Abstract
Emphasis on water reuse in agricultural sector receives a renewed interest to close the loop in circular economy, especially in dry and water-stressed regions. In this work, wastewater from cooperative smoked sheet rubber factory and the effluent (digestate) from its treatment system (anaerobic digester) were used as medium to grow purple non-sulfur bacteria (PNSB), Rhodopseudomonas palustris strain PP803, with wood vinegar supplement at mid-log growth phase to stimulate the release of 5-aminolevulinic acid (ALA), a plant growth promotor. Wastewater-to-digestate ratios (D:W) represented by soluble chemical oxygen demand (SCOD) were found to influence both the growth of R. palustris and synthesis of ALA. The highest ALA release of 16.02 ± 0.75 μM and the biomass accumulation of 1302 ± 78 mg/L were obtained from the medium SCOD of 4953 mg/L. Although retarding biomass accumulation by 28–36%, wood vinegar (WV) addition was proven to improve ALA release by 40%. Result suggested that SCOD of 3438 mg/L (75:25 D:W) contained sufficient carbon source for PNSB growth and was chosen to subsequently run the photo-bioreactor (PBR) to sustain R. palustris PP803 cells production. In continuous PBR operation, PNSB proliferation suffered from the low organic concentration in PBR at low organic loading. An organic loading increase to 1.21 g COD/L day was found to attain highest biomass concentration and longest PNSB dominant period over microalgea. In this study, a real-time monitoring protocol of PNSB and microalgae was specifically developed based on image color analysis at acceptable accuracy (R2 = 0.94). In the final assay, verification of the PBR-grown inoculant was conducted and ALA release efficiency was discussed under various wood vinegar dosages and dosing frequencies. This work has advanced our understandings closer to practical field application.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anaerobic digestion (AD), in which the organic pollutants are converted to energy in a form of biogas, is commonly used as a standard treatment process in various agro-industries. However, anaerobic digester effluent so-called digestate still contains excessive organic matters. In many cases, it is stored in open ponds that have limited treatment capability and generally do not produce the effluent meeting the regulated discharge limit. The conventional aerobic post-treatment poses substantial expenditure from electricity for intensive aeration. Hence, there is a need for alternatives to minimize the treatment cost or even upgrade such effluent to enable water reuse. Water reuse in agricultural sector is in great need due to uncharacteristically long drought and unpredictable climates in recent years as a result of global warming (Aguirre-Gutiérrez et al. 2019).
Converting wastewater to irrigation water will close the loop for circular economy. Utilization of large volume of wastewater and treated effluent is most feasible in agriculture such as direct use as a nutrient source for crops and vegetative plants (Chaiprapat and Sdoodee 2007) or for hydroponics and microalgae cultivation (Chaiprapat et al. 2017; Fuldauer et al. 2018). Harvesting and processing low-value microscopic cells usually incur too high of the costs (Mohd Udaiyappan et al. 2017) making it economically unjustified. Therefore, the use of wastewaters to cultivate organisms releasing useful active compounds is deemed a more practical alternative.
Over 200 cooperative smoked sheet rubber factories (CSRFs), located throughout Thailand particularly in the southern region, were established by the grassroots conglomeration of rubber farmers in local communities. The production process of rubber sheets generates wastewater that contains liquid serum from fresh rubber latex after rubber particles are harvested as well as washing and rinsing waters in the process. This serum is rich in organics, nutrients, and minerals (Chaiprapat and Sdoodee 2007). Like in many industries, CSRFs started to adopt AD for their wastewater treatment as a way to recover energy for their heating requirement (Chaiprapat et al. 2015). However, the effluent from their anaerobic digester still contained organic matters which can possibly be utilized for bacterial cultivation. Thus, CRSF was used as a model case for digester effluent enrichment for agricultural wastewater reuse.
Purple non-sulfur bacteria (PNSB) are versatile organisms that grow under various environmental conditions: photoautotrophic and photoheterotrophic in anaerobic-light or micro aerobic-light, or chemoheterotrophic in the aerobic-dark conditions. Many researchers reported that PNSB grow well in certain polluted waters while having a high pollutant removal efficiency, for example, food industry wastewater, soybean wastewater, sugar wastewater, and rubber wastewater. Besides, their ability to produce valuable substances such as bacteriochlorophyll, carotenoids, 5-aminolevulinic acid (ALA) and CoQ10 is well recognized (Liu et al. 2016a; Liu et al. 2016b; Lu et al. 2019b; Zhi et al. 2019). Among these valuable substances, ALA has received a great consideration since it has board agricultural applications as non-toxic herbicide, pesticide, abiotic stress tolerance regulator, and growth-promoting factor (Wu et al. 2019). Nunkaew et al. (2014) reported that the applications of ALA only 2.11–2.67 μM (10X diluted Rhodopseudomonas palustris supernatant) could stimulate the rice seedling growth even under saline stress. ALA was intracellularly synthesized via the Shemin pathway, relating to the two precursors (succinyl-CoA and glycine), by using aminolevulinic synthase (ALAS). Subsequently, enzyme aminolevulinic dehydratase (ALAD) combines 2 molecules of ALA to form porphobilinogen and further converts it to tetrapyrrole compounds such as vitamin B12 and bacteriochlorophyll. Levulinic acid (LA), whose structure is an analogue of ALA, plays an important role in suppressing ALAD activity (Nandi and Shemin 1968), resulting in an enhanced ALA accumulation (Saikeur et al. 2009) and subsequently secreting the excessive intracellular ALA. Wood vinegar (WV) found to contain LA around 9.5 mM is a much cheaper substance derived from a condensate liquid of biomass pyrolysis (by-product of charcoal production) and was proven in our previous work to enhance extracellular ALA production by 3.7–4.2-folds in batch PNSB cultivation, then only using CSRF wastewater as a medium (Nunkaew et al. 2018). In fact, the wastewater would enter anaerobic digestion to recover biogas first and only the digester effluent should be used for PNSB cultivation. Furthermore, PNSB could provide a certain degree of treatment (Lu et al. 2019a) for the digester effluent prior to use for irrigation. Therefore, employing PNSB to upgrade industrial digester effluent is viewed a feasible option to enhance and add value to water reuse.
