Abstract
Among ex situ remediation technologies, stabilization/solidification (S/S) provides for the addition of a binder to dredged materials in order to chemically immobilise the contaminants and improve mechanical behaviour of sediments. The simplest form of treatment is obtained by the addition of Portland cement or lime (calcium oxide), although other additives such as adsorbents may be added. Nevertheless, the success of the S/S treatment may be affected by the contaminants present or by the salt content in the water. In this study, experimental laboratory investigation was carried out on sediments carefully collected from the Mar Piccolo of Taranto in Southern Italy, contaminated by heavy metals, polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs); the goal was to explore the effectiveness of S/S treatment by using Portland cement/lime as binders, monitoring over time (28 days) the leaching of the different mixtures of treated sediment. It is noted that the Mar Piccolo of Taranto is one of the sites of national interest subject to remediation by the Italian government. Once taken within the first meter under the sea floor by a team of experienced divers, the samples were stored at a controlled temperature, characterised in terms of grain size and physical-chemical characteristics and treated by S/S laboratory tests. The results indicate that the addition of binders increased the pH of the mixtures with a consequent leachability of different metals. The mobility of the metals appeared to be governed also by the curing time. The performance of the mixtures in terms of immobilised metals was influenced by the presence of organic contaminants (e.g. organic matter, PAHs and PCBs). As a lesson, high organic matter and fine-grained particles can negatively affect the effectiveness of the S/S treatment in terms of metal immobilisation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sediments are dredged, from harbours and waterways, for maintenance of navigation, environmental remediation or both. Every year, about 200 million of m3 of sediments are dredged only in Europe, over half contaminated and expensive to manage (EMODnet 2017).
In most cases, dredged materials are classified as waste and disposed with high environmental risks. Fortunately, this tendency has been changing in the recent years, and there is a growing impetus for considering dredged sediments as a resource rather than a waste (Apitz 2010; Todaro et al. 2016). Conventions for the protection of the marine environment and some new European regulations concerning waste have been introduced to set guidelines for a proper management of dredged sediments and to prioritize the reuse, recycling and recovering of sediments.
Sediments management strategies may involve several technologies to dredging or excavation, transport, pre-treat, treat, and/or dispose sediments and treatment residues (Lofrano et al. 2016). The pollutants are extracted from the sediments degraded through a series of chemical, physical, biological or thermal methods in a specially designed reactor. Most ex situ remediation technologies developed for soil or waste can be used for dredged sediments. Among the ex situ technologies stabilization/solidification (S/S) can be, in some cases, the most economically and ecologically sustainable treatment (Barjoveanu et al. 2018; Drouiche et al. 2019). It is based on adding binders to dredged materials to immobilising the contaminants and improves mechanical behaviour of sediments (compressibility reduction and strength improvement). The treated sediments can be recycled as aggregates for road construction (Wang et al. 2012), cemented mortars (Couvidat et al. 2016), fill material and blocks (Wang et al. 2015), raw materials in brick production (Cappuyns et al. 2015; Messina et al. 2017) or, at least, can be disposed of in sanitary landfills for not hazardous waste.
The S/S techniques can be different and vary depending on the target to be reached for the sediment reuse. They are based on adding chemical compounds to dredged material in order to (i) chemically immobilise the contaminants, reducing the leachability and bioavailability, and (ii) to geomechanically stabilize the material for its reuse as new construction material. S/S treatments generally do not remove the contaminants from the dredged material since the pollutants are transformed into a less mobile and harmful species. The simplest form of treatment is obtained by the addition of either Portland cement or lime. Other additives can be used (i.e. low-cost adsorbents, bentonite, zeolites) depending on the type of contaminants, physical properties and composition of the dredged material and the desired performance to be obtained for the optimisation of its reuse (Todaro et al. 2019a). Nevertheless, the contaminants (e.g. organic matter, soluble salts and heavy metals) can interfere with the chemistry of binders, compromising the effectiveness of both the chemical and mechanical stabilization (Wang et al. 2013). Previous studies investigated pre-treatment methods aimed at removal of chlorides (and other soluble salts) carried out by means of a proper waste washing (Colangelo et al. 2012; Ferraro et al. 2019).
This study shows the results of an experimental investigation, that are still on-going, aimed to explore new alternatives for the sustainable management of real contaminated marine sediments of the first basin of the Mar Piccolo of Taranto, in Southern Italy. In detail, experimental laboratory tests have been carried out on sediments contaminated by heavy metals, polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs), to explore the sustainability of ex situ stabilization/solidification with traditional binders (i.e. Portland cement and lime). The effectiveness of the S/S processes has been verified by studying the leaching of metals from the sediment to the aqueous solution.
