Abstract
The present study investigates the effect of blending oxygenate namely diethylene glycol dimethyl ether (diglyme) with minor vegetable oil namely rubber seed oil (RSO), babassu oil (BSO), and their blends in various proportions (R75B25, R50B50, and R25B75) on NOx-smoke trade-off and other engine characteristics. The tests were conducted on a commercial twin cylinder compression–ignition (CI) engine commonly used in tractors. The potential of the blends with diglyme is assessed based on performance, emission, and combustion characteristics of the engine at different load conditions. The tests were conducted at a constant speed of 1500 rpm maintaining the original injection timing and pressure. Compared to diesel, RSO, and BSO, and their blends exhibited inferior combustion due to poor physical properties like high viscosity and density. This resulted in a lower brake thermal efficiency with increase in HC, CO, and smoke emissions compared to diesel at all the load conditions. The augmented effect is observed with increase in BSO proportion for the blends and neat BSO. The poor combustion of minor vegetable oil and its blends lead to lower NOx emission as a result of lower in-cylinder temperature. To improve the performance and NOx-smoke trade-off, diglyme (DGM) was added with all the test fuels with the optimum share of 20% (by volume). Addition of DGM, increased brake thermal efficiency by 2–7% for all the test fuels due to improved combustion as a result of additional fuel bound oxygen in DGM and improved fuel blend properties. DGM addition reduced smoke, HC, and CO emission drastically with a slight increase in NOx emission compared to minor vegetable oil blends. The study shows that addition of DGM showed a promising note in NOx-smoke trade-off without affecting the other engine parameters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The transportation sector, agricultural sector, and heavy off-road vehicles widely employ diesel engines due to their fuel efficiency and robustness. Exhaust emission from diesel engines is one of the many sources of environmental pollution causing health problems. Serious efforts have been made to reduce the effect of emissions generated by combustion of fossil fuels in the environment. Among the various emissions from CI engine, NOx and smoke are dominant posing serious problems to environment and ecosystem. NOx emission is responsible for smog, acid rain, and ground level ozone formation, while smoke or soot emissions are carcinogenic affecting the human health adversely (Walsh 1999). To abide by the stringent EURO norms, various after-treatment systems namely selective catalytic reduction (SCR) (Guan et al. 2014), selective non-catalytic reduction (SNCR) (Thiyagarajan et al. 2017b), and non-selective catalytic reduction (NSCR) (Subramanian et al. 2017) were studied to reduce NOx emissions. Diesel particulate filter (DPF) is presently employed to reduce soot emissions (Chen et al. 2017). However, a trade-off exists between NOx-smoke which affects the engine performance to a larger extent (Kumar et al. 2016). Few other methods also aimed at achieving better NOx-smoke trade-off without compromising the performance. Many of these efforts have been aimed at improving the overall combustion efficiency by acting on elements such as injection systems (Corcione et al. 2001), shaping combustion chamber (Vedharaj et al. 2015), fuel formulation (Thiyagarajan et al. 2017a), and additives (Geo et al. 2017). Others have developed advanced combustion modes such as homogeneous charge compression ignition (HCCI) (Zheng et al. 2015), premixed charge compression ignition (PCCI) (Srihari and Thirumalini 2017), and reactivity controlled compression ignition (RCCI) (Benajes et al. 2015).
