Abstract
Individuals with higher contaminant burdens are expected to be in poorer physical health and be of lower individual body condition and energetic status, potentially resulting in reduced ornamentation or increased asymmetry in bilateral features. The degree and magnitude of this effect also would be expected to vary by sex, as female birds depurate contaminants into eggs. We tested for relationships among mercury in feathers, sex, and elaborate feather ornaments that relate to individual quality in crested auklets (Aethia cristatella), small planktivorous seabirds in the North Pacific Ocean. We found no relationships between mercury and the size of individuals’ forehead crest or degree of measurement asymmetry in auricular plumes, both of which are favoured by intersexual selection. Females had significantly greater mercury concentrations than males (females. 1.02 ± 0.39 μg/g; males, 0.75 ± 0.32 μg/g); but concentrations were below that known to have physiological effects, as expected for a secondary consumer. Sex differences in overwintering area for this long-distance migrant species (more females in the Kuroshio Current Large Marine Ecosystem than males) could be the reason for this seemingly counterintuitive result between sexes. Further research relating mercury burden to overwintering ecology and diet contents would build on our results and further elucidate interrelationships between sex, sexually selected feather ornaments and contaminant burden.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury (Hg) is a pervasive global contaminant that is largely produced anthropogenically, and projected to increase into the future (Driscoll et al. 2013; Krabbenhoft and Sunderland 2013; Lamborg et al. 2014; Lindberg et al. 2007; Selin 2014; Streets et al. 2009). As a potent neurotoxin, it can have detrimental effects on wildlife, including changes in physiology, behaviour and survival (Ackerman et al. 2016b; Goutte et al. 2014; Heinz et al. 2009; Jackson et al. 2016; Thompson 1996; Weiner et al. 2003). Understanding which species are at risk from high concentrations of contaminants such as Hg and what factors influence those conditions is therefore an important goal for managers and conservation biologists (Golden and Rattner 2003; Provencher et al. 2014; Thompson 1996). Mercury contamination in oceans and its prevalence in marine food chains is related to atmospheric fallout of particulates originating mostly from Asian coal burning (Pacyna et al. 2006) and its subsequent transformation into toxic methylmercury (MeHg) (Sunderland et al. 2009).
Birds are effective monitors of Hg in the environment, because they can integrate signals over space and time, Hg in tissues is dietary in origin, and tissues can easily be sampled non-destructively (Monteiro and Furness 1995; Monteiro and Furness 2001; Monteiro et al. 1998). Birds regulate their Hg body burden by excreting the toxic form of Hg, MeHg into growing feathers (Bond and Diamond 2009), which are inert once fully grown. The Hg in feathers is bound to disulphide bonds and remains stable (Appelquist et al. 1984; Crewther et al. 1965), allowing for a retrospective examination of Hg exposure (Bond et al. 2015; Vo et al. 2011).
A variety of factors affect Hg concentrations in birds, including proximity to point sources (Finger et al. 2015; Jackson et al. 2011), trophic position and diet (Becker et al. 2002; Elliott and Elliott 2016), age class (Thompson et al. 1991), and sex (Robinson et al. 2012). Individuals closer to Hg sources, those at higher trophic positions, and adults tend to have higher Hg than individuals farther from sources, at lower trophic positions, and chicks. Males are generally thought to have higher Hg concentrations than females, as females can also eliminate Hg in eggs (Braune and Gaskin 1987b; Lewis et al. 1993; Monteiro and Furness 2001; Robinson et al. 2012).
