Abstract
The goals of this study were to analyze if there is a difference in the stable isotopic ratio (δ13C and δ15N) of macrobenthic species sampled at two sandy beaches (one close to a river mouth and the other far from any freshwater input) and to identify differences in the stable isotopic ratio (δ13C and δ15N) in different body parts of three representative species of two Brazilian sandy beach macrofaunas: the polychaete Hemipodia californiensis, the mollusk bivalve Donax hanleyanus, and the crustacean decapod Emerita brasiliensis. No significant differences were detected in the δ13C stable isotopic ratio between the two sites analyzed; however, in the δ15N stable isotopic ratio, a significant difference was observed. Regarding the intraspecific response of stable isotopic ratio, D. hanleyanus showed a significant difference in carbon among different body part structures, while a trend for significance was observed for nitrogen isotopes. The differences were significant for both isotopes in E. brasiliensis, and no differences were observed among the body part structures in H. californiensis. There were significant differences in E. brasiliensis carapaces with regard to the δ15N stable isotopic ratio between the muscle and the whole body. Although the δ13C and δ15N stable isotopic ratio differs significantly in the digestive tract, muscles, and whole body of D. hanleyanus, such differences were not enough to determine changes in their trophic levels and food sources. Similar stable isotopic ratios were observed in the whole body, proboscis, and teeth of H. californiensis, highlighting this species as the top predator. In conclusion, stable isotopic analysis of benthic trophic structure can be employed as a tool in coastal management plans or environmental impact studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The composition and structure of coastal ecosystems have been changing in the last few decades due to the increase of anthropogenic activities, global climate change, and settlement of invasive species (UNEP 2002; Liao et al. 2015). The presence of the macrobenthic community in coastal ecosystems is recognized by the fundamental role of environmental processes, such as sediment remobilization and biomass availability to higher trophic levels. However, macrobenthic ecology can be modified by multiple sources of environmental change, including the origins of nutrient supplies. Thus, understanding the trophic structure of sandy beaches is an important issue for management actions in these areas (Corbisier et al. 2014; Linden 2017; McLachlan and Brown 2006; Thrush et al. 2006).
Stable isotope analyses are a good laboratory tool in physiological and trophic ecology studies (Olsen et al. 2013), and the common isotopes are usually carbon (13C) and nitrogen (15N) (Manetta and Benedito-Cecilio 2003; Michener and Lajtha 2008; Bongiorni et al. 2016). Both isotopes undergo a consistent trophic enrichment pattern, autonomous of the organism position in the food web (Post 2002), and also present an increasing trend of about 3.4‰ for each trophic level in nitrogen, besides carbon enrichment at a smaller intensity of about 1.0‰. The isotope rate of C is used to trace the origin of primary sources of C in food webs, while the isotope ratio of N is used to determine the trophic level in organisms (Fry et al. 1999). These properties allow using these measurements to compare the chemical composition of the same species from different locations and to analyze the influence of environmental variables on the organism’s diet within the food web.
Furthermore, the stable isotopic ratio in individuals is a good alternative when a limited quantity of biomass samples is available for analysis. When organisms reach a sufficient size for separating body parts, this can be used for stable isotopic analyses, as in muscle and mantle for mollusks (Antonio et al. 2010), abdominal muscle segments in decapod crustaceans (Bergamino et al. 2011), and even bivalve hemolymphs (Gustafson et al. 2007). When the biomass in a single organism is not sufficient for separating the body parts for isotopic analyses, it is possible to use the whole body, or in case of very small organisms, pooling animals from the same species (Bergamino et al. 2011).
Attempts for solving discrepancies in stable isotopic ratio using different sampling preparations are always in progress. For example, for minimizing chances in changing the stable isotopic ratio from the food in the digestive tract, some authors place organisms in individual aquariums with filtered marine water for cleaning them, and in this way, the contents do not affect the stable isotopic ratio (Olsen et al. 2013). There are other important aspects of sampling preparation, such as acidification, distilled water use, and lipid and fat removal. For example, Mateo et al. (2008) compared results between the whole body of marine invertebrates to those using soft tissues, showing that the stable isotopic ratio of 13C and 15N changed depending on the method used.