To sustain the operation of ALA synthesis unit from industrial effluent, stock culture of the selected strain of microorganism must be maintained and transferred to the light-exposed pond for subsequent cultures. No previous work had yet to address this stock culture replenishment issue which will be a key to full-scale field application. The amount of substrate fed to the photo-bioreactors (PBRs) can be regulated by hydraulic retention time (HRT) and organic loading rate (OLR) which in turn control the pollutant removal rate and the biomass accumulation. Liu et al. (2016b) showed that PBRs fed with soybean wastewater had the highest biomass production and PNSB to total bacteria ratio at HRT 60 h, while a shortened HRT would lower PNSB to total bacteria fraction. In fact, competition and growth of microalgae, a photosynthetic organism that uses the visible light spectrum as an energy source and produces oxygen as a product, are inevitable and more of a concern in PBR operations. The open pond with natural light may be operated to sustain the growth of PNSB by a simple control strategy of organic loading and hydraulic retention time, which is to be proven in this work.
Thus, the objective of this study is to investigate the extracellular ALA production efficiency using various mixing ratios of digester effluent and raw wastewater from our model industry, CRSF, as growth medium. This research consisted of three main parts, firstly the batch culture assays for investigating PNSB growth and ALA release using combination of digester effluent (digestate) and CSRF raw wastewater, secondly the continuous operation of photo-bioreactor that mimicked the Rhodopseudomonas palustris stock culture pond in the field, and lastly the verification of the produced inoculant effectiveness under various WV dosing schemes. During the second experiment, a technique to simplify on-field quick approximation of PNSB dominance in photo-bioreactor was developed. Results from all three parts can be used as a guideline for ALA irrigation water production for field application.
Materials and methods
Wastewater collection and medium preparation
Wastewater and digestate from Ban Kao Rang CSRF located in Songkhla Province, Thailand, were used as cultivating media in this study. The anaerobic digester was operated under stable condition when its effluent was collected for use during the experiment. The samples were prepared by passing through a thin sheet fabric cloth with pore size approximately 0.3 mm to remove large particles before being analyzed for their characteristics according to the Standard Methods for Examination of Water and Wastewater (APHA et al. 2012). The prepared wastewaters were kept at 4 °C until use.
Starter culture preparation
The selected Rhodopseudomonas palustris strain PP803 from a previous study (Nunkaew et al. 2015) was designated as starter culture in this study according to the ability to grow and release ALA in rubber wastewater. It was cultivated in 120-mL serum bottles containing 90 mL glutamate-acetate (GA) broth medium, which composed of (g/L) sodium-L-glutamate 3.8, CH3COONa 5.44, yeast extract 2, KH2PO4 0.5, K2HPO4 0.5, (NH4)2HPO4 0.8, MgSO4•7H2O 0.2, CaCO2•2H2O 0.053, nicotinic acid 0.001, biotin 0.00001, MnSO4•5H2O 0.0012, ferric citrate 0.0025, and CaCl2•6H2O 0.0012, and pH adjusted to 6.8 ± 0.02. The culture was incubated under incandescent lamps at intensity 3500 lx in a controlled temperature of 30 ± 2 °C for 48 h. The light intensity was measured using a lux meter model Digicon LX-55. After 48 h, 10 mL of culture broth was transferred to serum bottles containing 90 mL of the sterilized CSRF wastewater, then incubated under the same condition. PNSB growth was measured based on turbidity using a spectrophotometer at wavelength 660 nm, from which the starter medium was prepared by diluting it to an optical density of 0.5 to obtain a cell density approximately 108 cells/mL (Nunkaew et al. 2018).
Experiment part I: batch culture of R. palustris PP803 under various digestate-to-wastewater ratios for ALA release, soluble chemical oxygen demand (SCOD) removal, and biomass growth
The prepared digestate was mixed with raw CSRF wastewater at vol/vol ratios of 4:1, 3:1, 2:1, and 1:1 (designated as D:W for digestate-to-wastewater ratio) which acquired SCOD of 2482, 3438, 4953, and 7220 mg/L, respectively. The prepared mixtures were used as PNSB cultivation media. Characteristics of each mixture are shown in Table 1. Thirty milliliters of the prepared starter culture was introduced into 270 mL of the non-sterilized medium and transferred to a 400-mL clear glass flat-side reactor. The initial pH was adjusted to 6.8 ± 0.1 with 1 N HCl or 1 N NaOH and loosely sealed with cotton.
The reactors were then placed inside a controlled temperature chamber at 30 ± 2 °C under light intensity 5000 lx at 12-h photoperiod provided by incandescent lamps. After 3 days of cultivation, all treatments were filled with WV 1.25% v/v, and the dose of WV was selected according to Nunkaew et al. (2018). The WV used in this study composed (mM) of methanol 478.6, acetic acid 611.0, propionic acid 30.4, butyric acid 12.4, valeric acid 0.6, LA 9.5, and phenol 15.9 (Nunkaew et al. 2018). The culture lasted for 168 h (7 days) as each reactor was carefully mixed twice a day. Mixing twice a day would mimic the limited mixing in digestate storage pond in a typical industrial setting, which in laboratory setting prevented biofilm development on sidewall in the culture reactors. Homogeneous sample of 10 mL was drawn daily from each reactor at the end of the light period to measure SCOD, pH, biomass concentration, and extracellular ALA concentration.
The results from the experiment were used to determine the effects of initial SCOD of the wastewater medium (SCODi) and time of cultivation (TC) on extracellular ALA production, SCOD removal, and biomass production. The most suitable mixing ratio that gave the optimal biomass production was selected and used in the continuous operation of PBR in the next experiment.
Experiment part II: continuous cultivation of R. palustris PP803 for culture maintenance
The continuous operation of PBRs to cultivate and maintain the PNSB culture was carried out using the mixing ratio determined from experiment part I. The PBRs were made of clear glass of a dimension (W × L × H) 15 cm × 21.7 cm × 17.1 cm. They were operated at an effective volume of 3 L. The PBRs were illuminated from only the topside by two 100-W incandescent lamps for consecutive 12-h light/12-h dark cycles. All side-walls were covered with two layers of black plastic sheet to prevent any light interference. Light intensity at the water surface was adjusted to 5000 lx (294.1 W/m2) by adjusting the distance from the lamps. Homogeneous mixing was performed only in light period by a magnetic stirrer. The PBRs were placed in the controlled temperature chamber at 30 ± 2 °C.
