Abstract
High-strength wastewaters after being digested for biogas production in anaerobic digesters still contain substantial nutrients and organics. The anaerobic digestates from four major industries in Thailand were tested with batch cultivation of Chlorella sp. for oil production potentials. Pig farm digestate was found most suitable as the growth medium generating 0.95 g/Lmedium (dry biomass), which was 1.16–3.06 times of other digestates tested. Considerable removals of nitrogen and phosphorus achieved were an added benefit to the goal of ultimate treatment of these wastewaters. Light intensity had strong influence on growth and heterotrophic metabolism up to 78 μmol/m2/s, while the dilution of digestate above 2.4× diminished growth potential and lipid production. A quadratic regression model was constructed to describe interaction of light intensity, dilution factor, and time of cultivation to lipid production with a satisfactory precision. Light intensity could influence fatty acid composition, although palmitic acid was found predominant at 47.1 %. The algae oil generated could potentially increase the total energy output from anaerobic digesters of a typical pig farm by 22 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fossil fuels such as coal, oil, and natural gas are the nonrenewable resource that cannot be regenerated in human time scale. Rigorous exploration and consumption have caused global warming, acid rain, and emissions of toxic compounds to the atmosphere. Bio-based fuels are becoming a crucial alternative source of carbon-neutral energy. Similar to many countries, the Thai government had initiated multiple subsidiary policies for small renewable power generators. Among various types, biogas technology was rigorously exploited as energy production and waste treatment unit, ever since anaerobic digestion for industrial wastewaters has flourished. So far, industries have installed over 1145 biogas systems across various sectors [1]. After the wastewaters are digested, the effluent or so called digestate is typically discharged to the other post treatment processes since it still does not meet the regulated industrial effluent standard. The industry is obligated to further treat their wastewater to the secondary effluent standard with no incentive. These digester effluents still contain various constituents that can be used as substrate and nutrients for microalgae production.
In tropical region, biodiesel from palm oil is of major importance for many economies as it can substitute or blend to the conventional diesel and help leverage fluctuation of domestic palm oil stock and palm fruit price. Biodiesel is traditionally produced from plant oils such as palm, coconut, and rapeseed, but these would compete with the human and animal consumption. Microalgae are regarded as the future players in oil and biomass production, as well as the potential sink for carbon due to their fast growing rate, ability to grow in various environments with great adaptability and varieties, and the synthesis of lipid within their cell mass [2]. They are able to grow in many waters both fresh and saline, particularly the polluted waters. It is noteworthy that these could be accomplished without competing with food crops for land and water [3]. The lipid synthesized can be extracted and refined into biodiesel, while the cell wall consisted mostly of cellulosic material can be converted to sugars and other valuable products [4, 5].
Many microalgae are mixotrophs that can sequestrate CO2 from atmosphere and organic compounds in liquid growth medium as carbon source [6, 7]. Since both can be assimilated simultaneously under either photosynthetic or respiratory metabolism, the organics in the digestate can be removed, while the produced oxygen from photosynthesis enhances aerobic oxidation of the remaining pollutants in the liquid [8]. Although light intensity is central for the photosynthesis reaction kinetics [9], the concentration and composition of the growing media are not less vital [10]. The interaction between these two parameters may play a role in the growth and metabolism of the microalgae in a specific medium. To maximize the energy production from industrial wastewaters, digester effluents after biogas is extracted through anaerobic fermentation are one of the most economical mediums for microalgae cultivation. Although many studies regarding the use of digester effluents for microalgae cultivation were reported [11–14], the study on the comparative application across various industrial digester effluents is still needed, particularly at the interaction of important growth and oil synthesis factors, such as light intensity and medium dilution. This present study attempted to identify appropriate digestate and its use in order to produce more energy in the form of bio-oil and biomass from digestate while removing organics and nutrients at the same time.
The objectives of this study were firstly to compare the performance of Chlorella sp. growing in the digestate from main industries in Thailand that installed biogas systems. Secondly, the selected digester effluent suitable for growth was further investigated for its ability to produce lipids and remove organic pollutant. Multiple regression was employed to examine the interactive relationship of light intensity, digestate dilution, and time of cultivation on the overall lipid production. Results from this work help reveal the potential of the existing biogas installed industries to engage in an algal cultivation for additional energy recovery from their wastewater.
Materials and Method
Algae and Culture Stock Preparation
Microalgae Chlorella sp. used in this study was obtained from the National Institute of Coastal Agriculture located in Songkhla Province, Thailand. It was cultured in multiple 500-mL glass flasks to increase cell biomass with the standard medium modified Chu 13. The medium was composed of (g/L) KNO3 0.2, K2HPO4 0.04, MgSO4·7H2O 0.1, CaCl2·2H2O 0.054, Fe citrate 0.01, citric acid 0.1, NaHCO3 0.036, and 1 mL of a microelement solution consisting of (g/L) H3BO3 2.85, MnCl2·4H2O 1.8, ZnSO4·7H2O 0.02, CuSO4·5H2O 0.08, CoCl2·6H2O 0.08, and Na2MoO4·2H2O 0.05 [15]. This procedure was carried out in a growth chamber, which was operated on artificial lighting at intensity 39 μmol/m2/s (3000 lx) in a 16-h photoperiod provided by Sylvania 4-ft. 36 W cool white fluorescent bulbs with a digital timer. The culture was bubbled with filtered air, continuously with coarse ceramic diffusers under a controlled room temperature of 25 ± 1 °C. Continuous agitation was provided by magnetic stirrer at 250 rpm. The microalgae were successively subcultured approximately every 2–3 weeks to maintain active cells. These steps were repeated to obtain sufficient microalgae biomass as stock solution for all experiments in this study. The microalgae stock culture was grown to concentrations just above 1 g/L indicated by optical density (OD) at 730 nm using spectrophotometer with a pre-determined standard curve. The culture was transferred and then diluted back to 1 g/L before used, where 100 mL (containing approximately 0.1 g dry cell) was placed into each reactor in the treatments. The said microalgae inoculant from the maintained stock was active and within the exponential growth phase.