Thus, stable isotopic ratio measurements can be used to compare, for example, differences between food preferences in species living in different places and to analyze the influence of environmental variables on the organism’s diet within the food web. The beach locations analyzed in the present study, i.e., close to the organically polluted Paraíba do Sul River’s mouth and to the upwelling zone of the South Atlantic Central Water (SACW) intrusion at Cabo Frio, could be considered as the most important environmental variables in determining the food sources, since the water physicochemical characteristics of these two locations are quite different, as previously reported (Corbisier et al. 2014; Carreira et al. 2015; Cordeiro et al. 2018).
There were two main objectives in this study: (i) to determine differences in the stable isotopic ratio (δ13C and δ15N) in macrobenthic species sampled at two sandy beaches (one close to a river mouth and another far from any freshwater input) and (ii) to identify differences in the stable isotopic ratio (δ13C and δ15N) in different body parts of three representative species of sandy beach macrofauna: the polychaete Hemipodia californiensis, the mollusk bivalve Donax hanleyanus, and the crustacean decapod Emerita brasiliensis.
Materials and methods
Sampling and preparation for analyses
The H. californiensis, D. hanleyanus, and E. brasiliensis species were collected from two sandy beaches in the state of Rio de Janeiro: Praia do Farol in São Thomé (PF), located at Paraíba do Sul River’s mouth, in the municipality of São João da Barra (21° 37′ S and 41° 00′ W), and Praia Grande (PG) in the municipality of Arraial do Cabo (22° 58′ S and 42° 01′ W). PF is a reflective beach with a high nutrient contribution from the continent provided by the Paraíba do Sul River. This local seasonality includes rainy and dry periods, and according to Krüger et al. (2006), the difference in the nutrient supply changes slightly between both seasons, and 94% to 95% of the dissolved inorganic nitrogen (DIN) in the river is related to NO3−, with concentrations of this compound ranging from 7.41 to 32.8 μM.
As PG is a dissipative beach, there is no continental influence, and it is affected by the upwelling at Cabo Frio; the main intrusion is from the SACW. According to Corbisier et al. (2014), the variations in the DIN values range from < 0.05 to 3.20 μM NH4+, from < 0.05 to 1.11 μM NO2−, and from < 0.05 to 11.93 μM NO3−. The organisms were sampled using a 20-cm-diameter PVC cylinder, buried at a 20 cm depth in the sediment. Two sampling campaigns were carried out on each beach in December 2016 (favorable to upwelling), and organisms from the same species were collected at different beach locations to achieve spatial representativeness from each beach. Thus, for comparison of stable isotopic ratio (δ13C and δ15N) in macrobenthic species sampled at two sandy beaches, 50 D. hanleyanus and 18 H. californiensis organisms were sampled at different locations and freeze-dried for the stable isotopic ratio determination in muscles; 14 D. hanleyanus, 25 E. brasiliensis, and 12 H. californiensis individuals were sampled to identify differences in the stable isotopic ratio (δ13C and δ15N) in different body parts. It should be mentioned that not all individuals were used in the analyses. Each one of the following body parts was thawed, weighed, and dissected: in Hemipodia, the proboscis teeth (4 per animal), proboscis (without contents), whole body with full digestive tract; in Donax, the adductor muscle, all viscera including gills, and whole body; and in Emerita, the carapace, muscle bundles below the carapace, and whole body. After the body parts were separated, the samples were immediately washed with distilled and deionized water, and the wet weight was determined after acidic decarbonation. After the frozen-lyophilization process of the body parts, the dry weight was also determined. The mean elemental composition of the C:N ratio of the samples used in this study was ≤ 3.5. In that context, the lipid extraction was not considered as a restriction on body part δ13C value interpretation (Kiljunen et al. 2006; Post et al. 2007).
