Abstract
In the present work, silver nanoparticles (AgNPs) synthetized with Cryptocarya alba (Peumo) leaf extract were studied. The fabrication method was fast, low cost, and eco-friendly, and the final properties of AgNPs were determined by experimental parameters, such as AgNO3 and Peumo extract concentrations used. Setting suitable experimental conditions, crystalline AgNPs with apparent spherical forms and average diameter around 3.5 nm were obtained. In addition, the capability of synthesized Peumo-AgNPs to remove methylene blue dye (MB) in aqueous solution as well as their catalytic effectiveness was also investigated. The results showed that green synthesized AgNPs can remove fast and effectively the MB dye from aqueous medium by itself, but better results were found acting like catalyst by using sodium borohydride (NaBH4) in the reaction. In addition, this green nanomaterial can be recycling several times maintaining initial properties for removal of MB. Thus, AgNPs synthetized with Peumo leaf extracts could be an excellent catalyst candidate for degradation of blue methylene dye in chemical industries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The last advances in material science and technology have converted nanotechnology as one of the most promising emerging technologies of the twenty-first century, improving industrial products and processes (Stark et al. 2015). Within this broad area of research, metal nanoparticles are one of the most studied nanostructures due to their excellent properties such as large surface area and high surface reactivity, which are useful in a wide variety of application fields (Hoseinzadeh et al. 2017; Schröfel et al. 2014; Qazi and Javaid 2016; Zhang et al. 2016). Noble metal nanoparticles such as Ag, Au, and Pt have showed interesting properties as localized surface plasmon resonance, high antimicrobial activity, and catalytic nature. Thus, these nanoparticles have been received a lot attention for their applications in a wide range of fields, including electronic, photonic, telecommunications, energy, biology, and biomedicine (Alex and Tiwari 2015; Daraee et al. 2016; Haider and Kang 2015; Nasrabadi et al. 2016).
Different approaches have been applied for the fabrication of noble metal nanoparticles such as chemical, physical, electrochemical, biological, and greener methods (Qazi and Javaid 2016). However, there is a growing interest to develop eco-friendly techniques to avoid the use of toxic and hazardous chemicals as well as toxic and hazardous waste. In this sense, the known green synthesis method to obtain nanoparticles is one of the most studied processes in the last decade, because it offers several advantages in comparison with other methods as it is a low-cost, eco-friendly, and single-step process; does not require any additional energy such as temperature or pressure; and easily scales up for industrial production (Abdelghany et al. 2018; Rafique et al. 2017). Different organisms and derived biocompounds from them have been explored in the synthesis of silver nanoparticles (AgNPs) including bacteria, algae, sugar, and plant extracts. However, plant-mediated synthesis of AgNPs by using extracts is the most popular methods, and numerous authors have explored with different part of plants such as roots, leaves, and fruits (Carmona et al. 2017a; Saravanakumar et al. 2017; Recio-Sánchez et al. 2016). Although the mechanism to form AgNPs by plant extracts remains unclear, many studies suggest that the biomolecules of the plant extract such as polysaccharides, polyphenols, and aldehydes can reduce silver ions to metallic silver state; and other bioproducts including proteins, enzymes, and amino acids can also stabilize them without the need of additional chemical. As a result, the biosynthesized AgNPs have shown high antimicrobial activity and also very efficiency catalytic activity to reduce different organic dyes in water (Francis et al. 2017; Khodadadi et al. 2017; Saha et al. 2017; Vidhu and Philip 2014).
The organic Azo dyes are widely fabricated and used to stain various materials in the textile, leather, paper, paint, and plastic industries (Singh et al. 2019). Thus, Azo dyes are a relevant source of water pollution, because they have been discharged continuously from these industries through their effluents. Environmental impacts related with organic dyes can be related with the bioaccumulation, toxicity, and the capacity to interfere with photosynthesis activity of autotroph organisms by blocking sunlight in the water column (Mani et al. 2019). Among others, methylene blue (MB) dye can be considered a ubiquitous pollutant in freshwaters because it has been commonly used in the fabrication of paints, fibers staining, chemical analysis, and biomedical applications (Karthik et al. 2016). In addition, several negative effects have been described for MB, including Serotonin syndrome (Zuschlag and Warren 2018), toxicity in neonates (Albert et al. 2003), and genotoxicity (Costa et al. 2016). In this sense, the presence of MB in untreated waters suggests not only environmental injuries but also potential health risks.