Digester Effluent Preparation
The modified Chu 13 medium and effluents from the anaerobic digester, hereafter referred to as digestate, from four major industries in Thailand were used as media to cultivate Chlorella sp. These digestates were collected from full-scale anaerobic digesters receiving their respective wastewaters, which were operated in stable condition in pig farm, seafood-processing plant, palm oil mill, and concentrated rubber latex factory. They are designated as PIG, SEA, PAL, and RUB, respectively. Characteristics of these media as received bases are listed in Table 1 as raw effluents. The collected effluents then passed through a thin sheet fabric cloth to remove large particles. The effluents were then autoclaved at 121 °C for 15 min to eliminate or limit microbial growth that would interfere carbon and nutrient uptake of the algae. The liquids were let cool down and characterized before diluted to the designed experimental conditions. This process was done within 24 h before use in the experiment to ensure freshness of the medium. Sample analyses were carried out according to the Standard Methods [16].
Experimental Design
Part I: Comparative Cultivation of Chlorella sp. in Standard Medium and Different Digestates
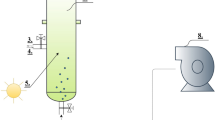
The microalgae were cultivated in the modified Chu 13 medium at light intensity 39, 78, and 117 μmol/m2/s (3000, 6000, and 9000 lux, respectively) in the growth chamber under the said temperature and photoperiod as standard medium references. For digestate cultivation, all autoclaved digestates were diluted to a total nitrogen (TN) concentration of 250 mg-N/L (Table 1). This was done to enable comparison of algal cultivation performance across the different digestates. One-hundred milliliters of stock culture containing 0.1 g microalgae was used to inoculate each reactor, in which the prepared digestate was filled to a final volume of 1 L. All four digestate cultivations were carried out in the growth chamber at 39 μmol/m2/s. To compensate for the evaporative loss of liquid medium, deionized water was refilled daily only to the level marked at each preceding liquid sampling point in order to avoid the error in cell concentration measurements. All treatments in this study consisted of at least three replicates.
Part II: Effects of Light Intensity, Dilution Factor, and Cultivating Time on Chlorella sp. Growth and Lipid Production
Only one digestate identified as most suitable in part I was chosen for use in experiments in part II. The selected digestate was used as medium to cultivate Chlorella sp. at light intensity 39, 78, and 117 μmol/m2/s under three dilutions at D1 (1:0 no dilution), D2 (1:1 dilution), and D3 (1:2 dilution). Deionized water was used as dilution water although in the large-scale application, the final effluent from the factories, which contain very low level of pollutants, can be used as dilution water. It was noted that the digestate used in part II was collected from different times but its characteristics were in close proximity with those in Table 1. Its main characteristics were chemical oxygen demand (COD) 632 mg/L, TN 472 mg/L, and total phosphorus (TP) 34.5 mg/L. Performance of algal cultivation was measured in terms of organic (as COD) removal, biomass growth, and lipid production. At least three replicates were employed in all treatments.
Sampling and Analytical Procedures
During the experiment, a sample of 30-mL homogeneous reactor content was drawn every 2 days over the course of the 32-day cultivation period. The sample was then centrifuged at 4000 rpm (rcf 1073×g) for 15 min, and the concentrated cell mass pellet was taken and washed with deionized water twice. The harvested cell mass was dried in an oven at 60 °C until constant weight and left to cool down in a desiccator before weight measurement. Dry cell mass concentration was then calculated and used to determine the microalgae growth rate according to the modified Gompertz equation (Eq. 1), which is widely used for microbial growth modeling [17, 18]. The algal net dry mass concentration was calculated from the total suspended solid concentration at time t (consisting of digestate particulates, inoculated algal cells, and algal growth cells) subtracted by the initial total suspended solid concentration (consisting of digestate particulates and inoculated algal cells). It was assumed that the particulates persisted in the digestate are recalcitrant. The supernatant of each sample was carefully taken from the centrifuge flask for the analyses of TN, TP, and COD according to the Standard Methods [16].
where DM(t) is dry mass concentration of algal growth cells (g/L) at time t, e is exp(1) = 2.71828, R m is maximum specific algal growth rate (g/L/day), DMmax is algal dry mass production potential (g/L), and λ is lag phase time (days). The parameters in this equation were estimated by least square method using Solver Function in Microsoft® Office Excel 2010.
The said harvested cell mass was subsequently analyzed for lipid content by the protocol adapted from Folch et al. [19]. The extraction of lipid was performed using chloroform/methanol (2:1 v/v) solvent with sonication by Elmasonic E30H (Elma-Han Schmidbauer GmbH & Co, Germany) for 30 min. The extracted lipid was centrifuged, and the supernatant collected was placed under a vacuum hood to remove the solvent. This extraction process was performed successively until no lipid appeared in the solvent.