Laboratory measurements
Elemental (C and N) and isotopic (δ13C and δ15N) compositions were determined by weighing approximate 0.5-mg samples in tin capsules and analyzed by a continuous-flow isotope-ratio mass spectrometer (Delta V Advantage; Thermo Scientific, Germany) coupled with an elemental analyzer (Flash 2000) (Fry 2006). The results are expressed in the conventional delta (δ) notation related to Pee Dee Belemnite for δ13C and atmospheric N2 for δ15N, according to Eq. (1), where Rsample and Rstandard are the corresponding ratios of rare to common isotopes (13C/12C and 15N/14N) in the sample and international standards, respectively (Peterson and Fry 1987). Samples were run using blank cups and known analytical isotope standards of urea of IVA Analysentechnik 330802174 (CH4N2O, Mw = 60, C = 20%, N = 46%) with the certified isotopic composition (δ13C = − 39.79‰ and δ15N = − 0.73‰). Data quality control was checked by performing a reference standard run (Elemental Microanalysis Protein Standard OAS of certified isotopic composition: δ13C = − 26.98‰ and δ15N = 5.94‰) after every ten samples. Reproducibility was based on triplicate analysis of samples as ± 0.2‰ for δ13C and δ15N.
Statistics
Two sites were compared by bi-factorial ANOVA considering beaches (PG and PF) and species (H. californiensis and D. hanleyanus) as orthogonal and fixed factors, using the stable isotopic ratio determined in the muscle of both species (Underwood 1997). One-way ANOVA was applied to compare the body structures on each species, considering different body structures as factors (Underwood 1997). Normality and homogeneity variances were verified by the Kolmogorov-Smirnov and Bartlett’s tests, respectively, and when these requirements were not met, transformations were applied to log10 (X + 1).
Results and discussion
The ecosystem data are analyzed by applying temporal and spatial scales and play a critical role in shaping our understanding of their structure and function (Levin 1992; Estes et al. 2018). A comparison between two specific places was intended in this study, and temporal replication of data was contemplated at different times when the organisms were sampled. The objective emphasized in this study was to verify if different sites or body part structures presented differences in the stable isotopic ratio of δ13C and δ15N.
Spatial comparison between Praia Grande and Praia do Farol beaches
The reflective beach of Praia do Farol in São Thomé is characterized by a high nutrient contribution from the continent, in the mouth of Paraíba do Sul River, while Praia Grande is a dissipative beach, without any continental influence, but it is affected by the upwelling at Cabo Frio (intrusion of the SACW). There were differences in the stable isotopic ratio (δ13C and δ15N) of macrobenthic species sampled at these two sandy beaches. Table 1 shows the mean stable isotopic ratio on the muscle of organisms sampled at PG and PF, while Table 2 shows the ANOVA results from these data.
No significant differences were detected for the δ13C stable isotopic ratio between the two sites; however, a significant difference was observed for the δ15N stable isotopic ratio (Table 2). In fact, the two sampled species at the PF beach showed lower δ15N stable isotopic ratio than the sampled organisms at the PG beach. While the δ13C stable isotopic ratio was similar in both species and sites, the δ15N stable isotopic ratio varied between the sites and species. The SE values were lower when the N value was higher regarding the measurement data quality.