The prepared starter culture of 300 mL was transferred to each PBR and pre-incubated for 2 days with the non-sterilized mixed medium made up of digestate and CSRF wastewater at the D:W determined in experiment part I. Initial pH was adjusted to 6.8 ± 0.1 with 1 N HCl or 1 N NaOH. Three PBRs were operated at different OLR, regulated by HRT at 5, 4, and 3 days, respectively. PBRs were fed with the mixed wastewater at the beginning of light period and withdrawn at the end of dark period by a peristaltic pump (Masterflex Easy-load model 751860, USA). During the operation, effluent from each PBR was collected and analyzed for SCOD, suspended solids (SS), and pH every 2 days. Microbial populations in PBR operated at 3-day HRT were monitored every 6 days by the most probable number (MPN) technique, which is a method to evaluate the number of viable cells of a target microbe in sample by means of replicate liquid broth in tenfold dilutions (Blodgett 2010). The populations in the other two PBRs were mathematically determined according to the protocol developed using image color pigment analysis, outlined in the “Image color analysis for microbial populations monitoring” section.
Experiment part III: verification of the PBR-derived PNSB and impacts of WV dosing schedule
PBR effluent from the most suitable OLR based on R. palustris PP803 cell concentration was used as an inoculant for yet another set of verification batch assays. This was done to imitate a successive batch operation of an open storage pond which would receive digester effluent and a portion of the untreated CSRF wastewater as substrate at the ratio used in the “Experiment part I: batch culture of R. palustris PP803 under various digestate-to-wastewater ratios for ALA release, soluble chemical oxygen demand (SCOD) removal, and biomass growth” section. Furthermore, three different WV addition patterns were tested composing of (1) one-time addition of 1.25% v/v at mid-log growth phase (day 3), (2) two-time additions of 0.625% v/v (half quantity of previous condition) at mid-log growth phase (day 3) and midway to the end point (day 5), and (3) two-time additions of 1.25% v/v at day 3 and day 5. The setup of this assay was identical to that in the “Experiment part I: batch culture of R. palustris PP803 under various digestate-to-wastewater ratios for ALA release, soluble chemical oxygen demand (SCOD) removal, and biomass growth” section.
Analytical methods and statistical analysis
For biomass concentration measurement, the samples collected from reactors were centrifuged at 4000 revolutions per minute (rpm) for 15 min to collect cell mass pellet at the bottom of the centrifuge tube. It was washed twice with distilled water. Washed cell mass was dried at 103 °C until constant weight and left to cool in a desiccator before weight measurement. Biomass growth was calculated from the cell concentration at time t subtracted by the initial cell concentration. The supernatant of each sample was taken from a centrifuge tube for SCOD analysis according to the Standard Methods (APHA et al. 2012). The concentration of extracellular ALA was measured according to a previously described method in Nunkaew et al. (2015).
PNSB and microalgae (MICA) populations were determined with the MPN technique by adapting the method described by Harada et al. (2001) with GA and BG-11 medium, respectively. Both mediums are usually used for PNSB and MICA culture. Compositions of GA medium are shown in starter culture preparation part (“Starter culture preparation” section). BG-11 was composed (g/L) of NaNO3 1.5, K2HPO4 0.04, MgSO4•7H2O 0.075, CaCl2•2H2O 0.036, citric acid 0.006, ferric ammonium citrate 0.006, EDTA 0.001, Na2CO3 0.02, and 1 mL of trace metal mix, which contained (g/L) H3BO3 2.868, MnCl2•4H2O 1.81, ZnSO4•7H2O 0.222, Na2MoO4•2H2O 0.39, CuSO4•5H2O 0.079, and Co(NO3)2•6H2O 0.0494. The PBR effluent was taken for a serial dilution in sterile normal saline solution. Two milliliters of appropriate dilution was transferred to 20-mL screw cap tube which contained 18 mL of GA or BG-11 medium. Both cultures were incubated under room temperature (30 °C) with continuous illumination using different light sources according to the spectra absorption of pigment in each microorganism. The GA culture (PNSB) was incubated under 3500 lx from incandescent lamps while the BG-11 culture (MICA) was incubated under 3500 lx from fluorescent lamps. After 2 weeks of incubation, the tubes that had changed to red color (cultured GA tubes) and green color (cultured BG-11 tubes) indicated as positive by which population numbers of PNSB and MICA were calculated.
In order to determine the effects of independent variables, means and standard deviations were compared using SPSS software version 23.0.0.0 with the one-way analysis of variance (ANOVA) and Scheffe’s multiple range test at α = 0.05.
Image color analysis for microbial populations monitoring
In this work, a quick estimation technique of relative MICA and PNSB populations by image pigment colors was developed. Image colors were used to quantify the tone of green and red of the culturing reactor because the PP803 strain possesses red pigment and MICA contains green chlorophyll pigment. Effluent from each PBR was transferred to 120-mL clear serum bottle before photographed with a digital camera in the light-controlled booth. In every photo session of the samples, the camera and light settings were identically arranged in a black box. The images were digitally evaluated for R (red) and G (green) values from random 10 locations by using color sampler tool in Adobe Photoshop CC2017. A correlation between PNSB and MICA populations from MPN were then determined by regressing the populations logarithmic ratio to pigment ratio. Details of the analysis will be explained in the “Development of image color pigment quantification technique for detection of PNSB and MICA populations in PBRs” section.
Results and discussion
Effects of digestate-to-wastewater ratio and cultivation time
A preliminary trial of R. palustris PP803 cultivation in digester effluent (digestate) and wastewater from Ban Kao Rang CSRF was performed to observe the characteristics of cell growth and determine the proper range of dilutions. When only digestate was used, it was found that the culture could produce minimal cell growth due to the low SCOD (carbon source) at only 167 mg/L (Table 1). The phenomenon was similarly found in the study of Meng et al. (2018) that photosynthetic bacteria treatment with initial chemical oxygen demand (COD) 100 and 300 mg/L showed virtually no change in biomass concentration at the endpoint, and suggested minimum COD for PNSB proliferation of around 500 mg/L. Therefore, the raw CSRF wastewater (non-treated) was brought to mix with digestate to stimulate strain PP803 growth. This mixing was intended to make use of the minimal portion of raw CSRF wastewater as it should go to AD for biogas production first. However, such strategy may not be necessary for industries with high remaining COD in digestate which depends largely on the COD of their raw wastewater and the efficiency of the digester. The effect of initial SCOD (SCODi) on ALA release, SCOD removal, and biomass accumulation from non-sterilized mixed wastewater of R. palustris PP803 culture were then investigated.