Crested auklets (Aethia cristatella) are small planktivorous seabirds breeding around the Bering and Okhotsk Seas, have a diet of mostly euphausiids and calanoid copepods, and lay a single egg each year (Bond et al. 2012; Jones 1993a). Crested auklets are socially monogamous and have elaborate sexually monomorphic feather and bill ornaments that are displayed during courtship (Jones et al. 2000). Their most prominent feather ornament is a conspicuous forehead crest that experiments showed to be a product of mutual sexual selection and paired white auricular plumes (Jones and Hunter 1993; Jones et al. 2000; Jones et al. 2004). Although Crested auklet males have a larger body size and proportionally larger bills than females, crest and auricular plume length are sexually monomorphic (Jones 1993b; Jones et al. 2000). Like many sexually selected traits, crested auklet crest length and the degree of measurement asymmetry of the auricular plumes are highly variable in expression across individuals of both sexes (Jones et al. 2000). This kind of variability in a sexually selected trait has been suggested to relate to its function as an indicator of individual quality in mate choice (Van Valen 1962; Zahavi 1975), in which individuals benefit either directly or indirectly by mating with healthy individuals as indicated by the expression of the sexually selected or more symmetrical trait (Spencer and MacDougall-Shackleton 2011). Nevertheless, there are few clues as to what aspect of quality crested auklet crests might signal as no relationships between body condition and survival have been found (Jones et al. 2000; Jones et al. 2004). There is also the question of why variability in crest length is greater in females than in males (Jones et al. 2000).
In other taxa, greater Hg concentrations have been associated with the degree of asymmetry of feather traits, though not in all cases (Evers et al. 2008; Herring et al. 2016). Here we aimed to test for relationships of mercury burden, sex, and sexually selected feather ornaments in this spectacularly ornamented sexually monomorphic seabird. We predicted that crested auklet males would have higher feather Hg than females because females can eliminate Hg in eggs and that individuals with longer crests and more symmetrical auricular plumes, being in better condition, would have lower feather Hg concentrations.
Methods
We collected feather samples from Sirius Point, Kiska Island in the western Aleutian Islands, Alaska (52° 08′N, 177° 36′E), in June and July 2009 (n = 28) and 2010 (n = 6); no individuals were sampled more than once. Birds were captured on the colony surface using noose carpets (Jones et al. 2004), aged (adult or subadult) following Bédard and Sealy (1984), and sex determined from bill morphology (Jones 1993b). We restricted our samples to adult birds and an equal number of females and males (n = 17 of each sex). Birds were weighed using an electronic balance (± 1 g), and we measured crest length (± 0.1 mm) and length of the auricular plumes (± 0.1 mm) using callipers (Jones et al. 2000; Jones et al. 2004). Measurement asymmetry of auricular plumes was calculated as: \( \sqrt{{\left(\mathrm{left}-\mathrm{right}\right)}^2} \); all measurements were performed by one individual (ALB).
Two breast feathers were plucked and placed in individual paper envelopes. Crested auklets replace breast feathers and feather ornaments prior to breeding as in other Aethia spp. auklets (Bédard and Sealy 1984; Bond et al. 2013; Pitocchelli et al. 2003; Pyle 2008). Feathers therefore represent the accumulation of Hg since the previous moult, the same period over which they can invest in ornamentation.
Feathers were placed in sterile glass scintillation vials, washed in a 2:1 (v/v) chloroform/methanol solution to remove external contamination (Borghesi et al. 2016), and air dried for 24 h at ambient room temperature. We analysed two feathers from each individual (Bond and Diamond 2008) using a DMA-80 (atomic absorption spectrometry; Milestone, Ltd) (Haynes et al. 2006). Feathers were placed in nickel boats and kept in place using glass capillary tubes and Nanopure deionized water. Method blanks consisting of capillary tubes and water were all below the level of detection (0.04 ng Hg).
We analysed three certified reference materials (CRMs) for quality assurance and control: lobster hepatopancreas (TORT-3, National Research Council of Canada; certified concentration ± expanded uncertainty (UCRM; Joint Committee for Guides in Metrology 2008): 0.292 ± 0.022 μg/g; recovery, 113 ± 2%, n = 8), dogfish muscle (DORM-4, National Research Council of Canada; certified concentration, 0.412 ± 0.036 μg/g; recovery, 106 ± 1%, n = 8), and human hair (IAEA-85, International Atomic Energy Agency; certified concentration, 23.20 ± 0.06 μg/g; recovery, 98 ± 1%, n = 5).