The D. hanleyanus and H. californiensis species are not clearly subject to the influence of marine organic matter origin in their δ13C stable isotopic ratio (Table 1) when comparing the stable isotopic ratio in the organisms at PG and PF. D. hanleyanus is a filtering bivalve, incorporating unicellular organisms with or without photosynthetic capacity, placing it at the base of the trophic web (Post 2002; Gustafson et al. 2007). Since there is no significant difference related to the δ13C isotopic signal among the D. hanleyanus individuals sampled at both beaches, we can infer that organisms do not have a differentiated influence on the organic matter source, namely the upwelling of the SACW at the PG beach or the supply of the river organic matter from the PF beach. However, a variation of δ13C stable isotopic ratio was reported from the same sources of organic matter (i.e., zooplankton, suspended organic matter, and phytoplankton). They are the most potential source of the δ13C stable isotopic ratio for the macrobenthic organisms (Corbisier et al. 2006; Petracco 2008; Franco 2013; Di Beneditto et al. 2013; Corbisier et al. 2014). Thus, according to our results, it is still important to determine if the influence of the different phenomena occurring in these two places is equivalent as a source of organic matter, at least for the δ13C stable isotopic ratio. Higher values were found in the PG-sampled organisms related to the δ15N stable isotopic ratio. That may be an indication of the greater influence of the SACW upwelling on the constitution of nitrogenous organic matter. Seasonal upwelling leads to strong and variable shifts, and they were reported in production sources cascading up food webs in the region. According to France (1994), the δ15N stable isotopic ratio of invertebrates reflects that both trophic-dietary and habitat source fractionation and the relative position of statistical mode are different for marine and freshwater organisms. Thus, if we consider the results from Table 1, organisms from the PG beach clearly showed marine food source influence, while organisms from the PF beach showed continental food source influence. Stable isotope analyses offer a reliable approach for defining these changes; however, in relation to generated data, small temporal variability sampling is likely to provide different information than longer ones, and thus, conclusions obtained from specific studies should be put into perspective (Corbisier et al. 2014).
Comparisons of stable isotopic ratio among species morphological structures
Three species were chosen due to the sampling facility, for easy separation of the body part structures, and mainly because they represent organisms feeding on sediment (e.g., Hemipodia) and in the water column (e.g., Donax and Emerita). Thus, these organisms play distinct ecological functions at different levels of the trophic web in sandy beaches. Hemipodia is a top predator (carnivorous) (Pinotti et al. 2014), and Donax and Emerita are both consumers (McLachlan and Brown 2006; Bergamino et al. 2011; Pinotti et al. 2014).
D. hanleyanus showed a significant difference for δ13C in the body part structures, but only a tendency to be significant for the δ15N stable isotopic ratio. In E. brasiliensis, the differences were significant for both isotopes, and in H. californiensis, no differences were observed among the body part structures (Tables 3 and 4).
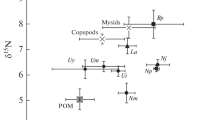
For D. hanleyanus, δ13C mean values ranged from − 16.7‰ in the muscle to − 17.9‰ in the whole animal, while δ15N ranged from 8.4‰ in the viscera to 9.2‰ in the muscle. For E. brasiliensis, the carapace displayed a notable reduction for both δ13C and δ15N, ranging from − 15.7 to − 18.9‰ for δ13C and from 3.2 to 8.1‰ for δ15N, respectively. In the case of H. californiensis, which presented no differences among its body part structures, the values for δ13C ranged from − 16.4% to − 17.2‰ and those for δ15N from 13.7 to 14.5‰ (Table 3).
The δ15N stable isotopic ratio of the bivalve D. hanleyanus was close to that of several other suspended bivalves. In E. brasiliensis, a suspensivore crustacean, the δ15N stable isotopic ratio is similar to that in other crustaceans with the same feeding habit (Corbisier et al. 2006; Han et al. 2015; Franco 2013). Finally, the δ15N isotopic signature of H. californiensis was similar to that of other predators, as polychaetes of the same class and other polychaetes, and even similar to carnivorous fishes, such as Trichiurus lepturus (Corbisier et al. 2006; Petracco 2008; Bergamino et al. 2011; Di Beneditto et al. 2013; Corbisier et al. 2014).
H. californiensis, a polychaete of the Glyceridae family, has developed jaws made up by four pointed teeth with the capacity to inject paralyzing poison produced by glands at the base of the teeth. Also, a long muscular proboscis, which can reach three fourths of the body, is everted for projecting the teeth and catching its prey. The internal digestive tract may be visible to the naked eye in the rest of the body because it is often filled (Fauchald and Jumars 1979). H. californiensis presented no significant difference for δ15N signal, but the values were lower than the ones presented by Bergamino et al. (2011) for the same species. However, our results corroborate with those of Corbisier et al. (2014) for several benthic predators, placing the polychaete in the same trophic position as an octopus or a carnivorous fish.