ALA production
It must be noted that concentration of WV application at 1.25% v/v was selected based on our previous study (Nunkaew et al. 2018) and WV was introduced to the culture at the mid-log growth phase (day 3). Results show that SCODi and WV dosing to the PNSB culture had a positive effect on ALA release (Fig. 1). The highest ALA concentration of 16.02 ± 0.75 μM was obtained at SCODi of 4953 mg/L (D:W 2:1) and TC at 4 days, which is 40% greater than its control set (without WV dosing). It was noticeable that ALA production at D:W 3:1 (SCODi 3438 mg/L), although posed slightly lower concentration, was not statistically different when compared across the means of all treatments (Fig. 1). The greater ALA release in the WV application sets confirmed the effectiveness of LA contained in WV to inhibit ALAD and enhance ALAS activity (Sasaki et al. 1987) and use of WV is a good low-cost scheme for ALA production. Based on the Shemin pathway that PNSB use for ALA synthesis, glycine and succinyl-CoA as precursors are converted to ALA by ALAS, and further converted to porphobilinogen by ALAD. To increase intracellular ALA concentration, ALAD must be inhibited. LA which is an ALA analogue when present in suitable amount will disrupt ALAD activity, hence, elevating the ALA synthesis.
Effect of initial SCOD of the digestate-wastewater media and WV addition on ALA release in 7-day cultivation time. Data plotted are at day 4 where ALA was highest in all treatments. Error bar represents the standard deviation of the mean (n = 3). Different letters indicate significant differences among treatments at α = 0.05
However, WV dosing to the lowest SCODi of 2483 mg/L culture as well as the highest SCODi of 7220 mg/L culture did not promote ALA release, and even caused slight decrease compared with the control. The weakening in ALA release at low of SCODi was due to the insufficient organic carbon source to support PNSB growth while also being competed by mixotrophic microalgae, capable of using both organic carbon and carbon dioxide for cell synthesis. In contrast, a decline in ALA release occurred at high SCOD medium was a result of the competition with heterotrophic fermentative bacteria residing in AD effluent. These organisms are reportedly having higher growth rate than the photoheterotrophic PNSB (Liu et al. 2016a). Hydrolytic or fermenting bacteria could be found to as high as 40% of the population in photo-bioreactor cultured using high-concentration digester effluent as substrate (Garcia et al. 2017). This competition was supported by the lowering pH to 5.44–6.51 which is known as a result of the accumulation of organic acids and dissolved carbon dioxide from the metabolism of the fermentative organisms at high organic loading in anaerobic digestion pathway (Palamae et al. 2018).
Based on Fig. 1, the sufficient amount of carbon source (SCOD) is a prerequisite to firstly stimulate PNSB growth while the appropriate amount of WV application will trigger an effective excretion of ALA to the medium. This release mechanism was carried out to keep ALA level in PNSB cells in balance for normal cell activity. In addition, daily measurement of ALA revealed that there was a slight degradation of ALA in the medium after it reached the maximum (around day 4) caused by cell re-uptake and conversion to tetrapyrrole compound, a precursor of bacteriochlorophyll (Sasaki et al. 1991). Thus, timing of WV application and lead time for maximal ALA release are critical to the optimal operation of this system. Nevertheless, environmental condition such as light intensity, temperature, and rain water dilution can also play a significant role in cell growth and metabolism in field application. Since the lead time after WV addition to maximal ALA release can be estimated, a simple measurement of PNSB growth can help determine an appropriate WV application timing.
Organic removal
High SCOD removal from the medium reduces organic load to soil when it is land applied because only nutrients and active biocompounds are needed for agricultural irrigation. The higher SCODi in the medium caused a slight decrease in SCOD removal but still reaching 93% efficiency at day 7 without WV addition (control set). TC positively affects SCOD removal as the longer TC provides time for degradation. After 5 days of cultivation, SCOD removal had subsided (Fig. 2a), exhibiting slower reaction kinetics in the lowering reactant concentration. It was noticed that the application of WV (on day 3) caused immediate suppression of heterotrophic SCOD degradation (no SCOD removal on day 4). The overall 7-day SCOD removal declined slightly as shown in Fig. 2b. This microbial retardation was primarily caused by a mild toxin from methanol which is the major composition found in WV (Nunkaew et al. 2018). This methanol was co-generated with WV in the pyrolysis of biomass from the methoxyl groups of uronic acid and the breakdown of methyl esters and/or ethers from decomposition of pectiln-like materials (Güllü and Demirbaş 2001). At low dosage, this inhibitory side effect could be corrected and outweighs the benefit from the ALA release. This LA when introduced suppressed ALAD activity to process ALA to tetrapyrroles and bacteriochlorophyll, thus disrupting light energy harvesting. This situation resulted in lower ATP molecules produced and less active cell metabolism.
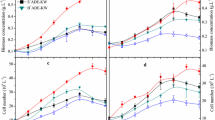
Biomass accumulation
Figure 3 demonstrates that the biomass accumulation was influenced by SCODi. The highest biomass accumulation was 1302 mg/L at SCODi 4953 mg/L (D:W 2:1). The treatments with high wastewater mix (SCODi 4953 and 7220 mg/L) showed longer lag phase in early stage (Fig. 3c, d) due to the more complex molecules such as protein and carbohydrate in the CSRF wastewater compared with much simpler molecules in digestate. Similar to SCOD removal, the application of WV caused immediate pause to biomass production on day 4 and repressed the overall (final) biomass accumulation (day 7) by 24.9–36.9% in treatments of SCODi 2482, 4953, and 7220 mg/L (Fig. 3a, c, d). However, PNSB biomass was able to quickly recover from WV dose in treatment SCODi 3438 mg/L (Fig. 3b) which is consistent with the almost unaffected SCOD removal profile (Fig. 2b). This SCOD concentration appeared to be an optimum for PNSB metabolic activity and growth. It was also observed that almost no SCOD in the medium was removed after day 5 (Fig. 2) but biomass growth still continued toward the final TC of 7 days (Fig. 3). That the cells utilized the stored carbon and nutrients earlier assimilated from the medium for growth (Chaiprapat et al. 2003) implies that the addition of WV can be done in a wider time window, i.e., days 3–4–5, as the majority of cells were still actively reproducing.