The fatty acid composition in the extracted oil was analyzed using a Hewlett–Packard gas chromatography (model 6850), with an FFAP capillary column (30 m length × 0.25 mm ID × 0.25 μm film thickness) and a flame ionization detector. Helium was used as carrier gas with the injector temperature of 290 °C and the detector temperature of 300 °C. The column temperature, at initial temperature of 210 °C for 12 min, was increased at the rate of 20 °C/min to 250 °C and held for 8 min. Sample preparations and analyses were done in the Biogas and Biorefinery Engineering Laboratory of the Civil Engineering Department, while the fatty acid composition in the extracted oil was analyzed at the Scientific Equipment Center, Prince of Songkla University.
Lipid content and cumulative lipid production were determined according to Eqs. 2 and 3, respectively.
where WL is the weight of the extracted lipids (g), and DM is the algal dry cell mass (g).
where V is the volume of medium sample that DM was derived from (L), and LC is the lipid content of sample (%).
The relationship of the variables (light intensity and medium concentration) to the cumulative lipid production was analyzed using multiple regression quadratic model (Eq. 4) with the Design Expert software version 6.0.8.
where Y is the predicted response, β 0 is a constant, β i is the linear coefficient, β ii is the squared coefficient, β ij is the cross-product coefficient, k is the number of studied variables, and X i is the actual values of the studied independent variables. The response of the lipid production was regressed with respect to light intensity (LI in μmol/m2/s), dilution factor (DF), and time of cultivation (TC in days).
Coefficients with statistical significance were indicated at p < 0.05. Response surface plots were generated with the Design Expert software version 6.0.8. In order to determine the effects of independent variables, means and standard deviations were compared using SPSS software version 11.0 with the one-way analysis of variance (ANOVA) and Duncan’s multiple range test.
Results and Discussion
Characteristics of Industrial Digester Effluents
The modified Chu 13 was used as a standard medium to grow Chlorella sp. as it contained only soluble constituents with balanced nutrients and high transparency that allows thorough light penetration. It served as a reference medium in comparison to the other digestates. Fresh digester effluent (digestate) was collected from each of the four respective industries during normal plant operations. Solids were screened out by a thin fabric clothe and analyzed within 24 h. Data designated as raw digestate in Table 1 could represent digestate characteristics of four major industries in Thailand after biogas extraction (digestion). It was noted that there was a slight change of the digestates properties in sterilization process by autoclave. pH of the diluted autoclave digestates noticeably went up slightly as CO2, a major component of carbonic acid, and was forced out of the solution, but all were still under pH 8.0, which is still suitable for microalgae cultivation. Nitrate was quite low as no nitrification occurred in the anaerobic digester due to the void of oxygen. It was evident that turbidity varied greatly among the digestates starting from only 21 nephelometric turbidity unit (NTU) in PIG to an undetectable level represented by a dark brown color in PAL. These characteristics could play a role in light-demanding cultures. C/N/P calculated from the ratio of COD/TN/TP also showed obvious variations. The noticeable one was the palm oil mill (PAL) digestate with particularly high COD to nutrients ratio.
Generally, ammonium tolerance limit of different algae species was reportedly around 1000 μmol NH4 +-N/L [20] equivalent to 14 mg-N/L, which is quite low. In batch culture, the reported medium concentrations are the initial concentrations, which would decline rather quickly as the culture progressed, exposing the algae to a lower concentration. Nevertheless, dilution of the digestate was deemed necessary to prevent acute toxic and enable overall optimal growth of microalgae. If the ammonium concentration is not too high, it would only retard or inhibit algal growth in an early stage, usually observed as lag phase. Earlier works on Chlorella sp. had employed wastewaters at high ammonia nitrogen medium with good growth and nutrient removal activities, for instance, 267 mg/L [21], 410 mg/L [22], and 1550 mg/L [23].
Growth of Chlorella sp. in Different Digestates
Results showed that the inoculated 0.1 g/L Chlorella sp. started to grow shortly after the start of cultivation represented by the data points in Fig. 1. There were some lags of biomass growth during the first few days before the exponential growth phase ensued. The culture appeared to enter a stationary phase at around 12 days. The dry mass concentration after day 22 exhibited a decline as the culture goes into the endogenous decay phase. LI positively affected the growth of Chlorella sp. Data from the first 22 days were used to fit the Gompertz model (Eq. 1), which gave high R 2 of 0.9685, 0.9074, and 0.9446, for LI 39, 78, and 117 μmol/m2/s, respectively. The modeled predictions are shown as dash lines in Fig. 1. It is noted that the data after day 22 were not included in the model, as the Gompertz model does not account for the decay phase. From the fitted model, the maximum dry mass concentrations achieved were 0.49, 0.58, and 0.67 g/L, which are equivalent to the net dry mass growth of 0.39, 0.48, and 0.57 g/L at LI 39, 78, and 117 μmol/m2/s, respectively. The lag time varied inversely with LI from 3.8 to 2.9 to only 2.0 days at 39 to 117 μmol/m2/s. Meanwhile, the maximum biomass growth rate (the maximum slope of each growth profile) was improved from 0.06 to 0.08 g/L/day when LI increased from 39 to 78 μmol/m2/s but did not increase further at 117 μmol/m2/s. Specific biomass growth potentials (per gram of nutrient consumed) were 3.5, 3.5, and 5.1 g/g TN utilized, and 19.6, 22.1, and 27.5 g/g TP utilized at LI 39, 78, and 117 μmol/m2/s, respectively. It was clear that light intensity promoted carbon assimilation and cell synthesis in mixotrophic growth, which more often could lead to a lower carbon to nutrient ratio within the cell. This situation, in long cultivating time, could prompt a nutrient starvation and subsequently higher lipid accumulation [24]. This matter will be revisited in the subsequent section in this paper.
