Abstract
Microplastics (MPs) are widely recognised as a contaminant of emerging concern in the marine environment. This work provides original data of the presence of MPs in coastal sediments along the Spanish Mediterranean continental shelf. Ten surface sediment samples were collected in order to document baseline microplastic distribution from Algeciras to Barcelona. Microplastics were extracted from bulk sediments by density separation. The number of microplastics per kilogramme of dry weight ranged from 45.9 ± 23.9 MPs/kg d.w. observed at Palma de Mallorca to 280.3 ± 164.9 MPs/kg d.w. noted at Málaga, with an average value of 113.2 ± 88.9 MPs/kg d.w. The lower limit is defined by the pore filter size used (1.2 μm). For all analysed locations, the dominant microplastic type was fibres (82.9%), followed by fragments, and the main colours were transparent and blue. Microplastic size distribution was presented; in the case of fragments, 85% was lower than 0.5 mm, and in the case of fibres, the three studied intervals (0.5–1, 1–2, 2–5 mm) had similar distribution (35, 34 and 31%, respectively). Attending to all available data, no statistically significant relationship (Spearman’s correlation) was found between microplastic average size and distance to the coast, the depth, density population and sediment grain size. Neither relationship was observed between these variables and microplastic concentration using the non-parametric Kruskal-Wallis H test. This study has confirmed the widespread distribution of MPs in surface sediments from the Spanish Mediterranean continental shelf, and these data are useful to define baselines for MPs in the Western Mediterranean region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The extensive use of plastics and their applications, e.g. agriculture, industry or packaging, favour their presence in all environmental compartments as a result of improper management at the end of this material’s life cycle (Jambeck et al. 2015). Marine plastic particles may be transported far away from its source (Derraik 2002; Andrady 2011; Cole et al. 2011) and/or fragmented by physical phenomena, photo-degradation, etc. in smaller plastics which spread easily (Barnes et al. 2009). These pieces are so-called microplastics (particles less than 5 mm according to Arthur et al. (2009)). This marine litter has its own characteristics (colour, shape, size, chemical composition, etc.) and comprises a very heterogeneous group.
Although microplastics were first mentioned in the 70s (Carpenter and Smith 1972), it was not until the 2000s when the scientific community became aware of the great problem posed by microplastics for the marine ecosystem. Nevertheless, studies are not equally distributed around the world. The bulk is concentrated in the USA, Western Europe (UK, France, Germany, Spain and Italy), Oceania and East Asia (Japan and South Korea) (Barboza and Gimenez 2015).
The seafloor sediments have been recognised as a major sink and have a high potential to accumulate microplastics (Martellini et al. 2018). Microplastic quantification is necessary in order to better understand the distribution and rates of accumulation. Also, their high residence time in the marine environment (even centuries) and their affinity to organic pollutants and metals sorbed onto microplastics may pose negative effects on marine organisms (Barboza and Gimenez 2015; Rocha-Santos and Duarte 2015; Mai et al. 2018). As Cannas et al. (2017) suggested, gathering as much information and data as possible informs us about the real state of health of our seas and, in consequence, helps to develop strategies to act.
The Mediterranean Sea is a semi-enclosed sea with a relatively small area compared to other large marine ecosystems worldwide. This particularity, together with other factors like high-density population, maritime traffic, fisheries, etc., makes it one of the areas most affected by plastic accumulation all over the world (Galgani et al. 2010; Deudero and Alomar 2015) with an estimated plastic concentration of 243,853 plastic pieces km2 in this basin. Out of which, 83% are microplastics (Cózar et al. 2015). Due to these high microplastic concentrations, the Mediterranean Sea was comparable to hot spots like the five subtropical gyres (Cózar et al. 2015; Martellini et al. 2018). However, information about the microplastic distribution in the Mediterranean sediments is still scarce.
Within this context, and in order to achieve information for the Marine Strategy Framework Directive (Descriptor 10) implementation in the Spanish Mediterranean region, the aim of the present study was to establish a baseline data about the abundance, distribution, size and composition of microplastics in surface sediments along the Spanish Mediterranean continental shelf.
Mediterranean seabed sediment samples were collected during two research cruises in autumn 2014 and 2015 on board the oceanographic vessel F.P. Navarro (Spanish Institute of Oceanography). Ten sampling locations (see Fig. 1) were chosen considering mainly depositional areas (sediments with high fraction lower than 63 μm) which corresponded to the use for the chemical pollution monitoring programme. Four of them belong to Estrecho-Alborán Demarcation (Marine Strategy Framework Directive, MSFD), Algeciras, Málaga, Castell de Ferro and Almería, and six belong to Levantino-Balear Demarcation, Cartagena, Benidorm, Benicarló, Vallcarca, Barcelona and Palma de Mallorca (Table 1). Barcelona, Cartagena and Algeciras are classified as ‘priority pollution hot spots’ in the Mediterranean Sea (UNEP/MAP/MEDPOL 2005).
