HB2 was significantly increased with addition of Zn under Cd stress and was highest under 5-mg/L Cd + 10-mg/L Zn treatment. For both populations, the root Cd concentrations were significantly increased by adding Zn under Cd stress.
Effect of different Cd and Zn additions to the nutrient solutions on the Cd concentration in leaf (A), stem (B), and root (C) in two populations of H. spectabile. The Zn concentration in leaf (D), stem (E), and root (F) are also given. Bars represent standard deviation (SD) of four replicates. Different letters on the bars for the same population show significant differences between the treatments
There were no significant differences in Zn concentrations in different tissues between HB1 and HB2 (Fig. 3). Addition of Cd significantly decreased the Zn concentration in stem of HB1 and HB2 as compared with 20-mg/L Zn. The root Zn concentrations of the two populations were also significantly decreased under Cd + Zn treatments in comparison with Zn single stress.
The Cd and Zn transfer factors in different parts of H. spectabile are shown in Fig. 4. The transfer factor from stem to leaf (TF1) for Cd in HB2 was significantly higher than that in HB1 under Cd stress conditions, but a sharp decrease was observed in HB2 with the addition of Zn (Fig. 4A). In contrast to HB2, the TF1 for Cd in HB1 significantly increased under Cd5 + Zn10 treatment. The transfer factor from root to stem (TF2) for Cd in HB1 was significantly higher than that in HB2 under 2-mg/L Cd but was greatly decreased with increased Cd stress or the addition of Zn (Fig. 4B). For HB2, the TF2 for Cd significantly increased by adding 10-mg/L Zn under 2- or 5-mg/L Cd stress but significantly decreased with addition of 20-mg/L Zn. There was no significant effect of different Zn or Cd + Zn treatments on the TF1 for Zn in either population of H. spectabile (Fig. 4C). The TF2 for Zn in HB1 was significantly decreased with increased Zn stress but was not affected by adding Cd under Zn stress (Fig. 4D).
Effect of different Cd and Zn additions to the nutrient solutions on the transfer factor (TF) for Cd and Zn in two populations of H. spectabile. Bars represent standard deviation (SD) of four replicates. Different letters on the bars for the same population show significant differences between the treatments. TF1, transfer factor from stem to leaf; TF2, transfer factor from root to stem
Metal accumulation
Results for Cd and Zn content in both populations of H. spectabile under various Cd or Cd + Zn treatments are presented in Fig. 5. The Cd accumulations in shoot and root were much higher in both populations under Cd and Cd + Zn treatments than in the control, while no significant differences were observed for HB1 under different Cd or Cd + Zn stress. In addition, the Cd accumulation in shoot and root of HB2 were significantly increased by adding 10-mg/L Zn under 2- or 5-mg/L Cd stress. Moreover, the Cd accumulation in aboveground part of HB2 was significantly increased by 188% and 170% under Cd2 + Zn10 and Cd5 + Zn10 treatments, respectively, and was significantly higher than in HB1.
Effect of different Cd and Zn additions to the nutrient solutions on the Cd accumulations in shoot and root in two populations of H. spectabile. The Zn accumulations in shoot and root are also given. Bars represent standard deviation (SD) of four replicates. Different letters on the bars for the same population show significant differences between the treatments
Zn accumulation in different tissues of both populations was greatly increased with increased Zn in medium (Fig. 5). The leaf Zn accumulation in HB1 was significantly increased by 71.3% under Cd2 + Zn20 treatment. Addition of Cd exhibited a negative effect on Zn accumulation in the stem of HB2, which was significantly decreased under Cd5 + Zn20 treatment as compared with 20-mg/L Zn. The Zn accumulation in the root of HB2 was significantly higher than in HB1, and that of both populations was significantly reduced by the addition of Cd.
