Abstract
Fucoidans (FUCs) are sulfated polysaccharides that have a wide range of bioactivities. The current study was designed to evaluate the antioxidant potential of FUC against microcystin-LR (MC-LR)-induced toxicity. Five mice groups (n = 8) were used. Group 1 received saline, Group 2 received oral FUC 100 mg/kg/day for 21 days, Group 3 received i.p. MC-LR 10 μg/kg/day for 14 days, Group 4 received MC-LR plus FUC 50 mg/kg/day, and Group 5 received MC-LR plus FUC 100 mg/kg/day. The present study showed that MC-LR administration was associated with significant increases (p < 0.01) in serum concentrations of hepatic (aspartate transferase, alanine transferase, and alkaline phosphatase), renal (urea and creatinine), and cardiac (creatine kinase and CK-MB) injury biomarkers, as well as serum lactate dehydrogenase, cholesterol, and pro-inflammatory cytokines (interleukins-1β and 6, and tumor necrosis factor-α), compared with the control group. Further, MC-LR-intoxicated mice exhibited significantly higher (p < 0.01) hepatic, renal, and cardiac tissue levels of malondialdehyde and nitric oxide, as well as lower tissue levels of reduced glutathione and activities of glutathione peroxidase, superoxide dismutase, and catalase enzymes in comparison with control mice. Treatment by FUC significantly ameliorated all the above-mentioned alterations in a dose-dependent manner with frequent restoration of the normal ranges in the FUC 100 mg/kg/day dose group. Moreover, treatment by FUC alone at 100 mg/kg/day was not associated with significant negative alterations in the assessed biochemical parameters, highlighting its safety at this dose. In conclusion, treatment by FUC significantly ameliorated organ injury, induced by MC-LR in mouse hepatic, renal, and cardiac tissues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microcystins (MCs) are cyclic heptapeptides, produced by different species of cyanobacteria that live in fresh waters. They are potent hepatotoxins that can cause severe human and livestock poisoning when their aquatic concentrations are high (Scoglio 2018). Acute MC exposure can cause severe hepatotoxicity, intrahepatic hemorrhage, hypoglycemia, and circulatory shock (van der Merwe 2015). Moreover, chronic exposure to low concentrations of MCs in food and drinking water has been linked to gastroenteritis, allergy, and hepatocellular and colorectal cancers (Svircev et al. 2017). Despite extensive research, no effective treatment for MC-induced hepatotoxicity has been specified to date.
Over 80 structural analogues of MCs have been identified; however, the most common, toxic, and extensively studied is microcystin-LR (MC-LR) (van der Merwe 2015). Different molecular mechanisms are involved in MC cytotoxicity, including inhibition of the serine/threonine-specific protein phosphatases as PP1, PP2A, CaMK2, and MAPKs (McLellan and Manderville 2017). This leads to several downstream events as increased production of reactive oxygen species (ROS), disturbed cell signaling and differentiation (Wang et al. 2019b), alterations in the cytoskeleton structure and dynamics (Huang et al. 2015), and impaired DNA repair (Zhang et al. 2018). Oxidative stress is a major player in MC-LR toxicity that induces cytotoxic damage directly or indirectly via the induction of apoptosis and pro-inflammatory cytokine release (Huang et al. 2015; Liu et al. 2018a).
Fucoidans (FUCs) are sulfated polysaccharides, present in the cell walls of brown seaweeds as Laminaria Japonicum, Fucus Vesicularis, and Cladosiphon okamuranus (Luthuli et al. 2019). These molecules have shown a wide range of bioactivities, including antithrombotic, antioxidant, antiviral, anticarcinogenic, anti-inflammatory, immunomodulatory, hypolipidemic, and cytoprotective effects (Wang et al. 2019c). The antioxidant and anti-inflammatory effects of FUCs have been shown in several in vitro experiments (in DPPH assays and LDL oxidation systems (Yuan and Macquarrie 2015; Zhao et al. 2011)), as well as in vivo studies on rats with diabetes (Wang et al. 2014), diabetic nephropathy (Xu et al. 2017), and hypoxia-induced injuries (Novoyatleva et al. 2019). Further, FUCs have shown cytoprotective effects against the toxicity of several xenobiotics, such as carbon tetrachloride (Boshy et al. 2017), acetaminophen (Wang et al. 2018), alcohol-induced liver damage (Lim et al. 2015), and isoproterenol-induced cardiotoxicity (Thomes et al. 2010). Due to these properties, FUCs have attracted the interest of several research groups over the past decades to optimize their use for preventive and therapeutic purposes.
To our knowledge, data are lacking on the chemoprotective potential of FUC against MC-LR toxicity in animal models. Therefore, the current study was designed to evaluate the antioxidant and anti-inflammatory effects of FUC against MC-LR-induced toxicity in the liver, heart, and kidneys of experimental mice.