Selection of digestate-to-wastewater ratio
Performances of R. palustris PP803 in ALA release, SCOD removal, and biomass accumulation were considered in a selection of an appropriate D:W as feed to PBR for continuous culture. The medium with SCODi 2483 mg/L contained insufficient carbon source to support PNSB biomass growth while the medium with SCODi 7220 mg/L suffered from competition with fermentative bacteria and lower light penetration. Although the control set of medium SCODi 4953 mg/L (D:W 2:1) gave highest biomass accumulation, the performance in ALA release and SCOD removal were equivalent to medium SCODi 3438 mg/L (D:W 3:1 or using only 25% of wastewater) which used less wastewater in the mixture. By the primary aim to preserve raw CSRF wastewater for biogas production in AD treatment system, the higher D:W of 3:1 (SCODi 3438 mg/L) was chosen to run the PBRs. It should be noted that the digestate in other industries may be of higher SCOD and probably does not need to mix with the raw wastewater. In the present study, it is demonstrated that raw wastewater can be diverted to mix with digestate to satisfy the carbon requirement for PNSB growth.
Operation of continuous PBRs for maintaining R. palustris PP803 starter culture
Effects of OLR on SCOD removal, biomass production, and PNSB dominance
In order to make use of R. palustris PP803, its stock culture supply must be sustained on site in PBRs. Three PBRs (PBR-5d, PBR-4d, and PBR-3d) were fed with non-sterile medium prepared by mixing digestate and CSRF wastewater at ratio 3 to 1 containing SCOD around 3438 mg/L (Table 1). It was chosen instead of the best condition for biomass production (1302 mg/L) at SCODi 4953 mg/L to avoid the undesired loss of SCOD to produce biogas in AD while still achieving high cell production (Fig. 3). Results show that PBR-5d and PBR-4d operated at OLR 0.71 and 0.94 g/L day, respectively, were able to remove over 85% of SCOD producing an effluent of SCOD 434–494 mg/L consistently. The high rate of SCOD removal from both PBRs was comparable with that of Liu et al. (2016b) which obtained 86.8% COD removal from acidic wastewater at HRT 96 h and OLR 0.84 g/L day; hence, confirming that HRT 4–5 days was sufficient for pollutant degradation by PNSB. At OLR 1.21 g/L day, PBR-3d achieved only 75.3% removal with effluent SCOD 936.0 mg/L (Table 2) but yielded highest biomass concentration at 884 mg/L, equivalent to biomass yield of 321 mgbiomass/g SCODremoved. PNSB themselves can grow well in a wide range of COD in the medium ranging from 500 to 40,000 mg/L (Meng et al. 2018), provided that there is enough light. As the COD concentration exposed to cells in a continuous reactor is represented by the concentration of effluent, it appeared that PBR-5d and PBR-4d could not sustain sufficient COD for the PNSB cells while PBR-3d, with its less COD removal efficiency, left enough COD for their growth. No effect from pH on PNSB growth was observed since all three PBRs were able to sustain pH of 7.62–7.69 that are within an optimal pH of 7–8 for COD removal and biomass production for PNSB (Liu et al. 2015). There was no issue of competition with fermentative bacteria here. All performance data in Table 2 are the average values from the last 5 points at each stable reactor operation.
Populations of PNSB and MICA were measured by MPN method for PBR-3d operation. In the first day, the ratio of PNSB cells to the total (PNSB+MICA) cells in the effluent based on MPN/L (designated as RMPN) was 0.5 and increased to 0.99 only 18 days into the operation, at which point PNSB population reached 6.38 log MPN/L while MICA still had 4.37 log MPN/L. Growth of MICA started to be noticeable on day 24, causing a decrease of RMPN. However, PNSB populations were still larger than 4 log MPN/L throughout the operation period (Fig. 4). PNSB could maintain high activity in PBR-3d for 30 days. Once the MICA started to grow, it would take only a few days to take over the culture. Honda et al. (2006) reported an 18-day period from start to total domination of MICA in PNSB culture. It was observed that the dynamics between PNSB and MICA populations corresponded to color of the liquid in PBR. The PBR effluent initially had a light brown color. It took 2 days to observe some pale pink color and gradually changed to red during days 12–24 because of the increasing PNSB photopigment content. The color then slowly turned to pale green at day 30 (Fig. 5a). In comparison, PBR-3d exhibited the ability to maintain PNSB dominance for as long as 30 days. The R. palustris PP803 culture, therefore, needs to be renewed approximately every 30 days to sustain the substantial PNSB stock in the inoculant PBR. Although growth of MICA seemed inevitable in extended open tank operation, the use of the infrared transmitting filter installation (Chitapornpan et al. 2012) in combination with the organic loading control as deployed in this study should greatly prolong the PNSB dominance period, which is under our future research plan.

Changes in color of the culture medium over time (a) and the regression model describing the correlation between the relative populations of PNSB and MICA and the color pigment green and red from medium photographs (b). Error bar represents the standard deviation of the mean (n = 10)
Fig. 5
Development of image color pigment quantification technique for detection of PNSB and MICA populations in PBRs
From the observation of changes in color of the culture in the reactor, which corresponded to the shift in populations of PNSB and MICA, numerical method to determine their relative population was sought. Figure 5 a shows the color of the culture (homogenous effluent from PBR-3d) throughout the operation period. The intensity of red color gradually increased and highest at day 18, which was in good agreement with the PNSB population trend. Meanwhile, the appearance of pale green color toward day 30 coincided with the blooming of the MICA (Fig.4). Due to the reddish color of PNSB and greenish color of MICA, R (red pigment) and G (green pigment) in the photograph of homogeneous culture sample were used as their respective representation. A relationship between R to G ratio (R:G) was trialed with their population numbers in various forms. It was found that the pigment colors are correlated well with log ratio of MPNPNSB:MPNMICA (Fig. 5b). Thus, photographic color analysis protocol was developed based on a linear empirical model. Data from reactor PBR-3d were linearly regressed according to Eq. 1, which gave an R2 of 0.94.
where MPNPNSB is most probable number of PNSB in sample (MPN/L), MPNMICA is most probable number of MICA in sample (MPN/L), R is the intensity of red pigment in the liquid sample image, and G is the intensity of green pigment in the liquid sample image. When log (MPNPNSB/MPNMICA) is greater than 0, population of PNSB is higher than MICA.