Map of Spanish Mediterranean Coast (including the Balearic Islands) showing sampling points. Green lines indicate bathymetry of the zone. Each line is an isobate. The isobates are at 50 and 500 m. AG, Algeciras; MA, Málaga; CF, Castell de Ferro; AL, Almería; CT, Cartagena; BD, Benidorm; BL, Benicarló; VL, Vallcarca; BA, Barcelona; PM, Palma de Mallorca
These locations covered the Spanish Mediterranean Coast from south to north and comprised a variety of coastal environments affected by different types and degrees of anthropogenic pressure. Several hot spots were considered such as Algeciras, one of the largest ports in Europe (https://www.icontainers.com/us/2016/12/22/top-15-ports-in-europe/) and the seaport with greater transit of goods in Spain is settled or Barcelona with more than 1.5 million inhabitants and very important industrial and port activities. Areas not directly affected by relevant pollution sources such as Castell de Ferro and Benicarló were also included in this study.
The sampling strategy was designed taking into account the highly heterogeneous distribution of microplastics in sediments (Wang and Wang 2018). Sediments were sampled using a stainless steel box-corer (0.17 m × 0.10 m), where only surface sediment layers (approx. 1.5 cm depth) were collected using a clean stainless steel spatula. Several replicates were conducted at each sampling point to achieve a representative sample (Wang and Wang 2018); specifically, four drags were pooled in each sampling point. The pooled sediment sample was subsequently homogenised in a stainless steel vessel so that between 3 and 5 kg of sediment were obtained per sampling point. Finally, homogenised sediment was distributed in clean aluminium containers and stored until their analysis at − 20 °C.
Once in the laboratory, aluminium trays with sediment samples were defrosted at room temperature. In order to determine water content, 3 g of sediment, in triplicate, was weighed in aluminium cups and dried at 70 °C for 24 h (Table 1). The highest water content was observed in Benidorm (47.2 ± 0.5%) whilst Vallcarca only presented 28.4 ± 0.5% of humidity.
Granulometric sediment composition was established, so an aliquot of sediments was analysed. The granulometric analysis was made with a Mastersizer 2000 laser particle size analyser (Malvern Instruments, Worcestershire, UK). Sediments were classified according to the Udden-Wentworth grain size classification: sand (63–2 mm), silt (4–63 μm) and clay (< 4 μm). In Table 1, sedimentary composition at the ten studied stations is shown. Sediment samples were mainly characterised by silt (Fig. 2). Only in three stations, Algeciras, Vallcarca and Palma de Mallorca, sand represented the main fraction, 65.90%, 68.35% and 79.67%, respectively. Benidorm showed the highest percentage of finer sediments (silt + clay) (99.37%). This result is in concordance with water content percentage (Table 1). As higher is the finer fraction, higher is water content and vice versa because sandy sediments are more permeable. In consequence, they retain less water than silt/clay sediments.
Different brine solutions could be used for density separation (see, e.g. Thompson et al. 2004; Liebezeit and Dubaish 2012; Nuelle et al. 2014); among all of them, NaCl (ρ ~ 1.2 g L−1) is the most extended in use because it is a relatively inexpensive salt, an eco-friendly product and no special precaution is necessary to adopt during laboratory processes (Galgani et al. 2013). In addition, the most common polymers present in the marine environment are usually polyethylene (PE), polypropylene (PP) and polystyrene (PS) with densities of 0.89–0.98, 0.85–0.92 and 1.04–1.06 g cm−3, respectively (Enders et al. 2015). Regardless, this procedural has an important limitation: plastics with densities above 1.2 g L−1 will probably not be recovered.
Nowadays, plastic is present everywhere; therefore, during the analytical process, it is very important to take special care to avoid cross-contamination (e.g. Browne et al. 2011; Woodall et al. 2014). Prior to analysis at the laboratory, work surfaces were cleaned with alcohol and hands and forearms scrubbed to prevent contamination from skin, hair and dirt particles (Lusher et al. 2014). During analytical procedure, various precautions were adopted: use 100% cotton fibre clothing, wear electrical blue lab coats, work inside a fume hood, wear nitrile gloves, rinse thoroughly with clean deionised water all apparatus prior to use, employ consumables directly from packaging and use zero plastic materials. Finally, after filtration, the collected samples were immediately covered and/or wrapped with aluminium foil (see, e.g. Cole et al. 2014). In addition, lab windows remained closed during experiments (Peng et al. 2017). Blanks of the analytical procedure were run (Graca et al. 2017), and results were blank-corrected according to Güven et al. (2017). Despite of these precautions microfibres, specially the lowest fraction (< 500 μm) could be overestimated (Lusher et al. 2017). Consequently, this microfibre fraction was excluded from data analyses.
The sediment sample (100 g) was introduced into a 2-L beaker; 250 mL of the hyper-saline solution of sodium chloride was added and stirred vigorously for 5–10 min (Frias et al. 2016). After mixing, the sample was left to stand for 30 min so heavy sediments would settle down whilst lighter materials would remain in suspension or float in surface solution. The supernatant was then filtered with a Millipore vacuum pump onto a Whatman® GF/C filter (1.2 μm pore and 47 mm diameter). Filters were placed in Petri dishes, dried in the dark and stored in a temperature-controlled environment (stable room temperature) to reduce storage degradation (Hidalgo-Ruz et al. 2012). This process was repeated three times (Fig. 3).
To evaluate cross-contamination, controls were run in parallel during the entire procedure. No fragment was observed in controls. In the case of fibres, transparent was the most common (2.4 ± 1.2 fibres), but other colours blue (1.3 ± 0.5 fibres), black (1.2 ± 0.4 fibres) and red (1.0 ± 0.0 fibres) were observed too. To counter contamination for the specific sampling site, the same number and colour fibres observed in controls were subtracted from the raw data.
