Abstract
Cadmium and mercury are non-biodegradable toxic metals that may cause many detrimental effects to the thyroid gland and blood. Vitamin C has been found to be a significant chain-breaking antioxidant and enzyme co-factor against metal toxicity and thus make them less available for animals. The current study was performed to find the effect of individual metals (cadmium and mercury), their co-administration, and the ameliorative effects of vitamin C on some of the parameters that indicate oxidative stress and thyroid dysfunction. Cadmium chloride (1.5 mg/kg), mercuric chloride (1.2 mg/kg), and vitamin C (150 mg/kg of body weight) were orally administered to eight treatment groups of the rabbits (1. control; 2. Vit C; 3. CdCl2; 4. HgCl2; 5. Vit C + CdCl2; 6. Vit C + HgCl2; 7. CdCl2 + HgCl2, and 8. Vit C + CdCl2 + HgCl2). After the biometric measurements of all experimental rabbits, biochemical parameters viz. triidothyronine (T3), thyroxine (T4), thyroid-stimulating hormone (TSH), and triglycerides were measured using commercially available kits. The results exhibited significant decline (p < 0.05) in mean hemoglobin, corpuscular hemoglobin, packed cell volume, T3 (0.4 ± 0.0 ng/ml), and T4 (26.3 ± 1.6 ng/ml) concentration. While, TSH (0.23 ± 0.01 nmol/l) and triglyceride (4.42 ± 0.18 nmol/l) were significantly (p < 0.05) increased but chemo-treatment with Vit C reduces the effects of Cd, Hg, and their co-administration but not regained the values similar to those of controls. This indicates that Vit C had a shielding effect on the possible metal toxicity. The Cd and Hg also found to accumulate in vital organs when measured by atomic absorption spectrophotometer. The metal concentration trend was observed as follows: kidney > liver > heart > lungs. It was concluded that Cd and Hg are toxic and tended to bioaccumulate in different organs and their toxic action can be subdued by vitamin C in biological systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Toxic metals are non-biodegradable and their existence in environment is a major concern as they cause severe damage to the animal body even at low dosage. Various risk factors are linked with human health regarding toxic metal entry into food chain (Sarwar et al. 2017; Tay et al. 2009). Both anthropogenic and natural sources can cause toxic metal pollution. These metals seep in sloppy areas and are running water carry them downhill or runaway into sea. During extraction activities, water reservoirs are contaminated more strongly (Akinhanmi et al. 2016; Pacyna et al. 2016). The consumption of these toxic metal–contaminated plants or animals play detrimental role in humans (Sundseth et al. 2017; Truby 2003). Toxic metals can affect male and female reproductive tracts, prostate gland, breast development, cancer metabolism, cardiovascular endocrinology, neuroendocrinology, and thyroid and also can cause obesity (Maffini et al. 2006). On the other hand, various degrees of abnormalities in male genitalia, unilateral or bilateral cryptorchidism from mild to severe hypospadias, feminization of males, and poorly developed testes are common manifestations in domestic animals like dogs, goats, and horses (Lange et al. 2002; Meyer et al. 2006). Toxic metals enter the digestive tract of terrestrial animals through food, in general, and then accumulate in various organs after transmission via blood (Javed and Usmani 2012). The blood parameters like hemoglobin (Hb), mean corpuscular hemoglobin concentration (MCHC), and packed cell volume (PCV) are used to evaluate the health status of animals (Javed and Usmani 2015). However, blood parameters alone usually do not give a reliable evaluation of toxic impacts of metals’ exposure (Okediran et al. 2016). Therefore, the study of other parameters like metal accumulation in different organs may need to consider.
Mercury (Hg) is a non-essential element in the nutrition of animals and plants. It is studied that mercury persists in the environmental systems and many environmental organizations have marked it as a major pollutant (Ibraheem et al. 2016). Mercury is one of the major contaminants in an aquatic environment (Pereira et al. 2009). It tends to bioaccumulate in tissues and organs of animals (Das et al. 2008).
