Abstract
Liver injury is one of the adverse effects of methotrexate (MTX). Ferulic acid (FA) is an antioxidant phytochemical that confers hepatoprotective efficacy; however, its effect against MTX hepatotoxicity remains unexplored. This study investigated the role of FA in modulating oxidative stress, inflammation, Nrf2/HO-1 signaling, and PPARγ in MTX-administered rats. Following oral FA supplementation for 15 days, rats received a single dose of MTX at day 16 and samples were collected at day 19. MTX provoked multiple histological manifestations, including degenerative changes, steatosis, inflammatory cells infiltration and hemorrhage, and altered serum transaminases, bilirubin, and albumin. Reactive oxygen species, lipid peroxidation, and nitric oxide were increased in the liver of rats that received MTX. FA prevented all histological alterations, ameliorated liver function markers, suppressed oxidative stress, and boosted antioxidants in MTX-induced rats. FA reduced serum TNF-α and IL-1β, and hepatic NF-κB p65, Bax, and caspase-3, whereas increased Bcl-2, Nrf2, NQO1, HO-1, and PPARγ. In conclusion, FA prevented MTX hepatotoxicity by activating Nrf2/HO-1 signaling and PPARγ, and attenuating oxidative stress, inflammation, and cell death.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drug-induced liver injury (DILI) induced by medications, herbs, and toxins is a prevalent cause of acute liver failure. Although it accounts for < 1% of the acute liver injury cases, DILI is one of the leading causes of drug withdrawals and attrition of drug candidates in drug development (Stevens and Baker 2009). Methotrexate (MTX) is an antimetabolite chemotherapeutic agent and immune system suppressant used clinically in the treatment of rheumatoid arthritis, neoplastic diseases, and other disorders. The clinical applications of MTX are often limited because of its untoward effects. MTX disrupts folate metabolism and hence its use is associated with multiple adverse effects, such as diarrhea, nausea, fever, and vomiting (Khan et al. 2012). Other side effects of MTX include liver and kidney injury (Mahmoud et al. 2018; Mahmoud et al. 2017c). Although its hepatotoxic mechanism is not fully explored, studies have pointed to the role of reactive oxygen species (ROS) and mitochondrial dysfunction. For instance, treatment of rat hepatocytes with MTX in vitro resulted in cytotoxicity mediated by decreased mitochondrial membrane potential (MMP) and increased ROS generation (Al Maruf et al. 2018). We have previously demonstrated hepatic oxidative/nitrative stress and diminished antioxidants in MTX-administered rodents (Mahmoud et al. 2017b; Mahmoud et al. 2017c).
Besides oxidative stress, MTX hepatotoxicity was associated with inflammation. ROS trigger nuclear factor-kappaB (NF-κB) and subsequently the release of pro-inflammatory mediators (Morgan and Liu 2011). In rodents, MTX elicited inflammation and hepatocyte apoptosis (Mahmoud et al. 2017c). In addition, suppressed nuclear factor (erythroid-derived 2)-like 2 (Nrf2) signaling and peroxisome proliferator activated receptor gamma (PPARγ) have been reported in MTX hepatotoxicity (Mahmoud et al. 2017b). Nrf2 is a transcription factor that protects against ROS-induced oxidative damage through activating the expression of antioxidant and cytoprotective proteins (Jaiswal 2004). Under quiescent conditions, Nrf2 is sequestered in the cytosol by Keap1, whereas dissociates on exposure to ROS. Following its nuclear translocation, the liberated Nrf2 binds to antioxidant response element (ARE) to activate cytodefensive genes (Jaiswal 2004; Satta et al. 2017). PPARγ is a ligand-inducible nuclear hormone receptor centrally involved in the regulation of adipogenesis and metabolism (Kim et al. 2015). Activated PPARγ dimerizes with retinoid X receptor (RXR) and binds to specific response element to regulate target gene expression (Barish et al. 2006). PPARγ activation can inhibit NF-κB and promote the expression of antioxidant enzymes (Yu et al. 2014), thereby attenuates oxidative damage. We have previously shown that upregulation of PPARγ mitigated liver injury (Aladaileh et al. 2019a), hepatocarcinogenesis (Mahmoud et al. 2017d), and hepatic fibrosis (Mahmoud et al. 2019). Therefore, co-activation of PPARγ and Nrf2 can effectively attenuate MTX hepatotoxicity by preventing oxidative stress and inflammation.
