Abstract
Chicoric acid (CA) is a natural antioxidant with promising hepatoprotective activity. We investigated the potential of CA to prevent methotrexate (MTX) hepatotoxicity, pointing to the role of Nrf2/HO-1 signaling and PPARγ. Rats received CA for 15 days and were then injected with MTX at day 16. Blood and tissue samples were collected for analysis at day 19. CA ameliorated liver function markers and mitigated histological alterations in MTX-induced rats. Pre-treatment with CA suppressed reactive oxygen species and lipid peroxidation and enhanced antioxidants in MTX-induced rats. Moreover, CA upregulated hepatic Nrf2, HO-1, NQO-1, and PPARγ, and attenuated inflammation. Consequently, CA inhibited apoptosis by increasing Bcl-2 expression and suppressing Bax, cytochrome c, and caspase-3 in MTX-administered rats. In conclusion, CA prevented oxidative stress, inflammation, and liver injury induced by MTX by activating Nrf2 /HO-1 signaling and PPARγ.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Methotrexate (MTX) is a chemotherapeutic dihydrofolate reductase inhibitor widely used in the treatment of several types of cancer, such as leukemia, osteosarcoma, lung cancer, and breast cancer (Farber 1966). It is used as an immunosuppressive agent to treat rheumatoid arthritis, psoriasis, and systemic lupus erythematosus. MTX disrupts folate metabolism and inhibits DNA synthesis (Rajagopalan et al. 2002), and its use is therefore associated with multiple adverse effects, including nausea, diarrhea, fever, and vomiting (Khan et al. 2012). In addition, the toxicity profile of MTX has been demonstrated to include nephrotoxicity (Perazella 2009) and hepatotoxicity (Conway and Carey 2017).
Drug-induced liver injury (DILI) is a serious side effect that can occur during the use of MTX (West 1997), thereby limiting its clinical applications. The mechanism of MTX hepatotoxicity is not fully understood; however, studies have highlighted the possible involvement of oxidative damage and mitochondrial dysfunction. MTX triggers cytotoxicity in rat hepatocytes by increasing the production of reactive oxygen species (ROS) and altering mitochondrial membrane potential (MMP) (Al Maruf et al. 2018). In experimental rats, MTX induced hepatic oxidative stress and reduced cellular antioxidants (Mahmoud et al. 2017b, c). In addition to oxidative damage, ROS can activate nuclear factor-kappaB (NF-κB) and the release of pro-inflammatory mediators (Morgan and Liu 2011). Inflammation and redox imbalance have also been associated with the apoptosis of hepatocytes following MTX administration (Mahmoud et al. 2017b, c).
Chicoric acid (CA) is a hydroxycinnamic acid that occurs in at least 63 genera and species, including Cichorium intybus L. and Echinacea purpurea (Lee and Scagel 2013). In plants, CA plays a defensive role to protect against insects and infection (Nishimura and Satoh 2006), and helps in wound healing after mechanical damage (Tomás-Barberán et al. 1997). Recent studies have demonstrated that CA possesses antioxidant and anti-inflammatory efficacies (Ding et al. 2019; Liu et al. 2017; Tsai et al. 2017). CA ameliorated antioxidant defenses in diabetic mice (Zhu et al. 2017), cerebral ischemia/reperfusion (I/R) injury (Jia et al. 2018), and in hepatocytes in vitro (Ma et al. 2018). Despite its potent antioxidant efficacy, nothing has been yet reported on its potential to suppress oxidative stress in MTX-administered rats. Therefore, we investigated the hepatoprotective effects of CA, emphasizing on oxidative stress and the possible involvement of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) signaling in MTX-administered rats. Nrf2 is a redox-sensitive factor conferring protection against oxidative stress by stimulating the expression of antioxidant defenses (Satta et al. 2017). Under normal cellular conditions, Nrf2 is sequestered in the cytoplasm by Kelch-like ECH-associated protein 1 (Keap1). ROS dissociate Nrf2/Keap-1 complex and the liberated Nrf2 translocates into the nucleus and elicits the transcription of multiple genes, including heme oxygenase (HO)-1, superoxide dismutase (SOD), and catalase (CAT) (Itoh et al. 1997). In addition, we evaluated the effect of CA on peroxisome proliferator-activated receptor gamma (PPARγ), a nuclear hormone receptor with central roles in carbohydrate metabolism, adipogenesis, and cell differentiation (Kim et al. 2015). Upon activation, PPARγ forms a heterodimer with retinoid X receptor (RXR) and controls the transcription of several genes through binding to a specific response element (Barish et al. 2006). It promotes the expression of antioxidant defense enzymes and inhibits the release of pro-inflammatory cytokines via NF-κB suppression (Hwang et al. 2005; Remels et al. 2009). Therefore, the activation of Nrf2 and PPARγ can mitigate inflammation, oxidative damage, and liver injury induced by MTX.