Filters were visually observed, and all potential microplastic particles were counted, photographed and measured under a Leica S8AP0 stereomicroscope equipped with a digital camera. Visual examination of microplastics is the most common method to identify microplastics in environmental samples, especially for high-volume samples because it reduces measuring effort (Galgani et al. 2013; Löder et al. 2015; Renner et al. 2018). However, it can have a relatively high error rate due to the examiner, the sample matrix, the particle shape and size and the microscope used (e.g. Lenz et al. 2015; Löder et al. 2015). In addition, the smaller the size of the particles, the easier it is to make mistakes. To avoid these problems, different protocols can be adopted (e.g. Norén 2007; Cole et al. 2011). Items which presented the following features were identified as potential microplastics: (i) no visible cellular or organic structures, (ii) fibres were equally thick throughout their length, (iii) coloured particles are homogenously coloured and (iv) transparent and white particles should be further confirmed to exclude an organic origin (Norén 2007).
Determining all microplastic polymer composition is time consuming. In addition, micro-FTIR is limited to microplastics higher than its aperture size (> 10–20 μm) (Huppertsberg and Knepper 2018). For these reasons, a subset of microplastics (30%) was analysed (see, e.g. Cózar et al. 2014 or Lusher et al. 2014) with FTIR microscope (Spotlight 200i FTIR microscope system equipped with Spectrum Two, PerkinElmer) in the reflectance mode. Fifty scans were taken to produce the spectra with wavelengths between 600 and 4000 cm−1 and with the spectral resolution of 4 cm−1. Spectra were compared to the spectra libraries supplied by PerkinElmer and with a customised spectra database that includes all weathered common polymers. For accurate identification of the polymer, the detection threshold was set to a match of at least 70% (see, e.g. Frias et al. 2016). A total of three polymer types were identified, PS, polyamide (PA) and polymethyl methacrylate (PMMA), polystyrene being the most abundant (50%) followed by PMMA (25%) and PA (15%). It was surprising that neither polypropylene nor polyethylene was observed, as it usually occurred (e.g. Frère et al. 2017). The presence of these light polymers in sediments could explain long-distance dispersal (Ryan 2015) and sedimentation processes (Chae et al. 2015).
According to Free et al. (2014) recommendations, plastic particles were classified in (i) fragment, a piece broke off or detached from a bigger one; (ii) fibre, something resembling a filament; (iii) pellet, a small, rounded or spherical body; (iv) film, a thin sheet or strip of plastic; and (v) foam, spongy material.
Following a similar colour classification, as used by Peng et al. (2017), microplastics can be divided into different colour categories: blue, black, brown, green, yellow, transparent and red. Grey particles were counted in the black category. In the yellow category, orange particles were counted. The transparent category consisted of colourless particles. The red group included pink and purple particles. Results were expressed as microplastics per kilogramme of dry sediment (MPs/kg d.w.) (Claessens et al. 2011).
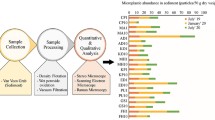
Microplastics were presented in all analysed samples from the Spanish Mediterranean inner continental shelf, with a wide range of distribution that goes from 46 to 280 MPs/kg d.w. The average concentration of microplastic particles observed at the different sampling stations is presented in Table 2 and Fig. 2. Overall, fibres (82.9%) were the most common type of microplastic found, followed far by fragments (12.2%), films (3.5%) and pellets (1.4%). The high microfibres observed in contrast to fragments content could be explained by the brine solution used for density separation in our experiment. Direct consequence, as Graca et al. (2017) suspected, is an underestimation of high-density microplastics. It is remarkable that only in Barcelona area all categories of microplastics were found, and in Benidorm, only fibre category showed up.
In order to carry out a comparison of the results obtained, it is necessary that the analytical procedures used are comparable (Zhao et al. 2014). Consequently, our results had been compared with those studies that used similar working protocols and units. In Changjiang Estuary, Peng et al. (2017) reported an average concentration of 121 ± 9 MPs/kg d.w., varying from 20 to 340 MPs/kg d.w., which is similar to the concentrations obtained in our study. However, microplastic concentration in sediments from other areas was lower than in the Mediterranean continental shelf, such as in the Singapore coast where it was 37 ± 24 MPs/kg d.w. (Nor and Obbard 2014); in bottom sediments in the Southern Baltic Sea where microplastic concentration ranged from 0 to 27 MPs/kg d.w. (Graca et al. 2017); in the UK estuarine sediments where Thompson et al. (2004) reported up to 31 MPs/kg d.w.; or along the Belgium coast, where Claessens et al. (2011) showed a microplastic abundance of 97.2 ± 18.6 MPs/kg d.w.. On the contrary, Cannas et al. (2017) reported concentrations much higher in the coast of south Tuscany (Tyrrhenian Sea) than in this study, with a maximum of 1069 microplastics per kg of dry sediment as well as Vianello et al. (2013) in the Venice Lagoon in Italy (672 to 2175 MPs/kg d.w.).
The ubiquitous distribution and accumulation of microplastics in some areas, rather than in others, depend on many factors, such as population density, freshwater inputs, water depth, sea bottom topography, waves, tides, flood events and water currents along the coast (see, e.g. Vianello et al. 2013; Woodall et al. 2014; Peng et al. 2017; Gündoğdu et al. 2018). Likewise, the source of microplastics in sediments is also related to the degradation of macroplastics (Andrady 2011; Cole et al. 2011; Cannas et al. 2017), the existence of sedimentation procedures related to ageing processes that modify the density and shape of particles (Cole et al. 2011; Chae et al. 2015), development of surficial biofilms (Cózar et al. 2014) or their incorporation into marine aggregates (Long et al. 2015). Filella (2015) pointed out that these kinds of processes could dominate microplastic environmental occurrence.