Cadmium is listed among the priority pollutants as well as a carcinogen of first category (IARC 2012). Cadmium causes cancerous effects on the lungs, testicles, and prostate, but there is insufficient data in the support of thyroid dysfunction due to cadmium poisoning. Cadmium seems to stimulate or activate various factors which lead to amplified cell proliferation and a decrease in normal apoptosis in thyroid cells (Buha et al. 2018). The liver, kidneys, and muscles are the most vital sites for cadmium accumulation. Biological half-life of cadmium is between 5 and 30 years because of its binding to molecules like MT and glutathione that are rich in sulfhydryl groups and thus excretes slowly. Cadmium exposure even at biologically low levels over time is related to surfeit toxic effects on the liver, kidneys, bones, testes, and the circulatory system (Matović et al. 2011; Matović et al. 2015; Mezynska and Brzóska 2018). Many researches indicate that cadmium has endocrine disrupting properties that suggest its probable role in diabetes mellitus (Edwards and Ackerman 2016) and oestrogenic activity (Pollack et al. 2011; Silva et al. 2012). Moreover, cadmium has been included in group 1 human carcinogen with adequate data for the lungs (Person et al. 2013; Hartwig 2013) and evidence for the prostate (Pal et al. 2017; Hartwig 2013), breast, and kidneys (Hartwig 2013). Some of the recent studies support the association of cadmium with other cancers like pancreatic (Buha et al. 2017), breast (Larsson et al. 2015), and urinary bladder cancer (Feki-Tounsi and Hamza-Chaffai 2014). Cadmium not only accumulates significantly in various organs but also has affinity to adversely affect the thyroid gland (Jancic and Stosic 2014). The presence of cysteine-rich proteins, metallothioneins (MT), enhances the Cd deposition in thyroid tissues as these MT bind with Cd and signify a potent intracellular cadmium detoxifier (Klaassen et al. 2009). People residing in contaminated areas have three times higher concentrations of metal(s) in their thyroid glands in comparison with those living in non-contaminated areas (Uetani et al. 2006). The thyroid hormones, including T3, T4, and TSH, are generally used as trustworthy gage for representation of the thyroid function in experimental animals and humans. The change in concentration of these hormones in serum indicates the disturbances in their glandular synthesis and/or secretion as well as disorders in their extra-thyroidal peripheral metabolism (Chaurasia et al. 1996; Kelly 2000). In short, the function of the thyroid gland is evaluated by measuring the levels of thyroid hormones such as T3 and T4 and pituitary TSH in serum. Being an imperative chain-breaking antioxidant and enzyme co-factor (Jurczuk et al. 2005; Wilson 2002), vitamin C efficiently removes hydroxyl, singlet oxygen, superoxide, hypochlorous acid, and water-soluble peroxyl radical (Smirnoff and Wheeler 2000). Vitamin C is not only a therapeutic agent against heavy metal toxicity; it can provide a remarkable solution to heavy metal toxicity and ameliorate toxicity (Bhattacharjee et al. 2003). Vitamin C binds to mercury ions (Hg+2) due to its nucleophilic properties and tends to reduce mercury-induced impairment. It further potentiates its detoxification process by eradicating or diminishing free radicals formed by mercury (Herbaczynska et al. 1995). Pratima and Anand (1998) have found that the treatment with ascorbic acid restored the metal-induced reduction in hepatic 5′D-I activity and serum triiodothyronine (T3) concentrations. The raised level of lipid peroxidation was also ameliorated by ascorbic acid. It supports that the defensive effect of ascorbic acid against cadmium-induced thyroid dysfunction is bestowed to its anti-oxidative action. Present study aims to assess the toxicological properties of cadmium chloride and mercuric chloride on biochemical/blood parameters (T3, T4, TSH, triglyceride, Hb, MCHC, packed cell volume) and bioaccumulation of metals in vital organs (lungs, liver, heart, and kidney). The ameliorating effects of vitamin C against metal toxicity were also evaluated using rabbits as experimental model.