Population dynamics between PNSB and MICA in PBR
By using the measured R:G from the images of homogeneous samples taken directly from each PBR, log MPNPNSB/MPNMICA (designated as log PNSB/MICA) of PBR could be derived. Thus, the instantaneous relative population of PNSB to MICA of PBRs operated at all 3 organic loadings was derived and plotted over the operation time course as shown in Fig. 6. Log P/M started to increase from the beginning until reaching maximum of 0.81 at day 10 in PBR-5d, 1.26 at day 10 in PBR-4d, and 2.12 at day 18 in PBR-3d. It is noted that log P/M = 0, an equal amount of PNSB and MICA, could be used as a termination point of the PNSB cultivation. PBR-3d was able to maintain the longest PNSB dominant period for 30 days, followed by PBR-4d (20 days) and PBR-5d (16 days), in order. The dominant period of PNSB in PBR operated at HRT-4d and 5d were reportedly shorter than 30 days based on Honda et al. (2006); thus, HRT is not a determinant parameter for PNSB growth but rather it is dependent on exposing concentration to cells which is more related to the organic loading to a continuous system. PNSB cell synthesis was suppressed in aerobic condition (Qin et al. 2017) as the hem reporter plastids exhibited low expression that lowered the bacteriochlorophyll level (Smart et al. 2004). That the PBRs in this study mimicked natural condition of an open pond with direct exposure to air at the liquid surface could have made the PNSB biomass less productive. This is particularly true at low medium concentration since oxygen in the air, which is not consumed in aerobic oxidation reactions at the upper liquid layers, can penetrate deeper into the liquid medium.
The increase of OLR not only lengthened PNSB dominant period but also stimulated PNSB population growth as demonstrated in Table 2. The shortened HRT was proven to benefit PNSB over other bacteria under light condition, but too short HRT (i.e., < 36 h) would then favor the heterotrophs (Liu et al. 2016b). It was the effluent SCOD, that both organisms exposed to, played an important role in controlling the PNSB growth and MICA population. SCOD from PBR-3d was consistently at a level above 900 mg/L compared with the other two reactors at only 400–500 mg/L. COD below 500 mg/L was deemed insufficient for PNSB growth as MICA became more competitive than PNSB. In addition, dissolve oxygen produced from MICA inhibited bacteriochlorophyll synthesis of most photosynthetic bacteria, as many hem genes which correlated to ALA and bacteriochlorophyll production are repressed under aerobic condition (Smart et al. 2004). This present work shows that MICA growth in PBRs was retarded by increasing OLR or decreasing HRT, although it cannot completely be suppressed. To raise OLR, increasing feed concentration may be a more desirable method to avoid the possible biomass washout if larger flow would be introduced to regulate HRT (OLR = CODinfluent/HRT).
With the use of the specifically developed calibration curve for estimating relative microbial cells (PNSB and MICA) based on MPN technique and the image color analysis, it was proven an adequate real-time technique for monitoring this population dominance. The technique was highly economical and able to effectively trace the stability of PNSB in the continuous culturing; hence, any undesirable changes that might occur will be resolved in time, in which a real-time PCR, requiring highly skilled personnel and very expensive equipment, is normally used (Liu et al. 2016a). For other applications, this technique is suitable for use in culture with color pigment producing microbes where quick approximation would suffice to serve the purpose for filed application. It should be noted that the characteristics of wastewater used to run photo-bioreactor may affect the calibration curve or regression model (Fig. 5b). Thus, the regression model should be developed for each case prior to use with the protocol outlined in this work.
Verification of PBR inoculant effectiveness and impacts of WV addition frequency on ALA release
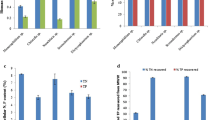
PBR-3d was selected from the previous experiment (“Effects of OLR on SCOD removal, biomass production, and PNSB dominance” section) as a representative inoculant culture for PNSB cultivation in succeeding batch reactors. This experiment was designed to test the effectiveness of the inoculant produced by PBR-3d for extracellular ALA production. Mixed liquor from PBR-3d operated at day 18 was taken to seed the cultivation flasks. Table 3 shows the release of maximal extracellular ALA by the PBR-grown inoculant with different WV application frequency and dosage. Note that the values reported in Table 3 were already deducted by the blank set (without PNSB inoculant). Results show that PBR-3d effluent could be sufficiently used as an inoculant even though there was a lower ALA by 30.9% (Run-II vs. Run-V) at a single WV dose of 1.250%. This was mainly caused by the existence of MICA in the culture as previously described. ALA release was, however, improving when WV addition was applied in different patterns, as clearly shown by ANOVA in Table 3. By doubling WV dosing (Run-III), extracellular ALA concentration increased to 12.61 ± 1.14 μM, which statistically was indifferent to the pure culture inoculant (Run-V). Repeated addition of WV induced ALA release and there was, interestingly, no observable MICA growth in all cultivation flasks during the cultivation (7 days). Results from Run-IV show that by splitting the dose of WV in half and creating an injection interval, the culture was more stimulated, and release ALA at 7.1% higher (p < 0.05) than the one-time application. Dosage and frequency of WV application clearly influenced ALA excretion. Although inhibition of WV to MICA growth is likely, it was not systematically proven in this work.