Studies were also done in using alternative fuels especially from plants, which can simultaneously develop power similar to diesel and reduce exhaust emissions. The methyl or ethyl ester of vegetable oils commonly known as biodiesel is one such alternative which is both environment-friendly and renewable (Joshi et al. 2017). Biodiesel use in diesel engines reduces hydrocarbon (HC), carbon monoxide (CO), and smoke emissions, but increases oxides of nitrogen emission (NOx). Numerous non-edible biodiesel were identified and tested in CI engine to explore the potential opportunities in replacing diesel (Subramanian et al. 2018; Kasiraman et al. 2016; Sajjadi et al. 2016). However, commercial use of these biodiesel in transportation or agriculture sector is not realized due to high-input cost for transesterification to convert the high-viscous vegetable oil to biodiesel (Agarwal et al. 2017). Unless, a government policy in subsidizing the price of biodiesel is in place, biodiesel use especially by farmers in tractors and genset application is difficult (de LT Oliveira et al. 2017). Hence, a feasible solution is to use minor vegetable oils available in the particular region as straight fuel which does not have much commercial use with less input cost. Among the various minor vegetable oils available in India, rubber seed oil and babassu oil were identified and taken for this research study. Rubber seed oil is mainly available in tropical regions of India with a production potential of 5000 tons per year (Ramadhas et al. 2005). Numerous studies were done in using RSO and improving its performance in CI engine (Geo et al. 2008; Geo et al. 2009; Bhovi et al. 2017). Babassu oil is extracted from babassu palm trees also available in tropical regions. The production potential is only 1000 tons per year (Demirbas 2009). While, few research works were done in analyzing babassu oil properties for diesel engine application (Oliveira et al. 2013), potential use of babassu oil in CI engine is not explored in the literature other than the preliminary work reported by authors previously to optimize the blend ratio of rubber seed oil and babassu oil (Varuvel et al. 2017).
Addition of oxygenated additives such as ethanol, dimethyl carbonate, and diglyme (DGM) can improve the low-temperature properties of biofuels with an enormous potential in reducing exhaust emissions. Most of oxygenates have been widely investigated; however, few investigations exist on the use of DGM. DGM is a colorless liquid with slight ether-like odor and relatively low toxicity and reactivity (Di et al. 2010). It is inter-soluble with diesel fuel, has high cetane number, and lower soot formation tendency (Bertoli et al. 1998). Use of DGM as an additive in diesel to reduce smoke or particulate matter (PM) emissions has generated interest among researchers as this method does not require the engine to be modified. Ren et al. (2008) observed no influence of oxygenates on NOx emission and thermal efficiency. However, PM emission from the engine exhaust is reduced considerably. Miyamoto et al. (1998) observed similar results wherein the increase in oxygen content in the blend reduced the smoke emission, and almost all the soot emissions were practically removed once the oxygen content reached 25–30%. The authors attributed it to the improvement in diffusion combustion phase along with the post-flame oxidation of soot. The authors also observed that the trade-off curve between NOx and smoke emission is flat, indicating that addition of oxygenates has little effect on NOx emission. Also, oxygenated fuels have greater tolerance to exhaust gas recirculation (EGR). The combined effect of diglyme and oxidation catalyst was studied by Tamanouchi et al. (1999). The authors observed a reduction in soluble organic fraction caused by oxidation catalyst and reduction in soot emission by DGM. Ren et al. (2007) in another study of various blends of diesel and diglyme observed a reduction in ignition delay and amount of heat released in premixed combustion phase with the increase of diglyme in the blend. The author also observed the center of heat release curve moving close to top dead center (TDC) along with an increase in thermal efficiency with increase in oxygen content.
Song et al. (2016) compared the combustion and emission characteristics of a multi-cylinder diesel engine fueled with blends of diesel with rapeseed methyl ester, diglyme, and butyl diglyme in various quantities. The authors found improvement in smoke emission with a penalty in NOx emission and fuel consumption. Also, no change in fuel spray development was observed; however, the ignition delay shortened. Gill et al. (2012) used diesel, 100% rapeseed methyl ester, 50% blend of diesel, and 50% rapeseed methyl ester, and 85% diesel and 15% diglyme in a single cylinder diesel engine. Authors extensively studied the particle number concentration and particle mass concentration at different loads. They observed lower particle number and mass concentration and a shift in size distribution to smaller mean diameter particles for oxygenates, with rapeseed methyl ester producing lowest particle number and mass concentration. Barrios et al. (2014) used a Volkswagen Euro 4 engine for comparing ethyl tert-butyl ether (ETBE) and diglyme blended in commercial diesel. Dramatic reduction in the total number of particles is observed for both oxygenates, especially when the ratio reaches 15%; however, ETBE is slightly better than DGM. DGM, on the other hand, is slightly better in reducing NOx emission as compared to ETBE maintaining similar fuel consumption.