Thus, in our study, it would be expected that Barcelona presented the highest concentration of microplastics due to its strong anthropogenic pressure (industry, urban nuclei and port). Nevertheless, values obtained are close to the average MP concentration, probably because microplastics were washed offshore by Besós and Lobregat river plumes and the hydrodynamic of the zone (e.g. Copernicus: http://www.copernicus.eu/) which conducted debris towards the end of the continental shelf. On the contrary, the microplastic concentration of sediments observed in Málaga is surprisingly high and could be explained by the hydrodynamic mixing processes. These results are in agreement with Vianello et al. (2013) and Peng et al. (2017) where microplastics are mainly associated with human activities, in addition to other factors, such as hydrodynamic and geographical conditions which strongly influence the distribution of microplastics and their final sinks.
Microfibre proportions were higher than 73% in all samples (mean value was 82.9%) achieving 100% in Benidorm, except for in the Barcelona location where only 37% of microplastics were microfibres. These results are in agreement with Fastelli et al. (2016) in the Aeolian archipelago’s islands, with a percentage of fibres > 85% of the identified microplastics. Also in the Tyrrhenian Sea, fibres were the most common shape category (> 88%) in analysed samples (Cannas et al. 2017). Furthermore, the microfibre was the most prevalent shape of microplastics in marine bottom sediments in Polish coast of the Baltic Sea (Graca et al. 2017), the Irish Continental Shelf (Martin et al. 2017), in the Central Adriatic Sea (Mistri et al. 2017) or in Changjiang Estuary (Peng et al. 2017), among others. This high ratio distribution of fibres should be due to their release from artificial goods during wash cycles (Napper and Thompson 2016), as machine filters and wastewater treatment plants are not specifically designed to retain them. Fibres are then transported via sewage outfalls to rivers and dumped sewage sludge (see, e.g. Gago et al. 2018).
Another important factor associated with the distribution of microfibres is their low weight and high potential for long-distance oceanic transport, in comparison to heavier microplastic particles (Ling et al. 2017). In fact, sampling areas in our study were located at several miles from the main urban and industrial wastewater effluent discharge where low-weight plastics, such as fibres, can be preferentially transported.
In this study, fragments, films and pellets represent 18% of the total microplastics. Cannas et al. Cannas et al. (2017) reported that fragments represented < 9% in each sample in the Tyrrhenian Sea. Similar results were shown in China by Peng et al. (2017) where only a small amount of microplastics was fragments (6%) and pellets (1%).
The predominant colours (Fig. 4) observed in fibres were transparent > blue > black > red > green > yellow > red. For fragments, the sequence was blue > transparent > black = green = red > brown. Colour films were transparent, yellow and blue. In the case of pellets, only transparent pellets were observed. Similar results were reported by Veerasingam et al. (2016) along the Chennai Coast (India) where pellets in sediments were white/transparent.
Among different microplastic colour in sediments, the highest percentage (64%) corresponded to transparent fibres. This result was similar to the levels observed in Southern Baltic Sea (73%, Graca et al. 2017) and higher than levels observed in China sediments from Changjiang Estuary (Peng et al. 2017) where 93% of microplastics were fibres and occurred in transparent colour in 42%. In the Tyrrhenian Sea, Guerranti et al. (2017) observed that items were dominated by white and clear colour. Fastelli et al. (2016) reported a more homogeneous distribution of colours in Aeolian Archipelago’s islands, being green and black the most represented (20% and 28%, respectively), whilst white, clear, red, blue and pink were found approximately in the same percentage (about 10% of the total number of debris). In the Tunisian coast (Abidli et al. 2018), blue fragments were the predominant colour. Apart from that, Frias et al. (2016) in Southern Portuguese coastal sediments pointed out that fibres collected occurred mainly in four different colours, red, green, blue and black, and fragments in two colours, blue and green, but no percentage was indicated. This colour distribution, as Cannas et al. (2017) have highlighted, may be due to the different origin of the plastic materials or to degradation processes in the marine environment.
In order to study microplastic size distribution, microplastics were measured with ImageJ software (Schneider et al. 2012). The size intervals were delimited as 500 μm to 1 mm, 1–2 mm and 2–5 mm. Fibres ranged between 503 and 4933 μm (fraction < 500 μm was excluded in this stud, see above). In the case of fragments (focusing our attention only in fragment length dimension), up to 85% fell in the fraction under 500 μm, ranging 38–854 μm. The microplastic size distribution is shown in Fig. 5. In this study, the most important fraction for fibres was 0.5–1 mm (61%). These results are in line with the Enders et al. (2015) research, who observed that microplastics larger than 100 μm settled out of the water column. Naji et al. (2017) observed that microplastic size category of 1–4.7 mm length represented 56% in littoral sediments from the Persian Gulf. In the Polish coast, Graca et al. (2017) observed relatively small (0.1–2.0 mm) microplastics were the predominant size (64%). Our results were lower than the values observed in the Aeolian Archipelago’s islands by Fastelli et al. (2016) in a fraction of 0.063–1 mm which represented 94.8%. It was surprising that no particles (excluding fibres) were found in the 2–5-mm size class in the Bay of Brest (Frère et al. 2017). A possible explanation of these results can be found in the work of Vianello et al. (2013) where microplastic biofouling favours incorporation of these materials into the sediment.