Ferulic acid (FA; 3-methoxy-4-hydroxycinnamic acid) is an antioxidant phytochemical found in the cell wall of rice, oats, and other plants (Saulnier and Thibault 1999). FA possesses multiple biological activities and is responsible for most of the health benefits of arabinoxylans (Fadel et al. 2018). Previous studies have demonstrated the protective efficacy of FA against diosbulbin B- (Niu et al. 2016), acetaminophen- (Yuan et al. 2016), formaldehyde- (Gerin et al. 2016), and fluoride-induced hepatotoxicity (Panneerselvam et al. 2013). However, the ameliorative potential of FA on MTX hepatotoxicity has not been investigated. Therefore, we scrutinized the hepatoprotective effect of FA, pointing to its ability to modulate PPARγ and Nrf2 signaling in MTX-induced rats.
Materials and methods
Experimental animals and treatments
Twenty-four male Wistar rats (150–160 g) aged 7–8 weeks were included in this study. The animals were obtained from the animal facility of the National Research Centre (Egypt), housed under standard conditions, and given free access to chow diet and water. The experimental protocol was approved by the Institutional Research Ethics Committee, Beni-Suef University (Egypt).
The rats were acclimatized for 1 week and then divided into 4 groups (n = 6). Group I received a single intraperitoneal (i.p.) injection of saline, whereas groups II–IV received 20 mg/kg MTX (Shanxi PUDE Pharmaceutical Company, China) at day 16 (Mahmoud et al. 2017c). Groups I and II received 0.5% carboxymethyl cellulose (CMC; Sigma, USA) orally for 15 days. Groups III and IV received 25 and 50 mg/kg FA (Sigma, USA) (Hassanzadeh et al. 2017), respectively, dissolved in 0.5% CMC orally for 15 days.
At day 19, the animals were sacrificed under thiopental (Eipico, Egypt) anesthesia, and blood and liver samples were collected for analyses. Pieces from the liver were homogenized in cold PBS (10% w/v), centrifuged, and the clear homogenates were collected for biochemical assays, whereas other samples were fixed in 10% neutral buffered formalin (NBF) for 48 h and others were kept at − 80 °C.
Biochemical assays
Assay of liver function markers
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyl transferase (γGT), bilirubin, and albumin were determined using Spinreact kits (Girona, Spain).
Assay of cytokines and caspase-3
Tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) were measured in serum using ELISA kit (R&D systems, USA), whereas caspase-3 was assayed in liver homogenate using Cusabio (Wuhan, China) ELISA kit.
Assay of oxidative stress and antioxidants
ROS, malondialdehyde (MDA), and nitric oxide (NO) were determined according to Mahmoud et al. (2017e), Ohkawa et al. (1979), and Grisham et al. (1996), respectively. Reduced glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) were assayed following the methods of Beutler et al. (1963), Marklund and Marklund (1974), Cohen et al. (1970), and Matkovics et al. (1998), respectively.
Histology and immunohistochemistry
The fixed liver samples were passed into a serial ascending grade of ethanol and xylene, embedded in paraffin, and then 5-μm sections were cut. The sections were stained with hematoxylin and eosin (H&E) and examined using a light microscope. To assess the expression of Bax, paraffin-embedded sections were cleared in xylene and rehydrated in descending grade of ethanol. The sections were immersed in distilled water followed by EDTA (pH 8). Following treatment with 0.3% hydrogen peroxide and blocking in 5% bovine serum albumin in tris-buffered saline (TBS), the sections were stained with rabbit anti-Bax (sc-526; Santa Cruz Biotechnology, USA). After washing, the sections were probed with the secondary antibody and incubated in diaminobenzidine (DAB; Dako) for 2 min followed by counterstaining with hematoxylin and then examined.
Gene expression
The gene expression levels of Bcl-2, Bax, Nrf2, HO-1, NQO-1, and PPARγ were assessed by qRT-PCR as previously described (Mahmoud et al. 2018). Total RNA was extracted using TRIzol (Invitrogen, USA), treated with DNase, and quantified, and samples with OD260/OD280 nm absorption ratio ≥ 1.8 were used of cDNA synthesis which has been amplified using SYBR green master mix and the set of primers listed in Table 1. The amplification was carried on using ABI 7500 RT-PCR System (Applied Biosystems, USA). The obtained amplification data were analyzed by the 2−ΔΔCt method (Livak and Schmittgen 2001) and normalized to β-actin.
Western blotting
The expression levels of Nrf2, PPARγ, and NF-κB p65 were determined as we previously described (Abd El-Twab et al. 2019). Briefly, liver samples were homogenized in RIPA buffer supplemented with proteinase inhibitors and protein concentration in the homogenate was determined using Bradford reagent (Bradford 1976). Fifty micrograms of protein was subjected to SDS-PAGE and transferred to PVDF membranes which were blocked by 5% milk in TBS-tween (TBST) for 1 h at room temperature. The blocked membranes were incubated with antibodies against Nrf2, NF-κB p65, PPARγ, or β-actin (Novus Biologicals, USA; cat. numbers: NBP1-32822, NB100-2176, NBP2-22106, and NB600-501, respectively) overnight at 4 °C. After washing in TBST, the membranes were incubated with secondary antibodies, washed, and then developed by enhanced chemiluminescence kit (BIO-RAD, USA). The blots were imaged, and the band intensity was determined using ImageJ (version 1.32j; NIH, USA).