In Fig. 2, net algal growth curves of PIG, SEA, PAL, and LAT under LI 39 μmol/m2/s in a 32-day period are compared side by side. PIG showed an obvious lag phase but had steeper log phase until arriving to the stationary plateau at day 22, approximately 6 days before SEA and LAT, which possessed minimal lag. PIG’s higher turbidity (189 NTU) may have affected phototrophic growth in the beginning. Its turbidity had decreased with a visible floc formation (a conglomeration of particulates), and PIG medium became slightly clearer approximately 7 days in the experiment. This occurrence coincided with the rapid rise in its growth curve to reach 0.95 g/L, which was 1.16, 1.23, and 3.06 times of SEA, LAT, and PAL, respectively, at the end of experiment. With regard to the reference medium (modified Chu 13) at 39 μmol/m2/s that achieved maximum dry mass concentration 0.49 g/L, only PAL has lower growth at around 0.4 g/L (Fig. 2). Digestates are deemed a suitable media for algal cultivation.
LAT and PAL cultures also showed some lags, while SEA virtually picked up the growth speed from the beginning of the experiment partly because of the higher light penetration characterized by its lowest turbidity among all the media tested. The growth rate (slope of the growth curve) in SEA, however, declined over the course of the experiment and reached stationary phase at around 28 days with the final net algal concentration of 0.79 g/L. Data from Table 1 and Fig. 2 indicated that the final cell concentrations had an inverse correlation with COD. This incidence was consistent with Lin et al. [25], where the media of COD 1280 mg/L caused a decline in Chlorella pyrenoidosa biomass growth and the dilution of the leachate was necessary for its optimal growth. Dilution of the growth medium at a certain level could be necessary to promote growth.
In PAL, the net dry mass growth was the lowest reaching only 0.31 g/L. One of the possible explanations is the low light penetration caused by its high turbidity. Phototrophic metabolism of the microalgae was inevitably limited. The dark brown color is a result of the tannin residue released during oil extraction. Tannin is the recalcitrant plant polyphenolic compound that binds to proteins and various other organic compounds, and it was released from the fiber of palm fruits in a hush oil extraction process with steam cooker. The microalgal growth in this medium, therefore, came largely from the heterotrophic metabolism or dark reaction [26]. Another possibility is toxicity from the small concentration of phenolic compounds in the PAL digestate [27]. It reached stationary phase in only 16 days and showed a decay after that.
This experiment demonstrated the Chlorella sp. growth characteristics in four digestates and clearly showed the best performance in PIG over the others, as well as the reference medium. Although ammonia was a more preferred form of nitrogen for algal growth [28], in this study, it is not in total agreement with growth as the other factors discussed could cumulatively become more predominant. This implies that the total characteristics of the medium are more important than an individual factor to determine medium suitability.
Nutrient Removal Potential
Nutrient uptake by microalgae for growth was considered secondary benefit for their cultivation in wastewaters. The digestates, which were subjected to posttreatment, were used as cultivating media, and their treatability by Chlorella sp. were evaluated. Although final effluent from the entire treatment system of each factory can be used as dilution water, in this study, DI water was used in order to limit the variations in the experiments. The removal of TN could be achieved as high as 89.0–93.7 % in a 32-day cultivation period for PIG, SEA, and LAT. With the low growth in PAL medium, TN removal reached a reasonable 72.6 %. TN and TP removal rates in PIG were slower than the other three media (Fig. 3), corresponding to the longest lag phase in PIG culture (Fig. 2). Removal of TP was in a range of 43.8–62.3 %. TP removal percentage was not directly interpreted in relation with biomass growth because the starting concentration was different. The reductions of TP concentration in 32-day cultivation were 12.4, 10.6, 7.7, and 4.6 mg/L for SEA, PIG, LAT, and PAL, respectively. SEA culture had uptaken the most TP, while the biomass growth was second to PIG possibly affected by its high COD concentration, which is consistent with the report from Lin et al. [25]. PAL contained very low TP at only 7.3 mg/L and high N/P ratio at 34:1, coupled with the low light penetration from its dark color; the growth rate was very limited. Phosphorus uptake in PAL occurred only in the first 8–10 days, and virtually, there was no TP removal after that, while TN and TP uptakes still proceeded in other media. In terms of nutrient removal, the final TN concentrations at 32 days were in a range of 15.6–58.6 mg/L, which was below the industrial effluent standard of 100 mg/L [29].