Materials and methods
Chemicals
Microcystin-LR was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Fucoidan (from Laminaria Japonica, 500 mg/capsule) was purchased from Absunutrix Lyfetrition (USA). All enzymatic assay kits were obtained from Biodiagnostics Co. (Cairo, Egypt), except for the kits of lactate dehydrogenase (LDH) (Randox Laboratories Ltd., UK), creatine kinase (CK) and CK-myoglobin binding (CK-MB) (Stanbio™, TX, USA) enzymes, interleukin (IL)-1β and IL-6 (Glory Science Co. Ltd., Del Rio, TX, USA), and tumor necrosis factor (TNF)-α (BioSource Inc., Camarillo, CA, USA).
Animals and experimental design
After approval of the ethics committee (Faculty of Veterinary Medicine, Suez Canal University, Egypt; approval number: 201622), 40 male Swiss-albino mice (weighing 22 to 27 g, 10 to 12 weeks old) were obtained from the Egyptian Organization for Biological Products and Vaccines and acclimatized for 1 week at optimal environmental conditions (12-h light-dark cycles and a temperature of 25 ± 2 °C). Mice were later divided into five groups. Group 1 received normal saline orally (control); Group 2 received FUC at a daily oral dose of 100 mg/kg bw for 21 days (Wei et al. 2017); Group 3 received MC-LR at a daily intraperitoneal (i.p) dose of 10 μg/kg bw for 14 days (Lone et al. 2017); Group 4 received FUC 50 mg/kg bw daily for 21 days plus MC-LR 10 μg/kg for 14 days, starting 7 days after the first FUC dose; and Group 5 received FUC 100 mg/kg bw daily for 21 days plus MC-LR 10 μg/kg for 14 days, starting 7 days after the first FUC dose (Fig. 1).
Summary of the antioxidant and anti-inflammatory effects of pretreatment with fucoidan 50 or 100 mg/kg bw daily for 21 days in mice exposed to microcystin-LR 10 μg/kg for 14 days, starting 7 days after the first FUC dose. ALT, alanine transferase; ALP, alkaline phosphatase; AST, aspartate transferase; CAT, catalase; CK, creatine kinase; CK-MB, CK-myoglobin binding; GPx, glutathione peroxidase; GSH, reduced glutathione; LDH, lactate dehydrogenase; IL, interleukin; MDA, malondialdehyde; NO, nitric oxide; SOD, superoxide dismutase; TNF, tumor necrosis factor
At the 21st day of the experiment, blood samples were taken from the retro-orbital plexus, and then, all mice were sacrificed by cervical decapitation under isoflurane anesthesia. Blood samples were initially allowed to clot and were centrifuged at 3000g for 15 min to separate clear serum samples. Then, the liver, heart, and kidneys of mice were extracted and 0.5 g of each organ was homogenized in potassium phosphate buffer (0.1 M, pH 7.4), then centrifuged at 5000 rpm for 30 min. The homogenate was frozen at − 80 °C for later biochemical analyses.
Serum biochemical assays
The obtained serum samples were used for the biochemical assays of alanine transferase (ALT) and aspartate transferase (AST) according to Reitman and Frankel (1957), alkaline phosphatase (ALP) according to Tietz et al. (1983) and creatinine and urea according to Larsen (1972) and Coulombe and Favreau (1963), respectively. To assess the serum activity of CK and CK-MB, we used Stanbio™ CK-NAC (UV-Rate)/CK-MB kits (TX, USA) according to the methods described by Szasz et al. (1979) and Wurzburg et al. (1976), respectively. Later, serum levels of LDH were measured according to Babson and Babson (1973), while serum total cholesterol was assessed according to Allain et al. (1974) and Richmond (1973).
Serum cytokine analysis
Commercially available ELISA kits were used to measure the serum concentrations of IL-1β, IL-6, and TNF-α according to the manufacturer’s instructions. The optical density was then read at 450 nm and the cytokine concentration was calculated from a standard curve, and then multiplied by the dilution factor.
Tissue biochemical assays
To evaluate lipid peroxidation in the hepatic, cardiac, and renal tissues in each mouse, we employed the methods of Mihara and Uchiyama (1978) to measure the tissue concentrations of malondialdehyde (MDA). While the levels of nitric oxide (NO) in the same tissues were measured according to Green et al. (1982). The non-enzymatic antioxidant reduced glutathione (GSH) was assessed as per Beutler et al. (1963). Later, the enzymatic activities of glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT) were determined according to the methods described by Paglia and Valentine (1967), Nishikimi et al. (1972), and Aebi (1984), respectively.