Physical and chemical properties of a fuel play a major role in the formation of either type of particle during combustion (Puzun et al. 2011), suggesting that addition of oxygenates leading to increase in oxygen in the air-fuel mixture can significantly change the size distribution and number of emitted particles. Engine and emission characteristics for blending jatropha biodiesel and diglyme with diesel was studied by Nabi and Hustad (2012). Blending biodiesel and DGM with diesel resulted in higher brake-specific fuel consumption compared to diesel due to their lower energy content. The author also observed both nucleation mode and accumulation mode particles for jatropha biodiesel, whereas only accumulation mode particles were observed with DGM biodiesel.
The literature clearly indicates that few experimental works were done with rubber seed oil as fuel in CI engine, with limited research done using babassu oil. The literature also shows that DGM is favorable for NOx-smoke trade-off characteristics due to higher oxygen content. The purpose of the study is to clarify the behaviors of the combustion and emission characteristics of a twin cylinder tractor engine fueled with blends of rubber seed oil and babassu oil with DGM as an oxygenate, whose properties are given in Table 1. Experiments were carried out using R75B25 (blend of 75% rubber seed oil and 25% babassu oil on volume basis), R50B50, R25B75, neat RSO, and BSO and diesel as a baseline. Tests were also conducted by adding 20% DGM to the abovementioned fuels and its blends.
Experimental setup
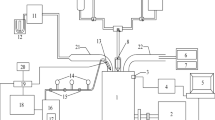
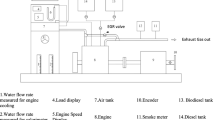
Tests were conducted on a Simpsons make S217 model twin cylinder compression–ignition engine employed in tractors, whose specification is given in Table 2. An eddy current dynamometer was used for loading the engine. AVL 5 gas analyzer was used to measure HC, CO, and NOx from the engine exhaust. Figure 1 shows the schematic representation of the experimental setup. The engine exhaust smoke opacity was measured using AVL 432c smoke meter based on light extinction technique. A three-way, two-position directional control valve was used for rapid switching between diesel and the test fuel.
The in-cylinder pressure was measured using a piezoelectric pressure sensor mounted on the engine cylinder head. Crank angle with the one-degree resolution was obtained using an optical shaft position encoder. The signal from both the sensors was acquired using a data acquisition system and stored on a personal computer using analog to digital converter. From the acquired data, peak pressure, occurrence of peak pressure, and heat release rate were calculated.
The tests were conducted at a constant speed of 1500 rpm with the load varying in steps of 25%. Volumetric air flow rate and fuel flow rate were measured using a U-tube manometer and a 50 cm3 burette/stopwatch, respectively. The engine was initially started with diesel and after attaining stable condition the fuel was switched to the test fuel using the three-way valve. The engine’s performance was evaluated in terms of brake thermal efficiency, exhaust gas temperature, and emission characteristics. All the tests were conducted three times and the average values are taken for analysis.
Results and discussion
Experiments were performed at a rated engine speed of 1500 rpm and at engine loads of 2.9 kW, 5.9 kW, 8.8 kW, and 11.8 kW. At each engine load, experiments were carried out using R75B25, R50B50, and R25B75, neat RSO, and BSO and diesel as a baseline. The effect of adding DGM to the blends on performance, emission, and combustion characteristics are analyzed.
Fatty acid composition of babassu and rubber seed oil
The properties of the vegetable oil are determined by the presence of each fatty acid in its molecule. The carbon chain length and the number of double bonds (unsaturation) affects the physical properties of the oil (Pinzi et al. 2013). Some of the properties significantly affected are viscosity, calorific value, and cetane number. The fatty acid composition of babassu and rubber seed oil is shown in Table 3. Viscosity of the fuel plays a major role in its atomization and proper mixing with the air. If the viscosity is more then the atomization will not be proper and more fuel will burn in premixed combustion phase resulting in high-combustion temperature. The viscosity of rubber seed oil is higher than babassu oil since more amount of fatty acids with longer chain lengths is present in RSO. Also, due to the presence of large amount of longer chain fatty acids, the calorific value of rubber seed oil is higher than BSO. The cetane number determines the ignition delay and combustion quality of the fuel. The higher is the cetane number, the lower is the ignition delay resulting in lower premixed combustion and lower combustion temperature. The cetane number is higher for the fuels with longer chain length and presence of more amount of saturated molecules (Ramos et al. 2009). RSO has a large amount of longer chain molecules; however, the degree of unsaturation is more. Whereas, BSO has large amount of saturated molecules but their chain length is smaller than RSO. Therefore, both the fuels have similar cetane number as shown in Table 1.