Although coagulation and/or filtration methods are some of approaches used for dye removal, these techniques are not sufficient to the complete elimination of colorants from residual waters, because they show aromatic structure that prevents their degradation (Khodadadi et al. 2017; Saha et al. 2017). Thus, the removal of persistent dyes from waters by using new and advance materials must be studied.
Cryptocarya alba, also known as Peumo, is a medicinal tree with wide geographical distribution in Argentina, Chile, and Peru. Peumo extracts have been widely used for different medicinal treatments, and their antioxidant capabilities have been also studied (Carmona et al. 2017b). These properties make it a potential candidate as an efficient bioresource for the synthesis of AgNPs with catalytic activity for removal organic colorants. In the present work, AgNPs were biosynthesized by using Peumo extract as reducing and stabilizing agent. In addition, the efficiency of Peumo extract to remove organic dye methylene blue (MB) and the catalytic effect of biosynthesized AgNPs obtained from green synthesis with Peumo extracts have been also analyzed.
Materials and methods
C. alba (Peumo) extracts
C. alba leaves were obtained from the botanical garden of Universidad Católica de Temuco, Temuco city, Chile (38° 42´ S, 72° 42´ W). To prepare Peumo aqueous extracts, 10 g of dried leaves were mixed during 4 h with 200 ml of distilled water in a Soxhlet extractor.
Green synthesis of Peumo-AgNPs
Green synthesis of AgNPs was performed by stirring 1% volume of C. alba extracts with different concentrations of aqueous solution of AgNO3 (Merck Company, Darmstadt, Germany), which were changed from 0.1 to 10 mM.
UV-vis spectroscopy
Measurements of UV-vis absorption spectra were carried out with a Shimadzu UV-mini 1240 model spectrophotometer operating with a 1-nm interval and 1-s of integration time, for a total range of wavelength from 300 to 800 nm.
Transmission electron microscopy
Morphology of biosynthesized AgNPs was analyzed by transmission electron microscopy (TEM) using a JEOLJEM-2011 instrument.
Hydrodynamic size and Z potential
The hydrodynamic size and Z potential were analyzed by dynamic light scattering (DLS) and laser Doppler velocimetry (LDV), respectively. The measurements were carried out with a Malvern Zetasizer Nano-ZS zen 3600 instrument.
X-ray diffraction
X-ray pattern profile was performed by a Rigaku X-Ray diffractometer Smartlab model, with goniometer Theta-Theta Bragg-Brentano geometry and solid-state detector D/teX Ultra 250 model (Rigaku Corporation, Japan). The instrumental alignment was checked against the NIST SMR 660c LaB6 powder standard and its optic configuration was employed Ni-filtered Cu radiation (30 kV and 40 mA), 0.5° divergence slit, 0.25° antiscatter slit, and both sides with 5° Soller slits. In preference, pattern was collected in the 30–90° range, counting 0.5°/sec per step of 0.01°. PDXL 2 v.2.7.3.0 software and ICDD 2018 PDF-4 reference database were used for search match phases.
X-ray photoelectron spectroscopy
XPS specta of biosynthetized AgNPs were obtained with a hemispherical analyzer (Physical Electronics 1257 system). The power of the twin anode (Mg and Al) with X-ray source was fixed at 200 W and Al Kα radiation (1486.6 eV) was used. The emission angle of sample stage was selected to 45°.
Removal of MB dye
On the one hand, to study the degradation rate of MB with Peumo, 0.5 mg of Peumo dry extracts were added to 10 ml of aqueous solution of MB with 3.1 × 10−5 M of concentration. On the other hand, to evaluate the potential application of AgNPs as catalyst for removal MB in water, 10 ml of aqueous solution of MB with 3.1 × 10−5 M of concentration were added to 10 ml of aqueous solution of NaBH4 with 3.0 × 10−3 M of concentration. Then, 3 mg of AgNPs biosynthesized with different conditions were added to the mixture. The resulting mixture was stirred at room temperature until the solution was colorless. The pH of the reaction mixture of MB and AgNPs showed pH values ranging from 6.7 to 6.2, but when NaBH4 was added also into the same mixture the pH increased until 8.1. The progress of the reaction was monitored by UV-vis spectroscopy. When the reaction was completed, the catalyst was washed with distilled water, sonicated, recovered by centrifugation, and finally air-dried. Then, the process was repeated to analyze the recyclability of the catalyst.