Materials and methods
Experimental animals
During the experiment, the rabbits of age 1 month (Oryctolagus cuniculus) were used as model animal. The other details of experimental animals, ethical statement, and chemicals were given in Ali et al. (2019)
Administration of cadmium and mercury
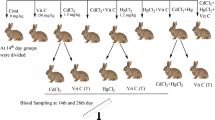
After an initial 2 weeks of acclimatization, 66 rabbits of both genders were randomly divided into eight groups, i.e., one control and seven treatment groups. The scheme of the experimental design is presented in Fig. 1 and the detailed procedure used for the administration of cadmium and mercury was also explained in our previous published study (Ali et al. 2019).
Hematological/biochemical parameters
The blood (5 ml) was collected aseptically through vein puncture from lateral saphenous and/or cephalic vein of each rabbit on the 14th and 28th day of experiment. The collected blood was gently transferred to an ImuMed® vacutainer 3 ml coated with EDTA.K3 and kept at 4 °C for further analyses. Analysis of hematological parameters viz. hemoglobin (Hb), packed cell volume, and mean corpuscular hemoglobin concentration (MCHC) was done in the “Hematology Unit” using the automated method with the automatic analyzer “Hematology autoanalyzer Sysmex KX-21N.” The mean MCHC, concentration of Hb in a given volume of packed red cells, was calculated by the following formula (Linne and Ringsrud 1999).
Blood samples were centrifuged (at 2000 rpm for 10 min for analysis of different parameters). The serum was separated, and aliquots were kept at − 20 °C until analysis (Naito 1984). Triiodothyronine (T3), triglycerides, thyroxine (T4), and thyroid-stimulating hormone (TSH) were analyzed using commercially available kits (Tables 1 and 2).
Estimation of metal accumulation in organs
After the completion of experiments, each sample of different treatment was euthanized by intravenous applied of sodium pentobarbital overdose and the heart was removed surgically and weighed on balance. The known weight of the heart was acid-digested and concentrations of mercury and cadmium were measured through atomic absorption spectrophotometer (Du Preez and Steyn 1992). More detail is given in our recent published study (Ali et al. 2019).
Data analysis
Data were presented as mean ± SEM. Normality of the data was calculated by Kolmogorov–Smirnov test and then analyzed statistically by one-way ANOVA, with “Dunnett’s Multiple Comparison Test,” to identify any significant differences among the group means. GraphPad Prism version 5.0 for windows (GraphPad Software, San Diego, CA, USA) was used for analysis. Probability value (p ≤ 0.05) was taken as significant.
Results
Effect on 3,3,5-triiodothyronine (T3) concentration
Oral administration of cadmium chloride, mercury chloride, and their combination in rabbit for 28 alternative days showed a declined trend in the level of serum T3. The level of T3 was determined for various groups. Level of serum T3 in the Cd exposed group (0.4 ± 0.0 ng/ml) showed the highest significant decrease while in the Hg exposed (0.4 ± 0.0 ng/ml) and combination of Cd + Hg group animals (0.3 ± 0.0 ng/ml), it showed a higher and the highest significant difference, respectively, as compared with that of the control group (0.7 ± 0.1 ng/ml) on the 14th day of experiment. In the prevention group (Cd + Vit C and Hg + Vit C), serum T3 level (0.6 ± 0.0 ng/ml) showed a little higher difference as compared with the respective exposed groups. The Cd + Hg + Vit C–treated group of rabbits (0.6 ± 0.0 ng/ml) showed a higher significant difference compared with the combination of Cd + Hg (0.3 ± 0.0 ng/ml) on above the same time of sampling (Fig. 2a).