Performance and emission characteristics
Figure 2 shows the brake thermal efficiency (BTE) with load for various blends of rubber seed oil and babassu oil, neat rubber and babassu oil with and without DGM, and base diesel. BTE is lower for neat RSO and BSO as compared to diesel; this can be attributed to higher viscosity and density resulting in poor atomization and slower heat release. Also, lower calorific value for RSO and BSO compared to diesel demands more fuel to be burnt for producing same power output. BTE increases with increase in load for all the fuel samples. Engine operation with diesel has the highest thermal efficiency for all loads, and the maximum efficiency achieved is 32.24%. Addition of DGM to the blend increased the BTE for all the blends at all loads, however, it is lower than diesel. At full load, due to the addition of DGM, BTE increased by 2.1%, 2.5%, 3.35%, 5.57%, and 6.78% for R75B25, R50B50, and R25B75, neat RSO, and BSO, respectively. The increase in BTE is due to the addition of DGM which further increases the oxygen content of the blends, thereby improving combustion, especially during diffusion combustion phase. It was also observed that with the increase of BSO in the blend the BTE decreased at all the loads, but the addition of DGM slightly improved the BTE.
Variation of exhaust gas temperature (EGT) with engine load for different blends of RSO and BSO, neat RSO, and BSO with and without DGM, and baseline diesel is shown in Fig. 3. EGT increases with increase in load for all the blends. At full load, EGT is 406 °C for diesel and 432 °C and 490 °C for RSO and BSO respectively without DGM. With the addition of 20% DGM, it reduces to 417 °C and 451 °C for RSO and BSO respectively. Neat RSO and BSO have poor volatility and higher viscosity resulting in the fuel burning in the later stage of combustion leading to inferior engine performance and higher exhaust gas temperature. Addition of DGM to the blends helps in reducing the EGT and improves engine performance. The higher cetane number of DGM reduces the ignition delay, thereby improving the premixed combustion phase which reduces the fuel burnt in the later stage of combustion thus reducing EGT. Also, the faster burning rate associated with DGM addition aided in reduced combustion phasing leading to lower EGT. In addition, BTE increases with the addition of DGM resulting in less use of fuel energy input for a given output.
The modulation of unburned hydrocarbon (HC) emission with engine load for various fuel blends is represented in Fig. 4. It is observed that HC emissions increase with an increase in engine load for all the tested fuel blends and it is lowest for engine operation with diesel at all loads. HC emissions at full load are 19 ppm, 32 ppm, and 49 ppm for diesel, neat RSO, and BSO respectively. Higher emissions of straight vegetable oils can be attributed to high viscosity and poor atomization characteristics causing incomplete combustion thus masking the oxidation of hydrocarbons. It is also observed that neat BSO engine operation resulted in the highest HC emissions at all loads. Increase in percentage of BSO in the blend also leads to increase in HC emissions at all loads. Addition of DGM to the blend leads to decrease in HC emission for the fuels at all loads. At full load, HC emissions for engine operation with diglyme decreased by 22.2%, 22.4%, 22.85%, 10.81%, and 9.5% for neat RSO and BSO, R75B25, R50B50, and R25B75 respectively as compared to engine operation without diglyme. Diglyme has high cetane number and low auto-ignition temperature causing shorter ignition delay, which improves combustion during premixed phase causing oxidation of the hydrocarbons. Also, availability of addition oxygen atoms in DGM aided in oxidation of HC emissions at high-combustion temperature.