Statistical methods
We assessed normality of Hg data using Shapiro-Wilk test (Shapiro and Wilk 1965) and then constructed a series of general linear models using year of collection (2009 or 2010) and sex (male or female) as predictors. We also included crest length and asymmetry of auricular plumes (and their interactions) to predict feather Hg, as they can also act as a signal of individual quality (Jones 1993a; Jones et al. 2000; Jones and Montgomerie 1991a; Jones and Montgomerie 1991b). Models were compared using Akaike’s Information Criteria adjusted for small sample size (AICc) using the package AICcmodavg (Mazerolle 2017); models with ΔAICc > 2 were not considered competitive. Model terms were considered significant when p < 0.05. We calculated the effect size using Hedge’s g (an unbiased estimator of the standardized mean difference) (Hedges 1982) using the package compute.es (Del Re 2013) in R 3.3.2 (R Core Team 2018). Differences in morphometrics were assessed using t tests. Data are presented as means ± SD.
Results
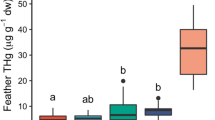
Data were normally distributed (Shapiro-Wilk W = 0.95, p = 0.09), so Hg data were not transformed. Males had longer auricular plumes than females (males, 33.1 ± 8.1 mm; females, 27.6 ± 6.7 mm; t32 = 2.17, p = 0.038), but crest length did not differ between sexes (36.3 ± 5.5 mm; t32 = − 1.48, p = 0.15). The model for predicting feather Hg that included sex received the most support (wi = 0.73); no other model had ΔAICc < 2, and models that included ornaments were not competitive (ΔAICc < 9.8; Table 1), so results are from the top-ranked model only. Feather Hg was significantly higher in females (1.02 ± 0.39 μg/g) than in males (0.75 ± 0.32 μg/g; t32 = −2.18, p = 0.037; Fig. 1). The effect size (± variance) of sex was g = − 0.73 ± 0.12 (95% confidence interval: 0.02–1.44), indicating a large effect size (Cohen 1988).
Discussion
We found higher Hg concentrations in female crested auklets than males at Kiska Island, counter to the hypothesis that females’ Hg burden should be lower as they can depurate Hg into their egg. Crested auklets lay a single egg, weighing approximately 14% of female body mass (260 g; Fraser et al. 1999; Jones 1993a). Previous studies that examined this hypothesis found that, though it was supported, depuration into eggs could not fully account for the differences in Hg between sexes (Ackerman et al. 2016a; Monteiro and Furness 2001). In some species, however, there is no significant relationship between Hg in females’ winter-grown breast feathers and Hg in their subsequent eggs, as the kinetics of Hg depend on the timing and pattern of feather moult (Ackerman et al. 2016a; Braune and Gaskin 1987a; Thompson et al. 1998).
The effect size of sex on feather Hg concentrations was in the 7th percentile of a recent review (Robinson et al. 2012), suggesting that our study is one of the few cases where the difference in feather Hg is so great between sexes and greater in females than males. This suggests either a dietary/physiological difference between the sexes or spatial segregation resulting in differential Hg exposure. Male and female crested auklets’ behaviour during the breeding differs markedly (Fraser et al. 2002; Wails 2016), and they are the most sexually dimorphic auk (Gaston and Jones 1998; Jones 1993b; Jones et al. 2000).