Statistical analysis
The obtained results are expressed as mean ± standard error of the mean (SEM). All statistical comparisons were determined by one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons using GraphPad Prism 7 software (San Diego, CA, USA). A P value < 0.05 was considered significant.
Results
FA prevents liver injury in MTX-administered rats
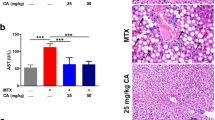
Rats received MTX showed an increase in ALT, AST, ALP, and γGT activities and bilirubin levels (P < 0.001; Fig. 1a–e), and decreased albumin levels (P < 0.001; Fig. 1f). Both FA doses ameliorated all liver function markers and showed a dose-dependent amelioration of AST.
The histological findings confirmed the protective effect of FA against MTX hepatotoxicity. While the control animals exhibited normal liver histology (Fig. 2a), MTX administration produced multiple alterations, including degenerative changes of hepatocytes, steatosis, activated Kupffer cells (KCs), inflammatory cellular infiltration, and cytoplasmic vacuolations (Fig. 2b). Both the 25 (Fig. 2c) and 50 mg/kg FA (Fig. 2d) doses prevented tissue injury triggered by MTX.
FA prevents MTX-induced histological alterations. Photomicrographs of sections in the liver of a control rats showing normal histological structure of the hepatic lobules, hepatocytes, and sinusoids; b rats that received MTX showing degenerative changes of hepatocytes (arrow heads), steatosis (black arrow), activated Kupffer cells and inflammatory cells infiltration (black arrow), and hemorrhage (green arrow); and MTX-induced rats pre-treated with 25 mg/kg (c) and 50 mg/kg FA (d) showing no histological alterations. (Scale bar 50 μm)
FA attenuates MTX-induced hepatic oxidative stress
Rats that received MTX exhibited a remarkable (P < 0.001) increase in liver ROS, MDA, and NO levels (Fig. 3a–c). Pre-treatment with either dose of FA reduced ROS, MDA, and NO in the liver of MTX-administered rats.
In contrast to the oxidative/nitrative stress markers, GSH, SOD, CAT, and GPx were significantly (P < 0.001) diminished by MTX (Fig. 4a–d). FA markedly alleviated liver GSH and enzymatic antioxidants in MTX-administered rats.
FA suppresses inflammation in MTX-administered rats
To demonstrate the effect of FA on the inflammatory response following MTX administration, hepatic NF-κB p65 and serum TNF-α and IL-1β were determined. MTX increased NF-κB p65 (Fig. 5a), TNF-α (Fig. 5b), and IL-1β (Fig. 5c) significantly (P < 0.001) in rats. Pre-treatment with either dose of FA reduced NF-κB p65, TNF-α, and IL-1β in MTX-administered rats.
FA prevents MTX-induced apoptosis
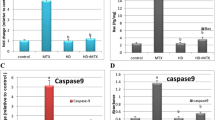
The modulatory effect of FA on apoptosis markers in rats was evaluated. MTX elicited a significant increase in hepatic Bax mRNA abundance (P < 0.001; Fig. 6a) and protein expression determined by immunohistochemistry (Fig. 6c). Apoptosis was confirmed by the decreased Bcl-2 mRNA (Fig. 6b), and increased Bax/Bcl-2 ratio (Fig. 6d) and caspase-3 (Fig. 6e). FA decreased Bax, both mRNA and protein, Bax/Bcl-2 ratio, and caspase-3, whereas upregulated Bcl-2 in the liver of MTX-administered rats. FA exerted a dose-dependent ameliorative effect on caspase-3.
FA prevents apoptosis in liver of MTX-induced rats. FA increased Bcl-2 mRNA abundance (b), and decreased the gene (a) and protein expression (c) of Bax, Bax/Bcl2 ratio (d), and caspase-3 (e) in liver of MTX-administered rats. Data are expressed as mean ± SEM, n = 6. *P < 0.05, **P < 0.01, and ***P < 0.001
FA activates Nrf2/ HO-1 signaling in liver of MTX-administered rats
The liver of MTX-administered rats exhibited a significant decrease in Nrf2 (Fig. 7a), HO-1 (Fig. 7c), and NQO1 (Fig. 7d) mRNA, an effect that was reversed in rats received FA. The declined Nrf2 gene expression was confirmed by western blotting where MTX diminished Nrf2 protein expression (Fig. 7b) and both doses of FA increased it significantly (P < 0.001).