Discussion
The most obvious characteristic of plants suffering from Cd stress is growth inhibition (Jamali et al. 2014). In this study, both populations of H. spectabile were tolerant of high levels of Cd and Zn stress without significant decline in the biomass of aerial parts, except that applying Cd plus Zn significantly decreased the leaf biomass of HB1. Various previous studies reported that the effects of Cd and Zn on plant growth varied with the plant genotype and the dose of Cd or Zn exposure (Rizwan et al. 2016). Although a relatively low level of Zn alleviated Cd-induced oxidative stress, the addition of high concentrations of Zn may make the inhibitory effect of Cd on plant growth aggressive (Ammar et al. 2015; Sarwar et al. 2014). The root biomasses for both populations of H. spectabile were relatively low, and the root/shoot ratios ranged from 0.003 to 0.016. It was known that the higher root/shoot ratio contributed in higher tolerance to heavy metals and their translocation to the shoot (Lavres et al. 2019; Lin et al. 2015; Tang et al. 2016; Xu et al. 2018; Yu et al. 2017). The results might be explained that the tillers of H. spectabile used in this study were based on cutting propagation without initial roots and were just cultured for 1 month under hydroponic condition including preculture. In contrast with hydroponic results, the root biomass of H. spectabile in our previous pot experiment accounted for 27–40% of total biomass (Guo et al. 2017). Similarly, the root biomasses of S. alfredii were quite low when cultured with Cd and Zn stress of hydroponic condition for 14 days and counted far lower of the total biomass than that of pot experiment (Li et al. 2009, 2011). Nevertheless, some information may still be obtained from the changes of root growth and morphology under different Cd and Zn stress. Root growth in our study was more severely affected by metal stress than aerial parts. Addition of Zn aggravated the stress of Cd on root growth inhibition of HB1; however, that of HB2 was rarely affected by adding low level of Zn (10 mg/L). Therefore, the interaction of Cd and Zn on the growth of H. spectabile varies among different populations and HB1 might be more sensitive to Cd and Zn stress than HB2.
The root morphological characteristics might reveal more information about the impact of Cd and Zn interaction on H. spectabile than does simply root biomass (Benáková et al. 2017; Li et al. 2009). Results from the present study showed that the RL, RSA, RV, and RT of both populations of H. spectabile were severely suppressed under high levels of Cd or Zn, while no significant inhibition was found with low levels of Cd or Zn. Moreover, the combined effects of Cd plus Zn aggravated the toxicity and significantly decreased the root morphological characteristics of both populations of H. spectabile, except by adding 10-mg/L of Zn under Cd stress. This observation was consistent with previous results (Cheng et al. 2018). A high level of Cd might lead to inhibition of mitosis in cells of root tips, decrease in root hair production, and the formation of asymmetrical epidermal and cortical cells, as well as intercellular air spaces (Benáková et al. 2017; Lux et al. 2011). An appropriate level of Zn, as a physically, chemically, and geologically similar element to Cd, alleviated Cd toxicity through the increase of antioxidative enzyme activity and enabled maintenance of normal plant growth under Cd stress (Balen et al. 2011; Hassan et al. 2005; Taspinar et al. 2011). However, a high level of Zn might interrupt the signaling pathways critical for induced accumulation of metallothioneins (MTs), which contributed to Cd accumulation and tolerance in roots (Sekhar et al. 2011). Despite these results, the interaction of Cd and Zn varied with different populations of H. spectabile, which was in accordance with a previous review (Rizwan et al. 2016). Furthermore, although the root biomass of both populations was significantly decreased with addition of Cd and/or Zn, the root morphological parameters of HB2 were less severely affected than those of HB1, which implied that HB2 might possess a more effective coping mechanism than HB1 in response to Cd and/or Zn stress.