Figure 5 portrays the variation of carbon monoxide (CO) emission for the tested fuel blends at various loads. CO emissions increased with increase in load for all the tested fuels. Diesel-fueled engine operation resulted in the lowest CO emissions at all loads. At full load, CO emissions are 0.08%, 0.24%, and 0.32% for diesel, neat RSO, and BSO respectively. CO emissions without DGM at full load are 0.27%, 0.27%, and 0.31% for R75B25, R50B50, and R25B75 respectively. It can be observed from Fig. 5 that increase in the percentage of BSO in the blend resulted in an increase in CO emissions. The blending of DGM resulted in a decrease in CO emissions for all the fuels at all loads. At full load, CO emissions observed are 0.21%, 0.28%, 0.24%, 0.25%, and 0.27% for RSO, BSO, R75B25, R50B50, and R25B75 respectively. The reason for the decrease in CO emission is the same as HC emissions. Another reason for the reduction of emissions could be the increased oxygen content caused by the addition of DGM which further oxidizes carbon monoxide.
The variation of oxides of nitrogen (NOx) emission from the engine exhaust with engine load is shown in Fig. 6. The major cause of increased NOx emissions is high gas temperature in the combustion chamber and presence of oxygenated fuel. From Fig. 6, it is observed that the NOx emissions increase with increase in engine load, as more fuel is burnt to produce the required power which increases the combustion temperature. Engine operation with diesel resulted in highest NOx emissions at all loads. However, engine operation with neat BSO resulted in the lowest NOx emissions at all loads, and as compared to diesel, it reduced by 25% at full load. In spite of inherent presence of oxygen in the fuel structure, the reduction in emissions is due to reduced premixed burning rate which tends to slow the heat release rate causing lower combustion temperature. As for the blends of RSO and BSO, it is observed that the increase in percentage of BSO in the blend resulted in the decrease in NOx emissions at all loads. NO emission at full load for R75B25, R50B50, and R25B75 are 934 ppm, 912 ppm, and 897 ppm respectively. Addition of diglyme to the blends increased NOx emission as evident in Fig. 6. However, with a blend of ultra-low sulfur diesel and diglyme, Di et al. (2010) observed NOx reduction. Similarly, Subramanian et al. (2018) also observed NOx reduction with camphor oil and diglyme blend. The authors based the reduction in NOx emissions on the fact that due to high cetane number of DGM, the ignition delay is reduced thus the amount of fuel burning in premixed phase is reduced; meanwhile, due to low heating value of DGM the heat released in premixed combustion phase is reduced resulting in lower combustion temperature. However, Gill et al. (2012) reported an increase in NOx emissions which is consistent with the results of this study. As stated earlier, with the addition of DGM the heat released is less and the combustion temperature is low, but the increase in the NOx emissions is mainly due to the increase in availability of oxygen in the combustion chamber.
The effect on smoke opacity under the influence of engine load and with and without DGM is depicted in Fig. 7. Smoke opacity increases with increase in engine load for all the fuels and it is lowest for diesel-fueled engine operation. At full load, smoke opacity for diesel, neat RSO, and BSO is 60.2%, 81.5%, and 91.2%, respectively; the increase in emissions is due to poor atomizing and mixture-forming tendency of the vegetable oils. Engine operation with blends of RSO and BSO resulted in smoke opacity lying between smoke emissions emitted during neat RSO and neat BSO engine operation. Also, addition reduced by 11%, 9.48%, 8.7%, 4.7%, and 3.3% for RSO, BSO, R75B25, R50B50, and R25B75 as compared to engine operation without diglyme addition. Soot particles are mainly formed during diffusion phase combustion and addition of DGM to the blends provides additional oxygen for combustion, which decreases the rich mixture regions and improves diffusion phase combustion resulting in oxidation of the already formed soot.
A trade-off exists between NOx and smoke emissions for conventional compression ignition engines. Oxygen deficiency in the combustion chamber results in increase in smoke emissions, whereas oxygen enrichment tends to increase NOx emission resulting in a trade-off between NOx and smoke emissions. DGM addition to the tested fuels influences the trade-off curve as shown in Fig. 8. It can be seen that addition of diglyme to the tested fuels lead to increase in NOx emissions and simultaneous reduction in smoke opacity. Similar trend was observed for all engine loads. The probable reason for the trend could be the high availability of oxygen present in both the vegetable oil and diglyme, which increases NOx emissions and simultaneously oxidizes soot particles. Exhaust gas recirculation is one such viable method which can reduce NOx emissions without much affecting other parameters as oxygen required for combustion is provided by the fuel.