We would expect differences in feather Hg if females and males differed in either their exposure or physiology. During the nonbreeding season, it is expected that Hg exposure (and therefore concentrations of Hg acquired) should be equal between the sexes as females are not laying eggs, and the physiological kinetics of Hg should be similar (Monteiro and Furness 2001). Crested auklet breast feathers are likely grown in the early spring (Pyle 2008); males and females differ in body size and also bill shape and size – with the larger males having more strongly hooked bills in summer (Jones 1993b). Crested auklet males and females take on different roles during chick rearing, with a greater role for females in chick provisioning and of males in chick guarding (Fraser et al. 2002), with strong differences in diurnal timing of colony attendance between the sexes (Wails 2016). Crested auklets are the only member of the family Alcidae for which individuals’ sex can be determined by examination of external characters and are the most sexually dimorphic auk (Gaston and Jones 1998; Jones 1993b; Jones et al. 2000). Male bill shape and size may be affected by intra- or intersexual selection because the bill is used for fighting as well as display (Gaston and Jones 1998), but the dimorphism could manifest in dietary differences between sexes (Mancini et al. 2013; Phillips et al. 2011) and therefore Hg exposure. Studies of crested auklet diet outside the breeding season are virtually unknown, save one specimen shot in January 1883 (Stejneger 1885), and a study of nine birds (2 adult males, 3 subadult males, 4 subadult females) collected in Unimak Pass in the winter of 1986–1987, which did not examine sex or age differences (Troy and Bradstreet 1991), though diet composition appears to be broadly similar to that of chicks in the breeding season, dominated by euphausiids (Bond et al. 2012). Why then did females in our sample have higher Hg? Hg in feathers could also represent some of the body burden acquired during the previous breeding season. Hg is eliminated via feathers from a body pool acquired several months previously. An understanding of nonbreeding dietary differences between male and female crested auklets is lacking and impedes our interpretation.
Sex differences in Hg could also arise from spatial segregation (Watanuki et al. 2016). Based on archival geolocation tracking data of birds from Buldir and Gareloi Island, Aleutian Islands between 2013 and 2015, significantly more females than males overwintered in the Kuroshio Current Large Marine Ecosystem (K. Robbins unpublished data). The Kuroshio Current Large Marine Ecosystem lies off the east cost of Japan (Di Lorenzo et al. 2013); Red-legged kittiwakes (Rissa brevirostris) wintering in the Kuroshio Current had the highest feather total Hg concentrations (Fleishman et al. 2019). Streaked Shearwaters (Calonectris leucomelas) wintering in different areas of the Pacific Ocean showed considerable variation in feather Hg concentrations (Watanuki et al. 2016), and a similar pattern may be present in crested auklets.
Crested auklet males and females do not differ significantly in crest length (i.e., they are sexually monomorphic for this ornament; Jones et al. 2000), even though females have a greater Hg burden. Notably, variability in crest size in crested auklets was found to be greater in females than in males (Jones et al. 2000). Feather Hg was also unrelated to the degree of measurement asymmetry of auklets’ auricular plumes, another possible indicator of individual quality. One possible explanation is that the Hg concentrations we observed were too low to cause any negative physiological effects. Among piscivores, including many seabirds, Hg concentrations of > 20 μg/g in feathers are the threshold at which when negative effects are likely to manifest (Ackerman et al. 2016b; Bond et al. 2015; Evers et al. 2014). Sublethal effects, however (such as ornament expression), are likely affected at lower concentrations, though the effect threshold is undoubtedly species specific; among birds, smaller species have lower Hg toxicity thresholds compared to larger species (Fuchsman et al. 2016). The maximum feather Hg concentration we measured was 1.69 μg/g; within individuals, Hg concentrations in feathers is typically greater than concentrations in blood, and though toxicity thresholds are highly variable (Ackerman et al. 2016b; Fuchsman et al. 2016), we conclude that crested auklets are not likely experiencing deleterious effects of Hg.
Our results indicate low concentrations of Hg in the feathers of a planktivorous seabird are unrelated to ornament expression, likely owing to the low concentrations we measured. Furthermore, we identified a significantly greater mercury burden in females compared to males that appears to be unrelated to expression of sexually selected ornaments, and was contrary to expectations, suggesting some unknown physiological or behaviour differences between sexes, which deserves further exploration. Measurement of Hg burden in feathers is not difficult or invasive and should be considered as an add-on for future seabird tracking studies, as these birds are wide-ranging top predators of the world’s oceans.