FA upregulates hepatic PPARγ in MTX-induced rats
MTX downregulated hepatic PPARγ, both mRNA (P < 0.01) and protein (P < 0.001), as represented in Fig. 8 a and b, respectively. FA increased liver PPARγ mRNA and protein expression in MTX-administered rats.
Discussion
FA is abundant in plant cell walls and represents the building block of lignocelluloses (Saulnier and Thibault 1999). We demonstrated the ability of FA to mitigate MTX hepatotoxicity, pointing to modulation of Nrf2 signaling and PPARγ.
Rats that received MTX exhibited liver injury evidenced by the elevated serum transaminases and bilirubin, and decreased albumin levels. Elevated transaminases represent a sensitive quantitative marker of hepatocyte damage. In addition, increased bilirubin and declined albumin point to impaired liver function. Therefore, altered liver function markers indicate hepatocyte injury triggered by MTX as previously reported (Famurewa et al. 2019; Khafaga and El-Sayed 2018; Mahmoud et al. 2017b; Mehrzadi et al. 2019). MTX hepatotoxicity was further supported by the histological findings. Microscopic examination revealed degenerative changes, KCs activation, steatosis, inflammatory cells infiltration, vacuolations, and hemorrhage. Accordingly, previous work from our lab (Mahmoud et al. 2017b; Mahmoud et al. 2017c) as well as others (Erdogan et al. 2015) showed similar findings, including hepatocyte degeneration, necrosis, inflammatory cells infiltration, sinusoidal dilatation, and other manifestations. Pre-treatment with FA ameliorated serum liver function markers and prevented all histological alterations, demonstrating its potent hepatoprotective efficacy. In support of our findings, FA attenuated liver injury induced by diosbulbin B (Niu et al. 2016), acetaminophen (Yuan et al. 2016), formaldehyde (Gerin et al. 2016), and fluoride (Panneerselvam et al. 2013). In these studies, FA ameliorated serum markers of liver function and alleviated the histological alterations, at least in part, by modulating inflammation and oxidative damage. Therefore, we investigated the effect of FA on oxidative/nitrative stress markers, antioxidant defenses, and inflammatory mediators.
Oxidative damage has been implicated in hepatic and renal injury elicited by MTX (Aladaileh et al. 2019b; Mahmoud et al. 2018). The current study showed an increase in hepatic ROS in rats exposed to MTX. Although the causes of surplus ROS generation by MTX are not fully explored, the role of mitochondrial dysfunction has been acknowledged. In this context, treatment of rat hepatocytes with MTX resulted in decreased MMP, and increased ROS generation, cytochrome c release, and cytotoxicity (Al Maruf et al. 2018). Increased ROS generation and apoptosis were observed in Jurkat T, EL4 T, and Raji B T lymphocytic cell lines treated with low doses of MTX (Herman et al. 2005). Increased infiltration of neutrophils in rats administered with MTX for 3 consecutive days (Kolli et al. 2009), and increased ROS levels, upregulated NADPH oxidase and mitochondrial dysfunction (Abd El-Twab et al. 2019; Heidari et al. 2018) following a single injection of MTX have been proposed as the main causes of renal oxidative stress. Additionally, depleted antioxidant machinery of the mitochondria has been indirectly implicated in MTX hepatotoxicity (Kolli et al. 2014).
Our results added support to the studies showing increased ROS generation in vivo in response to MTX. Excess ROS can induce deleterious effects, such as oxidative damage to cellular macromolecules, resulting in cell death (Fink and Cookson 2005). Accordingly, the lipid peroxidation (LPO) marker MDA and NO were increased in rats that received MTX. NO and superoxide radicals can react producing peroxynitrite, a potent radical that induces DNA breaks and cell death (McKim et al. 2003). The increased NO is directly attributed to upregulation of iNOS (El-Sheikh et al. 2015). Besides lipid peroxidation, ROS activate NF-κB which provokes inflammatory mediators, including TNF-α and IL-1β. In addition, peroxynitrite can activate NF-κB and stimulate the production of pro-inflammatory cytokines in KCs (Matata and Galiñanes 2002). The present study showed increased phosphorylation of hepatic NF-κB, along with elevated serum TNF-α and IL-1β. Hence, increased ROS generation provoked by MTX triggered an inflammatory response mediated via NF-κB.