The length and surface area of root are the most important parameters which determined the extent of root extension and the nutrients absorption range of root, respectively. Therefore, the interaction effect of Cd + Zn on root morphology was most pronounced in the distribution of RL and RSA with different root diameters (Figs. 1 and 2). In the present study, the total RL and RSA were mainly accounted for by roots with diameters 0.1–0.4 mm in both populations of H. spectabile. Moreover, the RL and RSA varied with population, the dose, and ratio of Zn and Cd exposure. The RL and RSA for root diameters 0.1–0.4 mm in HB1 showed a decreasing trend with increase in Cd and Zn stress, while the decreasing trend is relatively less on those of HB2 especially at low level of Cd and Zn. In addition, the interaction of Cd and Zn showed little additional toxicity effect on the RL and RSA of HB2 by adding low level of Zn as compared with single Cd stress, while addition of Zn significantly decreased root growth under Cd stress in HB1. These results indicate that the root system of HB2 might be more specialized in maintaining a large and widely branched root system that is able to cope with the toxic effects of Cd and Zn. This difference between the two H. spectabile populations in response to Cd and Zn might be ascribed to differences in plant hormones and exudates, consequently affecting root morphology and metal uptake (Li et al. 2009). Furthermore, the Cd/Zn showed lesser inhibitory effect on RL and RSA of HB2 than HB1, which allowed HB2 to preserve more root biomass available for Cd uptake, implying great potential for using HB2 in practical applications in phytoremediation of soils polluted by heavy metals.
The combined effects of Cd and Zn on the uptake and transport of the metals by plants are difficult to predict, despite being an active research topic (Cheng et al. 2018). In the present study, we found a synergistic effect of Cd plus Zn on Cd absorption by the roots in both populations of H. spectabile. As Zn is an essential component of antioxidant enzymes, adding Zn at low concentrations might decrease Cd-induced oxidative stress by increasing antioxidative enzyme activity in the roots (Balen et al. 2011; Hassan et al. 2005; Taspinar et al. 2011). Nevertheless, excessive addition of Zn aggravated the Cd-induced oxidative stress and enhanced root membrane permeability, thereby increasing the Cd absorption of the roots in H. spectabile (Cherif et al. 2011). In contrast to the results of moderate level of Zn increased in some cases of Cd concentrations of H. spectabile, Cd showed little effect on Zn concentrations and even inhibited Zn absorption of HB2 (Table S1 and Table S2). Moreover, adding of 10-mg/L Zn significantly increased the transfer factor of Cd from stem to leaf of HB1, while significantly decreased that of HB2. However, the opposite was observed for the transfer factor of Cd from root to stem. That means that in HB1, addition of Zn inhibited Cd transport from root to stem, while it facilitated that from stem to leaf, leading to increased Cd toxicity in leaves and inhibition of leaf growth. The results might be attributed, at least partly, to a relatively high level of Zn in the stem accelerating the transport of Cd to the leaf. Cd and Zn in protoplasts was proved to be compartmentalized in vacuoles, as a result of the activity of Cd and Zn transporters at the tonoplast, such as CAX, HMA, and CDF (Verbruggen et al. 2010). However, the vacuoles in the stem of HB1 might be saturated with sequestered Zn, leading to the expression of MTP1 being switched off, and so the excess Cd would be more likely to be transferred to the leaf (Hendrik and Kochian 2010). For HB2, in contrast, a low concentration of Zn promoted Cd transport from root to stem, as well as inhibited transfer into the leaf. It was reported that the translocation of Cd relies on the formation of complexes with phytochelatins (Cd-PC) and their sequestration in the vacuole of root cells, thereby limiting Cd translocation (Hassan et al. 2005). However, moderate addition of Zn might compete with Cd for PC to form Zn-PC complexes and thus increase the amount of free Cd, ultimately facilitating Cd translocation from root to stem (Sarwar et al. 2015). Furthermore, the substantial difference in Cd translocation between the two H. spectabile populations under the combined stress of Cd plus Zn might be attributed to a novel and specialized transporter in the plasma membrane system that is induced by addition of Zn (Li et al. 2009). Cd combined with Zn exhibited an antagonistic effect on Zn uptake in both populations of H. spectabile, and no difference was observed between the two populations. This result was in agreement with a previous report (Benáková et al. 2017). A possible reason for this effect might be that Zn and Cd share a common transporter at the root plasma membrane, which might have a higher affinity for Cd than Zn, consequently restricting Zn translocation into the root in the presence of Cd.