References
Ackerman JT, Eagles-Smith CA, Herzog MP, Hartman CA (2016a) Maternal transfer of contaminants in birds: Mercury and selenium concentrations in parents and their eggs. Environ Pollut 210:145–154. https://doi.org/10.1016/j.envpol.2015.12.016
Ackerman JT et al (2016b) Avian mercury exposure and toxicological risk across western North America: a synthesis. Sci Total environ 568:749–769. https://doi.org/10.1016/j.scitotenv.2016.03.071
Appelquist H, Asbirk S, Drabæk I (1984) Mercury monitoring: mercury stability in bird feathers. Mar Pollut Bull 15:22–24. https://doi.org/10.1016/0025-326x(84)90419-3
Becker PH, González-Solís J, Behrends B, Croxall J (2002) Feather mercury levels in seabirds at South Georgia: influence of trophic position, sex and age. Mar Ecol Prog Ser 243:261–269. https://doi.org/10.3354/meps243261
Bédard J, Sealy SG (1984) Moults and feather generations in the least. Crested and Parakeet Auklets J Zool (Lond) 202:461–488
Bond AL, Diamond AW (2008) High within-individual variation in total mercury concentration in seabird feathers. Environ Toxicol Chem 27:2375–2377. https://doi.org/10.1897/08-163.1
Bond AL, Diamond AW (2009) Total and methyl mercury concentrations in seabird feathers and eggs. Arch Environ Contam Toxicol 56:286–291. https://doi.org/10.1007/s00244-008-9185-7
Bond AL, Hobson KA, Branfireun BA (2015) Rapidly increasing methyl mercury in endangered ivory Gull (Pagophila eburnea) feathers over a 130-year record. Proc R Soc Lond B Biol Sci 282:20150032. https://doi.org/10.1098/rspb.2015.0032
Bond AL, Jones IL, Williams JC, Byrd GV (2012) Diet of auklet chicks in the Aleutian Islands, Alaska: similarity among islands, interspecies overlap, and relationships to ocean climate. J Ornithol 153:115–129. https://doi.org/10.1007/s10336-011-0704-3
Bond AL, Konyukhov NB, Jones IL (2013) Variation in primary moult in Least Auklets Condor 115:348–355 https://doi.org/10.1525/cond.2013.110062
Borghesi F, Migani F, Andreotti A, Baccetti N, Bianchi N, Birke M, Dinelli E (2016) Metals and trace elements in feathers: a geochemical approach to avoid misinterpretation of analytical responses. Sci Total Environ 544:476–494. https://doi.org/10.1016/j.scitotenv.2015.11.115
Braune BM, Gaskin DE (1987a) A mercury budget for the Bonaparte's Gull during autumn moult. Ornis Scand 18:244–250
Braune BM, Gaskin DE (1987b) Mercury levels in Bonaparte's gulls (Larus philadelphia) during autumn molt in the Quoddy region, New Brunswick, Canada. Arch Environ Contam Toxicol 16:539–549
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum Associates, Hillsdale
Crewther WG, Fraser RDB, Lennox FG, Lindley H (1965) The chemistry of keratins. Adv Protein Chem 20:191–303
Del Re AC (2013) compute.es: Compute Effect Sizes. R package version 0:2–2 http://CRAN.R-project.org/package=siar
Di Lorenzo E et al (2013) Synthesis of Pacific Ocean climate and ecosystem dynamics. Oceanography 26:68–81. https://doi.org/10.5670/oceanog.2013.76
Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N (2013) Mercury as a global pollutant: sources, pathways, and effects. Environ Sci Technol 47:4967–4983. https://doi.org/10.1021/es305071v
Elliott KH, Elliott JE (2016) Origin of sulfur in diet drives spatial and temporal mercury trends in seabird eggs from Pacific Canada 1968–2015. Environ Sci Technol 50:13380–13386. https://doi.org/10.1021/acs.est.6b05458
Evers DC et al (2008) Adverse effects from environmental mercury loads on breeding common loons. Ecotoxicology 17:69–81. https://doi.org/10.1007/s10646-007-0168-7