Data analysis
All values were expressed as the mean ± the standard error of the mean (SEM). The differences between the mean values of different groups were tested using the ANOVA, followed by post hoc Tukey’s test, performed by the SPSS software (version 22 for PC, IBM, Armonk, NY). The differences were considered statistically significant if p values were < 0.05.
Results
Serum biomarkers
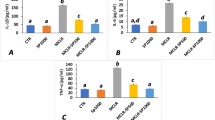
Mice, treated with FUC at 100 mg/kg/day for 21 days, exhibited comparable serum levels of liver injury biomarkers (AST, ALT, and ALP), cardiac injury biomarkers (CK and CK-MB), renal injury biomarkers (urea and creatinine), LDH, cholesterol, and pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) to mice in the control group. The administration of MC-LR at 10 μg/kg/day for 14 days was associated with significant increases (p < 0.01) in the serum values of all these parameters. Treatment of MC-LR-intoxicated mice with FUC at 50 or 100 mg/kg/day for 21 days was associated with significant reductions (p < 0.05) in the serum levels of the aforementioned parameters in comparison with the MC-LR-intoxicated group; however, only the 100 mg/kg/day dose could restore the serum levels to normal control ranges, except for IL-6 (p = 0.03 versus the control group) (Table 1 and Fig. 2).
The effects of fucoidan (at 50 and 100 mg/kg/day bw) against microcystin-LR (10 μg/kg/day bw) on the serum concentrations of interleukin 1β, IL-6, and tumor necrosis factor-α. The presented data are mean ± SEM (n = 8 per group). Different superscripts on columns indicate statistically significant differences at p < 0.05
Oxidant/antioxidant status in the hepatic tissue
Mice, receiving FUC alone at 100 mg/kg/day for 21 days, showed comparable values (p > 0.05) of MDA, NO, GSH, GPx, SOD, and CAT to control mice. However, MC-LR treatment at 10 μg/kg/day for 14 days was associated with significant elevations in the hepatic tissue MDA and NO concentrations (p < 0.001) and significant reductions in hepatic GSH concentration and antioxidant enzymatic activities (p < 0.01). Co-administration of MC-LR and FUC at either 50 or 100 mg/kg/day ameliorated MC-LR-induced alterations with restoration of the normal tissue concentration ranges of GSH, GPx, and CAT in the MC-LR-FUC 100 mg/kg/day group (Fig. 3).
The antioxidant effects of fucoidan (at 50 and 100 mg/kg/day bw) against microcystin-LR (10 μg/kg/day bw) on the hepatic tissue concentrations of malondialdehyde, nitric oxide, reduced glutathione, and antioxidant enzymatic activities. The presented data are mean ± SEM (n = 8 per group). Different superscripts on columns indicate statistically significant differences at p < 0.05
Oxidant/antioxidant status in the renal tissue
Interestingly, treatment with FUC alone at 100 mg/kg/day for 21 days was associated with significant increases in renal GPx (p = 0.03) and SOD (p = 0.01) enzyme activities, compared with control mice; otherwise, the concentrations of other parameters (MDA, NO, GSH, and CAT) were comparable between the two groups. In line with the findings in hepatic tissue samples, MC-LR administration at 10 μg/kg/day for 14 days was associated with significant alterations (p < 0.05) in the renal tissue levels of oxidant/antioxidant parameters. However, co-treatment with FUC at 50 or 100 mg/kg/day for 21 days alleviated these alterations, while the 100 mg/kg/day FUC dose restored the normal tissue concentration ranges of all parameters, except for NO and GSH (p = 0.02 and 0.04 versus control mice, respectively) (Fig. 4).
The antioxidant effects of fucoidan (at 50 and 100 mg/kg/day bw) against microcystin-LR (10 μg/kg/day bw) on the renal tissue concentrations of malondialdehyde, nitric oxide, reduced glutathione, and antioxidant enzymatic activities. The presented data are mean ± SEM (n = 8 per group). Different superscripts on columns indicate statistically significant differences at p < 0.05
Oxidant/antioxidant status in the cardiac tissue
Fucoidan treatment at 100 mg/kg/day for 21 days caused no significant alterations (p > 0.05) in cardiac tissue levels of MDA, NO, GSH, GPx, SOD, and CAT. On the other hand, MC-LR administration at 10 μg/kg/day for 14 days was associated with significant elevations (p < 0.05) in cardiac tissue levels of MDA and NO, as well as significant reductions of GSH concentration and activities of GPx, SOD, and CAT enzymes in the cardiac muscle in comparison to control mice. All these alterations were ameliorated by FUC treatment at 50 or 100 mg/kg/day for 21 days; however, only the 100 mg/kg/day dose could restore the levels of these parameters to normal control ranges, except for GSH and CAT (p = 0.02 and 0.01 versus control mice, respectively) (Fig. 5).