In summary, the poor performance and emission characteristics of neat RSO and BSO and its blends were improved with DGM addition. It is observed that among the various blends, RSO-BSO (50–50) blend with DGM is optimum in terms of performance and emission characteristics. The combustion parameters for the optimum blend combination compared to base fuels are presented in the following section.
Combustion characteristics
The combustion characteristics of diesel, RSO, BSO, and RSO50 + BSO50 with and without diglyme at full load are shown in Fig. 9. The poor combustion of RSO and BSO resulted in lower peak pressure compared to diesel. Peak pressure for diesel, RSO, and BSO are 91 bar, 84.7 bar, and 81 bar respectively. Peak pressure for both RSO and BSO occurred late compared to diesel with BSO in the farther end. The reason is due to longer ignition delay for these fuels due to poor atomization and vaporization which led to more heat wasted to exhaust than converted to useful energy as justified from previous studies (Geo et al. 2017) and performance results as seen in Figs. 2 and 3. Peak pressure for RSO50-BSO50 is 81 bar at full load which is similar to RSO and BSO. This is improved with addition of DGM. Peak pressure of RSO50-BSO50 + DGM20 is 85 bar and the peak occurred early. This is due to better atomization and vaporization leading to a more fuel-air mixture prepared for combustion. Also, the high cetane number of DGM aided in slightly early occurrence of peak pressure.
Heat release rate curve in Fig. 9 shows that compared to diesel, combustion is poor for RSO and BSO. It is clearly observed that for RSO and BSO, diffusion phase is dominant rather than premixed combustion phase. Generally, more heat is converted to energy in premixed combustion phase. Due to the slower start of combustion, the combustion phasing is shifted which reduced the BTE for both RSO and BSO. Peak heat release for diesel, RSO, and BSO is 74 J/oCA, 63 J/oCA, and 54 J/oCA at full load respectively. The high viscosity of both RSO and BSO increased the physical delay causing a delayed start of combustion. Peak heat release for RSO50-BSO50 is 60 J/oCA at full load. The peak heat release rate for blend occurs between RSO and BSO and about 19% less compared to diesel. Addition of DGM improved the combustion of RSO and BSO blend due to better atomization, vaporization, and mixing with air along with improved cetane number, the peak occurred much early, and combustion is faster as compared to other fuels as seen from the combustion phasing. Peak heat release for RSO and BSO blend with DGM addition is 65 J/oCA at full load.
Conclusions
The present study shows that compression ignition engines used in the agricultural sector can be operated with neat RSO and BSO available commonly in the tropical regions. Engine performance with RSO and BSO is poor as compared to diesel. BTE reduced by about 9–15% at full load for RSO, BSO, and their blends without DGM as compared to diesel. At all loads and for all the tested fuels without DGM, HC and CO emissions were higher as compared to diesel. NOx emissions are lower for the tested fuels without DGM due to inferior combustion leading to higher smoke opacity. Compared to RSO, the performance of BSO is poor and the performance improves with an increase in the concentration of RSO in the blend. Addition of DGM improved the combustion for all the test fuels. BTE is improved with reduced HC, CO, and smoke emissions. There is a slight penalty of NOx emissions with DGM addition for all the fuels. It is observed that considering NOx-smoke trade-off and other performance parameters of the engine, RSO50 + BSO50 + DGM20 is identified optimum.
This study shows that, instead of laborious transesterification process, a proven technique to improve the performance of vegetable oil, diglyme blending showed promising results. This study will aid the rural people to use the minor vegetable oil available in their region to replace conventional diesel.