Effects of Light Intensity and Dilution Factor
Biomass Growth
Results from part I clearly showed that PIG possessed more suitable characteristics over the other media, therefore, it was chosen for further investigations. Three dilutions of PIG were tested to find a suitable strength of pig farm digestate for Chlorella sp. cultivation. At 39 μmol/m2/s, there was almost no growth of Chlorella sp. in full-strength PIG (D1 in Fig. 4a). Higher LI clearly stimulated the growth across all dilutions even in D1 that showed an obvious increase of cell concentration of 0.49 and 0.63 g/L to reach 0.59 and 0.73 g/L (values calculated by Eq. 1) in 32-day cultivation at 78 and 117 μmol/m2/s, respectively (Fig. 4b, c). Growth in D1 was not only lower but also obviously possessed longer lag time due to the high concentration of substances and lower transparency. This was consistent with Travieso et al. [30] and Zhu et al. [31], which illustrated that the lower dilution of the piggery wastewater started to show growth inhibition at COD above 800 mg/L for C. vulgaris and 1900 mg/L for C. zofingiensis, respectively. The lag phase times derived from Gompertz model in D1 treatments were 16.1, 13.2, and 11.8 days at LI 39, 78, and 117 μmol/m2/s, respectively. Thus, dilution of the digestate was crucial for successful Chlorella sp. cultivation. When the digestate was diluted to 2× (D2) and 3× (D3), it was obvious that growth increased and lag time were substantially reduced. D2 was evidently the best dilution factor in this case, as D3 was too thin and required unnecessary dilution water.
It is interesting to note that dry mass growth increased with LI at a diminishing rate. Larger leap of cell concentration was observed as LI increased from 39 to 78 μmol/m2/s compared to 78 to 117 μmol/m2/s. These characteristics indicated the saturation type, where further increase in light energy would return lesser benefit. The maximum direct sunlight of around 826–1076 μmol/m2/s at around noontime [32] seems excessive, but the depth of pond as well as shading from the suspended cells or artificial shading above the pond surface could help cut down light intensity to a near desirable level. A more depth-wise consistent light exposure could be achieved in shallower pond but still subjected to variation in sunlight intensity throughout the day. Controlled light source is possible but with the expense of energy supply cost. It was observed at higher LI that the effects of medium concentration were reduced, evidenced by the narrower gap between D2 and D3 treatments, 0.24 g/L at LI 39–78 μmol/m2/s and 0.23 g/L at 78–117 μmol/m2/s, respectively. The results clearly suggested some interactions between these parameters. For other living organisms close to algae like cyanobacteria, growth rate on mixotrophic mode was higher than either strict phototrophic and heterotrophic ones [33]. Organic carbon source changed the metabolic response of cells to light. There is a synergistic interaction between the phototrophic and heterotrophic modes in mixotrophic growth. Since algae cannot grow in strict heterotrophic mode, light is still required for energy production for cell use in mixotrophic mode, but cell synthesis is dependent largely on organic carbon compounds [3]. It seems that in order to synthesize more cell mass, the energy (light) requirement for cell use increases. This fact could draw the connection between the media transparency and organic concentration in perspective.
Organic Removal
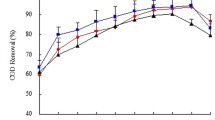
Organic removal from the medium was also observed (Table 2). The growth of algae took up COD from the solution for heterotrophic metabolism that was very apparent during the first 22 days. At low LI (39 μmol/m2/s), the biomass entered stationary phase at around 22 days as the soluble COD (SCOD) removal almost shut down, and there was even a little SCOD release back to the medium in D2, signifying perhaps the endogenous decay. However, at higher LI, growth continued beyond 22 days as seen in Table 2 with an additional SCOD uptake. The cultures entered stationary phase at some later time around 28–30 days. It can be deduced that LI could stimulate heterotrophic growth together with the phototropic one. Across the tested dilution factors, D2 always statistically gave the highest SCOD removal followed by D3 and D1, in order, whose trend was consistent with the dry mass growth characteristic. This synchronized pattern of the two parameters validated the importance of heterotrophic growth of Chlorella sp. However, D1 at any LI gave the highest SCOD uptake compared to other dilutions. Higher SCOD in its aqueous environment could have allowed slightly lower light penetration and induced a higher heterotrophic metabolism. The organic carbon uptake ratio in terms of gSCOD per gram cell at (D1, D2, D3) were (8.22, 0.16, 0.12) at 39 μmol/m2/s, (0.93, 0.18, 0.10) at 78 μmol/m2/s, and (0.53, 0.15, 0.07) at 117 μmol/m2/s, respectively. This suggested that the strength of the digestate medium dictates the contribution of algal cell synthesis pathways in the culture. With the correlation between organic uptakes and biomass growth in mixotrophic mode, the same rationales as described in the “Biomass Growth” section of light and dilution of the media could be used to describe the phenomena.
Lipid Content and Production
Lipid content along the course of experiment was measured and compared across different treatments in each time step (Table 2). The lipid level in Chlorella sp. cell was 12.3 % at the beginning and accumulated over time to as high as 32.7 % at day 22 in D2 at 117 μmol/m2/s. Overall, lipid content increased mostly during the first 22 days that was when the biomass growth started to enter stationary phase (Fig. 4). Improvement in lipid accumulation during the last 10 days was minimal. LI appeared to help lessen the effect of high-strength media (D1). It showed an obvious positive impact in the diluted media although without a definite statistical difference at α = 0.05 (no mutually exclusive difference among the means).
The positive influence of dilution to lipid content was found at early stage of growth. This was consistent with other researches that reported the stimulation of lipid accumulation in microalgae in nutrient deficiency state [24, 34]. In batch cultivation, the effect of low nutrients availability would inevitably occur toward the end of the batch where most nutrients are almost depleted with cell growth, as illustrated in Fig. 3. How fast the nutrients are uptaken depends on the growth rate, which depends on light as this experimental result demonstrated. The state of nutrient deficiency should be more relevant to the intercellular nutrient content, not the medium nutrient concentration. The delayed growth in relation to the pattern of nutrient depletion from the medium in this study coincides with the luxury uptake phenomena observed in aquatic plants [35, 36], as mathematically elaborated in Droop quota model [37]. This fact implies a required harvesting time after nutrient dosing to the culture for high lipid cell mass.