The antioxidant effects of fucoidan (at 50 and 100 mg/kg/day bw) against microcystin-LR (10 μg/kg/day bw) on the cardiac tissue concentrations of malondialdehyde, nitric oxide, reduced glutathione, and antioxidant enzymatic activities. The presented data are mean ± SEM (n = 8 per group). Different superscripts on columns indicate statistically significant differences at p < 0.05
Discussion
The present experiment showed that acute exposure to a high dose of MC-LR is associated with multiorgan injury, as manifested by the increased serum levels of hepatic, renal, and cardiac injury biomarkers in mice. Such tissue damage may be mediated by MC-LR-induced oxidative stress, resulting from increased ROS production and reduced activities of endogenous antioxidant enzymes. Treatment of MC-LR-intoxicated mice with FUC dose-dependently alleviated the tissue injury and underlying oxidative stress in all three organs.
Microcystins are primarily hepatotoxins. Following oral exposure, they are absorbed from the ileum, passed to the portal circulation through which they reach their target cells (hepatocytes), and are largely cleared from the blood (Bischoff 2001). Another study in rats showed that large portions of MCs are retained in the kidney, indicating that they can be directly excreted in the urine. Only small concentrations (as low as 0.2%) are distributed to other organs as the heart, intestine, spleen, and gonads (Wang et al. 2008). However, these small concentrations may have significant toxic effects as shown in the current study and prior ones. Further, the cytotoxicity in these organs is augmented secondary to the hepatotoxic effects of MC-LR (McLellan and Manderville 2017).
Oxidative stress is a major biochemical feature of MC toxicity. Microcystins were shown able to increase the generation of ROS in several in vitro systems as human hepatic cells (Liu et al. 2018b) and erythrocytes (Sicinska et al. 2006), fish cell lines (Puerto et al. 2009), and lymphocytes (Zhang et al. 2008), as well as in several in vivo studies in rat and mouse liver, heart, and reproductive system (Ersoy and Kizilay 2018; Lone et al. 2017). The involved mechanisms in MC-LR oxidative stress are numerous. In concordance with previous findings, this study has shown that MC-LR exposure increases the production of NO in different organs and inhibits the activities of endogenous antioxidant enzymes as GPx, SOD, and CAT (Abdel-Daim et al. 2019; Lone et al. 2017). Other studies reported that MC-LR suppresses the expression and activity of the glutathione S-transferase enzyme (Balsano et al. 2017) and increases the expression of CYP2E1 and NADPH oxidase (Nong et al. 2007). Further, Ding et al. reported that the first event that occurs in cultured rat hepatocytes after MCs exposure is a surge in the mitochondrial Ca+2 levels, which initiates the mitochondrial outer-membrane permeabilization transition, increasing ROS generation (Ding et al. 2001). Another group suggested that MC-LR induced inhibition of protein phosphatases as CaMK2 is involved in ROS generation (Krakstad et al. 2006); however, further research is needed to fully characterize the pathways of oxidative stress and its contributions to MC-LR organ toxicity.
In addition, this study showed that acute exposure to a high dose of MC-LR significantly increased the serum concentrations of pro-inflammatory cytokines as IL-1β, IL-6, and TNF-α. Similar findings have been reported in earlier studies (Elgawish et al. 2017; Lu et al. 2014). TNF-α plays a role in MC-induced liver damage and circulatory shock and can directly induce cytotoxicity in hepatic, glomerular, and renal tubular cells (Al-Lamki and Mayadas 2015; Kakino et al. 2018). Further, IL-6 and IL-1β can change the glomerular hemodynamics and increase the thickness of the glomerular basement membrane (Duran-Salgado and Rubio-Guerra 2014). Microcystins have been shown to increase the expression of other cytokines as IL-8, cytokine-induced neutrophil chemoattractant-2αβ, L-selectin, and β2-integrin (Chen et al. 2018; Kujbida et al. 2009).
Due to the relevance of oxidative stress in MC-induced organotoxicity, several studies have evaluated the potential of different antioxidant compounds in preventing or curing its toxicity. In this regard, the current study showed that FUC can ameliorate the hepatic, cardiac, and renal damages of MC-LR intoxication as manifested by the improvements in serum concentrations of tissue injury biomarkers. The antioxidant mechanism is probably the main player in this outcome although other mechanisms may have contributed to it. Further, it confirmed the safety of FUC at the 100 mg/kg/day dose on the functions of the three organs.