Interestingly, FA attenuated MTX-induced ROS generation, LPO, and NF-κB activation. The antioxidant effects of FA have been shown in previous studies of experimental liver injury. The antioxidant efficacy of FA could be explained by its dual ability to quench free radicals and enhance the cellular antioxidant defenses. A resonance stabilized phenoxy radical is formed during quenching free radicals by FA’s phenolic nucleus and unsaturated side chain. This radical can condensate with a ferulate radical to produce ROS scavengers (Graf 1992). The antioxidant efficacy of FA was supported by studies showing improvement of in vivo mitochondrial function (Perez-Ternero et al. 2017), and suppressed NADPH oxidase in human mononuclear cells (Perez-Ternero et al. 2017) and H2O2-induced rat vascular smooth muscle cells (Cao et al. 2015) following treatment with FA. In addition to its radical-scavenging activity, FA boosted hepatic antioxidant defenses in MTX-administered rats, thereby mitigated oxidative stress and its deleterious effects.
Next, we determined the effect of MTX on hepatic Nrf2/HO-1 pathway and the modulating role of FA. The administration of MTX resulted in diminished hepatic Nrf2 as described previously (Mahmoud et al. 2017c). Under mild oxidative stress conditions, ROS dissociate Nrf2 from Keap-1 to activate the transcription of antioxidant defenses, including NOQ-1 and HO-1. Therefore, diminished Nrf2 signaling results in increased ROS levels and oxidative stress. The suppressed Nrf2 signaling in this study was confirmed by the downregulation of hepatic NOQ-1 and HO-1 in rats received MTX. Although activated by ROS, sustained and excessive oxidative stress lead to suppression of Nrf2. Accordingly, previous studies have demonstrated the suppression of Nrf2/HO-1 signaling in liver, kidney, and endothelial cells under oxidative stress conditions (Aladaileh et al. 2019b; Mahmoud and Al Dera 2015; Mahmoud et al. 2017e). Interestingly, pre-treatment with FA activated Nrf2 signaling, boosted antioxidant defenses, and prevented excessive ROS generation. Nrf2 activation resulted in upregulation of antioxidant defenses, including HO-1 which catalyzes the degradation of heme into bilirubin which possesses radical scavenging properties (Siow et al. 1999). These findings added support to recent studies showing the involvement of Nrf2 in the protective effect of FA against inhibition of neurite outgrowth induced by lead acetate in vitro (Yu et al. 2016) and γ-radiation-induced oxidative DNA damage in mice (Das et al. 2017). Herein, our study introduced a new information on the role of Nrf2 signaling in the protective mechanism of FA against MTX hepatotoxicity. In addition to mitigating oxidative stress, Nrf2 might mediate, at least in part, the anti-inflammatory effect of FA. Activation of Nrf2 mitigates ROS generation, and suppresses NF-κB and inflammation in experimental liver injury (Mahmoud et al. 2017a; Mahmoud et al. 2017d). In the same context, the lack of Nrf2 in mouse primary cultured astrocytes was associated with NF-κB activation (Pan et al. 2012). Recently, Lampiasi and Montana studied the crosstalk between Nrf2 and NF-κB in the presence of FA in vitro (Lampiasi and Montana 2018). They showed the ability of FA to regulate both NF-κB and Nrf2, and mimic the NF-κB-dependent transcription blocker BMS (Lampiasi and Montana 2018), demonstrating the potent anti-inflammatory efficacy of FA.
Given its dual efficacy to diminish oxidative stress and inflammation, FA prevented hepatocytes death triggered by MTX. MTX provoked apoptosis as evidenced by increased Bax and caspase-3, and declined Bcl-2. Bax provokes cell death by increasing the release of mitochondrial cytochrome c which subsequently activates caspase-3 (Almeida et al. 2000). FA suppressed the expression of pro-apoptosis markers and upregulated Bcl-2. The anti-apoptosis efficacy of FA is a direct result of diminished oxidative injury and inflammation mediated via Nrf2 activation. In addition to Nrf2 activation, we assumed that PPARγ plays a role in the hepatoprotective mechanism of FA. Previous studies from our laboratory have demonstrated suppressed hepatic PPARγ expression following MTX administration (Mahmoud et al. 2017b; Mahmoud et al. 2017c). Here, MTX reduced hepatic PPARγ, an effect that was reversed by FA. Activation of PPARγ has been reported to suppress NADPH oxidase, NF-κB, and pro-inflammatory cytokines (Remels et al. 2009). Inhibition of NF-κB by PPARγ could be explained in terms of direct physical interactions, sequestration of NF-κB co-activators, and transcriptional control of NF-κB target genes. Besides, induced expression of antioxidant genes is an established effect of PPARγ activation (Yu et al. 2014), thereby suppressing ROS generation and NF-κB activation. Moreover, the dual activation of PPARγ and Nrf2 has been associated with protection against liver injury and hepatocarcinogenesis (Mahmoud and Al Dera 2015; Mahmoud et al. 2017b; Mahmoud et al. 2017d).