Materials and methods
Experimental design
Male Wistar rats (150–160 g), obtained from the National Research Centre (NRC, Giza, Egypt), were housed under standard conditions and given a standard chow diet and water ad libitum.
CA (Chengdu Purify Phytochemicals Ltd., China) was dissolved in 0.5% carboxymethyl cellulose (CMC) and MTX (Shanxi PUDE Pharmaceutical Co., China) was dissolved in physiological saline. After acclimatization for 1 week, the animals were allocated into 4 groups, six rats per group (n = 6), as follows:
-
Group I: received vehicle for 15 days and a single intraperitoneal (i.p.) injection of physiological saline at day 16. This group served as a control.
-
Group II: received vehicle for 15 days and a single injection of MTX (20 mg/kg) at day 16.
-
Group III: received CA (25 mg/kg) orally for 15 days and a single i.p. dose of MTX at day 16.
-
Group IV: received CA (50 mg/kg) orally for 15 days and a single i.p. injection of MTX at day 16.
The doses of CA were selected according to previous studies demonstrating its antioxidant efficacy in vivo (Ding et al. 2019; Jia et al. 2018; Zhu et al. 2017), and MTX-induced liver injury was established as previously described (Mahmoud et al. 2017c).
Collection and preparation of samples
At day 19, overnight fasted rats were sacrificed under thiopental (Eipico, Egypt) anesthesia and blood and liver samples were collected. Pieces from the liver were fixed in 10% neutral buffered formalin (NBF) for 48 h and others were kept at − 80 °C for RNA and protein isolation. Samples from the frozen liver were homogenized in cold PBS (10% w/v) for the determination of glutathione (GSH), SOD, CAT, glutathione peroxidase (GPx), cytochrome c, caspase-3, malondialdehyde (MDA), and nitric oxide (NO). For Western blotting, other frozen samples were homogenized in RIPA buffer containing proteinase inhibitors and protein content was measured by Bradford reagent (Bradford 1976).
Assay of liver function, inflammation, and apoptosis markers
Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) were assayed using Spinreact kits (Spinreact, Girona, Spain). ELISA kits were used for measuring tumor necrosis factor alpha (TNF-α; R&D systems, USA), cytochrome c (Cusabio, Wuhan, China), and caspase-3 (Cusabio, Wuhan, China).
Assay of oxidative stress and antioxidant markers
ROS (Mahmoud et al. 2017d, e), MDA (Ohkawa et al. 1979), NO (Grisham et al. 1996), GSH (Beutler et al. 1963), SOD (Marklund and Marklund 1974), CAT (Cohen et al. 1970), and GPx (Matkovics et al. 1998) were assayed in the liver homogenates of the control and experimental rats.
Histology and immunohistochemistry
The fixed liver samples were processed for paraffin embedding and 5-μm sections were cut. The sections were stained with hematoxylin and eosin (H&E) for examination using light microscopy. The effect of MTX and CA on the expression of Bcl-2-associated X protein (Bax) was determined using immunohistochemical staining (Mahmoud et al. 2017c). Briefly, the sections were blocked in 3% hydrogen peroxide (H2O2) and then probed with anti-Bax (Santa Cruz Biotechnology, USA; 1:1000 dilution) overnight at 4 °C. After washing, the sections were incubated with biotinylated secondary antibody (Santa Cruz Biotechnology, USA) at 1:1000 dilution and then washed. The slides were incubated with streptavidin/peroxidase conjugate followed by diaminobenzidine, counterstained with hematoxylin, and then examined.
Gene expression
RNA was isolated using TRIzol reagent (Invitrogen, USA), quantitated and reverse transcribed into cDNA which was amplified by SYBR Green master mix and the primers listed in Table 1. The 2−ΔΔCt method (Livak and Schmittgen 2001) was used to analyze obtained amplification data followed by normalization to β-actin.
Western blotting
A total of 50 μg protein from each sample was separated using SDS-PAGE followed by transfer to PVDF membranes. After blocking, the membranes were washed and incubated with primary antibodies against Nrf2, PPARγ, and β-actin (Novus Biologicals, USA) overnight at 4 °C. After washing with TBST, secondary antibodies (Novus Biologicals, USA) were added to the membranes for 1 h at room temperature. All antibodies were used at 1:1000 dilution. ImageJ (version 1.32j; NIH, USA) was used to quantify the band intensity and results were normalized to β-actin.