Sediments are known to have the potential to accumulate microplastics (Zalasiewicz et al. 2016) and other pollutants in marine environment (Woodall et al. 2014). Grain size classification allows us to assess the link between the grain size and microplastic concentration through statistical analyses (Fastelli et al. 2016). In order to determine if microplastic abundance is related to sediment grain size, a Spearman’s correlation was applied. The significance level for statistical analyses was 0.05. Based on this analysis, MP concentrations showed no relationship with grain size in the analysed sediments (ρ = 0.527, p > 0.05). Other researchers pointed out that there is a non-clear relationship between microplastic concentration and sediment grain size distribution (e.g. Browne et al. 2011; Nor and Obbard 2014; Alomar et al. 2016; Graca et al. 2017; Peng et al. 2017). Cluzard et al. (2015) argued that physical processes determine the fate of microplastics within sediments, Blaskovic et al. (2017) remarked that MPs do not follow silt dynamics and Alomar et al. (2016) suggested that aggregation with organic matter might play an important role.
Using the Spearman’s correlation analysis, other environmental factors were also evaluated in relation to MP concentration, the distance to the coast (DC), the depth of the sediment (DS) and density population (DP). The distance to the coast was calculated using the coordinates of the sampling point and the shortest distance to the coast in a geographical information system. The significant level was, in all cases, higher than 0.05 (0.16, 0.19 and 0.64, respectively), so we concluded that no relationship was observed between these variables and microplastics concentration with our database, nor for specific fractions (fibre and fragment). The software used was SPSS Statistics.
In order to verify if there are significant differences between microplastic concentration in the two Mediterranean Demarcations, Levantino-Balear and Estrecho-Alborán, the Student t test of means comparison was applied (t = 0.88, p = 0.40). This result lets us conclude that there are no significant differences between the two demarcations.
Shapiro-Wilk test showed a non-normal data distribution (ρ = 0.804, p < 0.05), so the non-parametric Kruskal-Wallis H test was used for testing whether samples originate from the same distribution. Based on results, there were no statistically significant differences among all areas (p > 0.05).
Conclusions
This study offers an insight into the microplastic occurrence and spatial distribution in the Spanish Mediterranean coastal shelf. Microplastics were found in all of the analysed surface sediment samples, which ratify the widespread distribution of microplastics along the Spanish Mediterranean coast. Plastic reaches marine environment by different sources: runoffs, direct discharges, accidental episodes, tourism, etc. The occurrence of microplastics in sediments could be a valuable tool to estimate the repercussion on the environment, as the gas exchange or life on the seabed (Katsanevakis et al. 2007).
As Renner et al. (2018) have remarked, it is very complicated to obtain representative conclusions due to the heterogeneous distribution of MP in the environment, suggesting that microplastics may be available for different trophic levels (Wright et al. 2013; Ruiz-Orejón et al. 2016). So, further studies are necessary to quantify and identify microplastics which now make up a significant part of sediments. In addition, systematic monitoring over time of the microplastics present in the area will allow us to identify, with greater precision, the possible sources and transport mechanisms of marine litter. This information would let us develop actions to reach the objectives established by the Marine Strategy Framework Directive.
The values reported in this study will be useful for other countries to define baselines in the Mediterranean region. Our methodologies are in line with state of the art in this discipline. Nevertheless, further studies are needed to understand the pathways, interactions and consequences of microplastic pollution in the marine environment.
References
Abidli S, Antunes JC, Ferreira JL, Lahbib Y, Sobral P, Trigui El Menif N (2018) Microplastics in sediments from the littoral zone of the north Tunisian coast (Mediterranean Sea). Estuar Coast Shelf Sci 205:1–9. https://doi.org/10.1016/j.ecss.2018.03.006
Alomar C, Estarellas F, Deudero S (2016) Microplastics in the Mediterranean Sea: deposition in coastal shallow sediments, spatial variation and preferential grain size. Mar Environ Res 115:1–10. https://doi.org/10.1016/j.marenvres.2016.01.005
Andrady AL (2011) Microplastics in the marine environment. Mar Pollut Bull 62:1596–1605. https://doi.org/10.1016/j.marpolbul.2011.05.030