D. hanleyanus is a filtering bivalve feeding by capturing suspended particulate matter in the water column. There is a system in its gills like eyelashes that select particles compatible with the opening of the mouth, while the larger particles are led to pseudofeces production. The data obtained for δ15N indicate this species is a secondary consumer, and the variations found for the structures did not indicate a trophic position variation based on the values found in the literature (Bergamino et al. 2011). The reduced values in the viscera, compared to those in the muscle, could be a result of the unassimilated material in the inhalant siphon.
E. brasiliensis presented a marked reduction for δ15N signal in the carapace compared to the muscle, with almost a three times lower mean value. Also, Yokoyama et al. (2005) reported that the carapaces present much lower values of δ15N than other parts of crustaceans. The lower values of δ15N found in carapaces were similar to those found by Kogure (2004) in benthic diatoms, phytoplankton, and zoobenthos. The δ15N stable isotopic ratio found in the muscle of this species is similar to those obtained by other authors. That is in benthic crustaceans with the same feeding habits as E. brasiliensis (Corbisier et al. 2006; Han et al. 2015). There were differences found in the δ13C isotopic signals in this species, especially when the whole organism was compared to specific body part structures that possibly occurred because the complete digestive tract was analyzed in the organism. Thus, the food that was not assimilated by the organism may affect the stable isotopic ratio, whereas in the muscle, the stable isotopic ratio reflects only what the organism has already assimilated. The difference may have occurred because both organic and inorganic carbons are present in their exoskeletons in carapaces, and this fact may reflect a decrease in the carbon isotope signal (Mateo et al. 2008) (Tables 3 and 4). The bivalve mollusk D. hanleyanus presented a similar situation to E. brasiliensis, in which the carbon isotopic signal was more enriched in the whole organism, probably because the digestive tract was filled with undigested matter. H. californiensis did not follow this pattern, suggesting that predators do not have this differentiation. Here, it should be noted that the stable isotopic ratio is species-specific and tissue-specific and that the accepted fractionation values may not be universally applicable (Yokoyama et al. 2005). Therefore, it is possible to identify contrasts in the stable isotopic ratio according to the body structure, as well as to the sampled location (Table 5).
Conclusions
Stable isotope analyses are a good tool in trophic ecology studies related to the chemical composition of the same species found in different locations, making it possible to analyze the influence of environmental variables on the organism’s diet. Also, isotope ratio quantification can be a good alternative analysis when little organic matter is available for testing. No significant differences were detected for the δ13C stable isotopic ratio between the two studied sites (i.e., PF and PG); however, for the δ15N stable isotopic ratio, a significant difference was observed. Also, it was possible to identify differences in the stable isotopic ratio according to the body structure. Significant differences were detected in the δ13C and δ15N isotopic signals for D. hanleyanus and E. brasiliensis. According to the δ13C isotope analysis, D. hanleyanus and H. californiensis displayed a great impact of marine organic matter in their feeding habits. More specifically, as there is no significant difference related to the δ13C isotopic signal among individuals of D. hanleyanus, we could infer that two beaches do not have different contributions of organic matter, and in both, the diet organism contribution is predominantly from zooplankton. However, the values obtained for δ15N stable isotopic ratio indicated that the variations found for the different structures did not show trophic position variations. E. brasiliensis presented a marked reduction in the δ15N ratio in the carapace compared to muscle, probably due to the sample acidification. Its average value was almost three times lower. The difference found between δ13C stable isotopic ratio in this species showed a significant difference comparing its body parts (complete digestive tract, muscle, and carapace), which may have occurred because of both organic carbon and the remaining inorganic carbon in the exoskeletons, reflecting the decrease in the δ13C stable isotopic ratio. The results also indicated a similar stable isotopic ratio in the whole body (including digestive tract), proboscis, and teeth of H. californiensis, highlighting this species as a top predator. In conclusion, there is an indication of the nutrient supply origin in different morphological structures allowing to understand the trophodynamic of benthic communities, which can be employed as a tool in coastal management plans or environmental impact studies.