To investigate the interaction of the sediments’ characteristics (i.e. grain size distribution, organic matter and water content) with the binders, three sediment samples were treated by using different contents of Portland cement and calcium oxide.
Materials and methods
Contaminated marine sediments
The sediments used in this research were taken from the Mar Piccolo (Fig. 1a), one of most polluted areas in Europe, declared as “at high risk of environmental crisis” by the Italian government (Labianca et al. 2018; Vitone et al. 2016; Todaro et al. 2019b). The three sediment samples (named S6P, S7P and S14P) were taken in proximity of First Bay (Fig. 1b).
The samples of sediment were taken, within the first meter below seafloor, by an expert team of scuba divers by means of direct push sampling methodology. For this purpose, a new sampling device was specially designed. It consists of 1-m thin tube in polyvinyl chloride (PVC, 0.08-m external diameter) with a screw cap with internal seal for the top while and a pressure cap with drain holes at the bottom. The sampler was equipped with a cutting edge, a screw cap with internal seal for the top and a pressure cap with drain holes for the bottom, the latter to be installed by scuba divers soon after the extraction. The sampler components are reported in Fig. 2.
The samples were characterised by the composition and physical-chemical properties reported in Table 1. They are essentially fine-grained soils, for which clay fraction, CF, varies between 40.25 (S6P sample) and 51.66% (S9P sample); sand fraction, SF, between 4.12 (S14P sample) and 14.26% (S16P sample); and silt fraction, MF, ranges from 41.26% of the S7P sample to 45.49% of the S6P sample. The sediments are silty clays (S-C) and clayey silts (C-S), and their clay fraction is mainly represented by illite-smectite clay minerals (Sollecito et al. 2019). The chemical characterisation (Table 2) showed that they were polluted by heavy metals (e.g. As, Hg, Pb, Cu and Zn), PAHs and PCBs.
The contents of contaminants in the tables are compared with the corresponding limits set by both site-specific law (ICRAM 2004; in italics in Table 2) and Italian National Law (Legislative Decree 152 2006; in bold in Table 2).
Binders
Portland cement CEM I 42.5 (Italcementi S.p.A., Italy) was used in this study. It is a fine grey powder produced by grinding Portland cement clinker, a limited amount of calcium sulphate and up to 5% minor constituents. The main chemical constituents of clinker are CaO, SiO2, Al2O3 and Fe3O3.
In this study, the type of lime used is calcium oxide (CaO), commonly known as quicklime (the product was obtained from Unicalce S.p.A.). The stabilizer is commercially produced finely ground with 99% passing 75-μm sieve and 97% passing 45-μm sieve. Hydrated lime, the slaked type of oxide, is equally effective but economically unattractive because much lighter and more voluminous than its reactive type.
The experimental programme consisted into the preparation and chemical analysis of 3 mixtures for each sediment sample; the mixtures have been obtained by setting binders’ content of 15% to the dry sediments. The water to dry material ratio of the mixtures was imposed equal to 1 (i.e. same as the water to binder ratio determined for optimum workability). Table 3 shows the mixture design for S/S treatments.
Laboratory tests for S/S treatments
Several specimens were prepared by thorough mechanical mixing of the sediments with the additives. All the materials were initially mixed for 5 min with a standard mixer, and then, a steel trowel was used to ensure a homogeneous paste. In the casting phase, the prepared mixture was introduced into a hemisphere moulds.
The samples, in the curing phase, were kept at 20 ± 5 °C and 80% humidity and then put on tests at different setting times, for monitoring the progressive changes (generally, 1 h, 7, 14 and 28 days).
Leaching test, according to the EN standard 12,457-2, was performed. This procedure can only be applied to granular wastes and sludges which have a particle size below 4 mm and cannot be used for evaluating the leaching grade of the non-polar organic compounds. For several samples, a 40 g portion was sampled and transferred to a polyethylene bottle. Demineralized water was added with a solid-liquid ratio of 1:10 by weight, and the bottles were keep in rotation at 12 rpm for 24 h using Rotax 6.8 (Velp Scientifica). After 24 h, a short retention time was given to the extraction vessels for the settlement of suspended coarse solids; then, the leachate was filtered for the removal of suspended solids. This test procedure produced an eluate which has been both physically and chemically characterised according to appropriate standard methods. In particular, the eluates were consequently divided into an appropriate number of parts for the different chemical analysis, after having been acidified at a pH equal to 2, through nitric acid, as prescribed by the regulatory for the determination of the metals in outline.