It is highly beneficial when the CSRF can maintain PNSB culture and implement the ALA synthesis unit (batch) to produce every 5–6 days the ALA-laden irrigating water, in addition to the plant nutrients it already contained. The liquid rich in ALA could be used to irrigate rubber plantation, rice field, or other plantation which are typically surrounding the CSRF. Many vegetative plants have chlorophyll content increased when treated with a trace of ALA (Al-Qurashi and Awad 2011) which enhanced photosynthesis rate. Irrigating water containing ALA around 0.2 μM was reportedly effective in enhancing rice growth (Nunkaew et al. 2014), panicle weight, number of grain per panicle, and grain yield (Kantachote et al. 2016). In addition, increase in root dry weight and shoot dry weight of germinated rice seed was attained under normal condition as well as arsenic stress condition (Nookongbut et al. 2018). ALA application on Hevea brasiliensis (rubber tree) could lead to increased chlorophyll and photosynthesis rate which is related to plant growth and latex production (Rao et al. 1998).
Conclusion
This work showed the utilization of anaerobic digester effluent using CSRF as a model industry for extracellular ALA synthesis. Due to the dilute effluent steam, CSRF wastewater was added at digestate-to-wastewater ratio 1:3 (SCOD 3438 mg/L) to reach most suitable PNSB’s carbon requirement, growth, and ALA production in batch culture system. In continuous PBR operation, adequate OLR of at least 1.21 g/L day was found essential for effective PNSB culture maintenance in relation to microalgae growth. The rapid assessment protocol was developed in this work using image color analysis that allowed a simple on-field microbial dominance monitoring in PBR. The PNSB harvested from the PBR effluent was later proved effective inoculant in subsequent culturing. Lastly, the half-dose with split injections of WV to the PNSB culture could significantly enhance ALA release. These findings can lead to the possible production scheme of ALA containing irrigation water from industrial digester effluent. Further works should be expanded to reuse digester effluent from different industries while response of economic crops to ALA containing effluent is worth exploring.
Abbreviations
- AD:
-
Anaerobic digestion
- ALA:
-
5-Aminolevulinic acid
- ALAD:
-
Aminolevulinic dehydratase
- ALAS:
-
Aminolevulinic synthase
- COD:
-
Chemical oxygen demand
- CSRF:
-
Cooperative smoked sheet rubber factory
- D:W:
-
Digestate-to-wastewater ratio
- HRT:
-
Hydraulic retention time
- LA:
-
Levulinic acid
- MICA:
-
Microalgae
- MPN:
-
Most probable number
- OLR:
-
Organic loading rate
- PBR:
-
Photo-bioreactor
- PNSB:
-
Purple non-sulfur bacteria
- R:G:
-
Pigment red to pigment green ratio
- RMPN :
-
Ratio of PNSB to total (PNSB + MICA) in the effluent based on MPN/L
- SCOD:
-
Soluble chemical oxygen demand
- TC:
-
Time of culturing
- TCOD:
-
Total chemical oxygen demand
- WV:
-
Wood vinegar
References
Aguirre-Gutiérrez J, Oliveras I, Rifai S, Fauset S, Adu-Bredu S, Affum-Baffoe K, Baker TR, Feldpausch TR, Gvozdevaite A, Hubau W, Kraft NJB, Lewis SL, Moore S, Niinemets Ü, Peprah T, Phillips OL, Ziemińska K, Enquist B, Malhi Y (2019) Drier tropical forests are susceptible to functional changes in response to a long-term drought. Ecol Lett 22:855–865
Al-Qurashi AD, Awad MA (2011) 5-Aminolevulinc acid increases tree yield and improves fruit quality of ‘Rabia’ and ‘Sukkariat-Yanbo’ date palm cultivars under hot arid climate. Sci Hortic 129:441–448
APHA, AWWA, WEF (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association, Washington, D.C.
Blodgett R (2010) BAM Appendix 2: most probable number from serial dilutions. https://www.fda.gov/food/laboratory-methods-food/bam-appendix-2-most-probable-number-serial-dilutions.
Chaiprapat S, Cheng J, Classen JJ, Ducoste JJ, Liehr SK (2003) Modeling nitrogen transport in duckweed pond for secondary treatment of swine wastewater. J Environ Eng 129:731–739
Chaiprapat S, Rakngam I, Suksaroj T (2015) Evaluation of biogas production enhancement by co-digestion and biogas to firewood replacement in small cooperative rubber sheet factories (phase 2). Final Report, Thailand Research Fund, Thailand (in Thai),
Chaiprapat S, Sasibunyarat T, Charnnok B, Cheirsilp B (2017) Intensifying clean energy production through cultivating mixotrophic microalgae from digestates of biogas systems: effects of light intensity, medium dilution, and cultivating time. Bioenerg Res 10:103–114
Chaiprapat S, Sdoodee S (2007) Effects of wastewater recycling from natural rubber smoked sheet production on economic crops in southern Thailand. Resour Conserv Recycl 51:577–590
Chitapornpan S, Chiemchaisri C, Chiemchaisri W, Honda R, Yamamoto K (2012) Photosynthetic bacteria production from food processing wastewater in sequencing batch and membrane photo-bioreactors. Water Sci Technol 65:504–512
Fuldauer LI, Parker BM, Yaman R, Borrion A (2018) Managing anaerobic digestate from food waste in the urban environment: evaluating the feasibility from an interdisciplinary perspective. J Clean Prod 185:929–940
Garcia GPP, Diniz RCO, Bicalho SK, Franco V, Pereira AD, Brandt EF, Etchebehere C, Chernicharo CAL, de Araujo JC (2017) Microbial community and sulphur behaviour in phototrophic reactors treating UASB effluent under different operational conditions. Int Biodeterior Biodegradation 119:486–498
Güllü D, Demirbaş A (2001) Biomass to methanol via pyrolysis process. Energ Convers Manage 42:1349–1356
Harada N, Nishiyama M, Matsumoto S (2001) Inhibition of methanogens increases photo-dependent nitrogenase activities in anoxic paddy soil amended with rice straw. FEMS Microbiol Ecol 35:231–238
Honda R, Fukushi K, Yamamoto K (2006) Optimization of wastewater feeding for single-cell protein production in an anaerobic wastewater treatment process utilizing purple non-sulfur bacteria in mixed culture condition. J Biotechnol 125:565–573