The effects of Cd and Zn, when in combination, were reported to show very different impacts on plant growth, root morphology characteristics, oxidative stress, and metal transport and accumulation (Cheng et al. 2018; Cherif et al. 2011; Cojocaru et al. 2016). The influence of Zn on Cd accumulation of S. alfredii varied in different ecotypes: Zn significantly increased Cd accumulation in a Cd/Zn hyperaccumulating ecotype but decreased that of a non-hyperaccumulating ecotype (Li et al. 2009). The results of the present study also showed obvious differences between the two populations of H. spectabile in response to the combined effect of Cd and Zn stress. A moderate level of Zn significantly increased Cd accumulation in the root and shoot of HB2, while it had little effect in HB1. In contrast, a high concentration of Zn showed no significant influence on Cd accumulation in either H. spectabile population. Therefore, not only the varietal differences but also the individual concentrations of Cd and Zn should be taken into account when attempting to clarify the interactions of Cd and Zn (Benáková et al. 2017). Furthermore, addition of Cd decreased Zn accumulation in the root in both populations of H. spectabile, whereas Zn accumulation in the root of HB2 was significantly higher than that of HB1 under the different Cd or Zn treatments. A higher Zn content might contribute to alleviating the oxidative stress induced by Cd and enhance Cd tolerance (Aravind et al. 2009), consequently enabling the higher Cd accumulation of HB2.
Conclusions
Our results indicated that the interactions of Cd and Zn in H. spectabile depended on the different individual concentrations of Cd and Zn as well as different populations, although both two tested populations showed relative tolerance to high levels of Cd and Zn. The HB2 population appears to be more specialized than HB1 in maintaining normal root growth and root morphological characteristics, enabling it to cope with the toxic effects of Cd and Zn. The interactions of Cd and Zn not only affected the absorption of Cd but also significantly affected the accumulation of Cd in different tissues of H. spectabile. A low level of Zn led to more Cd accumulating in the stem of HB2, while it distributed more Cd in leaf and root of HB1. We found strong positive interactions between Zn and Cd on Cd accumulation by adding low level of Zn in HB2, while it had little effect in HB1. In conclusion, HB2 showed great potential for application in phytoremediation of soil contaminated with both Cd and Zn, warranting further investigation under field condition.
References
Ammar WB, Zarrouk M, Nouairi I (2015) Zinc alleviates cadmium effects on growth, membrane lipid biosynthesis and peroxidation in Solanum lycopersicum leaves. Biologia 70(2):198–207
Aravind P, Prasad MNV (2003) Zinc alleviates cadmium-induced oxidative stress in Ceratophyllum demersum L.: a free floating freshwater macrophyte. Plant Physiol Biochem 41:391–397
Aravind P, Prasad MNV (2004) Zinc protects chloroplasts and associated photochemical functions in cadmium exposed Ceratophyllum demersum L., a freshwater macrophyte. Plant Sci 166:1321–1327
Aravind P, Prasad MNV, Malec P, Waloszek A, Strzałka K (2009) Zinc protects Ceratophyllum demersum L. (free-floating hydrophyte) against reactive oxygen species induced by cadmium. J Trace Elem Med Biol 23:50–60
Balen B, Tkalec M, Šikić S, Tolić S, Cvjetko P, Pavlica M, Vidaković-Cifrek Ž (2011) Biochemical responses of Lemna minor experimentally exposed to cadmium and zinc. Ecotoxicology. 20:815–826