Statistical analysis
The obtained results are expressed as means ± standard error of the mean (SEM). The statistical comparisons were made by one-way ANOVA followed by Tukey’s test using GraphPad Prism 7 (La Jolla, CA, USA). A P value < 0.05 indicated a statistical significance.
Results
CA attenuates MTX-induced liver injury in rats
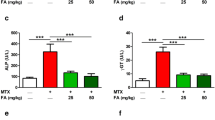
ALT, AST, and ALP were determined to evaluate the hepatoprotective effect of CA (Fig. 1). MTX-administered rats showed significantly elevated serum ALT, AST, and ALP (P < 0.001). In contrast, both doses of CA alleviated ALT (P < 0.01), AST (P < 0.001), and ALP (P < 0.001).
CA prevents MTX-induced liver injury in. CA alleviated serum levels of a ALT, b AST, and c ALP in MTX-administered rats. Data are expressed as mean ± SEM, n = 6. **P < 0.01 and ***P < 0.001. d Photomicrographs of sections in the liver of control rats showing normal histological structure of the hepatic lobules, hepatocytes, and sinusoids. MTX-induced rats showed degenerative changes of hepatocytes (red arrow), fatty infiltration (black arrow), activated Kupffer cells and inflammatory cells infiltration (green arrow), and hemorrhage (blue arrow). Pre-treatment of the MTX-induced rats with 25 mg/kg and 50 mg/kg CA prevented all histological alterations. (Scale bar 50 μm)
The histological findings supported the hepatoprotective efficacy of CA as represented in Fig. 1d. The control group showed normal structure of the hepatic lobules, hepatocytes, and sinusoids. In contrast, multiple histological alterations, including degenerative changes of hepatocytes, cytoplasmic vacuolations, fatty infiltrations, activated Kupffer cells (KCs), inflammatory cells infiltration, hemorrhage, and others were observed in rats received MTX. Both doses of CA prevented the histological alterations in MTX-intoxicated rats.
CA attenuates hepatic oxidative stress in MTX-induced rats
MTX-intoxicated rats exhibited significantly (P < 0.001) elevated hepatic ROS (Fig. 2a), MDA (Fig. 2b), and NO levels (Fig. 2c). CA ameliorated effectively (P < 0.001) hepatic ROS, MDA, and NO. The effect of CA on ROS levels was dose-dependent as represented in Fig. 2a.
CA alleviates cellular antioxidants in liver of MTX-induced rats
To evaluate the effect of CA on the cellular antioxidants, we determined GSH and activity of the antioxidant enzymes (Fig. 3). MTX administration decreased GSH content (Fig. 3a), SOD (Fig. 3b), CAT (Fig. 3c), and GPx (Fig. 3d) in the liver of rats (P < 0.001). CA boosted antioxidant defenses in MTX-administered rats.
CA suppresses inflammation and apoptosis in MTX-induced rats
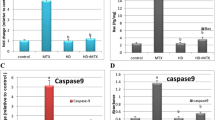
To assess the effect of CA pre-treatment on the inflammatory response and apoptosis following MTX administration, we determined serum and liver TNF-α and the expression of pro- and anti-apoptosis markers. MTX injection increased serum and liver TNF-α (P < 0.001; Fig. 4a), an effect that was significantly reversed in 25 and 50 mg/kg CA supplemented animals (P < 0.001). Hepatic Bax mRNA was significantly increased in MTX-administered rats (P < 0.001; Fig. 4b). The upregulation of Bax was confirmed by immunohistochemical staining which showed a noticeable increase in Bax in rats received MTX (Fig. 4c). In addition, Bcl-2 mRNA was decreased (P < 0.01; Fig. 4d), whereas Bax/Bcl-2 ratio (Fig. 4e), cytochrome c (Fig. 4f), and caspase-3 (Fig. 4g) were markedly increased (P < 0.001). CA decreased Bax mRNA abundance (P < 0.01), Bax immunostaining, Bax/Bcl-2 ratio (P < 0.001), cytochrome c (P < 0.001), and caspase-3 (P < 0.001), and up-regulated Bcl-2 (P < 0.01).