References
Agarwal AK, Gupta JG, Dhar A (2017) Potential and challenges for large-scale application of biodiesel in automotive sector. Prog Energy Combust Sci 61:113–149
Atabani AE, Silitonga AS, Ong HC, Mahlia TMI, Masjuki HH, Badruddin IA, Fayaz H (2013) Non-edible vegetable oils: a critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renew Sust Energ Rev 18:211–245
Barrios CC, Martin C, Dominguez-Saez A, Alvarez P, Pujadas M, Casanova J (2014) Effects of the addition of oxygenated fuels as additives on combustion characteristics and particle number and size distribution emissions of a TDI diesel engine. Fuel 132:93–100
Benajes J, Pastor JV, García A, Monsalve-Serrano J (2015) The potential of RCCI concept to meet EURO VI NOx limitation and ultra-low soot emissions in a heavy-duty engine over the whole engine map. Fuel 159:952–961
Bertoli C, Del Giacomo N, Beatrice C (1998) Evaluation of combustion behavior and pollutants emission of advanced fuel formulations by single cylinder engine experiments (no. 982492). SAE technical paper
Bhovi M, Banapurmath NR, Khandal SV, Yaliwal VS (2017) Effect of hydrogen and hydrogen enriched compressed natural gas induction on the performance of rubber seed oil methy ester fuelled common rail direct injection (CRDi) dual fuel engines. European Journal of Sustainable Development Research 1(2):07
Chen CY, Lee WJ, Wang LC, Chang YC, Yang HH, Young LH, Lu JH, Tsai YI, Cheng MT, Mwangi JK (2017) Impact of high soot-loaded and regenerated diesel particulate filters on the emissions of persistent organic pollutants from a diesel engine fueled with waste cooking oil-based biodiesel. Appl Energy 191:35–43
Corcione FE, Merola SS, Vaglieco BM, Formisano G (2001) In-cylinder optical analysis of CRDI diesel engine combustion (No. 2001-24-0027). SAE Technical Paper
de LT Oliveira G, McKay B, Plank C (2017) How biofuel policies backfire: misguided goals, inefficient mechanisms, and political-ecological blind spots. Energy Policy 108:765–775
Demirbas A (2009) Biofuels securing the planet’s future energy needs. Energy Convers Manag 50(9):2239–2249
Di Y, Cheung CS, Huang Z (2010) Experimental investigation of particulate emissions from a diesel engine fueled with ultralow-sulfur diesel fuel blended with diglyme. Atmos Environ 44(1):55–63
Geo VE, Nagarajan G, Nagalingam B (2008) A comparative combustion analysis of rubber seed oil and its methyl ester in a DI diesel engine (No. 2008-01-1386). SAE Technical Paper
Geo VE, Nagarajan G, Kamalakannan J, Nagalingam B (2009) Experimental investigations to study the characteristics of rubber-seed-oil-fueled diesel engine supplemented with diethyl ether. Energy Fuel 23(1):533–538
Geo VE, Sonthalia A, Nagarajan G, Nagalingam B (2017) Studies on performance, combustion and emission of a single cylinder diesel engine fuelled with rubber seed oil and its biodiesel along with ethanol as injected fuel. Fuel 209:733–741
Gill SS, Tsolakis A, Herreros JM, York APE (2012) Diesel emissions improvements through the use of biodiesel or oxygenated blending components. Fuel 95:578–586
Guan B, Zhan R, Lin H, Huang Z (2014) Review of state of the art technologies of selective catalytic reduction of NOx from diesel engine exhaust. Appl Therm Eng 66(1–2):395–414
Joshi G, Pandey JK, Rana S, Rawat DS (2017) Challenges and opportunities for the application of biofuel. Renew Sust Energ Rev 79:850–866
Kasiraman G, Geo VE, Nagalingam B (2016) Assessment of cashew nut shell oil as an alternate fuel for CI (compression ignition) engines. Energy 101:402–410
Kumar BR, Saravanan S, Rana D, Nagendran A (2016) Use of some advanced biofuels for overcoming smoke/NOx trade-off in a light-duty DI diesel engine. Renew Energy 96:687–699
Miyamoto N, Ogawa H, Nurun NM, Obata K, Arima T (1998) Smokeless, low NOx, high thermal efficiency, and low noise diesel combustion with oxygenated agents as main fuel (No. 980506). SAE technical paper
Nabi MN, Hustad JE (2012) Influence of oxygenates on fine particle and regulated emissions from a diesel engine. Fuel 93:181–188