Results and discussion
AgNPs were synthetized by mixing aqueous solution of AgNO3 with Peumo extract. Biocompounds of the plant extract reduce Ag+ ions to Ag0 and induce the formation of AgNPs. Localized surface plasmon resonance of AgNPs induces a color change in the solutions and allows to monitor the formation of AgNPs from UV-vis absorption spectra. Figure 1 shows the UV-vis absorption spectra measured at different time-points with fixed experimental conditions of 1% v/v of Peumo extract and 1 mM concentration of AgNO3. Peumo extract spectrum showed absorption band in the UV region (300–370 nm) related to the absorbance of all components from the aqueous extract. After 30 min of stirring, a clear additional absorption band with center at 455 nm can be observed associated with the localized surface plasmon resonance property of AgNPs, which confirms the successfully biosynthesis of AgNPs (Mann et al. 2017). For high reaction time-points, the intensity of this band increased, suggesting an increase in the amount of AgNPs synthetized. The process was completed before 24 h, when the absorbance spectrum remains unaffected.
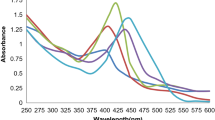
Although other plant extracts can reduce Ag+ in less time, this reaction time was quite efficient in comparison with other works (Carmona et al. 2017a, b; Khalil et al. 2014). Figure 2 shows UV-vis absorption spectra of biosynthesized AgNPs using different concentration of AgNO3, fixing Peumo extract concentration to 1% v/v, and stirring during 2 h. Typical localized surface plasmon resonance absorption band between 350 and 550 nm of wavelength was observed for all the concentrations studied. The intensity of the absorption bands increased with the concentration of AgNO3 indicating that high concentrations of AgNO3 allowed the synthesis of great amounts of green AgNPs.
To obtain information about appearance and diameter of biosynthesized AgNPs, TEM images were recorded. Figure. 3 a and b show representative TEM images and particle diameter distribution of AgNPs formed with 1 mM and 2 mM of AgNO3 concentration, respectively. The results obtained with 1 mM of AgNO3 indicated that most of AgNPs showed apparent spherical shapes and some levels of agglomeration, with an average diameter around 3.2 nm. Similar shapes were obtained with 2 mM of AgNO3, but high mean diameters of AgNPs (~ 26 nm) were found under these experimental conditions. The average diameters of AgNPs obtained for the rest of AgNO3 concentrations studied in this work are summarized in Table 1. For lesser concentrations of AgNO3, AgNPs biosynthesized reached smaller sizes than high concentrations. For 0.1 mM of AgNO3, more than the 80% of AgNPs were a size lower than 10 nm. However, the average size was 16.1 nm because a high agglomeration of particles was observed. When the concentration of AgNO3 was increased in the fabrication process, the particle size also increases, and for 2 mM of AgNO3, green AgNPs with an average diameter of 26 nm were obtained. However, for upper concentrations (5 mM and 10 mM) the average diameter decreased to 16.9 and 15.7 nm, respectively.
Hydrodynamic size and stability of AgNPs were measured by DLS and LDV techniques, respectively (see Table 1). In all the cases, the average diameters observed were larger than TEM results, since metallic nanoparticles tend to agglomerate easily in aqueous media (Recio-Sánchez et al. 2016). It can be observed that as the concentration of AgNO3 was increased, hydrodynamic diameter of AgNPs was also raised, except for 10 mM. Likewise, zeta potential measurements for all studied AgNPs showed negative charge. The negative charge can be explained from the nucleation process of the green synthesis and from the induced charge of residual OH– groups from Peumo extract molecules linked on the surface of AgNPs (Chung et al. 2016). Thus, the results suggest an incipient stability, which was high for 1 mM of AgNO3.