Arthur C, Baker J, Bamford H (eds) (2009) Proceedings of the International Research Workshop on the Occurrence, Effects and Fate of Microplastic Marine Debris. Sept 9–11, 2008. NOAA Technical Memorandum NOS-OR&R-30
Barboza LGA, Gimenez BCG (2015) Microplastics in the marine environment: current trends and future perspectives. Mar Pollut Bull 97:5–12. https://doi.org/10.1016/j.marpolbul.2015.06.008
Barnes DKA, Galgani F, Thompson RC, Barlaz M (2009) Accumulation and fragmentation of plastic debris in global environments. Philos Trans R Soc B 364:1985–1998. https://doi.org/10.1098/Rstb.2008.0205
Blaskovic A, Fastelli P, Cizmek H, Gerranti C, Renzi M (2017) Plastic litter in sediments from the Croatian marine protected area of the natural park of Telascica bay (Adriatic Sea). Mar Pollut Bull 114:583–586. https://doi.org/10.1016/j.marpolbul.2016.09.018
Browne MA, Crump P, Niven SJ, Teuten EL, Tonkin A, Galloway T, Thompson R (2011) Accumulation of microplastic on shorelines worldwide: sources and sinks. Environ Sci Technol 45:9175–9179. https://doi.org/10.1021/es201811s
Cannas S, Fastelli P, Guerranti C, Renzi M (2017) Plastic litter in sediments from the coasts of south Tuscany (Tyrrhenian Sea). Mar Pollut Bull 119:372–375. https://doi.org/10.1016/j.marpolbul.2017.04.008
Carpenter EJ, Smith KL (1972) Plastics on Sargasso Sea surface. Science 175:1240–1241. https://doi.org/10.1126/science.175.4027.1240
Chae DH, Kim IS, Kim SK, Song YK, Shim WJ (2015) Abundance and distribution characteristics of microplastics in surface seawaters of the Incheon/Kyeonggi coastal region. Arch Environ Contam Toxicol 69:269–278. https://doi.org/10.1007/s00244-015-0173-4
Claessens M, de Meester S, Van Landuyt L, De Clerck KD, Janssen CR (2011) Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar Pollut Bull 62:2199–2204. https://doi.org/10.1016/j.marpolbul.2011.06.030
Cluzard M, Kazmiruk T, Kazmiruk V, Bendell LI (2015) Intertidal concentrations of microplastics and their influence on ammonium cycling as related to the shellfish industry. Arch Environ Contam Toxicol 69:310–319. https://doi.org/10.1007/s00244-015-0156-5
Cole M, Lindeque P, Halsband C, Galloway TS (2011) Microplastics as contaminants in the marine environment: a review. Mar Pollut Bull 62:2588–2597. https://doi.org/10.1016/j.marpolbul.2011.09.025
Cole M, Webb H, Lindeque PK, Fileman ES, Halsband C, Galloway TS (2014) Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci Rep 4:4528. https://doi.org/10.1038/srep04528
Cózar A, Echevarría F, González-Gordillo JI, Irigoien X, Úbeda B, Hernández-León S, Palma AT, Navarro S, García-de-Lomas J, Ruiz A, Fernández-de-Puelles ML, Duarte CM (2014) Plastic debris in the open ocean. Proc Natl Acad Sci U S A 111:10239–10244. https://doi.org/10.1073/pnas.1314705111
Cózar A, Sanz-Martín M, Martí E, González-Gordillo JI, Úbeda B, Gálvez JA, Irigoien X, Duarte CM (2015) Plastic accumulation in the Mediterranean Sea. PLoS One 10:e0121762. https://doi.org/10.1371/journal.pone.0121762
Derraik JGB (2002) The pollution of the marine environment by plastic debris: a review. Mar Pollut Bull 44:842–852. https://doi.org/10.1016/S0025-326X(02)00220-5
Deudero S, Alomar C (2015) Mediterranean marine biodiversity under threat: reviewing influence of marine litter on species. Mar Pollut Bull 98:58–68. https://doi.org/10.1016/j.marpolbul.2015.07.012
Enders K, Lenz R, Stedmon CA, Nielsen TG (2015) Abundance, size and polymer composition of marine microplastics ≥10 mm in the Atlantic Ocean and their modeled vertical distribution. Mar Pollut Bull 100:70–81. https://doi.org/10.1016/j.marpolbul.2015.09.027
Fastelli P, Blaskovic A, Bernardi G, Romeo T, Cizmek H, Andaloro F, Russo GF, Guerranti C, Renzi M (2016) Plastic litter in sediments from a marine area likely to become protected (Aeolian Archipelago’s islands, Tyrrhenian Sea). Mar Pollut Bull 113:526–529. https://doi.org/10.1016/j.marpolbul.2016.08.054
Filella M (2015) Questions of size and numbers in environmental research on microplastics: methodological and conceptual aspects. Environ Chem 12:527–538. https://doi.org/10.1071/EN15012
Free CM, Jensen OP, Mason SA, Eriksen M, Williamson NJ, Boldgiv B (2014) High-levels of microplastic pollution in a large, remote, mountain lake. Mar Pollut Bull 85:156–163. https://doi.org/10.1016/j.marpolbul.2014.06.001
Frère L, Paul-Pont I, Rinnert E, Petton S, Jaffré J, Bihannic I, Soudant P, Lambert C, Huvet A (2017) Influence of environmental and anthropogenic factors on the composition, concentration and spatial distribution of microplastics: a case study of the Bay of Brest (Brittany, France). Environ Pollut 225:211–222. https://doi.org/10.1016/j.envpol.2017.03.023
Frias JPGL, Gago J, Otero V, Sobral P (2016) Microplastics in coastal sediments from Southern Portuguese shelf waters. Mar Environ Res 114:24–30. https://doi.org/10.1016/j.marenvres.2015.12.006