The ultimate parameter in this present study was the lipid production in terms of mass of lipid accumulated per liter of medium used. It was clear that the D2 was the best dilution in that regard as it simultaneously gave the highest cell production and lipid content. LI appeared to play a significant role as it also related directly to the biomass production. Overtime, the batch cultivation of only 22 days would be sufficient for the optimal lipid production throughput, as the improvement was insignificant. Increase of light energy did not seem to give a clear improvement beyond 78 μmol/m2/s in D1 and D3, but at an appropriate dilution of D2, LI could still enhance lipid production as suggested by a statistical difference between 78 and 117 μmol/m2/s treatments. The maximum lipid production was found at 212.5 mg/Lmedium under LI 117 at D2. Relationship of the parameters with lipid production and interaction can be illustrated by multiple regression and surface plots in the “Regression Model of Cumulative Lipid Production” section.
In our estimation, the oil production is 425 goil/m3 digestate, which is equivalent to 17.4 MJ/m3 digestate under the assumption of 41 MJ/kgoil from Chlorella oil [38]. With a biogas yield from typical pig farms of 3.5 m3/m3 wastewater at CH4 content 60 % [39] equivalent to approximately 22 MJ/m3 biogas, the biogas energy production from pig farm wastewater is estimated to be 77 MJ/m3 of wastewater. Hence, the algae cultivation from digestate could increase the total energy production by as high as 22 %. In a medium pig farm of around 1600 pigs, which generates wastewater approximately 600 m3/day, the algal oil energy output could be roughly 3.13 TJ/year or 76,500 kgoil at 300 days of operation. It must be noted that these estimates do not account for the energy used for sterilizing the medium, which was done to eliminate the influence of bacterial interference to algal cultivation in the experiment. For industrial scale application, energy for medium sterilization is not economically feasible. Thus, some variations from these figures may persist when raw digestate is to be used. In addition, there would be 876 g of algal cell sludge after oil extraction per 1 m3 of the digestate. This is equivalent to 157,680 kg/year on dry weight basis for fertilizer or fuel. Alternatively, this sludge could be used to co-digest with pig manure in the main digester for more biogas production that could increase the system’s efficiency and sustainability.
Regression Model of Cumulative Lipid Production
Full factorial experiment was employed to find the relationship among light intensity (LI), time of cultivation (TC), and dilution factor (DF) to lipid production. There were nine experiments with four time-wise samplings in triplicate runs, totaling of 108 data points. Quadratic regression was derived as shown in Table 3. The model F test returned a very low p value of <0.0001 with the adjusted R 2 of 0.8158. This model is statistically significant and able to explain the variability at 82 % of the response. Precision and reliability of the conducted experiments were affirmed by the low coefficient of variance (CV). Only the terms TC × TC and LI × DF were not statistically significant, but all terms were retained in the model to obtain high accuracy for analysis (Eq. 5).
The response surfaces of lipid production were generated with the three studied parameters as variables (Fig. 5). Figure 5a shows that both LI and TC positively promoted lipid production. The evenly distributed gap at 20 mg/Lmedium of the two adjacent contours indicated that these two parameters had neither significant synergistic nor antagonistic effects on the model. In Fig. 5b, TC had shown obvious impact on the change of lipid production, while the effect of DF was peak near the midrange of the surface curvature. This optimal DF for PIG was observed again in Fig. 5c. It is noted that at 22 days in cultivation, the lipid production reached at least 80 % of the total in all treatments. Longer cultivation provided insignificant incentive. DF in the midrange at around 2 seemed to contain the optimal in this medium and LI beyond 117 μmol/m2/s would give the diminishing return on lipid production (Fig. 5c), following Monod kinetics pattern as reported in Chojnacka and Marquiz-Rocha [40]. By a simple search algorithm using the MATLAB® program package, the highest lipid production predicted by the model (Eq. 5) within the range of studied variables was 202.6 at LI 117 μmol/m2/s, DF 2.4, and TC 32 days. Optimal DF was found within the studied range, while ranges of LI and TC did not contain the optimal values. The p value can be used to indicate dominance of variable to the model prediction. The terms TC, DF2, and TC*LI are highly significant to the model (p < 0.0001), while the least influential is the interaction term LI*DF with small coefficient (b9) and large p value. This optimal DF is near the dilution factor of 2 (D2) used in the experiment. The space between contours was widening in DF–LI plot (Fig. 5c), indicating a smaller and smaller change per unit of LI toward the lipid production of 160 mg/Lmedium contour. Beyond such contour, the surface was projected to level off and slowly approaching the optimal. In field condition, average intensity of sunlight would has been more than sufficient for lipid production optimum. Light is normally not a limitation in outdoor open pond system with transparent medium. However, interference from particulates in real digester effluent in filed condition could hinder the light penetration; thus, the separation of solids, probably through practical rock or gravel filter or precipitation, should be engaged. In this regard, the dilution water should be a clear liquid that can also help lower the solid concentration in the digestate.