Benáková M, Ahmadi H, Dučaiová Z, Tylová E, Clemens S, Tůma J (2017) Effects of Cd and Zn on physiological and anatomical properties of hydroponically grown Brassica napus plants. Environ Sci Pollut Res 24:20705–20716
Cheng M, Kopittke PM, Wang A, Sale PW, Tang C (2018) Cadmium reduces zinc uptake but enhances its translocation in the cadmium-accumulator, Carpobrotus rossii, without affecting speciation. Plant Soil 430:219–231
Cherif J, Mediouni C, Ammar WB, Jemal F (2011) Interactions of zinc and cadmium toxicity in their effects on growth and in antioxidative systems in tomato plants (Solarium lycopersicum). J Environ Sci 23:837–844
Choppala G, Saifullah, Bolan N, Bibi S, Iqbal M, Rengel Z, Kunhikrishnan A, Ashwath N, Ok YS (2014) Cellular mechanisms in higher plants governing tolerance to cadmium toxicity. Crit Rev Plant Sci 33:374–391
Cojocaru P, Gusiatin ZM, Cretescu I (2016) Phytoextraction of Cd and Zn as single or mixed pollutants from soil by rape (Brassica napus). Environ Sci Pollut Res 23:10693–10701
Emamverdian A, Ding Y, Mokhberdoran F, Xie Y (2015) Heavy metal stress and some mechanisms of plant defense response. Sci World J 2015:1–18
Green CE, Chaney RL, Bouwkamp J (2017) Increased zinc supply does not inhibit cadmium accumulation by rice (Oryza sativa L.). J Plant Nutr 40:869–877
Guo JM, Lei M, Yang JX, Yang J, Wan XM, Chen TB, Zhou XY, Gu SP, Guo GH (2017) Effect of fertilizers on the Cd uptake of two sedum species (Sedum spectabile Boreau and Sedum aizoon L.) as potential Cd accumulators. Ecol Eng 106(Part):409–414
Guo JM, Yang J, Yang JX, Chen TB, Guo L (2018) Subcellular cadmium distribution and antioxidant enzymatic activities in the leaves of four Hylotelephium spectabile populations exhibit differences in phytoextraction potential. Int J Phytoremediat 21(3):209–216
Hajiboland R, Amirazad F (2010) Growth, photosynthesis and antioxidant defense system in Zn-deficient red cabbage plants. Plant Soil Environ 56:209–217
Hänsch R, Mendel RR (2009) Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr Opin Plant Biol 12:259–266
Hassan MJ, Zhang G, Wu F, Wei K, Chen Z (2005) Zinc alleviates growth inhibition and oxidative stress caused by cadmium in rice. J Plant Nutr Soil Sci 168(2):255–261
Hendrik K, Kochian LV (2010) Transcriptional regulation of metal transport genes and mineral nutrition during acclimatization to cadmium and zinc in the Cd/Zn hyperaccumulator, Thlaspi caerulescens (Ganges population). New Phytol 185:114–129
Jamali N, Ghaderian SM, Karimi N (2014) Effects of cadmium and zinc on growth and metal accumulation of Mathiola flavida boiss. Environ Eng Manag J 12:2037–2944
Lavres J, Rabêlo FHS, Capaldi FR, dos Reis AR, Rosssi ML, Franco MR, Azevedo RA, Abreu-Junior CH, de Lima Nogueira N (2019) Investigation into the relationship among Cd bioaccumulation, nutrient composition, ultrastructural changes and antioxidative metabolism in lettuce genotypes under Cd stress. Ecotoxicol Environ Saf 170:578–589
Li T, Yang X, Lu L, Islam E, He Z (2009) Effects of zinc and cadmium interactions on root morphology and metal translocation in a hyperaccumulating species under hydroponic conditions. J Hazard Mater 169:734–741
Li T, Di Z, Yang X, Sparks DL (2011) Effects of dissolved organic matter from the rhizosphere of the hyperaccumulator Sedum alfredii on sorption of zinc and cadmium by different soils. J Hazard Mater 192:1616–1622
Lin L, Ning B, Liao M, Ren Y, Wang Z, Liu Y, Cheng J, Luo L (2015) Youngia erythrocarpa, a newly discovered cadmium hyperaccumulator plant. Environ Monit Assess 187:4205
Lux A, Martinka M, Vaculík M, White PJ, Lux A (2011) Root responses to cadmium in the rhizosphere: a review. J Exp Bot 62:21–37