The antioxidant and anti-inflammatory effects of FUC have been shown before in multiple investigations. For example, FUCs could protect the liver against the xenotoxicity of carbon tetrachloride (Boshy et al. 2017), acetaminophen (Wang et al. 2018), alcohol (Lim et al. 2015), and concanavalin A (Li et al. 2016). Such protection was mediated by antioxidant (increased endogenous antioxidant expression), anti-inflammatory (suppressed CYP2E1, inducible nitric oxide synthase, and COX-II expression, as well as release of IL-1β and TNF-α), antiapoptotic (reduced expression of Bax, cleaved caspase-3, cleaved caspase-8, and cleaved caspase-9), and antifibrotic (reduced expression of transforming growth factor β1) mechanisms (Hong et al. 2012; Li et al. 2016; Lim et al. 2015). Similar chemopreventive efficacies were reported for FUC in the amelioration of acute kidney injury (Wang et al. 2019a), chronic kidney disease (Wang et al. 2012), and diabetic nephropathy (Xu et al. 2017). Our findings confirm these results and extend them by showing the hepato- and nephroprotective effects of FUC against MC-LR toxicity.
Further, FUCs could ameliorate the severity of ischemia-reperfusion myocardial injury (Li et al. 2011), isoproterenol-induced myocardial infarction (Thomes et al. 2010), and autoimmune myocarditis in rats (Tanaka et al. 2011). These effects were explained by the amelioration of oxidative stress, cytokine release (TNF-α, IL-6, IL-10), neutrophil and macrophage myocardial tissue infiltration, and hyperlipidemia (Li et al. 2011; Tanaka et al. 2011; Thomes et al. 2010). According to our analysis, FUC administration significantly ameliorated MC-LR-induced elevation of serum cholesterol. Similar findings were reported in hypercholesterolemic rodents and patients (Huang et al. 2010). FUC can enhance the negative charges on the cell surfaces, and thereby reducing cholesterol levels in the serum (Li et al. 2008).
Regarding the antioxidant potential of FUC, our data showed that FUC reduces the production of the lipid peroxide MDA and NO and increases the activity of endogenous antioxidant enzymes as GPx, SOD, and CAT. Similar findings on the antioxidant capacity of FUC against other xenobiotics were published before (Boshy et al. 2017; Wang et al. 2018). Moreover, other studies showed that FUC can exert free radical scavenging effects and even inhibit the production of hydroxyl and superoxide radicals (Ajisaka et al. 2016). In addition, Yang et al. reported that FUC inhibited the production of NO in lipopolysaccharide-stimulated RAW264.7 cells and that this is probably mediated by suppressing the activation of activator protein-1 (Yang et al. 2006).
Interestingly, our study showed that FUC administration significantly reduced the serum concentrations of multiple pro-inflammatory cytokines as IL-1β, IL-6, and TNF-α. This confirms the results, published earlier in other models (Park et al. 2011). This may be explained by the reported effects of FUC on other molecules that regulate the activities of these cytokines and their secreting immune cells as NF-κB and IFN-γ (Choi et al. 2010; Li et al. 2011). These findings may also be secondary to the amelioration of oxidative stress in FUC-treated mice. Other anti-inflammatory mechanisms have been reported for FUCs as well, such as inhibition of COX-2 and iNOS expression (Cui et al. 2010; Park et al. 2011) and reducing leucocyte infiltration (Zhou et al. 2018). However, further research on the anti-inflammatory effects of different FUCs is recommended. Another interesting finding was the dose-dependent efficacy of FUC; this indicates that the 100 mg/kg/day dose is more promising for further investigation and potential applications.
To recapitulate, acute exposure to MC-LR caused marked hepatic, renal, and cardiac tissue injuries, probably through increasing the production of ROS and pro-inflammatory cytokines and impairing the endogenous antioxidant defenses. However, treatment by FUC was associated with significant antioxidant and anti-inflammatory effects in all three organs in a dose-dependent manner.