References
Antonio ES, Kasai A, Ueno M, Kurikawa Y, Tsuchiya K, Toyohara H, Ishihi Y, Yokoyama H, Yamashita Y (2010) Consumption of terrestrial organic matter by estuarine molluscs determined by analyses of their stable isotopes and cellulase activity. Estuar Coast Shelf Sci 86:401–407
Bergamino L, Lercari D, Defeo O (2011) Food web structure of sandy beaches: temporal and spatial variation using stable isotope analyses. Estuar Coast Shelf Sci 91:536–543
Bongiorni L, Fiorentino F, Auriemma R, Aubry FB, Camatti E, Camin F, Nasi F, Pansera M, Ziller L, Grall J (2016) Food web of a confined and anthropogenically affected coastal basin (the Mar Piccolo of Taranto) revealed by carbon and nitrogen stable isotopes analyses. Environ Sci Pollut Res 23:2725–12738
Carreira RS, Cordeiro LGMS, Oliveira DRP, Baêta A, Wagener ALR (2015) Source and distribution of organic matter in sediments in the SE Brazilian continental shelf influenced by river discharges: an approach using stable isotopes and molecular markers. J Mar Syst 141:80–89
Corbisier TN, Soares LSH, Petti MAV, Muto EY, Silva MHC, McClelland J, Valiela I (2006) Use of isotopic signatures to assess the food web in a tropical shallow marine ecosystem of Southeastern Brazil. Aquat Ecol 40:381–390
Corbisier TN, Petti MAV, Soares LS, Muto EY, Bromberg S, Valiela I (2014) Trophic structure of benthic communities in the Cabo Frio upwelling system (southeastern Brazilian shelf): a temporal study using stable isotope analyses. Mar Ecol Prog Ser 512:23–38
Cordeiro LGMS, Wagener ALR, Carreira RS (2018) Organic matter in sediments of a tropical and upwelling influenced region of the Brazilian continental margin (Campos Basin, Rio de Janeiro). Org Geochem 120:86–98
Di Beneditto APM, Rezende CE, Camargo PB, Kehrig HA (2013) Trophic niche comparison between two predators in northern Rio de Janeiro State, Brazil: a stable isotopes approach. Biota Neotrop 13:29–33
Estes L, Elsen PR, Treuer T, Ahmed L, Caylor K, Chang J, Choi JJ, Ellis E (2018) The spatial and temporal domains of modern ecology. Nat Ecol Evol 2:819–826. https://doi.org/10.1038/s41559-018-0524-4
Fauchald K, Jumars PA (1979) The diet of worms: a study of polychaete feeding guilds. Oceanogr Mar Biol 17:193–284
France RL (1994) Nitrogen isotopic composition of marine and freshwater invertebrates. Mar Ecol Prog Ser 115:205–207
Franco MAL (2013) Uso de ferramentas múltiplas na investigação do impacto de um recife artificial sobre uma assembleia de peixes transientes no norte do estado do Rio de Janeiro. Doctoral dissertation Thesis, North Rio de Janeiro University, RJ, Brazil
Fry B (2006) Stable isotope ecology. Springer, New York
Fry B, Mumford PL, Tam F, Fox DD, Warren GL, Havens KE, Steinman AD (1999) Trophic position and individual feeding histories of fish from Lake Okeechobee, Florida. Can J Fish Aquat Sci 56:590–600
Gustafson L, Showers W, Kwak T, Levine J, Stoskopf M (2007) Temporal and spatial variability in stable isotope compositions of a freshwater mussel: implications for biomonitoring and ecological studies. Oecologia 152:140–150
Han E, Park HJ, Bergamino L, Choi KS, Choy EJ, Yu OH, Kang CK (2015) Stable isotope analyses of a newly established macrofaunal food web 1.5 years after the Hebei Spirit oil spill. Mar Pollut Bull 90:167–180
Kiljunen M, Grey J, Sinisalo T, Harrod C, Immonen H, Jones RI (2006) A revised model for lipid-normalizing δ13C values from aquatic organisms, with implications for isotope mixing models. J Appl Ecol 43:1213–1222
Kogure Y (2004) Stable carbon and nitrogen isotope analyses of the sublittoral benthic food web structure of an exposed sandy beach. Bul Biogeog Soc Jpn 59:15–25