Qiu RL, Thangavel P, Hu PJ, Senthilkumar P, Ying RR, Tang YT (2011) Interaction of cadmium and zinc on accumulation and sub-cellular distribution in leaves of hyperaccumulator Potentilla griffithii. J Hazard Mater 186:1425–1430
Rizwan M, Ali S, Abbas T, Zia-ur-Rehman M, Hannan F, Keller C, Al-Wabel M, Ok YS (2016) Cadmium minimization in wheat: a critical review. Ecotoxicol Environ Saf 130:43–53
Sarwar N, Bibi S, Ahmad M, Ok Y (2014) Effectiveness of zinc application to minimize cadmium toxicity and accumulation in wheat (Triticum aestivum L.). Environ Earth Sci 71:1663–1672
Sarwar N, Ishaq W, Farid G, Shaheen MR, Imran M, Geng M, Hussain S (2015) Zinc-cadmium interactions: impact on wheat physiology and mineral acquisition. Ecotoxicol Environ Saf 122:528–536
Sekhar K, Priyanka B, Reddy VD, Rao KV (2011) Metallothionein 1 (CcMT1) of pigeonpea (Cajanus cajan, L.) confers enhanced tolerance to copper and cadmium in Escherichia coli and Arabidopsis thaliana. Environ Exp Bot 72:131–139
Tang X, Pang Y, Ji P, Gao P, Nguyen TH, Tong Y (2016) Cadmium uptake in above-ground parts of lettuce (Lactuca sativa L.). Ecotoxicol Environ Saf 125:102–106
Taspinar MS, Agar G, Alpsoy L, Yildirim N, Bozari S, Sevsay SM, Sinan T (2011) The protective role of zinc and calcium in Vicia faba seedlings subjected to cadmium stress. Toxicol Ind Health 27:73–80
Tran TA, Popova LP (2013) Functions and toxicity of cadmium in plants: recent advances and future prospects. Doga Turk J Bot 37:1–13
Verbruggen N, Hermans C, Schat H (2010) Molecular mechanisms of metal hyperaccumulation in plants. New Phytol 181:759–776
Wang Y, Wang X, Wang C, Peng F, Wang R, Xiao X, Zeng J, Kang H, Fan X, Sha L, Zhang H, Zhang Y (2017) Transcriptomic profiles reveal the interactions of Cd/Zn in dwarf polish wheat (Triticum polonicum L.) roots. Front Physiol 8:1–13
Wei S, Zhou QX (2006) Phytoremediation of cadmium-contaminated soils by Rorippa globosa using two-phase planting (5 pp). Environ Sci Pollut Res 13:151–155
Xu Z, Mei X, Tan L, Li Q, Wang L, He B, Guo S, Zhou C, Ye H (2018) Low root/shoot (R/S) biomass ratio can be an indicator of low cadmium accumulation in the shoot of Chinese flowering cabbage (Brassica campestris L. ssp. chinensis var. utilis Tsen et Lee) cultivars. Environ Sci Pollut Res 25:36328–36340
Yang J, Guo JM, Yang JX (2018) Cadmium accumulation and subcellular distribution in populations of Hylotelephium spectabile (Boreau) H. Ohba. Environ Sci Pollut Res 25:30917–30927
Yu R, Xia S, Liu C, Zhang Z, Shi G (2017) Variations in root morphology among 18 herbaceous species and their relationship with cadmium accumulation. Environ Sci Pollut Res 24:4731–4740
Acknowledgments
We thank Huw Tyson, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
This research was financially supported by the National Key R&D Program of China (2018YFC1802604), the National Natural Science Foundation of China (NSFC Nos. 41771509, 41771510, 41907125), the China Postdoctoral Science Foundation (2019M650827), and Major Science and Technology Projects of Zhejiang Province (2020C02024).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 5509 kb)
Rights and permissions
About this article
Cite this article
Guo, J., Guo, Y., Yang, J. et al. Effects and interactions of cadmium and zinc on root morphology and metal translocation in two populations of Hylotelephium spectabile (Boreau) H. Ohba, a potential Cd-accumulating species. Environ Sci Pollut Res 27, 21364–21375 (2020). https://doi.org/10.1007/s11356-020-08660-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08660-0