Abbreviations
- ALT:
-
alanine transferase
- ALP:
-
alkaline phosphatase
- AST:
-
aspartate transferase
- CAT:
-
catalase
- CK:
-
creatine kinase
- CK-MB:
-
CK-myoglobin binding
- FUC:
-
fucoidan
- GPx:
-
glutathione peroxidase
- GSH:
-
reduced glutathione
- LDH:
-
lactate dehydrogenase
- IL:
-
interleukin
- MC-LR:
-
microcystin-LR
- MDA:
-
malondialdehyde
- NO:
-
nitric oxide
- SOD:
-
superoxide dismutase
- TNF:
-
tumor necrosis factor
References
Abdel-Daim MM, Sayed AA, Abdeen A, Aleya L, Ali D, Alkahtane AA, Alarifi S, Alkahtani S (2019) Piperine enhances the antioxidant and anti-inflammatory activities of thymoquinone against microcystin-LR-induced hepatotoxicity and neurotoxicity in mice. Oxid Med Cell Long:2019
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ajisaka K, Yokoyama T, Matsuo K (2016) Structural characteristics and antioxidant activities of fucoidans from five brown seaweeds. J Appl Glycosci:jag. JAG-2015_024
Allain CC, Poon LS, Chan CS, Richmond W, Fu PC (1974) Enzymatic determination of total serum cholesterol. Clin Chem 20:470–475
Al-Lamki RS, Mayadas TN (2015) TNF receptors: signaling pathways and contribution to renal dysfunction. Kidney Int 87:281–296
Babson AL, Babson SR (1973) Kinetic colorimetric measurement of serum lactate dehydrogenase activity. Clinical chemistry 19, 766–769
Balsano E, Esterhuizen-Londt M, Hoque E, Lima SP (2017) Responses of the antioxidative and biotransformation enzymes in the aquatic fungus Mucor hiemalis exposed to cyanotoxins. Biotechnol Lett 39:1201–1209
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Bischoff K (2001) The toxicology of microcystin-LR: occurrence, toxicokinetics, toxicodynamics, diagnosis and treatment. Vet Hum Toxicol 43:294–297
Boshy M, Abdelhamidb F, Richab E, Ashshia A, Gaitha M, Qustya N (2017) Attenuation of CCl4 induced oxidative stress, immunosuppressive, hepatorenal damage by fucoidan in rats. J Clin Toxicol 7:2161–0495.1000348
Chen L, Liu X, Pan Z, Liu S, Han H, Zhao C, Tang X (2018) The role of IL-8/CXCR2 signaling in microcystin-LR triggered endothelial cell activation and increased vascular permeability. Chemosphere 194:43–48
Choi JI, Raghavendran HR, Sung NY, Kim JH, Chun BS, Ahn DH, Choi HS, Kang KW, Lee JW (2010) Effect of fucoidan on aspirin-induced stomach ulceration in rats. Chem Biol Interact 183:249–254
Coulombe JJ, Favreau L (1963) A new simple semimicro method for colorimetric determination of urea. Clin Chem 9:102–108
Cui YQ, Zhang LJ, Zhang T, Luo DZ, Jia YJ, Guo ZX, Zhang QB, Wang X, Wang XM (2010) Inhibitory effect of fucoidan on nitric oxide production in lipopolysaccharide-activated primary microglia. Clin Exp Pharmacol Physiol 37:422–428
Ding WX, Shen HM, Ong CN (2001) Pivotal role of mitochondrial Ca(2+) in microcystin-induced mitochondrial permeability transition in rat hepatocytes. Biochem Biophys Res Commun 285:1155–1161
Duran-Salgado MB, Rubio-Guerra AF (2014) Diabetic nephropathy and inflammation. World J Diabetes 5:393–398
Elgawish RA, Yoshimura Y, Isobe N (2017) Microcystin-leucine-arginine modulates the expression patterns of proinflammatory cytokines and an apoptotic gene in chicken liver. J Poult Sci 0170054
Ersoy O, Kizilay G (2018) Effects of fucoidan on diabetic rat testicular tissue. Biotechn Histochem 93:277–285
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 126:131–138
Hong SW, Lee HS, Jung KH, Lee H, Hong SS (2012) Protective effect of fucoidan against acetaminophen-induced liver injury. Arch Pharm Res 35:1099–1105
Huang L, Wen K, Gao X, Liu Y (2010) Hypolipidemic effect of fucoidan from Laminaria japonica in hyperlipidemic rats. Pharm Biol 48:422–426
Huang X, Chen L, Liu W, Qiao Q, Wu K, Wen J, Huang C, Tang R, Zhang X (2015) Involvement of oxidative stress and cytoskeletal disruption in microcystin-induced apoptosis in CIK cells. Aquat Toxicol 165:41–50
Kakino S, Ohki T, Nakayama H, Yuan X, Otabe S, Hashinaga T, Wada N, Kurita Y, Tanaka K, Hara K (2018) Pivotal role of TNF-α in the development and progression of nonalcoholic fatty liver disease in a murine model. Horm Metab Res 50:80–87
Krakstad C, Herfindal L, Gjertsen BT, Boe R, Vintermyr OK, Fladmark KE, Doskeland SO (2006) CaM-kinaseII-dependent commitment to microcystin-induced apoptosis is coupled to cell budding, but not to shrinkage or chromatin hypercondensation. Cell Death Differ 13:1191–1202