CA attenuates inflammation and apoptosis in the liver of MTX-induced rats. Pre-treatment with CA decreased a serum and liver TNF-α levels, down-regulated b Bax mRNA, c Bax protein (arrows), e Bax/Bcl-2 ratio, f cytochrome c, and g caspase-3, and d increased Bcl-2 abundance. Data are expressed as mean ± SEM, n = 6. **P < 0.01 and ***P < 0.001
CA upregulates hepatic Nrf2/HO-1 signaling in MTX-intoxicated rats
MTX administration decreased Nrf2 mRNA (P < 0.001; Fig. 5a) and protein (P < 0.01; Fig. 5b) in the liver of rats. MTX-induced suppression of Nrf2 signaling was confirmed by the reduced NQO-1 (Fig. 5c) and HO-1 (Fig. 5d). CA increased the expression of Nrf2, NQO-1, and HO-1 in the liver of MTX-intoxicated rats. The 50 mg/kg dose of CA increased the gene expression levels of Nrf2 significantly (P < 0.01) when compared with the lower dose (Fig. 5a).
CA increases hepatic PPARγ expression in MTX-induced rats
PPARγ mRNA and protein were reduced significantly in the liver of MTX-intoxicated rats (P < 0.001). Both doses of CA alleviated hepatic PPARγ mRNA and protein in MTX-administered rats as depicted in Fig. 6a, b.
Discussion
CA is a hydroxycinnamic acid with promising hepatoprotective efficacy. Previous reports have demonstrated the protective efficacy of CA on oxidative stress induced by H2O2 (Ma et al. 2018) and high glucose (Zhu et al. 2017), and FFA-induced metabolic deregulations (Guo et al. 2018) in hepatocytes in vitro. The ameliorative effect of CA on oxidative stress in vivo has been demonstrated in a murine model of cerebral I/R injury (Jia et al. 2018). In addition, CA suppressed inflammation and acute hepatic steatosis in mice (Landmann et al. 2014). Recent studies have shown the efficacy of CA to prevent MTX-induced acute kidney injury via attenuation of oxidative stress and inflammation (Abd El-Twab et al. 2019). Although CA has demonstrated several beneficial effects, its potential to prevent MTX-induced liver injury has not been investigated yet. Herein, we evaluated the effect of CA supplementation on oxidative stress, inflammation, and apoptosis in the liver of MTX-administered rats, pointing to the role of Nrf2 signaling and PPARγ.
MTX administration triggered liver injury and increased serum ALT, AST, and ALP. Altered circulating levels of these enzymes indicate hepatocyte damage and represent a sensitive marker of liver injury. Accordingly, these liver function markers were elevated in serum of MTX-intoxicated rats (Mahmoud et al. 2017b, c). The histological examination supported the biochemical findings where degenerative changes of hepatocytes, inflammatory cells infiltration, hemorrhage, fatty infiltration, and KCs activation were observed in the MTX-administered rats. Accordingly, similar histological alterations have been reported in MTX-administered rodents (Erdogan et al. 2015; Mahmoud et al. 2017b, c; Moghadam et al. 2015). Oral supplementation of CA ameliorated liver function markers and prevented all histological alterations provoked by MTX, demonstrating a potent hepatoprotective effect. Few studies have shown the in vivo hepatoprotective effect CA. For instance, CA attenuated both alcoholic (Landmann et al. 2014) and non-alcoholic steatohepatitis (Kim et al. 2017) in mice, at least in part, by modulating inflammation. Herein, our results confer new information that CA can prevent MTX-induced liver injury in rats.
Elevated ROS levels play a role in MTX hepato- and nephrotoxicity as previously described (Abd El-Twab et al. 2016; Mahmoud et al. 2018, 2017b, c). Therefore, we investigated the effect of CA on ROS levels, lipid peroxidation (LPO), and antioxidants in rat liver following MTX administration. MTX-induced rats exhibited oxidative stress evidenced by increased ROS and MDA. Increased ROS levels can provoke oxidative damage to different cell components, resulting in cell death (Fink and Cookson 2005). Accordingly, hepatic LPO was increased along with NO levels in MTX-administered rats. NO, generated via inducible NO synthase (iNOS) upregulation (El-Sheikh et al. 2015), can react with superoxide radicals producing peroxynitrite which induces oxidative DNA damage and cell injury (McKim et al. 2003).