Fatty Acid Composition Profile
The profile of fatty acids in the oil extracted from Chlorella sp. in D2 experiment is shown in Table 4, in comparison with other media and crude palm oil. The most abundant fatty acids found were confined in the C16 and C18 species, namely, palmitic acid (C16:0), stearic acid (C18:0), and oleic acid (C18:1), in order. LI appeared to have an impact on fatty acid production, particularly the oleic acid as its synthesis shifted by 7.2 times from 20.9 % at 39 μmol/m2/s to only 2.9 % at 117 μmol/m2/s to other fatty acids. Meanwhile, linoleic acid (C18:2) swing from 5.03 to 1.07 % was also noticeable. At high LI of 117 μmol/m2/s, the majority of the fatty acids produced in this study were palmitic and stearic acids at a combination of 58.0 % of the total, constituting 84.4 % of the detectable acids. Oleic acid (C18:1) synthesis could be linked to the heterotrophic pathway under low LI, while palmitic acid (C16:0) synthesis system is more active at high LI. Compared to the other wastewater media, C16 and C18 acids were still the highest composition in common. However, it was obvious that our fatty acids profile is more similar to crude palm oil especially at low light intensity. This could provide an easy transformation to other end uses with the existing technology of palm oil products.
Conclusion
This work demonstrated the usefulness of the anaerobic digester effluent after the wastewater is digested for biogas generation. Pig farm (PIG) digestate showed highest potential for the microalgae biomass growth, while the growth in palm oil mill digestate was suppressed. Further investigation using PIG medium showed that light intensity and dilution above 78 μmol/m2/s and 2×, respectively, gave weakening stimulus effects on lipid production and SCOD removal. The quadratic model constructed was able to describe the relationship among main cultivating parameters with satisfactory precision. Results also suggested that light intensity could have an influence on fatty acid synthesis pathway and composition. Algal oil produced could be a sizable source of energy apart from the main energy from biogas of the wastewater digestion.
References
Thaibiogas. (2015) Thailand Energy Policy and Planning Office. 2015. http://www.thaibiogas.com/index2.php. Accessed 20 Mar 2015
Ahmad AL, Yasin NHM, Derek CJC, Lim JK (2011) Microalgae as a sustainable energy source for biodiesel production: a review. Renew Sust Energ Rev 15:584–593
Prajapati SK, Kaushik P, Malik A, Vijay VK (2013) Phycoremediation coupled production of algal biomass, harvesting and anaerobic digestion: possibilities and challenges. Bitechnol Adv 31:1408–1425
Mirsiaghi M, Reardon KF (2015) Conversion of lipid-extracted Nannochloropsis salina biomass into fermentable sugars. Algal Res 8:145–152
Vardon DR, Sharma BK, Blazina GV, Rajagopalan K, Strathmann TJ (2012) Thermochemical conversion of raw and defatted algal biomass via hydrothermal liquefaction and slow pyrolysis. Bioresour Technol 109:178–187
Abreu AP, Fernandes B, Vicente AA, Teixeira J, Dragone G (2012) Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source. Bioresour Technol 118:61–66
Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31:1043–1049
Venkata MS, Rohit MV, Chiranjeevi P, Chandra R, Navaneeth B (2015) Heterotrophic microalgae cultivation to synergize biodiesel production with waste remediation: progress and perspectives. Bioresour Technol 184:169–178
Singh SP, Singh P (2015) Effect of temperature and light on the growth of algae species: a review. Renew Sust Energ Rev 50:431–444
George B, Pancha I, Desai C, Chokshi K, Paliwal C, Ghosh T, Mishra S (2014) Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus—a potential strain for bio-fuel production. Bioresour Technol 171:367–374
Passero M, Cragin B, Coats E, McDonald A, Feris K (2015) Dairy wastewaters for algae cultivation, polyhydroxyalkanoate reactor effluent versus anaerobic digester effluent. Bioenerg Res 8:1647–1660
Prajapati SK, Kumar P, Malik A, Vijay VK (2014) Bioconversion of algae to methane and subsequent utilization of digestate for algae cultivation: a closed loop bioenergy generation process. Bioresour Technol 158:174–180
Singh M, Reynolds DL, Das KC (2011) Microalgal system for treatment of effluent from poultry litter anaerobic digestion. Bioresour Technol 102:10841–10848
Xia A, Murphy JD (2016) Microalgal cultivation in treating liquid digestate from biogas systems. Trends Biotechnol 34:264–275
Dumrattana P, Tansakul P (2006) Cultivation of the hydrocarbon-rich alga, Botyococcus braunii in secondary treated effluent from a sea food processing plant. Songklanakarin J Sci Tech 28:99–105
APHA, AWWA, WPCF (2012) Standard methods for examination of water and wastewater. American Public Health Association, Washington, DC.