Evers DC et al (2014) Historic and contemporary mercury exposure and potential risk to yellow-billed loons (Gavia adamsii) breeding in Alaska and Canada. Waterbirds 37:147–159
Finger A, Lavers JL, Dann P, Nugegoda D, Orbell JD, Robertson B, Scarpaci C (2015) The little penguin (Eudyptula minor) as an indicator of coastal trace metal pollution. Environ Pollut 205:365–377
Fleishman AB et al (2019) Wintering in the western subarctic Pacific increases mercury contamination of red-legged kittiwakes. Environ Sci Technol 53:13398–13407. https://doi.org/10.1021/acs.est.9b03421
Fraser GS, Jones IL, Hunter FM (2002) Male-female differences in parental care in monogamous Crested Auklets Condor 104:413–423. https://doi.org/10.1650/0010-5422(2002)104[0413:MFDIPC]2.0.CO;2
Fraser GS, Jones IL, Williams JC, Hunter FM, Scharf L, Byrd GV (1999) Breeding biology of crested auklets at Buldir and Kasatochi Islands. Alaska Auk 116:690–701
Fuchsman PC, Brown LE, Henning MH, Bock MJ, Magar VS (2016) Toxicity reference values for methylmercury effects on avian reproduction: Critical review and analysis. Environ Toxicol Chem 36:294–319. https://doi.org/10.1002/etc.3606
Gaston AJ, Jones IL (1998) The auks: Alcidae. Oxford University Press, New York
Golden NH, Rattner BA (2003) Ranking terrestrial vertebrate species for utility in biomonitoring and vulnerability to environmental contaminants. Rev Environ Toxicol 176:67–136
Goutte A et al (2014) Demographic consequences of heavy metals and persistent organic pollutants in a vulnerable long-lived bird, the wandering albatross. Proc R Soc Lond B biol Sci 281:20133313
Haynes S, Gragg R, Johnson E, Robinson L, Orazio C (2006) An evaluation of a reagentless method for the determination of total mercury in aquatic life. Water Air Soil Pollut 172:359–374
Hedges LV (1982) Estimation of effect size from a series of independent experiments. Psychol Bull 92:490–499
Heinz GH, Hoffman DJ, Klimstra JD, Stebbins KR, Kondrad SL, Erwin CA (2009) Species differences in the sensitivity of avian embryos to methylmercury. Arch Environ Contam Toxicol 56:129–138. https://doi.org/10.1007/s00244-008-9160-3
Herring G, Eagles-Smith CA, Ackerman JT (2016) Mercury exposure may influence fluctuating asymmetry in waterbirds. Environ Toxicol Chem 36:1599–1605. https://doi.org/10.1002/etc.3688
Jackson A et al (2016) Mercury risk to avian piscivores across western United States and Canada. Sci Total environ 568:685–696. https://doi.org/10.1016/j.scitotenv.2016.02.197
Jackson AK et al (2011) Mercury exposure in terrestrial birds far downstream of an historical point source. Environ Pollut 159:3302–3308. https://doi.org/10.1016/j.envpol.2011.08.046
Joint Committee for Guides in Metrology (2008) Evaluation of measurement data — Guide to the expression of uncertainty in measurement. JCGM 100:2008. ISO 50461. JCGM Working Group 1, Geneva
Jones IL (1993a) Crested auklet (Aethia cristatella). In: Poole A, Gill F (eds) The Birds of North America, no. 70. The birds of North America Inc., Philadelphia
Jones IL (1993b) Sexual differences in bill shape and external measurements of crested auklets Wilson Bull 105:525-529
Jones IL, Hunter FM (1993) Mutual sexual selection in a monogamous seabird. Nature 362:238–239
Jones IL, Hunter FM, Fraser GS (2000) Patterns of variation in ornaments of Crested Auklets Aethia cristatella. J Avian Biol 31:119–127
Jones IL, Hunter FM, Robertson GJ, Fraser GS (2004) Natural variation in the sexually selected feather ornaments of crested auklets (Aethia cristatella) does not predict future survival. Behav Ecol 15:332–337