Kantachote D, Nunkaew T, Kantha T, Chaiprapat S (2016) Biofertilizers from Rhodopseudomonas palustris strains to enhance rice yields and reduce methane emissions. Appl Soil Ecol 100:154–161
Liu S, Li X, Zhang G, Zhang J (2015) Optimization of influencing factors on biomass accumulation and 5-aminolevulinic acid (ALA) yield in Rhodobacter sphaeroides wastewater treatment. J Microbiol Biotechnol 25:1920–1927
Liu S, Zhang G, Zhang J, Li X, Li J (2016a) Performance, 5-aminolevulinic acid (ALA) yield and microbial population dynamics in a photobioreactor system treating soybean wastewater: effect of hydraulic retention time (HRT) and organic loading rate (OLR). Bioresour Technol 210:146–152
Liu S, Zhang G, Zhang J, Li X, Li J (2016b) Performance, carotenoids yield and microbial population dynamics in a photobioreactor system treating acidic wastewater: effect of hydraulic retention time (HRT) and organic loading rate (OLR). Bioresour Technol 200:245–252
Lu H, Peng M, Zhang G, Li B, Li Y (2019a) Brewery wastewater treatment and resource recovery through long term continuous-mode operation in pilot photosynthetic bacteria-membrane bioreactor. Sci Total Environ 646:196–205
Lu H, Zhang G, Zheng Z, Meng F, Du T, He S (2019b) Bio-conversion of photosynthetic bacteria from non-toxic wastewater to realize wastewater treatment and bioresource recovery: a review. Bioresour Technol 278:383–399
Meng F, Yang A, Zhang G, Zhang P, Ye J (2018) Benchmark study of photosynthetic bacteria bio-conversion of wastewater: carbon source range, fundamental kinetics of substrate degradation and cell proliferation. Bioresour Technol Reports 1:31–38
Mohd Udaiyappan AF, Abu Hasan H, Takriff MS, Sheikh Abdullah SR (2017) A review of the potentials, challenges and current status of microalgae biomass applications in industrial wastewater treatment. J Water Process Eng 20:8–21
Nandi D, Shemin D (1968) δ-Aminolevulinic acid dehydratase of Rhodopseudomonas spheroides III. Mechanism of porphobilinogen synthesis. J Biol Chem 243:1236–1242
Nookongbut P, Kantachote D, Megharaj M, Naidu R (2018) Reduction in arsenic toxicity and uptake in rice (Oryza sativa L.) by As-resistant purple nonsulfur bacteria. Environ Sci Pollut Res 25:36530–36544
Nunkaew T, Kantachote D, Chaiprapat S, Nitoda T, Kanzaki H (2018) Use of wood vinegar to enhance 5-aminolevulinic acid production by selected Rhodopseudomonas palustris in rubber sheet wastewater for agricultural use. Saudi J Bio Sci 25:642–650
Nunkaew T, Kantachote D, Kanzaki H, Nitoda T, Ritchie RJ (2014) Effects of 5-aminolevulinic acid (ALA)-containing supernatants from selected Rhodopseudomonas palustris strains on rice growth under NaCl stress, with mediating effects on chlorophyll, photosynthetic electron transport and antioxidative enzymes. Electron J Biotechnol 17:19–26
Nunkaew T, Kantachote D, Nitoda T, Kanzaki H (2015) Selection of salt tolerant purple nonsulfur bacteria producing 5-aminolevulinic acid (ALA) and reducing methane emissions from microbial rice straw degradation. Appl Soil Ecol 86:113–120
Palamae S, Choorit W, Dechatiwongse P, Zhang D, Antonio del Rio-Chanona E, Chisti Y (2018) Production of renewable biohydrogen by Rhodobacter sphaeroides S10: a comparison of photobioreactors. J Clean Prod 181:318–328
Qin L, Liu Q, Meng Q, Fan Z, He J, Liu T, Shen C, Zhang G (2017) Anoxic oscillating MBR for photosynthetic bacteria harvesting and high salinity wastewater treatment. Bioresour Technol 224:69–77
Rao PS, Saraswathyamma CK, Sethuraj MR (1998) Studies on the relationship between yield and meteorological parameters of Para rubber tree (Hevea brasiliensis). Agric For Meteorol 90:235–245
Saikeur A, Choorit W, Prasertsan P, Kantachote D, Sasaki K (2009) Influence of precursors and inhibitor on the production of extracellular 5-aminolevulinic acid and biomass by Rhodopseudomonas palustris KG31. Biosci Biotechnol Biochem 73:987–992
Sasaki K, Ikeda S, Nishizawa Y, Hayashi M (1987) Production of 5-aminolevulinic acid by photosynthetic bacteria. J Ferment Technol 65:511–515
Sasaki K, Tanaka T, Nishizawa Y, Hayashi M (1991) Enhanced production of 5-aminolevulinic acid by repeated addition of levulinic acid and supplement of precursors in photoheterotrophic culture of Rhodobacter sphaeroides. J Ferment Bioeng 71:403–406
Smart JL, Willett JW, Bauer CE (2004) Regulation of hem gene expression in Rhodobacter capsulatus by redox and photosystem regulators RegA, CrtJ, FnrL, and AerR. J Mol Biol 342:1171–1186
Wu Y, Liao WB, Dawuda MM, Hu LL, Yu JH (2019) 5-Aminolevulinic acid (ALA) biosynthetic and metabolic pathways and its role in higher plants: a review. Plant Growth Regul 87:357–374
Zhi R, Yang A, Zhang G, Zhu Y, Meng F, Li X (2019) Effects of light-dark cycles on photosynthetic bacteria wastewater treatment and valuable substances production. Bioresour Technol 274:496–501
Acknowledgments
Research facility of the Biogas and Biorefinery Research Laboratory at the Faculty of Engineering of Price of Songkla University was very much appreciated.
Funding
This research was financially supported by the Thailand Research Fund (TRF) and Thai Rubber Latex Cooperative Ltd. (Nam Yang Thai Coop. Co., Ltd.) through the Research and Researcher for Industries (RRI) Grant No. MSD5710062. The authors would also like to thank partial supports from Annual Research Budget of the Prince of Songkla University (PSU) contract no. ENG570183S and the Graduate School of Prince of Songkla University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ta Yeong Wu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hosakul, P., Kantachote, D., Saritpongteeraka, K. et al. Upgrading industrial effluent for agricultural reuse: effects of digestate concentration and wood vinegar dosage on biosynthesis of plant growth promotor. Environ Sci Pollut Res 27, 14589–14600 (2020). https://doi.org/10.1007/s11356-020-08014-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08014-w