Gibson AM, Bratchell N, Roberts TA (1988) Predicting microbial growth: growth responses of salmonellae in a laboratory medium as affected by pH, sodium chloride and storage temperature. Int J Food Microbiol 6:155–178
McClure PJ, de W. Blackburn C, Cole MB, Curtis PS, Jones JE, Legan JD, Ogden ID, Peck MW, Roberts TA, Sutherland JP (1994) Modelling the growth, survival and death of microorganisms in foods: the UK food micromodel approach. Int J Food Microbiol 23:265–275
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 266:497–509
Collos Y, Berges JA (2004) Nitrogen metabolism in phytoplankton, Encyclopedia of Life Support Systems (EOLSS). EOLSS Publishers, Oxford
Hongyang S, Yalei Z, Chunmin W, Xuefei Z, Jinpeng L (2011) Cultivation of Chlorella pyrenoidosa in soybean processing wastewater. Bioresour Technol 102:9884–9890
Aslan S, Kapdan IK (2006) Batch kinetics of nitrogen and phosphorus removal from synthetic wastewater by algae. Ecol Eng 28:64–70
Khan M, Yoshida N (2008) Effect of L-glutamic acid on the growth and ammonium removal from ammonium solution and natural wastewater by Chlorella vulgaris NTM06. Bioresour Technol 99:572–582
Li Y, Han F, Xu H, Mu J, Chen D, Feng B, Zeng H (2014) Potential lipid accumulation and growth characteristic of the green alga Chlorella with combination cultivation mode of nitrogen (N) and phosphorus (P). Bioresour Technol 174:24–32
Lin L, Chan GYS, Jiang BL, Lan CY (2007) Use of ammoniacal nitrogen tolerant microalgae in landfill leachate treatment. Waste Manag 27:1376–1382
Jacob-Lopes E, Scoparo CHG, Lacerda LMCF, Franco TT (2009) Effect of light cycles (night/day) on CO2 fixation and biomass production by microalgae in photobioreactors. Chem Eng Process 48:306–310
Saifuddin N, Saltanat A, Refal H (2014) Enhancing the removal of phenolic compounds from palm oil mill effluent by enzymatic pre-treatment and microwave-assisted extraction. Chem Sci Trans 3:1083–1093
Li Y, Chen YF, Chen P, Min M, Zhou W, Martinez B, Zhu J, Ruan R (2011) Characterization of a microalga Chlorella sp. well adapted to highly concentrated municipal wastewater for nutrient removal and biodiesel production. Bioresour Technol 102:5138–5144
PCD (2015) Water Pollution Regulation, Pollution Control Department of Thailand. http://www.pcd.go.th/download/en_regulation.cfm?task=s3. Accessed 26 April 2015
Travieso L, Benítez F, Sánchez E, Borja R, Martín A, Colmenarejo MF (2006) Batch mixed culture of Chlorella vulgaris using settled and diluted piggery waste. Ecol Eng 28:158–165
Zhu L, Wang Z, Shu Q, Takala J, Hiltunen E, Feng P, Yuan Z (2013) Nutrient removal and biodiesel production by integration of freshwater algae cultivation with piggery wastewater treatment. Water Res 47:4294–4302
Somporn C, Kamtuo A, Theerakulpisut P, Siriamornpun S (2012) Effect of shading on yield, sugar content, phenolic acids and antioxidant property of coffee beans (Coffea Arabica L. cv. Catimor) harvested from north-eastern Thailand. J Sci Food Agric 92:1956–1963
Ye H, Jia S, Dai Y (2009) Growth characteristics of the cyanobacterium Nostoc flagelliforme in photoautotrophic, mixotrophic and heterotrophic cultivation. J Appl Phycol 21:127–133
Feng P, Deng Z, Fan L, Hu Z (2012) Lipid accumulation and growth characteristics of Chlorella zofingiensis under different nitrate and phosphate concentrations. J Biosci Bioeng 114:405–410
Chaiprapat S, Cheng JJ, Classen JJ, Liehr SK (2005) Role of internal nutrient storage in duckweed growth for swine wastewater treatment. T ASABE 48:2247–2258
Powell N, Shilton AN, Pratt S, Chisti Y (2008) Factors influencing luxury uptake of phosphorus by microalgae in waste stabilization ponds. Environ Sci Technol 42:5958–5962
Droop MR (1973) Some thoughts on nutrient limitation in algae 1. J Phycol 9:264–272
Xu H, Miao X, Wu Q (2006) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126:499–507
DEDE (2006) Technology for biogas production and utilization. Department of alternative Energy Development and Efficiency, Bangkok, Thailand
Chojnacka K, Marquez-Rocha FJ (2004) Kinetic and stoichiometric relationships of the energy and carbon metabolism in the culture of microalgae. Biotechnology 3:21–23
Farooq W, Lee YC, Ryu BG, Kim BH, Kim HS, Choi YE, Yang JW (2013) Two-stage cultivation of two Chlorella sp. strains by simultaneous treatment of brewery wastewater and maximizing lipid productivity. Bioresour Technol 132:230–238
Yang L, Tan X, Li D, Chu H, Zhou X, Zhang Y, Yu H (2015) Nutrients removal and lipids production by Chlorella pyrenoidosa cultivation using anaerobic digested starch wastewater and alcohol wastewater. Bioresour Technol 181:54–61
Ramos MJ, Fernández CM, Casas A, Rodríguez L, Pérez Á (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 100:261–268
Acknowledgments
This research was financially supported by the Annual Research Budget of the Prince of Songkla University (PSU) contract no. ENG570183S and Graduate School of PSU, Thailand. The authors would like to recognize the full support for research facility of the Biogas and Biorefinery Research Laboratory, Faculty of Engineering, Prince of Songkla University, Thailand.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaiprapat, S., Sasibunyarat, T., Charnnok, B. et al. Intensifying Clean Energy Production Through Cultivating Mixotrophic Microalgae from Digestates of Biogas Systems: Effects of Light Intensity, Medium Dilution, and Cultivating Time. Bioenerg. Res. 10, 103–114 (2017). https://doi.org/10.1007/s12155-016-9780-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-016-9780-9