Gago J, Carretero O, Filgueiras AV, Viñas L (2018) Synthetic microfibers in the marine environment: a review on their occurrence in seawater and sediments. Mar Pollut Bull 127:365–376. https://doi.org/10.1016/j.marpolbul.2017.11.070
Galgani F, Fleet D, Franeker J, Van Katsanevakis S, Maes T, Oosterbaan L, Poitou I, Hanke G, Thompson R, Amato E, Janssen C (2010) Marine Strategy Framework Directive: Task Group 10 Report Marine Litter. https://doi.org/10.2788/86941
Galgani F, Hanke G, Werner S, De Vrees L (2013) Marine litter within the European Marine Strategy Framework Directive. ICES J Mar Sci 70:1055–1064. https://doi.org/10.1093/icesjms/fst122
Graca B, Szewc K, Zakrzewska D, Dolega A (2017) Sources and fate of microplastics in marine and beach sediments of the Southern Baltic Sea—a preliminary study. Environ Sci Pollut Res 24:7650–7661. https://doi.org/10.1007/s11356-017-8419-5
Guerranti C, Cannas S, Scopetani C, Fastelli P, Cincinelli A, Renzi M (2017) Plastic litter in aquatic environments of Maremma Regional Park (Tyrrhenian Sea, Italy): contribution by the Ombrone river and levels in marine sediments. Mar Pollut Bull 117:366–370. https://doi.org/10.1016/j.marpolbul.2017.02.021
Gündoğdu S, Çevik C, Ayat B, Aydoğan B, Karaca S (2018) How microplastics quantities increase with flood events? An example from Mersin Bay NE Levantine coast of Turkey. Environ Pollut 239:342–350. https://doi.org/10.1016/j.envpol.2018.04.042
Güven O, Gökdag K, Jovanovic B, Kideys AE (2017) Microplastic litter composition of the Turkish territorial waters of the Mediterranean Sea, and its occurrence in the gastrointestinal tract of fish. Environ Pollut 223:286–294. https://doi.org/10.1016/j.envpol.2017.01.025
Hidalgo-Ruz V, Gutow L, Thompson RC, Thiel M (2012) Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ Sci Technol 46:3060–3075. https://doi.org/10.1021/es2031505
Huppertsberg S, Knepper TP (2018) Instrumental analysis of microplastics—benefits and challenges. Anal Bioanal Chem 410:6343–6352. https://doi.org/10.1007/s00216-018-1210-8
Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL (2015) Plastic waste inputs from land into the ocean. Science 347:768–771. https://doi.org/10.1126/science.1260352
Katsanevakis S, Verriopoulos G, Nicolaidou A, Thessalou-Legaki M (2007) Effect of marine litter on the benthic megafauna of coastal soft bottoms: a manipulative experiment. Mar Pollut Bull 54:771–778. https://doi.org/10.1016/j.marpolbul.2006.12.016
Lenz R, Enders K, Stedmon CA, Mackenzie DMA, Nielsen TG (2015) A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement. Mar Pollut Bull 100:82–91. https://doi.org/10.1016/j.marpolbul.2015.09.026
Liebezeit G, Dubaish F (2012) Microplastics in beaches of the East Frisian Islands Spiekeroog and Kachelotplate. Bull Environ Contam Toxicol 89:213–217. https://doi.org/10.1007/S00128-012-0642-7
Ling SD, Sinclair M, Levi CJ, Reeves SE, Edgar GJ (2017) Ubiquity of microplastics in coastal seafloor sediments. Mar Pollut Bull 121:104–110. https://doi.org/10.1016/j.marpolbul.2017.05.038
Löder MGJ, Kuczera M, Mintenig S, Lorenz C, Gerdts G (2015) Focal plane array detector-based micro-Fourier-transform infrared imaging for the analysis of microplastics in environmental samples. Environ Chem 12:563–581. https://doi.org/10.1071/EN14205
Long M, Moriceau B, Gallinari M, Lambert C, Huvet A, Raffray J, Soudant P (2015) Interactions between microplastics and phytoplankton aggregates: impact on their respective fates. Mar Chem 175:39–46. https://doi.org/10.1016/j.marchem.2015.04.003
Lusher AL, Burke A, O’Connor I, Officer R (2014) Microplastic pollution in the Northeast Atlantic Ocean: validated and opportunistic sampling. Mar Pollut Bull 88:325–333. https://doi.org/10.1016/j.marpolbul.2014.08.023
Lusher AL, Welden NA, Sobral P, Cole M (2017) Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Anal Methods 9:1346–1360. https://doi.org/10.1039/C6AY02415G
Mai L, Bao LJ, Shi L, Wong CS, Zeng EY (2018) A review of methods for measuring microplastics in aquatic environments. Environ Sci Pollut Res 25:11319–11332. https://doi.org/10.1007/s11356-018-1692-0
Martellini T, Guerranti C, Scopetani C, Ugolini A, Chelazzi D, Cincinelli A (2018) A snapshot of microplastics in the coastal areas of the Mediterranean Sea. Trends Anal Chem 109:173–179. https://doi.org/10.1016/j.trac.2018.09.028
Martin J, Lusher A, Thompson RC, Morley A (2017) The deposition and accumulation of microplastics in marine sediments and bottom water from the Irish continental shelf. Sci Rep 7:10772. https://doi.org/10.1038/s41598-017-11079-2
Mistri M, Infantini V, Scoponi M, Granata T, Moruzzi L, Massara F, De Donati M, Munari C (2017) Small plastic debris in sediments from the Central Adriatic Sea: types, occurrence and distribution. Mar Pollut Bull 124:435–440. https://doi.org/10.1016/j.marpolbul.2017.07.063