The exact mechanisms underlying increased ROS generation triggered by MTX are not fully understood. However, in vitro studies have pointed to the involvement of mitochondrial dysfunction. Rat hepatocytes treated with MTX showed decreased MMP, increased ROS levels, cytochrome c release, and cell death (Al Maruf et al. 2018). Similarly, increased ROS generation and cell death were demonstrated in different lymphocytic cell lines in response to low doses of MTX (Herman et al. 2005). In vivo studies have also added support to the role of MTX in triggering ROS generation. For instance, the kidney of rats received a single dose of MTX-exhibited mitochondrial dysfunction (Heidari et al. 2018), upregulated nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Arab et al. 2018) and elevated ROS levels (Abd El-Twab et al. 2019). In addition, depletion of the mitochondrial antioxidants machinery has been indirectly implicated in MTX toxicity (Kolli et al. 2014). Accordingly, hepatic GSH, SOD, CAT, and GPx were declined in MTX-administered rats in the present study. Besides oxidative stress, MTX resulted in inflammation as shown by the increased serum and liver TNF-α. These findings supported previous studies showing increased serum pro-inflammatory cytokines in MTX-administered rats (Abd El-Twab et al. 2016; Mahmoud et al. 2018, 2017b, c). Given that ROS can activate NF-κB which elicits the transcription of pro-inflammatory mediators, increased TNF-α in the present study might be a direct consequence of MTX-induced excessive ROS production. In addition, peroxynitrite can activate NF-κB and stimulate KCs leading to the release of pro-inflammatory cytokines (Matata and Galiñanes 2002). Furthermore, MTX has been recently reported to increase ROS generation and activate the NF-κB/NLRP3 inflammasome axis in rat kidney (Abd El-Twab et al. 2019).
Interestingly, CA suppressed ROS generation, LPO, and inflammation, and boosted hepatic antioxidants in MTX-administered rats. The antioxidant and anti-inflammatory effects of CA have been previously demonstrated both in vitro and in vivo. Ma et al. have recently reported the ameliorative effect of CA on oxidative stress induced by H2O2 in hepatocytes (Ma et al. 2018). CA ameliorated oxidative stress in high glucose-induced hepatocytes and inhibited inflammation in diabetic mice (Zhu et al. 2017). In lipopolysaccharide (LPS)-induced KCs, pre-treatment with CA downregulated iNOS and TNF-α gene expression (Landmann et al. 2014). In addition, CA suppressed hepatic iNOS protein expression in a mouse model of acute hepatic steatosis (Landmann et al. 2014). Moreover, CA prevented hepatocyte apoptosis in MTX-administered rats in the present investigation. MTX triggered Bax, cytochrome c, and caspase-3 expression, and suppressed Bcl-2. Bax provokes apoptosis via stimulating mitochondrial cytochrome c release and activation of caspase-3 (Almeida et al. 2000), whereas Bcl-2 inhibits this process (Mahmoud et al. 2014; Yang et al. 1997). Given that the balance between pro- and anti-apoptosis proteins determines the cell fate (Almeida et al. 2000), apoptosis has been demonstrated by the increased Bax/Bcl-2 ratio in MTX-intoxicated rats. CA suppressed hepatic Bax, cytochrome c, and caspase-3, and increased Bcl-2 in MTX-administered rats.
To reveal the underlying mechanism of the antioxidant, anti-inflammatory, and anti-apoptosis efficacies of CA, we evaluated its effect on Nrf2 signaling. CA activated Nrf2/HO-1 signaling in the liver of MTX-administered rats. Although activated by ROS, Nrf2 was suppressed in liver of MTX-administered rats in the present study. This is explained by the prolonged excessive generation of ROS as previously reported in multiple in vivo and in vitro studies (Abd El-Twab et al. 2016; Mahmoud and Al Dera 2015; Mahmoud et al. 2017b, c, d, e). The ability of CA to activate Nrf2 signaling has been recently reported in H2O2-induced hepatocytes where it increased the expression of Nrf2 and HO-1 mRNA (Ma et al. 2018). CA upregulated renal expression of Nrf2, NQO-1, and HO-1 in MTX-administered rats as we reported recently (Abd El-Twab et al. 2019). In animal models of liver injury, Nrf2 activation mitigated ROS generation, NF-κB activation, and pro-inflammatory cytokines (Mahmoud et al. 2017a, e). The suppressed inflammation following Nrf2 activation was supported by the study of Pan et al. who reported NF-κB activation in mouse primary astrocytes lacking Nrf2 (Pan et al. 2012). In addition to Nrf2, CA increased the expression of hepatic PPARγ in MTX-administered rats. PPARγ activation has been reported to induce the expression of antioxidant enzymes (Yu et al. 2014) and suppress NADPH oxidase and NF-κB (Hwang et al. 2005; Remels et al. 2009). Furthermore, the dual activation of PPARγ and Nrf2 has been associated with suppressed oxidative stress and inflammation in rat models of hepatotoxicity, fibrogenesis, and hepatocarcinogenesis (Mahmoud and Al Dera 2015; Mahmoud et al. 2019, 2017b, e).