Conclusions
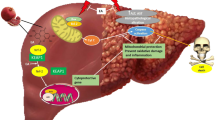
This investigation confers new information on the efficacy of FA in preventing MTX hepatotoxicity. FA prevented surplus production of ROS, inflammatory responses, and cell death provoked by MTX through activating Nrf2/HO-1 signaling and PPARγ (Fig. 9). Therefore, FA represents an effective hepatoprotective phytochemical; however, further studies are needed to elucidate the precise underlying mechanisms.
A proposed schematic diagram illustrating the protective mechanism of FA against MTX-induced liver injury. MTX, methotrexate; ROS, reactive oxygen species; Nrf2, nuclear factor (erythroid-derived 2)-like 2; NF-κB, nuclear factor-kappaB; Bax, Bcl-2-associated X protein; Keap-1, Kelch like-ECH-associated protein 1; ARE, antioxidant response element; PPARγ, peroxisome proliferator activated receptor gamma; RXR, retinoid X receptor
References
Abd El-Twab SM, Hussein OE, Hozayen WG, Bin-Jumah M, Mahmoud AM (2019) Chicoric acid prevents methotrexate-induced kidney injury by suppressing NF-κB/NLRP3 inflammasome activation and up-regulating Nrf2/ARE/HO-1 signaling. Inflamm Res 68:511–523
Al Maruf A, O’Brien PJ, Naserzadeh P, Fathian R, Salimi A, Pourahmad J (2018) Methotrexate induced mitochondrial injury and cytochrome c release in rat liver hepatocytes. Drug Chem Toxicol 41:51–61
Aladaileh SH, Abukhalil MH, Saghir SAM, Hanieh H, Alfwuaires MA, Almaiman AA, Bin-Jumah M, Mahmoud AM (2019a) Galangin activates Nrf2 signaling and attenuates oxidative damage, inflammation, and apoptosis in a rat model of cyclophosphamide-induced hepatotoxicity. Biomolecules 9:346
Aladaileh SH, Hussein OE, Abukhalil MH, Saghir SAM, Bin-Jumah M, Alfwuaires MA, Germoush MO, Almaiman AA, Mahmoud AM (2019b) Formononetin upregulates Nrf2/HO-1 signaling and prevents oxidative stress, inflammation, and kidney injury in methotrexate-induced rats. Antioxidants (Basel, Switzerland):8–430
Almeida OF, Conde GL, Crochemore C, Demeneix BA, Fischer D, Hassan AH, Meyer M, Holsboer F, Michaelidis TM (2000) Subtle shifts in the ratio between pro- and antiapoptotic molecules after activation of corticosteroid receptors decide neuronal fate. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 14:779–790
Barish GD, Narkar VA, Evans RM (2006) PPAR delta: a dagger in the heart of the metabolic syndrome. J Clin Invest 116:590–597
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cao YJ, Zhang YM, Qi JP, Liu R, Zhang H, He LC (2015) Ferulic acid inhibits H2O2-induced oxidative stress and inflammation in rat vascular smooth muscle cells via inhibition of the NADPH oxidase and NF-kappaB pathway. Int Immunopharmacol 28:1018–1025
Cohen G, Dembiec D, Marcus J (1970) Measurement of catalase activity in tissue extracts. Analytical Biochemistry Analytical Biochemistry 34:30–38
Das U, Manna K, Khan A, Sinha M, Biswas S, Sengupta A, Chakraborty A, Dey S (2017) Ferulic acid (FA) abrogates gamma-radiation induced oxidative stress and DNA damage by up-regulating nuclear translocation of Nrf2 and activation of NHEJ pathway. Free Radic Res 51:47–63
El-Sheikh AA, Morsy MA, Abdalla AM, Hamouda AH, Alhaider IA (2015) Mechanisms of thymoquinone hepatorenal protection in methotrexate-induced toxicity in rats. Mediat Inflamm 2015:859383
Erdogan E, Ilgaz Y, Gurgor PN, Oztas Y, Topal T, Oztas E (2015) Rutin ameliorates methotrexate induced hepatic injury in rats. Acta Cir Bras 30:778–784
Fadel A, Mahmoud AM, Ashworth JJ, Li W, Ng YL, Plunkett A (2018) Health-related effects and improving extractability of cereal arabinoxylans. Int J Biol Macromol 109:819–831
Famurewa AC, Folawiyo AM, Epete MA, Igwe EC, Okike PI, Maduagwuna EK (2019) Abrogation of hepatic damage induced by anticancer drug methotrexate by Zobo (Hibiscus sabdariffa extract) supplementation via targeting oxidative hepatotoxicity in rats. Journal of dietary supplements 16:318–330
Fink SL, Cookson BT (2005) Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun 73:1907–1916
Gerin F, Erman H, Erboga M, Sener U, Yilmaz A, Seyhan H, Gurel A (2016) The effects of ferulic acid against oxidative stress and inflammation in formaldehyde-induced hepatotoxicity. Inflammation 39:1377–1386