Krüger GCT, de Carvalho CEV, Suzuki MS (2006) Dissolved nutrient, chlorophyll-a and DOC dynamic under distinct riverine discharges and tidal cycles regimes at the Paraiba do Sul River estuary, RJ, Brazil. J Coast Res 39:724–730
Levin AS (1992) The problem of pattern and scale in ecology. Ecology 73:1943–1967
Liao E, Lu W, Yan X-H, Jiang Y, Kidwell A (2015) The coastal ocean response to the global warming acceleration and hiatus. Sci Rep 5:16630. https://doi.org/10.1038/srep16630
Manetta G, Benedito-Cecilio E (2003) Aplicação da técnica de isótopos estáveis na estimativa da taxa de turnover em estudos ecológicos: uma síntese. Acta Sci Biol Sci 25:121–129
Mateo MA, Serrano O, Serrano L, Michener RH (2008) Effects of sample preparation on stable isotope ratio of carbon and nitrogen in marine invertebrates: implications for food web studies using stable isotopes. Oecologia 157:105–115
McLachlan A, Brown AC (2006) Ecology of sandy shores. Academic, New York
Michener R, Lajtha K (2008) Stable isotopes in ecology and environmental science, 2nd edn. Blackwell Publishing Ltd, Oxford
Olsen YS, Fox SE, Hofmann L, Valiela I (2013) Benthic community composition and faunal stable isotopic signatures differ across small spatial scales in a temperate estuary. Mar Environ Res 86:12–20
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Ver Ecol Syst 18:293–320
Petracco M (2008) Produção secundária da macrofauna bentônica da zona entremarés no segmento norte da praia do Una, litoral sul do Estado de São Paulo. Tese de Doutorado, Instituto Oceanográfico, Universidade de São Paulo, 236p
Pinotti RM, Minasi DM, Colling LA, Bemvenuti CE (2014) A review on macrobenthic trophic relationships along subtropical sandy shores in southernmost Brazil. Biota Neotrop 14(3):e20140069
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
Post DM, Layman CA, Arrington DA, Takimoto G, Quattrochi J, Montaña CG (2007) Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152:179–189
Thrush SF, Hewitt JE, Gibbs M, Lundquist C, Norkko A (2006) Functional role of large organisms in intertidal communities: community effects and ecosystem function. Ecosystems 9:1029–1040
Underwood AJ (1997) Experiments in ecology: their logical design and interpretation using analyses of variance. Cambridge University Press, Cambridge
UNEP (2002) Oceans and coastal areas. Coastal threats [online] Cited November 2004. Available at http://earthwatch.unep.net/oceans/coastalthreats.php
van der Linden PRA (2017) A trait-based approach to investigate macrobenthic community functioning in estuarine and coastal ecosystems. Universidade de Coimbra. Tese de doutoramento. Available at http://hdl.handle.net/10316/79651. 18 Oct 2018
Yokoyama H, Tamaki A, Harada K, Shimoda K, Koyama K, Ishihi Y (2005) Variability of diet-tissue isotopic fractionation in estuarine macrobenthos. Mar Ecol Prog Ser 296:115–128
Funding
This study received financial support from the Universidade Estadual do Norte Fluminense (Brazil). T.C.M. Almeida received grants from Brazilian agencies FAPERJ (no. 102.004/2013) and CNPq (no. 158504/2014-3).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Almeida, T.C.M., Rocha, P.F.P., Zalmon, I.R. et al. Is there an indication of the origin of nutrient supply in different morphological structures of macrofauna at two different Brazilian southeastern sandy beaches? Comparison by C and N stable isotopes. Environ Sci Pollut Res 26, 33023–33029 (2019). https://doi.org/10.1007/s11356-019-06376-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06376-4