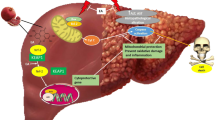
In conclusion, these results confer new information on the mechanism underlying the protective effect of CA on MTX hepatotoxicity. CA enhanced the antioxidant defenses, prevented MTX liver injury, and mitigated excessive ROS generation, LPO, inflammation, and apoptosis in rats. The dual activation of Nrf2 and PPARγ represents, at least in part, the mechanism underlying the hepatoprotective efficacy of CA as illustrated in Fig. 7. Therefore, CA could be supplemented as an adjuvant treatment to prevent liver injury provoked by MTX, pending further investigations to determine its exact mechanisms of action.
A proposed schematic diagram illustrating the protective efficacy of CA on MTX-hepatotoxicity. ROS, reactive oxygen species; Nrf2, nuclear factor (erythroid-derived 2)-like 2; Keap-1, Kelch like-ECH-associated protein 1; ARE, antioxidant response element; PPARγ, peroxisome proliferator activated receptor gamma; RXR, retinoid X receptor; PPRE, PPAR response element NF-κB, nuclear factor-kappaB; Bax, Bcl-2-associated X protein
References
Abd El-Twab SM, Hozayen WG, Hussein OE, Mahmoud AM (2016) 18beta-glycyrrhetinic acid protects against methotrexate-induced kidney injury by up-regulating the Nrf2/ARE/HO-1 pathway and endogenous antioxidants. Ren Fail 39:1516–1527
Abd El-Twab SM, Hussein OE, Hozayen WG, Bin-Jumah M, Mahmoud AM (2019) Chicoric acid prevents methotrexate-induced kidney injury by suppressing NF-κB/NLRP3 inflammasome activation and up-regulating Nrf2/ARE/HO-1 signaling. Inflamm Res 68:511–523
Al Maruf A, O’Brien PJ, Naserzadeh P, Fathian R, Salimi A, Pourahmad J (2018) Methotrexate induced mitochondrial injury and cytochrome c release in rat liver hepatocytes. Drug Chem Toxicol 41:51–61
Almeida OF, Conde GL, Crochemore C, Demeneix BA, Fischer D, Hassan AH, Meyer M, Holsboer F, Michaelidis TM (2000) Subtle shifts in the ratio between pro- and antiapoptotic molecules after activation of corticosteroid receptors decide neuronal fate. FASEB J 14:779–790
Arab HH, Salama SA, Maghrabi IA (2018) Camel milk attenuates methotrexate-induced kidney injury via activation of PI3K/Akt/eNOS signaling and intervention with oxidative aberrations. Food Funct 9:2661–2672
Barish GD, Narkar VA, Evans RM (2006) PPAR delta: a dagger in the heart of the metabolic syndrome. J Clin Invest 116:590–597
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cohen G, Dembiec D, Marcus J (1970) Measurement of catalase activity in tissue extracts. Anal Biochem 34:30–38
Conway R, Carey JJ (2017) Risk of liver disease in methotrexate treated patients. World J Hepatol 9:1092–1100
Ding H, Ci X, Cheng H, Yu Q, Li D (2019) Chicoric acid alleviates lipopolysaccharide-induced acute lung injury in mice through anti-inflammatory and anti-oxidant activities. Int Immunopharmacol 66:169–176
El-Sheikh AA, Morsy MA, Abdalla AM, Hamouda AH, Alhaider IA (2015) Mechanisms of thymoquinone hepatorenal protection in methotrexate-induced toxicity in rats. Mediat Inflamm 2015:859383
Erdogan E, Ilgaz Y, Gurgor PN, Oztas Y, Topal T, Oztas E (2015) Rutin ameliorates methotrexate induced hepatic injury in rats. Acta Cir Bras 30:778–784
Farber S (1966) Chemotherapy in the treatment of leukemia and Wilms’ tumor. Jama 198:826–836
Fink SL, Cookson BT (2005) Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun 73:1907–1916
Grisham MB, Johnson GG, Lancaster JR Jr (1996) Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol 268:237–246
Guo R, Zhao B, Wang Y, Wu D, Wang Y, Yu Y, Yan Y, Zhang W, Liu Z, Liu X (2018) Cichoric acid prevents free-fatty-acid-induced lipid metabolism disorders via regulating Bmal1 in HepG2 cells. J Agric Food Chem 66:9667–9678
Heidari R, Ahmadi A, Mohammadi H, Ommati MM, Azarpira N, Niknahad H (2018) Mitochondrial dysfunction and oxidative stress are involved in the mechanism of methotrexate-induced renal injury and electrolytes imbalance. Biomed Pharmacother 107:834–840
Herman S, Zurgil N, Deutsch M (2005) Low dose methotrexate induces apoptosis with reactive oxygen species involvement in T lymphocytic cell lines to a greater extent than in monocytic lines. Inflamm Res 54:273–280