Jones IL, Montgomerie R (1991a) Least auklet ornaments: do they function as quality indicators? Behav Ecol Sociobiol 30:43–52
Jones IL, Montgomerie R (1991b) Mating and remating of least auklets (Aethia pusilla) relative to ornamental traits. Behav Ecol 2:249–257
Krabbenhoft DP, Sunderland EM (2013) Global change and mercury. Science 341:1457–1458. https://doi.org/10.1126/science.1242838
Lamborg CH et al (2014) A global ocean inventory of anthropogenic mercury based on water column measurements. Nature 512:65–69. https://doi.org/10.1038/nature13563
Lewis SA, Becker PH, Furness RW (1993) Mercury levels in eggs, tissues, and feathers of Herring gulls Larus argentatus from the German Wadden Sea coast. Environ Pollut 80:293–299
Lindberg S et al (2007) A synthesis of progress and uncertainties in attributing the sources of mercury in deposition. Ambio 36:19–32. https://doi.org/10.1579/0044-7447(2007)36[19:asopau]2.0.co;2
Mancini PL, Bond AL, Hobson KA, Duarte LS, Bugoni L (2013) Foraging segregation in tropical and polar seabirds: testing the intersexual competition Hypothesis. J Exp Mar Biol Ecol 449:186–193. https://doi.org/10.1016/j.jembe.2013.09.011
Mazerolle MJ (2017) AICcmodavg - model selection and multimodel inference based on (Q)AIC(c) R package version 2.1-1
Monteiro LR, Furness RW (1995) Seabirds as monitors of mercury in the marine environment. Water Air Soil Pollut 80:851–870
Monteiro LR, Furness RW (2001) Kinetics, dose-response, and excretion of methylmercury in free-living adult Cory's shearwaters environ. Sci Technol 35:739–746. https://doi.org/10.1021/es000114a
Monteiro LR, Granadeiro JP, Furness RW (1998) Relationship between mercury levels and diet in Azores seabirds. Mar Ecol Prog Ser 166:259–266
Pacyna EG, Pacyna JM, Steenhuisen F, Wilson S (2006) Global anthropogenic mercury emission inventory for 2000. Atmos Environ 40:4048–4063. https://doi.org/10.1016/j.atmosenv.2006.03.041
Phillips RA, McGill RAR, Dawson DA, Bearhop S (2011) Sexual segregation in distribution, diet and trophic level of seabirds: insights from stable isotope analysis. Mar Biol 158:2199–2208
Pitocchelli J, Piatt JF, Carter HR (2003) Variation in plumage, molt, and morphology of the whiskered auklet (Aethia pygmaea) in Alaska. J Field Ornithol 74:90–98
Provencher JF, Mallory ML, Braune BM, Forbes MR, Gilchrist HG (2014) Mercury and marine birds in Arctic Canada: effects, current trends, and why we should be paying closer attention. Environ Rev 22:244–255
Pyle P (2008) Identification guide to north American birds. Part 2. Slate Creek press, Bolinas
R Core Team (2018) R: a language and environment for statistical computing. Version 3.4.4 [computer program]. R Foundation for Statistical Computing, Vienna, Austria
Robinson SA, Lajeunesse MJ, Forbes MR (2012) Sex differences in mercury contamination of birds: testing multiple hypotheses with meta-analysis. Environ Sci Technol 46:7094–7101. https://doi.org/10.1021/es204032m
Selin NE (2014) Global change and mercury cycling: challenges for implementing a global mercury treaty. Environ Toxicol Chem 33:1202–1210. https://doi.org/10.1002/etc.2374
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52:591–611
Spencer KA, MacDougall-Shackleton SA (2011) Indicators of development as sexually selected traits: the developmental stress hypothesis in context. Behav Ecol 22:1–9. https://doi.org/10.1093/beheco/arq068
Stejneger L (1885) Results of ornithological explorations in the Commander Islands and in Kamtschatka Bull US Nat Mus 29:1–382
Streets DG, Zhang Q, Wu Y (2009) Projection of global mercury emissions in 2050. Environ Sci Technol 43:2983–2988