Analysis of serum T3 in the treatment groups on day 14 (a) and day 28 (b) of experiments. Abbreviation and keys: Cont. stands for control, Vit C for vitamin C, Cd for cadmium, Hg for mercury, P for prevention, T for treatment. “*” indicates the significant difference between the control and cadmium, mercury, cadmium + mercury treatment groups; “#” represents the significant difference between the cadmium and cadmium + vitamin C prevention groups; “@” indicates the significant difference between the cadmium and cadmium + vitamin C treatment groups; “~” represents the significant difference between the mercury and mercury + vitamin C prevention groups; “^” indicates the significant difference between the mercury and mercury + vitamin C treatment groups; “&” represents the significant difference between the cadmium + mercury and cadmium + mercury prevention groups, “%” indicates the significant difference between the cadmium + mercury and cadmium + mercury treatment groups. Each bar of graph represents the mean value and SEM of six replications. Statistical icons: ~ p ≤ 0.05; **, ~~, ^^, ##, && p ≤ 0.01; ***, &&&, %%% p ≤ 0.001
On the 28th day of exposure, the level of serum T3 in Hg (0.46 ± 0.03 ng/ml) showed higher while the Cd (0.38 ± 0.02 ng/ml) and Cd + Hg group (0.32 ± 0.03 ng/ml) showed the highest significant decline as compared with the respective control group (0.74 ± 0.07 ng/ml). Chemo-prevention with cadmium, i.e., Cd + vit (0.68 ± 0.04 ng/ml), showed the highest significant difference while Chemo-treatment with cadmium, i.e., Cd + vit (0.64 ± 0.03 ng/ml), showed a higher significant difference as compared with the Cd exposure group (0.38 ± 0.02 ng/ml). Chemo-prevention of mercury, i.e., Hg + vit (0.72 ± 0.04 ng/ml), and chemo-treatment of mercury, i.e., Hg + vit (0.72 ± 0.03 ng/ml), both showed a higher significant difference as compared with Hg (0.46 ± 0.03 ng/ml) at the 28th day. Chemo-prevention and chemo-treatment of metal combination, i.e., Cd + Hg + vit (0.62 ± 0.02 ng/ml) and Cd + Hg + vit (0.65 ± 0.05 ng/ml), showed the highest significant difference in comparison with the combination of Cd + Hg (0.32 ± 0.03 ng/ml) on the 28th day (Fig. 2b). Serum T3 level that was significantly decreased in all heavy metal–exposed groups became comparable with that of the blank control group in both the preventive and treatment groups.
Effect on thyroxine (T4) concentration
Oral administration of cadmium chloride, mercury chloride, and their co-administration for 28 alternative days declined the level of serum T4. Level of serum T4 in Cd (26.3 ± 1.6 ng/ml), Hg (21.3 ± 1.1 ng/ml), and co-administration of Cd + Hg (27.3 ± 1.3 ng/ml) showed a comparable significant decrease with that of the control group (42.2 ± 1.7 ng/ml) sampled at 14th day of experiment. Prevention of Cd + vit (36.0 ± 2.1 ng/ml) and Hg + vit (31.5 ± 1.2 ng/ml) showed a higher significant increase in the serum level of T4 as compared with their respective Cd (26.3 ± 1.6 ng/ml) and Hg (21.3 ± 1.1 ng/ml) exposed rabbits but that was less than the respective blank control. Similarly, the Cd + Hg + vit (35.8 ± 2.1 ng/ml) group showed a significant increase compared with the combination of Cd + Hg (27.3 ± 1.3 ng/ml) on day 14 (Fig. 3a).
Analysis of serum T4 in the treatment groups on day 14 (a) and day 28 (b) of experiments. For abbreviation and keys, see Fig. 2
At the 28th day, the level of serum T4 in the Cd (25.8 ± 1.4 ng/ml), Hg (23.2 ± 1.6 ng/ml), and Cd + Hg group (27.3 ± 1.3 ng/ml) showed the highest significant decline as compared with the control group (45.5 ± 1.5 ng/ml). Chemo-prevention with cadmium, i.e., Cd + Vit C (38.2 ± 1.5 ng/ml), showed the highest significant difference while chemo-treatment with cadmium, i.e., Cd + Vit C (35.7 ± 1.2 ng/ml), showed a higher significant difference as compared with Cd (25.8 ± 1.4 ng/ml). Chemo-prevention of mercury, i.e., Hg + Vit C (37.3 ± 1.1 ng/ml), showed the highest significant difference and chemo-treatment of mercury, i.e., Hg + Vit C (32.0 ± 1.2 ng/ml), showed a significant difference as compared with Hg (23.2 ± 1.6 ng/ml) at the 28th day. Chemo-prevention and chemo-treatment of metal combination, i.e., Cd + Hg + Vit C (37.5 ± 1.2 ng/ml) and Cd + Hg + Vit C (35.3 ± 1.5 ng/ml), showed a higher significant difference and a significant difference respectively in comparison with the combination of Cd + Hg (27.3 ± 1.3 ng/ml) on the 28th day (Fig. 3b).