The crystallinity nature of AgNPs was studied from X-ray diffraction (XRD) measurements. Figure 4 shows the XRD pattern of AgNPs synthesized with 1 mM concentration of AgNO3. In the diffractogram, five different distinctive peaks of metallic silver (according to ICDD PDF card 03-065-2871) can be observed at 38.28°, 44.40°, 64.57°, 77.48°, and 82.32°, which correspond to (111), (200), (220), (311), and (222) lattice plane of the cubic silver (space group Fm-3 m). According to the pattern profile analysis, the peaks showed interesting broadening attributed to nanostructured powder. Thus, the XRD pattern showed that biosynthesized AgNPs with Peumo aqueous extract were crystalline. Similar results were found for AgNPs obtained with other concentrations of AgNO3.
In order to investigate the chemical surface of AgNPs, XPS measurements were also carried out. Figure 5 a shows survey spectra for AgNPs synthesized at different concentration of AgNO3. Only C, O, Ag, and Au (Au signal comes from the substrate) elements were detected on the survey spectra, while N signal was not detected, supporting the formation of AgNPs. Figure 5 b shows the Ag 3d band measured for all the samples. This band was fitted with a doublet placed at 368.3 eV in the case of 0.1 mM, 1 mM, 2 mM, and 5 mM of AgNO3 samples. This doublet, which is related to the binding energy of metallic Ag (Kaushik 1991), had an area ratio of 2/3 and a spin-orbit separation (sos) of 6 eV. In the case of the 10 mM sample, an extra doublet in the high energy side was needed. This doublet was observed at 370.9 eV with a sos of 6 eV. This new contribution was related to Ag–O bonds. Gozdziewska et al. (2015) suggested that this bond can be formed between unpaired electrons of nitroxides and conduction electrons from the silver surface.
The catalytic activity of AgNPs in the reduction of different dyes, such as 4-nitrofenol (4-NP), MB, and Rodamine-B (RhB), has been widely studied, and biosynthesized AgNPs have been demonstrated as very efficient catalytic element, because of its easy, low cost, and eco-friendly fabrication process (Khodadadi et al. 2017; Saha et al. 2017; Vidhu and Philip 2014). The catalytic ability of these nanoparticles had been usually investigated by using the reducing chemical NaBH4 as a model reaction. In the case of MB, it is a dye widely used in biology and chemistry, which can be accumulated and it could be hazardous for the environment. MB shows a blue color in oxidized state, but it can be reduced to the colorless compound called leucomethylene blue reduction action of NaBH4. This chemical reduction process can be monitored and characterized by UV-vis absorption spectra, in which a reduction of the absorbance peak of MB at 664 nm of wavelength shows this removal (Khodadadi et al. 2017).
In the present study, the activity of Peumo extract to reduce MB in water was investigated initially. The high antioxidant activity of this extract was able to reduce MB as Fig. 6 a indicates. UV-vis spectrum was recorded as a function of time when 0.5 mg of dry Peumo extract was added to 10 ml of aqueous solution of MB with 3.1 × 10−5 M. It can be observed that MB showed a maxima absorption band centered around 664 nm of wavelength, which is its typical absorption in aqueous solution. The degradation of MB can be easily followed by the reduction of the absorption maxima band at 664 nm.
After 30 min, Peumo extract was able to reduce almost the half of the MB. However, when AgNPs biosynthesized with a concentration of 1 mM of AgNO3 were assessed in similar experimental conditions by using 0.5 mg, the reduction rate of MB was clearly higher than Peumo extract alone (Fig. 6b), and after 30 min the removal was almost completed. When AgNPs concentration was increased, the rate of reduction also increases, and with 3 mg of AgNPs was able to reduced MB in just 1 min, as it can be observed in Fig. 6c. Finally, Fig. 6 d shows the monitoring reduction of MB with 3 mg of green Peumo-AgNPs with 2 mM of AgNO3, and they need almost 10 min to complete the reaction. Thus, AgNPs synthesized with 1 mM concentration of AgNO3 showed the most effectiveness removal activity than the other concentrations studied here. The above can be attributed that AgNPs formed with 1 mM of AgNPs showed better properties related with the physical-chemical behavior in water medium such as hydrodynamic size, stability, and charge. In addition, AgNPs caped with –OH groups from phenol compounds of Peumo extracts can participate also as reducing agents facilitating the removal of MB without the use of other chemical with similar function as NaBH4 (Carmona et al. 2017a, b).