Graf E (1992) Antioxidant potential of ferulic acid. Free Radic Biol Med 13:435–448
Grisham MB, Johnson GG, Lancaster JR Jr (1996) Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol 268:237–246
Hassanzadeh P, Arbabi E, Atyabi F, Dinarvand R (2017) Ferulic acid exhibits antiepileptogenic effect and prevents oxidative stress and cognitive impairment in the kindling model of epilepsy. Life Sci 179:9–14
Heidari R, Ahmadi A, Mohammadi H, Ommati MM, Azarpira N, Niknahad H (2018) Mitochondrial dysfunction and oxidative stress are involved in the mechanism of methotrexate-induced renal injury and electrolytes imbalance. Biomed Pharmacother 107:834–840
Herman S, Zurgil N, Deutsch M (2005) Low dose methotrexate induces apoptosis with reactive oxygen species involvement in T lymphocytic cell lines to a greater extent than in monocytic lines. Inflamm Res 54:273–280
Jaiswal AK (2004) Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med 36:1199–1207
Khafaga AF, El-Sayed YS (2018) Spirulina ameliorates methotrexate hepatotoxicity via antioxidant, immune stimulation, and proinflammatory cytokines and apoptotic proteins modulation. Life Sci 196:9–17
Khan ZA, Tripathi R, Mishra B (2012) Methotrexate: a detailed review on drug delivery and clinical aspects. Expert Opin Drug Deliv 9:151–169
Kim JH, Song J, Park KW (2015) The multifaceted factor peroxisome proliferator-activated receptor gamma (PPARgamma) in metabolism, immunity, and cancer. Arch Pharm Res 38:302–312
Kolli VK, Abraham P, Isaac B, Selvakumar D (2009) Neutrophil infiltration and oxidative stress may play a critical role in methotrexate-induced renal damage. Chemotherapy. 55:83–90
Kolli VK, Natarajan K, Isaac B, Selvakumar D, Abraham P (2014) Mitochondrial dysfunction and respiratory chain defects in a rodent model of methotrexate-induced enteritis. Hum Exp Toxicol 33:1051–1065
Lampiasi N, Montana G (2018) An in vitro inflammation model to study the Nrf2 and NF-kappaB crosstalk in presence of ferulic acid as modulator. Immunobiology 223:349–355
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 25:402–408
Mahmoud AM, Al Dera HS (2015) 18β-Glycyrrhetinic acid exerts protective effects against cyclophosphamide-induced hepatotoxicity: potential role of PPARγ and Nrf2 upregulation. Genes Nutr 10:41
Mahmoud AM, Germoush MO, Alotaibi MF, Hussein OE (2017a) Possible involvement of Nrf2 and PPARγ up-regulation in the protective effect of umbelliferone against cyclophosphamide-induced hepatotoxicity. Biomed Pharmacother 86:297–306
Mahmoud AM, Hozayen WG, Ramadan SM (2017b) Berberine ameliorates methotrexate-induced liver injury by activating Nrf2/HO-1 pathway and PPARgamma, and suppressing oxidative stress and apoptosis in rats. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 94:280–291
Mahmoud AM, Hussein OE, Hozayen WG, Abd El-Twab SM (2017c) Methotrexate hepatotoxicity is associated with oxidative stress, and down-regulation of PPARgamma and Nrf2: protective effect of 18beta-glycyrrhetinic acid. Chem Biol Interact 270:59–72
Mahmoud AM, Mohammed HM, Khadrawy SM, Galaly SR (2017d) Hesperidin protects against chemically induced hepatocarcinogenesis via modulation of Nrf2/ARE/HO-1, PPARgamma and TGF-beta1/Smad3 signaling, and amelioration of oxidative stress and inflammation. Chem Biol Interact 277:146–158
Mahmoud AM, Wilkinson FL, Jones AM, Wilkinson JA, Romero M, Duarte J, Alexander MY (2017e) A novel role for small molecule glycomimetics in the protection against lipid-induced endothelial dysfunction: involvement of Akt/eNOS and Nrf2/ARE signaling. Biochim Biophys Acta 1861:3311–3322
Mahmoud AM, Germoush MO, Al-Anazi KM, Mahmoud AH, Farah MA, Allam AA (2018) Commiphora molmol protects against methotrexate-induced nephrotoxicity by up-regulating Nrf2/ARE/HO-1 signaling. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 106:499–509
Mahmoud AM, Hozayen WG, Hasan IH, Shaban E, Bin-Jumah M (2019) Umbelliferone ameliorates CCl4-induced liver fibrosis in rats by upregulating PPARgamma and attenuating oxidative stress, inflammation, and TGF-beta1/Smad3 signaling. Inflammation 42:1103–1116