Effect on thyroid-stimulating hormone concentration
Oral administration of cadmium chloride, mercury chloride, and their co-administration for 28 alternative days declined the level of serum TSH. Level of serum TSH in Cd (0.17 ± 0.01 nmol/l), Hg (0.19 ± 0.01 nmol/l), and co-administration of Cd + Hg (0.17 ± 0.01 nmol/l) showed the highest significant difference as compared with the control group (0.06 ± 0.01 nmol/l) at the 14th day. Prevention of Cd + vit (0.08 ± 0.00 nmol/l) and Hg + Vit C (0.13 ± 0.01 nmol/l) showed a higher significant difference as compared with Cd (0.17 ± 0.01 nmol/l) and Hg (0.19 ± 0.01 nmol/l) respectively. Cd + Hg + Vit C (0.13 ± 0.01 nmol/l) showed higher significant differences compared with the combination of Cd + Hg (0.17 ± 0.01 nmol/l) at the 14th day (Fig. 4a).
Analysis of serum TSH in the treatment groups on day 14 (a) and day 28 (b) of experiments. For abbreviation and keys, see Fig. 2
At the 28th day, the level of serum TSH in the Cd (0.19 ± 0.01 nmol/l), Hg (0.23 ± 0.01 nmol/l), and Cd + Hg group (0.20 ± 0.01 nmol/l) showed the highest significant increase as compared with the control group (0.09 ± 0.01 nmol/l). Chemo-prevention with cadmium, i.e., Cd + Vit C (0.11 ± 0.01 nmol/l), showed the highest significant difference while chemo-treatment with cadmium, i.e., Cd + Vit C (0.14 ± 0.01 nmol/l), showed only a significant difference as compared with Cd (0.19 ± 0.01 nmol/l). Chemo-prevention of mercury, i.e., Hg + Vit C (0.13 ± 0.01 nmol/l), showed the highest significant difference and chemo-treatment of mercury, i.e., Hg + Vit C (0.17 ± 0.01 nmol/l), showed a higher significant difference as compared with Hg (0.23 ± 0.01 nmol/l) at the 28th day. Chemo-prevention and chemo-treatment of metal combination, i.e., Cd + Hg + Vit C (0.13 ± 0.01 nmol/l) and Cd + Hg + Vit C (0.15 ± 0.01 nmol/l), showed a higher significant difference and merely a significant difference respectively in comparison with the combination of Cd + Hg (27.3 ± 1.3 nmol/l) on the 28th day (Fig. 4b).
Effect on triglycerides concentration
Oral administration of cadmium chloride, mercury chloride, and their co-administration for 28 alternative days elevated the level of TG. Level of TG in Cd (3.8 ± 0.1 nmol/l), Hg (3.6 ± 0.2 nmol/l), and co-administration of Cd + Hg (4.3 ± 0.1 nmol/l) showed the highest significant difference as compared with the control group (1.8 ± 0.1 nmol/l) at the 14th day. Prevention of Cd + Vit C (3.0 ± 0.1 nmol/l) and Hg + Vit C (2.8 ± 0.1 nmol/l) showed a higher significant difference as compared with Cd (3.8 ± 0.1 nmol/l) and Hg (3.6 ± 0.2 nmol/l) respectively. Cd + Hg + Vit C (3.5 ± 0.1 nmol/l) showed a significant difference as compared with the combination of Cd + Hg (4.3 ± 0.1 nmol/l) at the 14th day (Fig. 5a).