The soluble concentrations of heavy metals of interest (i.e. As, Co, Cr, Cu, Ni, Pb, V and Zn) were analysed by using ICP-OES (Inductively Coupled Plasma-Optical Emission Spectrometry, iCAP 7000 Series-Thermo Scientific) in accordance with EPA method 200.8 (EPA 1994). Liquid samples, after acidification (pH = 2) and filtering (0.45-μm sterile acetate nitrate filter), were directly analysed by ICP-OES, and the analysis directly produces the values of the concentrations in milligramme per litre.
Results and discussion
A first consideration emerging from the analysis of the results is the totally absence of organic contaminants in the liquid samples: PAHs and PCBs concentrations in all the leached samples were not detectable (Limit Of Detection, LOD, is approximately 0.001 mg/l). However, these contaminants may have a detrimental effect on the properties of treated materials slowing down the hydration process of the binders (Hasegawa et al. 2016; Kogbara 2014).
Focusing on the heavy metals, the most important processes are sorption and precipitation, both strongly depending on the pH conditions. Figure 3 shows the effect of the binders on the pH of the specimens, after different curing time.
When binders are added, there is a substantial pH increase with respect to that of the natural sediments. After 24 h (execution of the first leaching test), the pH of the mixtures is equal to approximately 13: the pH values varying between 12.2 (MIX A, sediment sample S14P) and 13.1 (MIX C, sediment samples S6P). Longer curing time tends to reduce the pH of the eluates; however, the values are still higher than those of the untreated sediment: the pH values varying between 10.3 (MIX A, sample S14) and 12.6 (MIX C, sample S7P). It can be observed that the addition of cement (MIX A) involves the lower increase in pH with the respect to lime. The solubility of silica and alumina greatly increase at elevated pH levels (12–13), which can lead to an increase in pozzolanic reactions. Under the attack of OH− in such a high pH solution, a mineralogical breakdown takes place in the matrix. Silicate or aluminosilicate network formers (from the sediment) are also depolymerized and dissolve into solution, producing two primary cementing agents, C-S-H and C-A-H, which have important roles in improving the mechanical properties of the specimens (Silitonga et al. 2010). However, high pH values may increase the leaching of some metals (Desogus et al. 2012; Stegemann and Zhou 2009; Cao et al. 2008).
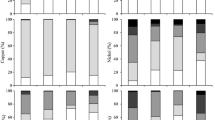
In Fig. 4, the concentrations detected into the leached fluid of the chosen heavy metals respect to the different binder/sediment relationship are reported for the three investigated sediment samples. The concentrations of Hg are not reported because they always resulted to be lower than the instrumental detection limit (about 0.001 mg/l).
With respect to As (Fig. 4a), the pattern of release varies with the sediment sample: for S6P, as shows its lowest solubility at low pH values (except for the MIX A); for S7P and S14P its solubility remains almost constant with curing time.
The increasing release of Co with the decrease of the pH (i.e. with the increase of the curing time) can be observed in Fig. 4b.
A similar behaviour is found for Cu, Ni, V and Zn concentrations. However, the maximum concentrations in the leached fluid of Co, V and Cu are found after 28 days; for Ni and Zn, the maximum of the release curves is 14 days. This shows a positive effect of the curing time that blocks, at least, reduces the mobility of metals. The concentrations of Cr and Pb are reduced with curing time (i.e. with the reduction of pH values).
It could be noted that, in general, the addition of binders is found to highly increase the leaching characteristics of the mixture, which decrease with curing time only for some metals. For almost all the metals, except for the As and Zn, the leached concentrations increase after the treatment. In particular, an enhanced of chemical characteristic has been obtained for As (exceptions: MIX A sample S6P and MIX C sample S14P) and Zn (exceptions: mixtures of sample S6P).
It is possible to calculate the rate of leaching of metals as a ratio of the content of an element in eluate after 28 days (C28 in milligramme per litre) to its total content in the untreated sediments (CSED in milligramme per kilogramme) (Fig. 5). This coefficient provides information about both the amount and rate of leaching of a given element from the treated sediment.
The data indicate that the highest amounts of As, Co, Ni and V are released from sample S14P, whereas the highest amounts of Cr and Pb are released from sample S7P and the highest amounts of Cu and Zn are released from sample S6P.
According to the literature (Kogbara et al. 2012; Todaro et al. 2018; Pan et al. 2019), the main factors affecting mobility of metals from bottom sediments include pH and organic matter content. Moreover, the rate of solution permeating through sediments is also connected with their grain size distribution. Permeability of solutions seeping through sediments is connected with the finest fraction, which seals the spaces between coarser fractions, and favours chemical reduction and therefore element leaching. It follows that the relatively high mobility of metals from S14P (sample with the highest value of organic matter content and the minimum values of sand fraction), despite the low total content of metals, can depend on its composition.