In this work, the catalytic reaction of MB degradation was performed with some variations of pH ranging from 6.2 to 8.1 values in the solution mixture. In the sense, previous studies indicate that neutral and basic pH favor the removal conditions of MB, since under these pH conditions, the MB is a cationic molecule that allow the formation of electrostatic interaction between their positively charged ions and the negatively charged AgNPs (Qian et al. 2018). Thus, high pH values in the catalytic reaction solution improve the capacity of AgNPs to remove MB.
In order to demonstrate and compare with other studies the advantages of Peumo-AgNPs for the reduction of MB dye, comparable experiment conditions were carried out by using the reducing chemical NaBH4 as a model reaction. Figure 7 a summarized absorption spectra as function of time reduction of MB by adding NaBH4, when 10 ml of aqueous solution of MB with 3 × 1·10−5 M concentration was mixed with 10 ml of NaBH4 at 3 × 10−3 M. As can be observed, the reduction was very slow and after 30 min just a small part of MB was reduced. When 3 mg of AgNPs biosynthesized with 1 mM concentration of AgNO3 were added to the mixture, MB was reduced in less than a minute (Fig. 7b). Similar results were obtained with AgNPs fabricated with 2 mM concentration of AgNO3. To show the efficiency of these green catalysts, a comparison between these findings and reported data of reduction time for 100% MB dye reduction with other systems is shown in Table 2. These findings demonstrated that Peumo-AgNPs showed an extremely efficient catalytic activity to reduce MB dye in water.
Additionally, efficient catalysts with heterogeneous properties are especially important for commercial and industrial applications, where recoverability and reusability must be their main advantages. In this sense, the recyclability of green Peumo-AgNPs was also studied here and the results indicated that green AgNPs can be easily recovered from the reaction solution by centrifugation. Then, the recovered catalyst was evaluated again in the reduction of MB with the same initial experimental conditions. As it can be observed in Table 3, the high stability of the catalysts allowed unaltered catalytic activity of MB removal almost constant up to 5 cycles for AgNPs fabricated with 1 mM concentration of AgNO3. These results clearly showed that biosynthesized AgNPs with Peumo extract are a promising catalyst for reduction organic dyes as MB in water.
Conclusions
Silver nanoparticles with apparent spherical shape and crystalline nature were successfully biosynthesized by using C. alba leaf extract. The process involves a fast, easy, low cost, and eco-friendly alternative method for the synthesis of silver nanoparticles. In addition, by simply changing the concentration of AgNO3 and plant extract in aqueous solution, the diameter and stability of biosynthesized nanoparticles can be modified and controlled from 3.5 to 25 nm.
In this study, also the capacity to reduce MB dye by C. alba extracts was explored, and it was shown than the high antioxidant activity of this extract was able to reduce MB in aqueous solution. In addition, green synthetized AgNPs with C. alba have shown a suitable efficiency catalytic activity to reduce MB in aqueous solution without the use of other reducing chemicals. However, with the use of the inorganic NaBH4 as reducing agent, MB dye was completely degraded in less than 1 min, when only 3 mg of biosynthesized AgNPs was added. In addition, the catalyst can be easily recovered and reused for several cycles. These results showed the usefulness of this kind of green synthetized AgNPs in dye degradation, promoting their application for industrial effluents containing organic colorants.