Kujbida P, Hatanaka E, Vinolo MA, Waismam K, Cavalcanti DM, Curi R, Farsky SH, Pinto E (2009) Microcystins -LA, -YR, and -LR action on neutrophil migration. Biochem Biophys Res Commun 382:9–14
Larsen K (1972) Creatinine assay by a reaction-kinetic principle. Clin Chim Acta 41:209–217
Li B, Lu F, Wei X, Zhao R (2008) Fucoidan: structure and bioactivity. Molecules (Basel, Switzerland) 13:1671–1695
Li C, Gao Y, Xing Y, Zhu H, Shen J, Tian J (2011) Fucoidan, a sulfated polysaccharide from brown algae, against myocardial ischemia-reperfusion injury in rats via regulating the inflammation response. Food Chem Toxicol 49:2090–2095
Li J, Chen K, Li S, Liu T, Wang F, Xia Y, Lu J, Zhou Y, Guo C (2016) Pretreatment with fucoidan from Fucus vesiculosus protected against ConA-induced acute liver injury by inhibiting both intrinsic and extrinsic apoptosis. PLoS One 11:e0152570
Lim JD, Lee SR, Kim T, Jang SA, Kang SC, Koo HJ, Sohn E, Bak JP, Namkoong S, Kim HK, Song IS, Kim N, Sohn EH, Han J (2015) Fucoidan from Fucus vesiculosus protects against alcohol-induced liver damage by modulating inflammatory mediators in mice and HepG2 cells. Mar Drugs 13:1051–1067
Liu H, Zhang S, Zhang X, Huang H, Wu J, Wang Y, Yuan L, Liu C, Zeng X, Cheng X (2018a) Oxidative stress mediates microcystin-LR-induced endoplasmic reticulum stress and autophagy in KK-1 cells and C57BL/6 mice ovaries. Front Physiol 9:1058
Liu W, Wang L, Zheng C, Liu L, Wang J, Li D, Tan Y, Zhao X, He L, Shu W (2018b) Microcystin-LR increases genotoxicity induced by aflatoxin B1 through oxidative stress and DNA base excision repair genes in human hepatic cell lines. Environ Pollut 233:455–463
Lone Y, Bhide M, Koiri RK (2017) Amelioratory effect of coenzyme Q10 on potential human carcinogen microcystin-LR induced toxicity in mice. Food Chem Toxicol 102:176–185
Lu YF, Liu J, Wu KC, Qu Q, Fan F, Klaassen CD (2014) Overexpression of Nrf2 protects against microcystin-induced hepatotoxicity in mice. PLoS One 9:e93013
Luthuli S, Wu S, Cheng Y, Zheng X, Wu M, Tong H (2019) Therapeutic effects of Fucoidan: a review on recent studies. Mar Drugs 17:487
McLellan NL, Manderville RA (2017) Toxic mechanisms of microcystins in mammals. Toxicol Res 6:391–405
Mihara M, Uchiyama M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–278
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46:849–854
Nong Q, Komatsu M, Izumo K, Indo HP, Xu B, Aoyama K, Majima HJ, Horiuchi M, Morimoto K, Takeuchi T (2007) Involvement of reactive oxygen species in microcystin-LR-induced cytogenotoxicity. Free Radic Res 41:1326–1337
Novoyatleva T, Kojonazarov B, Owczarek A, Veeroju S, Rai N, Henneke I, Böhm M, Grimminger F, Ghofrani HA, Seeger W (2019) Evidence for the fucoidan/P-selectin axis as a therapeutic target in hypoxia-induced pulmonary hypertension. Am J Respir Crit Care Med 199:1407–1420
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Park HY, Han MH, Park C, Jin CY, Kim GY, Choi IW, Kim ND, Nam TJ, Kwon TK, Choi YH (2011) Anti-inflammatory effects of fucoidan through inhibition of NF-kappaB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem Toxicol 49:1745–1752
Puerto M, Pichardo S, Jos A, Cameán AM (2009) Oxidative stress induced by microcystin–LR on PLHC-1 fish cell line. Toxicol in Vitro 23:1445–1449
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63
Richmond W (1973) Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clin Chem 19:1350–1356
Scoglio S (2018) Microcystins in water and in microalgae: do microcystins as microalgae contaminants warrant the current public alarm? Toxicol Rep 5:785–792
Sicinska P, Bukowska B, Michalowicz J, Duda W (2006) Damage of cell membrane and antioxidative system in human erythrocytes incubated with microcystin-LR in vitro. Toxicon 47:387–397
Svircev Z, Drobac D, Tokodi N, Mijovic B, Codd GA, Meriluoto J (2017) Toxicology of microcystins with reference to cases of human intoxications and epidemiological investigations of exposures to cyanobacteria and cyanotoxins. Arch Toxicol 91:621–650
Szasz G, Waldenstrom J, Gruber W (1979) Creatine kinase in serum: 6. Inhibition by endogenous polyvalent cations, and effect of chelators on the activity and stability of some assay components. Clin Chem 25:446–452