Hwang J, Kleinhenz DJ, Lassegue B, Griendling KK, Dikalov S, Hart CM (2005) Peroxisome proliferator-activated receptor-gamma ligands regulate endothelial membrane superoxide production. Am J Physiol Cell Physiol 288:C899–C905
Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I (1997) An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236:313–322
Jia L, Chen Y, Tian YH, Zhang G (2018) MAPK pathway mediates the anti-oxidative effect of chicoric acid against cerebral ischemia-reperfusion injury in vivo. Exp Ther Med 15:1640–1646
Khan ZA, Tripathi R, Mishra B (2012) Methotrexate: a detailed review on drug delivery and clinical aspects. Expert Opin Drug Deliv 9:151–169
Kim JH, Song J, Park KW (2015) The multifaceted factor peroxisome proliferator-activated receptor gamma (PPARgamma) in metabolism, immunity, and cancer. Arch Pharm Res 38:302–312
Kim M, Yoo G, Randy A, Kim HS, Nho CW (2017) Chicoric acid attenuate a nonalcoholic steatohepatitis by inhibiting key regulators of lipid metabolism, fibrosis, oxidation, and inflammation in mice with methionine and choline deficiency. Mol Nutr Food Res 61(5)
Kolli VK, Natarajan K, Isaac B, Selvakumar D, Abraham P (2014) Mitochondrial dysfunction and respiratory chain defects in a rodent model of methotrexate-induced enteritis. Hum Exp Toxicol 33:1051–1065
Landmann M, Kanuri G, Spruss A, Stahl C, Bergheim I (2014) Oral intake of chicoric acid reduces acute alcohol-induced hepatic steatosis in mice. Nutrition 30:882–889
Lee J, Scagel C (2013) Chicoric acid: chemistry, distribution, and production. Frontiers in Chemistry 1:40
Liu Q, Chen Y, Shen C, Xiao Y, Wang Y, Liu Z, Liu X (2017) Chicoric acid supplementation prevents systemic inflammation-induced memory impairment and amyloidogenesis via inhibition of NF-kappaB. FASEB J 31:1494–1507
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408
Ma J, Li M, Kalavagunta PK, Li J, He Q, Zhang Y, Ahmad O, Yin H, Wang T, Shang J (2018) Protective effects of cichoric acid on H2O2-induced oxidative injury in hepatocytes and larval zebrafish models. Biomed Pharmacother 104:679–685
Mahmoud AM, Al Dera HS (2015) 18β-Glycyrrhetinic acid exerts protective effects against cyclophosphamide-induced hepatotoxicity: potential role of PPARγ and Nrf2 upregulation. Genes Nutr 10:1–13
Mahmoud AM, Ahmed OM, Galaly SR (2014) Thymoquinone and curcumin attenuate gentamicin-induced renal oxidative stress, inflammation and apoptosis in rats. EXCLI J 13:98–110
Mahmoud AM, Germoush MO, Alotaibi MF, Hussein OE (2017a) Possible involvement of Nrf2 and PPARγ up-regulation in the protective effect of umbelliferone against cyclophosphamide-induced hepatotoxicity. Biomed Pharmacother 86:297–306
Mahmoud AM, Hozayen WG, Ramadan SM (2017b) Berberine ameliorates methotrexate-induced liver injury by activating Nrf2/HO-1 pathway and PPARgamma, and suppressing oxidative stress and apoptosis in rats. Biomed Pharmacother 94:280–291
Mahmoud AM, Hussein OE, Hozayen WG, Abd El-Twab SM (2017c) Methotrexate hepatotoxicity is associated with oxidative stress, and down-regulation of PPARgamma and Nrf2: protective effect of 18beta-glycyrrhetinic acid. Chem Biol Interact 270:59–72
Mahmoud AM, Wilkinson FL, Jones AM, Wilkinson JA, Romero M, Duarte J, Alexander MY (2017d) A novel role for small molecule glycomimetics in the protection against lipid-induced endothelial dysfunction: involvement of Akt/eNOS and Nrf2/ARE signaling. Biochim Biophys Acta 1861:3311–3322
Mahmoud AM, Wilkinson FL, McCarthy EM, Moreno-Martinez D, Langford-Smith A, Romero M, Duarte J, Alexander MY (2017e) Endothelial microparticles prevent lipid-induced endothelial damage via Akt/eNOS signaling and reduced oxidative stress. FASEB J 31:4636–4648
Mahmoud AM, Germoush MO, Al-Anazi KM, Mahmoud AH, Farah MA, Allam AA (2018) Commiphora molmol protects against methotrexate-induced nephrotoxicity by up-regulating Nrf2/ARE/HO-1 signaling. Biomed Pharmacother 106:499–509
Mahmoud AM, Hozayen WG, Hasan IH, Shaban E, Bin-Jumah M (2019) Umbelliferone ameliorates CCl4-induced liver fibrosis in rats by upregulating PPARgamma and attenuating oxidative stress, inflammation, and TGF-beta1/Smad3 signaling. Inflammation 42:1103–1116