References
Abdelghany TM, Al-Rajhi AM, Al Abboud MA, Alawlaqi MM, Magdah AG, Helmy EA, Mabrouk AS (2018) Recent advances in green synthesis of silver nanoparticles and their applications: about future directions. A review. BioNanoScience 8:5–16
Albert M, Lessin MS, Gilchrist BF (2003) Methylene blue: dangerous dye for neonates. J Pediatr Surg 38:1244–1245
Alex S, Tiwari A (2015) Functionalized gold nanoparticles: synthesis, properties and applications—a review. J Nanosci Nanotechnol 15:1869–1894
Atarod M, Nasrollahzadeh M, Sajadi SM (2016) Euphorbia heterophylla leaf extract mediated green synthesis of Ag/TiO2 nanocomposite and investigation of its excellent catalytic activity for reduction of variety of dyes in water. J Colloid Interface Sci 462:272–279
Carmona ER, Benito N, Plaza T, Recio-Sánchez G (2017a) Green synthesis of silver nanoparticles by using leaf extracts from the endemic Buddleja globosa hope. Green Chem Lett Rev 10:250–256
Carmona ER, Reyes-Díaz M, Parodi J, Inostroza-Blancheteau C (2017b) Antimutagenic evaluation of traditional medicinal plants from South America Peumus boldus and Cryptocarya alba using Drosophila melanogaster. J Toxicol Environ Health A 80:208–217
Chung IM, Park I, Seung-Hyun K, Thiruvengadam M, Rajakumar G (2016) Plant-mediated synthesis of silver nanoparticles: their characteristic properties and therapeutic applications. Nanoscale Res Lett 11:40
Costa SRD, Monteiro MDC, da Silva Júnior FMR, Sandrini JZ (2016) Methylene blue toxicity in zebrafish cell line is dependent on light exposure. Cell Biol Int 40:895–905
Daraee H, Eatemadi A, Abbasi E, Fekri Aval S, Kouhi M, Akbarzadeh A (2016) Application of gold nanoparticles in biomedical and drug delivery. Artif Cells Nanomed Biotechnol 44:410–422
Francis S, Joseph S, Koshy EP, Mathew B (2017) Green synthesis and characterization of gold and silver nanoparticles using Mussaenda glabrata leaf extract and their environmental applications to dye degradation. Environ Sci Pollut Res 24:17347–17357
Gan Z, Zhao A, Zhang M, Tao W, Guo H, Gao Q, Mao R, Liu E (2013) Controlled synthesis of Au-loaded Fe3O4@C composite microspheres with superior SERS detection and catalytic degradation abilities for organic dyes. Dalton Trans 42:8597–8605
Gozdziewska M, Cichowicz G, Markowska K, Zawada K, Megiel E (2015) Nitroxide-coated silver nanoparticles: synthesis, surface physicochemistry and antibacterial activity. RSC Adv 5:58403–58415
Haider A, Kang IK (2015) Preparation of silver nanoparticles and their industrial and biomedical applications: a comprehensive review. Adv Mater Sci Eng 2015:165257
Hoseinzadeh E, Makhdoumi P, Taha P, Hossini H, Stelling J, Amjad Kamal M (2017) A review on nano-antimicrobials: metal nanoparticles, methods and mechanisms. Curr Drug Metab 18:120–128
Karthik R, Muthezhilan R, Jaffar Hussain A, Ramalingam K, Rekha V (2016) Effective removal of methylene blue dye from water using three different low-cost adsorbents. Desalin Water Treat 57:10626–10631
Kaushik VK (1991) XPS core level spectra and Auger parameters for some silver compounds. J Electron Spectrosc Relat Phenom 56:273–277
Khalil MM, Ismail EH, El-Baghdady KZ, Mohamed D (2014) Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab J Chem 7:1131–1139
Khodadadi B, Bordbar M, Nasrollahzadeh M (2017) Achillea millefolium L. extract mediated green synthesis of waste peach kernel shell supported silver nanoparticles: application of the nanoparticles for catalytic reduction of a variety of dyes in water. J Colloid Interface Sci 493:85–93
Mani S, Chowdhary P, Bharagava RN (2019) Textile wastewater dyes: toxicity profile and treatment approaches. In: Emerging and eco-friendly approaches for waste management. Springer, Singapore, pp 219–244
Mann D, Nascimento-Duplat D, Keul H, Möller M, Verheijen M, Xu M, Paul Urbach H, Adam AJL, Buskens P (2017) The influence of particle size distribution and shell imperfections on the plasmon resonance of Au and Ag nanoshells. Plasmonics 12:929–945