Naji A, Esmaili Z, Mason SA, Vethaak AD (2017) The occurrence of microplastic contamination in littoral sediments of the Persian Gulf, Iran. Environ Sci Pollut Res 24:20459–20468. https://doi.org/10.1007/s11356-017-9587-z
Napper IE, Thompson RC (2016) Release of synthetic microplastic plastic fibres from domestic washing machines: effects of fabric type and washing conditions. Mar Pollut Bull 112:39–45. https://doi.org/10.1016/j.marpolbul.2016.09.025
Nor NHM, Obbard JP (2014) Microplastics in Singapore’s coastal mangrove ecosystems. Mar Pollut Bull 79:278–283. https://doi.org/10.1016/j.marpobul.2013.11.025
Norén, F (2007) Small plastic particles in coastal Swedish waters. KIMO Sweden Report, 1–11
Nuelle MT, Dekiff JH, Remy D, Fries E (2014) A new analytical approach for monitoring microplastics in marine sediments. Environ Pollut 184:161–169. https://doi.org/10.1016/J.ENVPOL.2013.07.027
Peng G, Zhu B, Yang D, Su L, Shi H, Li D (2017) Microplastics in sediments of the Changjiang Estuary, China. Environ Pollut 225:283–290. https://doi.org/10.1016/j.envpol.2016.12.064
Renner G, Schmidt TC, Schram J (2018) Analytical methodologies for monitoring micro(nano)plastics: which are fit for purpose? Curr Opin Env Sci Health 1:55–61. https://doi.org/10.1016/j.coesh.2017.11.001
Rocha-Santos T, Duarte AC (2015) A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. TrAC-Trend anal Chem 65:47–53. https://doi.org/10.1016/j.trac.2014.10.011
Ruiz-Orejón LF, Sardá R, Ramis-Pujol J (2016) Floating debris in the Central and Western Mediterranean Sea. Environ Res 120:136–144. https://doi.org/10.1016/j.marenvres.2016.08.001
Ryan PG (2015) Does size and buoyancy affect the long-distance transport of floating debris. Environ Res Lett 10:084019. https://doi.org/10.1088/1748-9326/10/8/084019
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John AWG, McGonigle D, Russell AE (2004) Lost at sea: where is all the plastic? Science 304:838. https://doi.org/10.1126/SCIENCE.1094559
UNEP/MAP/MEDPOL (2005) Sampling and analysis techniques for the eutrophication monitoring strategy of MED POL. MAP Technical Reports Series No. 163. UNEP/MAP, Athens (English)
Veerasingam S, Mugilarasan M, Venkatachalapathy R, Vethamony P (2016) Influence of 2015 flood on the distribution and occurrence of microplastic pellets along the Chennai coast, India. Mar Pollut Bull 109:196–204. https://doi.org/10.1016/j.marpolbul.2016.05.082
Vianello A, Boldrin A, Guerriero P, Moschino V, Rella R, Sturaro A, Da Ros L (2013) Microplastic particles in sediments of Lagoon of Venice, Italy: first observations on occurrence, spatial patterns and identification. Estuar Coast Shelf Sci 130:54–61. https://doi.org/10.1016/j.ecss.2013.03.02
Wang W, Wang J (2018) Investigation of microplastics in aquatic environments: an overview of the methods used, from field sampling to laboratory analysis. Trends Anal Chem 108:195–202. https://doi.org/10.1016/j.trac.2018.08.026
Woodall LC, Sanchez-Vidal A, Canals M, Paterson GLJ, Coppock R, Sleight V, Calafat A, Rogers AD, Narayanaswamy BE, Thompson RC (2014) The deep sea is a major sink for microplastic debris. R Soc Open Sci 1:140317. https://doi.org/10.1098/rsos.140317
Wright SL, Rowe D, Thompson RC, Galloway TS (2013) Microplastic ingestion decreases energy reserves in marine worms. Curr Biol 23:R1031–R103333. https://doi.org/10.1016/j.cub.2013.10.068
Zalasiewicz J, Waters CN, Ivar do Sul J, Corcoran PL, Barnosky AD, Cearreta A, Edgeworth M, Gałuszka A, Jeandel C, Leinfelder R, McNeill JR, Steffen W, Summerhayes C, Wagreich M, Williams M, Wolfe AP, Yonan Y (2016) The geological cycle of plastics and their use as a stratigraphic indicator of the Anthropocene. Anthropocene 13:4–17. https://doi.org/10.1016/j.ancene.2016.01.002
Zhao S, Zhu L, Wang T, Li D (2014) Suspended microplastics in the surface water of the Yangtze Estuary System, China: first observations on occurrence, distribution. Mar Pollut Bull 86:562–568. https://doi.org/10.1016/j.marpolbul.2014.06.032
Acknowledgments
This research has been supported by the Spanish Inter-Ministerial Science and Technology Commission through the “IMPACTA” project (CICYT, CTM2013-48194-C3-1-R), “BASEMAN” project (PCIN-2015170-CO2-02), “2-2 ESMARME” project and by the European Union through the European Regional Development Fund (ERDF).
The authors would like to thank the crew and scientists of F.P. Navarro (Spanish Institute of Oceanography) for providing assistance with sampling. Their hard work and contribution were of great worth to this research. We are also grateful to the participants of COMU for their assistance in sample collection.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Filgueiras, A.V., Gago, J., Campillo, J.A. et al. Microplastic distribution in surface sediments along the Spanish Mediterranean continental shelf. Environ Sci Pollut Res 26, 21264–21273 (2019). https://doi.org/10.1007/s11356-019-05341-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05341-5