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of Pyrogallol and a convenient assay for superoxide dismutase. FEBS European Journal of Biochemistry 47:469–474
Matata BM, Galiñanes M (2002) Peroxynitrite is an essential component of cytokines production mechanism in human monocytes through modulation of nuclear factor-kappa B DNA binding activity. J Biol Chem 277:2330–2335
Matkovics B, Szabo L, Varga IS (1998) Determination of enzyme activities in lipid peroxidation and glutathione pathways (in Hungarian). Laboratoriumi Diagnosztika 15:248–249
McKim SE, Gäbele E, Isayama F, Lambert JC, Tucker LM, Wheeler MD, Connor HD, Mason RP, Doll MA, Hein DW, Arteel GE (2003) Inducible nitric oxide synthase is required in alcohol-induced liver injury: studies with knockout mice. Gastroenterology 125:1834–1844
Mehrzadi S, Mehrabani M, Malayeri AR, Bakhshayesh M, Kalantari H, Goudarzi M (2019) Ellagic acid as a potential antioxidant, alleviates methotrexate-induced hepatotoxicity in male rats. Acta Chir Belg 119:69–77
Morgan MJ, Liu ZG (2011) Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res 21:103–115
Niu C, Sheng Y, Zhu E, Ji L, Wang Z (2016) Ferulic acid prevents liver injury induced by Diosbulbin B and its mechanism. Bioscience trends 10:386–391
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pan H, Wang H, Wang X, Zhu L, Mao L (2012) The absence of Nrf2 enhances NF-kappaB-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediat Inflamm 2012:217580
Panneerselvam L, Subbiah K, Arumugam A, Senapathy JG (2013) Ferulic acid modulates fluoride-induced oxidative hepatotoxicity in male Wistar rats. Biol Trace Elem Res 151:85–91
Perez-Ternero C, Werner CM, Nickel AG, Herrera MD, Motilva MJ, Bohm M, Alvarez de Sotomayor M, Laufs U (2017) Ferulic acid, a bioactive component of rice bran, improves oxidative stress and mitochondrial biogenesis and dynamics in mice and in human mononuclear cells. J Nutr Biochem 48:51–61
Remels AH, Langen RC, Gosker HR, Russell AP, Spaapen F, Voncken JW, Schrauwen P, Schols AM (2009) PPARgamma inhibits NF-kappaB-dependent transcriptional activation in skeletal muscle. Am J Physiol Endocrinol Metab 297:E174–E183
Satta S, Mahmoud AM, Wilkinson FL, Yvonne Alexander M, White SJ (2017) The role of Nrf2 in cardiovascular function and disease. Oxidative Med Cell Longev 2017:9237263
Saulnier L, Thibault J-F (1999) Ferulic acid and diferulic acids as components of sugar-beet pectins and maize bran heteroxylans. J Sci Food Agric 79:396–402
Siow RC, Sato H, Mann GE (1999) Heme oxygenase-carbon monoxide signalling pathway in atherosclerosis: anti-atherogenic actions of bilirubin and carbon monoxide? Cardiovasc Res 41:385–394
Stevens JL, Baker TK (2009) The future of drug safety testing: expanding the view and narrowing the focus. Drug Discov Today 14:162–167
Yu Y, Wu Y, Wen G, Yang W (2014) Effect of pioglitazone on the expression of TLR4 in renal tissue of diabetic rats. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 30:793–797
Yu CL, Zhao XM, Niu YC (2016) Ferulic acid protects against Lead acetate-induced inhibition of neurite outgrowth by upregulating HO-1 in PC12 cells: involvement of ERK1/2-Nrf2 pathway. Mol Neurobiol 53:6489–6500
Yuan J, Ge K, Mu J, Rong J, Zhang L, Wang B, Wan J, Xia G (2016) Ferulic acid attenuated acetaminophen-induced hepatotoxicity though down-regulating the cytochrome P 2E1 and inhibiting toll-like receptor 4 signaling-mediated inflammation in mice. Am J Transl Res 8:4205–4214
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University for supporting this research through the Fast-track Research Funding Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Mohamed Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mahmoud, A.M., Hussein, O.E., Hozayen, W.G. et al. Ferulic acid prevents oxidative stress, inflammation, and liver injury via upregulation of Nrf2/HO-1 signaling in methotrexate-induced rats. Environ Sci Pollut Res 27, 7910–7921 (2020). https://doi.org/10.1007/s11356-019-07532-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07532-6