Oliveira LE, Giordani DS, Paiva EM, De Castro HF, Da Silva MLCP (2013) Kinetic and thermodynamic parameters of volatilization of biodiesel from babassu, palm oil and mineral diesel by thermogravimetric analysis (TG). J Therm Anal Calorim 111(1):155–160
Pinzi S, Rounce P, Herreros JM, Tsolakis A, Dorado MP (2013) The effect of biodiesel fatty acid composition on combustion and diesel engine exhaust emissions. Fuel 104:170–182
Puzun A, Wanchen S, Guoliang L, Manzhi T, Chunjie L, Shibao C (2011) Characteristics of particle size distributions about emissions in a common-rail diesel engine with biodiesel blends. Procedia Environ Sci 11:1371–1378
Ramadhas AS, Jayaraj S, Muraleedharan C (2005) Biodiesel production from high FFA rubber seed oil. Fuel 84(4):335–340
Ramos MJ, Fernandez CM, Casas A, Rodriguez L, Perez A (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 100:261–268
Ren Y, Huang Z, Miao H, Jiang D, Zeng K, Liu B, Wang X (2007) Effect of the addition of diglyme in diesel fuel on combustion and emissions in a compression− ignition engine. Energy Fuel 21(5):2573–2583
Ren Y, Huang Z, Miao H, Di Y, Jiang D, Zeng K, Liu B, Wang X (2008) Combustion and emissions of a DI diesel engine fuelled with diesel-oxygenate blends. Fuel 87(12):2691–2697
Sajjadi B, Raman AAA, Arandiyan H (2016) A comprehensive review on properties of edible and non-edible vegetable oil-based biodiesel: composition, specifications and prediction models. Renew Sust Energ Rev 63:62–92
Song H, Quinton KS, Peng Z, Zhao H, Ladommatos N (2016) Effects of oxygen content of fuels on combustion and emissions of diesel engines. Energies 9(1):28
Srihari S, Thirumalini S (2017) Investigation on reduction of emission in PCCI-DI engine with biofuel blends. Renew Energy 114:1232–1237
Subramanian T, Varuvel EG, Martin LJ, Beddhannan N (2017) Effect of lower and higher alcohol fuel synergies in biofuel blends and exhaust treatment system on emissions from CI engine. Environ Sci Pollut Res 24(32):25103–25113
Subramanian T, Varuvel EG, Ganapathy S, Vedharaj S, Vallinayagam R (2018) Role of fuel additives on reduction of NO X emission from a diesel engine powered by camphor oil biofuel. Environ Sci Pollut Res, 1–10
Tamanouchi M, Akimoto T, Aihara S, Morihisa H (1999) Effects of DGM and oxidation catalyst on diesel exhaust emissions (No. 1999-01-1137). SAE Technical Paper
Thiyagarajan S, Geo VE, Leenus JM, Nagalingam B (2017a) Experimental investigation to reduce CO2 emission in a single cylinder CI engine using low carbon fuel blend with Karanja oil methyl ester and amine injection in the exhaust manifold. Int J Global Warm 13(3–4):278–295
Thiyagarajan S, Geo VE, Martin LJ, Nagalingam B (2017b) Selective non-catalytic reduction (SNCR) of CO2 and NO emissions from a single-cylinder CI engine using chemical absorbents. Emission Control Science and Technology 3(3):233–242
Varuvel EG, Subramanian T, Khatri P (2017) Effect of diglyme addition on performance and emission characteristics of hybrid minor vegetable oil blends (rubber seed and babassu oil) in a tractor engine–an experimental study. Biofuels, 1–9
Vedharaj S, Vallinayagam R, Yang WM, Saravanan CG, Lee PS (2015) Optimization of combustion bowl geometry for the operation of kapok biodiesel–diesel blends in a stationary diesel engine. Fuel 139:561–567
Walsh MP (1999) Assessing transportation-related air pollution in major cities. J Urban Technol 6(1):1–24
Zheng M, Han X, Asad U, Wang J (2015) Investigation of butanol-fuelled HCCI combustion on a high efficiency diesel engine. Energy Convers Manag 98:215–224
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Varuvel, E.G., Sonthalia, A., Subramanian, T. et al. NOx-smoke trade-off characteristics of minor vegetable oil blends synergy with oxygenate in a commercial CI engine. Environ Sci Pollut Res 25, 35715–35724 (2018). https://doi.org/10.1007/s11356-018-3484-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3484-y