For the beneficial reuse of contaminated marine sediments, the leaching of each metal has to be lower than law limits. In Italy, the chemical parameters must be under the threshold levels defined by the Italian Ministerial Decree 5/2/1998 (Ministerial Decree 1998). Table 4 shows the compliance of metal concentrations with law limits after 28 days of curing time.
In general, the addition of binders to the contaminated marine sediments shows a negative effect on decreasing the mobility of heavy metals. However, only Ni, Cu and Pb (in MIX B and MIX C for sample S7P) are released with concentrations higher than the law limits.
A key to interpret the results could be the following: (1) for sediments with minimal organic contamination (S14P), the mix with 15% (MIX A) of cement exhibited a better performance while maintaining the lowest concentration values of metals in the eluates, for 62.5% of the metals analysed; (2) for sediments with organic and inorganic contamination, it was observed that in 50% of cases MIX C (15% lime) is the most suitable.
Finally, it has been possible to observe how the immobilisation efficiency of the mixtures based on only lime or cement is always the highest when compared to MIX B (7.5% cement and 7.5% lime). Untreated sediments showed a low tendency to release the contaminants in solution. However, following the addition of the binders, due to the consequent increase in pH values, a significant increase of release is observed.
Conclusions
With reference to the case study of the Mar Piccolo first basin sediments, the results indicate that, for untreated sediments, the release of contaminants after contact with distilled water was very limited. Untreated sediments showed a low tendency to release the contaminants in solution. This was due to the low metals’ solubility and to the stability of their solid phases under slight basic conditions (8.8 < pH ˂ 9.2). Following the addition of the binders (Portland cement/calcium oxide), due to the consequent increase towards high pH values (> 10), a significant increase of release was observed. In particular, mobility of the metals appeared to be mainly governed by pH and curing time. It has to be underlined that the performance of the mixtures was also conditioned by the presence of organic contaminants (i.e. organic matter, PAHs and PCBs) that interfered with the hydration of binders compromising the effectiveness of metal immobilisation and development of hardening. Furthermore, even grain size distribution may have interfered with the chemistry of the binder. As a lesson, high organic matter and fine-grained particles could negatively affect the effectiveness of the S/S treatment in terms of metal immobilisation.
References
Apitz SE (2010) Waste or resource? Classifying and scoring dredged material management strategies in terms of the waste hierarchy. J Soils Sediments 10:1657–1668
Barjoveanu G, De Gisi S, Casale R, Todaro F, Notarnicola M, Teodosiu C (2018) A life cycle assessment study on the stabilization/solidification treatment processes for contaminated marine sediments. J Clean Prod 201:391–402
Cao X, Dermatas D, Xu X, Shen G (2008) Immobilization of lead in shooting range soils by means of cement, quicklime, and phosphate amendments. Environ Sci Pollut Res 15:120–127
Cappuyns V, Deweirt V, Rousseau S (2015) Dredged sediments as a resource for brick production: possibilities and barriers from a consumers’ perspective. Waste Manag 8:372–380
Colangelo F, Cioffi R, Montagnaro F, Santoro L (2012) Soluble salt removal from MSWI fly ash and its stabilization for safer disposal and recovery as road basement material. Waste Manag 32:1179–1185
Couvidat J, Benzaazoua M, Chatain V, Bouamrane A, Bouzahzah H (2016) Feasibility of the reuse of total and processed contaminated marine sediments as fine aggregates in cemented mortars. Constr Build Mater 112:892–902
Desogus P, Manca P, Orrù G, Zucca A (2012) Stabilization–solidification treatment of mine tailings using Portland cement, potassium dihydrogen phosphate and ferric chloride hexahydrate. Miner Eng 45:47–54
Drouiche MC, Moussaceb K, Joussein E, Bollinger JC (2019) Stabilization/solidification by hydraulic binders of metal elements from landfill leachate. Nova Biotechnol Chim 18:72–83
EMODnet (2017). The European Marine Observation and Data Network. http://www.emodnet.eu/ (accessed 26 September 2019)
EPA (1994). Method 200.7: Determination of metals and trace elements in water and wastes by inductively coupled plasma - atomic emission spectrometry. Environmental monitoring systems laboratory office of research and development U. S. Environmental Protection Agency, Cincinnati, OH