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Matata BM, Galiñanes M (2002) Peroxynitrite is an essential component of cytokines production mechanism in human monocytes through modulation of nuclear factor-kappa B DNA binding activity. J Biol Chem 277:2330–2335
Matkovics B, Szabo L, Varga IS (1998) Determination of enzyme activities in lipid peroxidation and glutathione pathways (in Hungarian). Laboratoriumi Diagnosztika 15:248–249
McKim SE, Gäbele E, Isayama F, Lambert JC, Tucker LM, Wheeler MD, Connor HD, Mason RP, Doll MA, Hein DW, Arteel GE (2003) Inducible nitric oxide synthase is required in alcohol-induced liver injury: studies with knockout mice. Gastroenterology 125:1834–1844
Moghadam AR, Tutunchi S, Namvaran-Abbas-Abad A, Yazdi M, Bonyadi F, Mohajeri D, Mazani M, Marzban H, Los MJ, Ghavami S (2015) Pre-administration of turmeric prevents methotrexate-induced liver toxicity and oxidative stress. BMC Complement Altern Med 15:246
Morgan MJ, Liu ZG (2011) Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res 21:103–115
Nishimura H, Satoh A (2006) Antimicrobial and nematicidal substances from the root of chicory (Cichorium intybus). In: Inderjit , Mukerji KG (Editors), Allelochemicals: Biological Control of Plant Pathogens and Diseases. Springer Netherlands, Dordrecht, pp. 177–180
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pan H, Wang H, Wang X, Zhu L, Mao L (2012) The absence of Nrf2 enhances NF-kappaB-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediat Inflamm 2012:217580
Perazella MA (2009) Renal vulnerability to drug toxicity. Clin J Am Soc Nephrol 4:1275–1283
Rajagopalan PTR, Zhang Z, McCourt L, Dwyer M, Benkovic SJ, Hammes GG (2002) Interaction of dihydrofolate reductase with methotrexate: ensemble and single-molecule kinetics. Proc Natl Acad Sci U S A 99:13481–13486
Remels AH, Langen RC, Gosker HR, Russell AP, Spaapen F, Voncken JW, Schrauwen P, Schols AM (2009) PPARgamma inhibits NF-kappaB-dependent transcriptional activation in skeletal muscle. Am J Physiol Endocrinol Metab 297:E174–E183
Satta S, Mahmoud AM, Wilkinson FL, Yvonne Alexander M, White SJ (2017) The role of Nrf2 in cardiovascular function and disease. Oxidative Med Cell Longev 2017:9237263
Tomás-Barberán FA, Gil MI, Castañer M, Artés F, Saltveit ME (1997) Effect of selected browning inhibitors on phenolic metabolism in stem tissue of harvested lettuce. J Agric Food Chem 45:583–589
Tsai KL, Kao CL, Hung CH, Cheng YH, Lin HC, Chu PM (2017) Chicoric acid is a potent anti-atherosclerotic ingredient by anti-oxidant action and anti-inflammation capacity. Oncotarget 8:29600–29612
West SG (1997) Methotrexate hepatotoxicity. Rheum Dis Clin N Am 23:883–915
Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X (1997) Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science (New York, N.Y.) 275:1129–1132
Yu Y, Wu Y, Wen G, Yang W (2014) Effect of pioglitazone on the expression of TLR4 in renal tissue of diabetic rats. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2014 Aug 30(8):793–797
Zhu D, Zhang X, Niu Y, Diao Z, Ren B, Li X, Liu Z, Liu X (2017) Cichoric acid improved hyperglycaemia and restored muscle injury via activating antioxidant response in MLD-STZ-induced diabetic mice. Food Chem Toxicol 107:138–149
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at Princess Nourah Bint Abdul Rahman University for supporting this research through the Fast-track Research Funding Program.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The experimental protocol and procedures were approved by the Institutional Research Ethics Committee of Beni-Suef University (Egypt).
Consent for publication
Not applicable.
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hussein, O.E., Hozayen, W.G., Bin-Jumah, M.N. et al. Chicoric acid prevents methotrexate hepatotoxicity via attenuation of oxidative stress and inflammation and up-regulation of PPARγ and Nrf2/HO-1 signaling. Environ Sci Pollut Res 27, 20725–20735 (2020). https://doi.org/10.1007/s11356-020-08557-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08557-y