Nasrabadi HT, Abbasi E, Davaran S, Kouhi M, Akbarzadeh A (2016) Bimetallic nanoparticles: preparation, properties, and biomedical applications. Artif Cells Nanomed Biotechnol 44:376–380
Qazi UY, Javaid R (2016) A review on metal nanostructures: preparation methods and their potential applications. ANP 5:27–43
Qian WC, Luo XP, Wang X, Guo M, Li B (2018) Removal of methylene blue from aqueous solution by modified bamboo hydrochar. Ecotoxicol Environ Saf 157:300–306
Rafique M, Sadaf I, Rafique MS, Tahir MB (2017) A review on green synthesis of silver nanoparticles and their applications. Artif Cells Nanomed Biotechnol 45:1272–1291
Recio-Sánchez GR, Castilla CL, Gómez NB, García A, Marcos R, Carmona ER (2016) Leaf extract from the endemic plant Peumus boldus as an effective bioproduct for the green synthesis of silver nanoparticles. Mater Lett 183:255–260
Saha J, Begum A, Mukherjee A, Kumar S (2017) A novel green synthesis of silver nanoparticles and their catalytic action in reduction of methylene blue dye. Sustain Environ Res 27:245–250
Saravanakumar A, Peng MM, Ganesh M, Jayaprakash J, Mohankumar M, Jang HT (2017) Low-cost and eco-friendly green synthesis of silver nanoparticles using Prunus japonica (Rosaceae) leaf extract and their antibacterial, antioxidant properties. Artif Cells Nanomed Biotechnol 45:1165–1171
Schröfel A, Kratošová G, Šafařík I, Šafaříková M, Raška I, Shor LM (2014) Applications of biosynthesized metallic nanoparticles–a review. Acta Biomater 10:4023–4042
Singh RP, Singh PK, Gupta R, Singh RL (2019) Treatment and recycling of wastewater from textile industry. In: Advances in biological treatment of industrial waste water and their recycling for a sustainable future. Springer, Singapore, pp 225–266
Sreekanth TVM, Jung MJ, Eom IY (2016) Green synthesis of silver nanoparticles, decorated on graphene oxide nanosheets and their catalytic activity. Appl Surf Sci 361:102–106
Stark WJ, Stoessel PR, Wohlleben W, Hafner A (2015) Industrial applications of nanoparticles. Chem Soc Rev 44:5793–5805
Veisi H, Azizi S, Mohammadi P (2018) Green synthesis of the silver nanoparticles mediated by Thymbra spicata extract and its application as a heterogeneous and recyclable nanocatalyst for catalytic reduction of a variety of dyes in water. J Clean Prod 170:1536–1543
Vidhu VK, Philip D (2014) Catalytic degradation of organic dyes using biosynthesized silver nanoparticles. Micron 56:54–62
Yang X, Zhong H, Zhu Y, Jiang H, Shen J, Huang J, Li C (2014) Highly efficient reusable catalyst based on silicon nanowire arrays decorated with copper nanoparticles. J Mater Chem A 2:9040–9047
Zhang Y, Zhu P, Chen L, Li G, Zhou F, Lu DD, Wong CP (2014) Hierarchical architectures of monodisperse porous Cu microspheres: synthesis, growth mechanism, high-efficiency and recyclable catalytic performance. J Mater Chem A 2:11966–11973
Zhang XF, Liu ZG, Shen W, Gurunathan S (2016) Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci 17:1534
Zuschlag ZD, Warren MW (2018) Serotonin toxicity and urinary analgesics: a case report and systematic literature review of methylene blue-induced serotonin syndrome. Psychosomatics 59:539–546
Acknowledgments
The authors of this work gratefully thank the technical support of Tanya Plaza and the FONDEQUIP 160152 Project for XRD measurements.
Funding
We also thank the UCT 2017RE-EC-03, UCT Bio-Nanomaterials Research Group N°201GI-CI-01, Fondecyt 11150322, and Conicyt PAI 79170079 projects for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Recio-Sánchez, G., Tighe-Neira, R., Alvarado, C. et al. Assessing the effectiveness of green synthetized silver nanoparticles with Cryptocarya alba extracts for remotion of the organic pollutant methylene blue dye. Environ Sci Pollut Res 26, 15115–15123 (2019). https://doi.org/10.1007/s11356-019-04934-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04934-4