Ferraro A, Farina I, Race M, Colangelo F, Cioffi R, Fabbricino M (2019) Pre-treatments of MSWI fly-ashes: a comprehensive review to determine optimal conditions for their reuse and/or environmentally sustainable disposal. Rev Environ Sci Biotechnol 1-19
Hasegawa H, Rahman I, Rahman M (2016) Environmental remediation technologies for metal-contaminated soils. Springer, Tokyo
ICRAM (2004) Values of intervention for sediments of areas strongly anthropized with reference to the site of reclamation of national interest in Taranto (in Italian). Central Institute for Scientific and Technological Research applied to the Sea, Rome
Kogbara RB (2014) A review of the mechanical and leaching performance of stabilized/solidified contaminated soils. Environ Res 22:66–86
Kogbara RB, Al-Tabbaa A, Yi Y, Stegemann JA (2012) pH-dependent leaching behaviour and other performance properties of cement-treated mixed contaminated soil. J Environ Sci 24:1630–1638
Labianca C, De Gisi S, Notarnicola M (2018) Assessing the correlation between contamination sources and environmental quality of marine sediments using multivariate analysis. Environ Eng Manag J 17(10):2391–2399
Legislative Decree 152 (2006). Environmental regulations, (in Italian). Italian Official Journal, No. 88, 04/04/2006
Lofrano G, Libralato G, Minetto D, De Gisi S, Todaro F, Conte B, Calabrò D, Quatraro L, Notarnicola M (2016) In situ remediation of contaminated marine sediment: an overview. Environ Sci Pollut R 24(6):5189–5206
Messina F, Ferone C, Molino A, Roviello G, Colangelo F, Molino B, Cioffi R (2017) Synergistic recycling of calcined clayey sediments and water potabilization sludge as geopolymer precursors: upscaling from binders to precast paving cement-free bricks. Constr Build Mater 133:14–26
Ministerial Decree (1998). Identification of not-hazardous waste subjected to simplified recovery procedures (in Italian). Italian Official Journal No. 88, 16/04/1998
Pan Y, Rossabi J, Pan C, Xie X (2019) Stabilization/solidification characteristics of organic clay contaminated by lead when using cement. J Hazard Mater 362:132–139
Sollecito F, Vitone C, Miccoli D, Plötze M, Puzrin AM, Cotecchia F (2019) Marine sediments from a contaminated site: geotechnical properties and chemo-mechanical coupling processes. Geosci. 9(8):333
Silitonga E, Levacher D, Mezazigh S (2010) Utilization of fly ash for stabilization of marine dredged sediments. Eur J Environ Civ Eng 14:253–265
Stegemann JA, Zhou Q (2009) Screening tests for assessing treatability of inorganic industrial wastes by stabilisation/solidification with cement. J Hazard Mater 161:300–306
Todaro F, De Gisi S, Notarnicola M (2016) Contaminated marine sediments: waste or resource? An overview of treatment technologies. Procedia Environ Sci Eng Manag 3(3):157–164
Todaro F, De Gisi S, Notarnicola M (2018) Sustainable remediation technologies for contaminated marine sediments: preliminary results of an experimental investigation. Environ Eng Manag J 17(10):2465–2471
Todaro F, Vitone C, Notarnicola M (2019a) Stabilization and recycling of contaminated marine sediments. E3S Web Conf 92 11004
Todaro F, De Gisi S, Labianca C, Notarnicola M (2019b) Combined assessment of chemical and ecotoxicological data for the management of contaminated marine sediments. Environ Eng Manag J 18(10):2287–2296
Vitone C, Federico A, Puzrin AM, Ploetze M, Carrassi E, Todaro F (2016) On the geotechnical characterization of the polluted submarine sediments from Taranto. Environ Sci Pollut Res 23:535–553
Wang D, Abriak NE, Zentar R, Xu W (2012) Solidification/stabilization of dredged marine sediments for road construction. Environ Technol 33:95–101
Wang D, Abriak NE, Zentar R, Chen W (2013) Effect of lime treatment on geotechnical properties of Dunkirk sediments in France. Road Mater Pavement 14:485–503
Wang L, Kwok JSH, Tsang DCW, Poon C (2015) Mixture design and treatment methods for recycling contaminated sediment. J Hazard Mater 283:623–632
Acknowledgements
The experimental activities described were funded by the Special Commissioner of the Italian government for urgent measures of reclamation, environmental improvements and redevelopment of the Taranto area (South of Italy). The authors gratefully acknowledge UNICALCE S.p.A for providing the additives used for the research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Todaro, F., De Gisi, S. & Notarnicola, M. Contaminated marine sediment stabilization/solidification treatment with cement/lime: leaching behaviour investigation. Environ Sci Pollut Res 27, 21407–21415 (2020). https://doi.org/10.1007/s11356-020-08562-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08562-1