Sunderland EM, Krabbemhoft DP, Moreau JW, Strode SA, Landing WM (2009) Mercury sources, distribution, and bioavailability in the North Pacific Ocean: insights from data and models. Glob Biogeochem Cycles 23:–GB2010. https://doi.org/10.1029/2008GB003425
Thompson DR (1996) Mercury in birds and terrestrial mammals. In: Beyer WN, Heinz GH, Redmon-Norwood AW (eds) Environmental contaminants in wildlife: interpreting tissue concentrations. SETAC Special Publications. CRC Press, New York
Thompson DR, Bearhop S, Speakman JR, Furness RW (1998) Feathers as a means of monitoring mercury in seabirds: insights from stable isotope analysis. Environ Pollut 101:193–200
Thompson DR, Hamer KC, Furness RW (1991) Mercury accumulation in great skuas Catharacta skua of known age and sex, and its effects upon breeding and survival. J Appl Ecol 28:672–684
Troy DM, Bradstreet MSW (1991) Marine bird abundance and habitat use. In: Truitt JC, Kertell K (eds) Marine birds and mammals of the Unimak pass area: abundance, habitat use and vulnerability. OCS study MMS 91-0038. LGL Alaska research associates, Inc., Anchorage, pp 5.1–5.70
Van Valen L (1962) A study of fluctuating asymmetry. Evolution 16:125–162
Vo A-TE, Bank MS, Shine JP, Edwards SV (2011) Temporal increase in organic mercury in an endangered pelagic seabird assessed by century-old museum specimens. Proc Natl Acad Sci U S A 108:7466–7471. https://doi.org/10.1073/pnas.1013865108
Wails C (2016) Movement, colony attendance, and social behaviour of prospecting least and crested auklets. University of New Brunswick, M.Sc. thesis
Watanuki Y et al (2016) Feather mercury concentration in streaked shearwaters wintering in separate areas of Southeast Asia. Mar Ecol Prog Ser 546:263–269
Weiner JG, Krabbemhoft DP, Heinz GH, Scheuhammer AM (2003) Ecotoxicology of mercury. In: Hoffman DJ, Rattner BA, Burton GA Jr, Cairns J Jr (eds) Handbook of ecotoxicology, 2nd edition, 2nd edn. CRC Press, New York, pp 409–463
Zahavi A (1975) Mate selection—A selection for a handicap. J Theor Biol 53:205–214. https://doi.org/10.1016/0022-5193(75)90111-3
Acknowledgements
We thank E.E. Penney and D.W. Pirie-Hay for assistance in the field, L. Flehr and T. Jardine for Hg analyses, and the Alaska Maritime National Wildlife Refuge, J.C. Williams, G.V. Byrd, and the captain and crew of the MV Tiglax for transport and logistical support. The Natural Science and Engineering Research Council of Canada (Postdoctoral Fellowship to ALB, Discovery Grant to ILJ), Alaska Maritime National Wildlife Refuge, North Pacific Research Board, and Northern Scientific Training Program of Aboriginal Affairs and Northern Development Canada supported out research financially. Data are available on figshare at https://doi.org/https://doi.org/10.6084/m9.figshare.11807370. Comments from anonymous reviewers improved this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This research was approved by the Memorial University of Newfoundland Institutional Animal Care Committee (protocol 09-01-IJ) and conducted under US Federal Bird Banding Permit 22,181, US Fish and Wildlife Service Migratory Bird Permit MB176119-1, and Canadian Wildlife Service Possession Permit SP2696.
Additional information
Editorial Responsibility: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bond, A.L., Jones, I.L. Relationships between mercury burden, sex, and sexually selected feather ornaments in crested auklet (Aethia cristatella). Environ Sci Pollut Res 27, 16640–16645 (2020). https://doi.org/10.1007/s11356-020-08219-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08219-z