Tanaka K, Ito M, Kodama M, Tomita M, Kimura S, Hoyano M, Mitsuma W, Hirono S, Hanawa H, Aizawa Y (2011) Sulfated polysaccharide fucoidan ameliorates experimental autoimmune myocarditis in rats. J Cardiovasc Pharmacol Ther 16:79–86
Thomes P, Rajendran M, Pasanban B, Rengasamy R (2010) Cardioprotective activity of Cladosiphon okamuranus fucoidan against isoproterenol induced myocardial infarction in rats. Phytomedicine 18:52–57
Tietz NW, Burtis CA, Duncan P, Ervin K, Petitclerc CJ, Rinker AD, Shuey D, Zygowicz ER (1983) A reference method for measurement of alkaline phosphatase activity in human serum. Clin Chem 29:751–761
van der Merwe D (2015) Chapter 31 - cyanobacterial (blue-green algae) toxins. In: Gupta RC (ed) Handbook of toxicology of chemical warfare agents (second edition). Academic Press, Boston, pp 421–429
Wang Q, Xie P, Chen J, Liang G (2008) Distribution of microcystins in various organs (heart, liver, intestine, gonad, brain, kidney and lung) of Wistar rat via intravenous injection. Toxicon 52:721–727
Wang J, Wang F, Yun H, Zhang H, Zhang Q (2012) Effect and mechanism of fucoidan derivatives from Laminaria japonica in experimental adenine-induced chronic kidney disease. J Ethnopharmacol 139:807–813
Wang J, Liu H, Li N, Zhang Q, Zhang H (2014) The protective effect of fucoidan in rats with streptozotocin-induced diabetic nephropathy. Mar Drugs 12:3292–3306
Wang Y-q, J-g W, M-j T, J-g G, Zhang W (2018) Fucoidan alleviates acetaminophen-induced hepatotoxicity via oxidative stress inhibition and Nrf2 translocation. Int J Mol Sci 19:4050
Wang J, Geng L, Yue Y, Zhang Q (2019a) Use of fucoidan to treat renal diseases: a review of 15 years of clinic studies. Prog Mol Biol Transl Sci 163:95–111
Wang Q, Liu Y, Guo J, Lin S, Wang Y, Yin T, Gregersen H, Hu T, Wang G (2019b) Microcystin-LR induces angiodysplasia and vascular dysfunction through promoting cell apoptosis by the mitochondrial signaling pathway. Chemosphere 218:438–448
Wang Y, Xing M, Cao Q, Ji A, Liang H, Song S (2019c) Biological activities of fucoidan and the factors mediating its therapeutic effects: a review of recent studies. Mar Drugs 17:183
Wei H, Gao Z, Zheng L, Zhang C, Liu Z, Yang Y, Teng H, Hou L, Yin Y, Zou X (2017) Protective effects of fucoidan on Abeta25-35 and d-gal-induced neurotoxicity in PC12 cells and d-gal-induced cognitive dysfunction in mice. Mar Drugs 15
Wurzburg U, Hennrich N, Lang H, Prellwitz W, Neumeier D, Knedel M (1976) Determination of creatine kinase-MB in serum using inhibiting antibodies (author’s transl). Klin Wochenschr 54:357–360
Xu Y, Zhang Q, Luo D, Wang J, Duan D (2017) Low molecular weight fucoidan ameliorates the inflammation and glomerular filtration function of diabetic nephropathy. J Appl Phycol 29:531–542
Yang JW, Yoon SY, Oh SJ, Kim SK, Kang KW (2006) Bifunctional effects of fucoidan on the expression of inducible nitric oxide synthase. Biochem Biophys Res Commun 346:345–350
Yuan Y, Macquarrie D (2015) Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr Polym 129:101–107
Zhang H, Zhang J, Chen Y, Zhu Y (2008) Microcystin-RR induces apoptosis in fish lymphocytes by generating reactive oxygen species and causing mitochondrial damage. Fish Physiol Biochem 34:307–312
Zhang K, Ma H, Yan P, Tong W, Huang X, Chen DD (2018) Electrochemical detection of microcystin-LR based on its deleterious effect on DNA. Talanta 185:405–410
Zhao X, Wang JF, Xue CH (2011) The inhibitory effects of fucoidans from laminaria japonica on oxidation of human low-density lipoproteins. Advanced Materials Research. Trans Tech Publ, pp 2067-2071
Zhou M, Ding Y, Cai L, Wang Y, Lin C, Shi Z (2018) Low molecular weight fucoidan attenuates experimental abdominal aortic aneurysm through interfering the leukocyte-endothelial cells interaction. Mol Med Rep 17:7089–7096
Funding
This work was supported by the Deanship of Scientific Research at King Saud University (Research group no.: RGP-018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
AlKahtane, A.A., Abushouk, A.I., Mohammed, E.T. et al. Fucoidan alleviates microcystin-LR-induced hepatic, renal, and cardiac oxidative stress and inflammatory injuries in mice. Environ Sci Pollut Res 27, 2935–2944 (2020). https://doi.org/10.1007/s11356-019-06931-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06931-z