Analysis of serum tryglycerides in the treatment groups on day 14 (a) and day 28 (b) of experiments. For abbreviation and keys, see Fig. 2. Effects of cadmium and mercury chloride and therapeutic role of vitamin C on hematological parameters
At the 28th day, the level of serum TG in the Cd (4.42 ± 0.18 nmol/l), Hg (3.90 ± 0.10 nmol/l), and Cd + Hg group (4.77 ± 0.16 nmol/l) showed the highest significant increase as compared with the control group (1.74 ± 0.08 nmol/l). Chemo-prevention with cadmium, i.e., Cd + Vit C (3.05 ± 0.21 nmol/l), showed the highest significant difference while chemo-treatment with cadmium, i.e., Cd + Vit C (3.52 ± 0.14 ± 1.2 nmol/l), showed a higher significant difference as compared with Cd (4.42 ± 0.18 nmol/l). Chemo-prevention of mercury, i.e., Hg + Vit C (2.87 ± 0.16 nmol/l), showed a higher significant difference and chemo-treatment of mercury, i.e., Hg + Vit C (3.02 ± 0.16 nmol/l), showed merely a significant difference as compared with Hg (3.90 ± 0.10 nmol/l) at the 28th day. Chemo-prevention and chemo-treatment of metal combination, i.e., Cd + Hg + Vit C (3.70 ± 0.08 nmol/l), showed the highest significant difference and Cd + Hg + Vit C (4.00 ± 0.09 nmol/l) showed a simply significant difference in comparison with the combination of Cd + Hg (4.77 ± 0.16 nmol/l) on the 28th day (Fig. 5b).
Effect on hemoglobin
Oral administration of cadmium chloride, mercury chloride, and their co-administration for 28 alternative days decreased the level of Hb. The level of Hb in Cd (10.6 ± 0.6 g/dl) and Hg (9.3 ± 0.6 g/dl) showed the highest significant difference, and the co-administration of Cd + Hg (13.1 ± 0.4 g/dl) showed a higher significant difference as compared with the control group (14.4 ± 0.5 g/dl) at the 14th day. Prevention groups, i.e., Cd + Vit C (13.4 ± 0.6 g/dl), Hg + Vit C (12.1 ± 0.5 g/dl), and Cd + Hg + Vit C (13.9 ± 0.4 g/dl), showed a significant difference as compared with Cd (10.6 ± 0.6 g/dl) and Hg (9.3 ± 0.6 g/dl), and the combination of Cd + Hg (13.1 ± 0.4 g/dl) showed a significant difference as compared with control at the 14th day (Fig. 6a).
Analysis of serum Hb in the treatment groups on day 14 (a) and day 28 (b) of experiments. For abbreviation and keys, see Fig. 2
At the 28th day, the level of hemoglobin in Cd (9.9 ± 0.3 g/dl) and Hg (10.2 ± 0.5 g/dl) showed the highest while the Cd + Hg group (11.1 ± 0.4 g/dl) showed a higher significant decrease as compared with the control group (14.0 ± 0.4 g/dl). Chemo-prevention with cadmium, i.e., Cd + Vit C (13.4 ± 0.6 g/dl), showed the highest significant difference while chemo-treatment with cadmium, i.e., Cd + Vit C (12.4 ± 0.5 nmol/l), showed a higher significant difference as compared with Cd (9.9 ± 0.3 g/dl). Chemo-prevention of mercury, i.e., Hg + Vit C (13.9 ± 0.4 g/dl), showed the highest significant difference and chemo-treatment of mercury, i.e., Hg + Vit C (12.9 ± 0.2 g/dl), showed a higher significant difference as compared with Hg (10.2 ± 0.5 g/dl) at the 28th day. Chemo-prevention and chemo-treatment of metal combination, i.e., Cd + Hg + Vit C (13.4 ± 0.3 g/dl) and Cd + Hg + Vit C (13.5 ± 0.3 g/dl), showed simply a significant difference in comparison with the combination of Cd + Hg (11.1 ± 0.4 g/dl) on the 28th day (Fig. 6b).
Effect on mean corpuscular hemoglobin concentration
Oral administration of cadmium chloride, mercury chloride, and their co-administration for 28 alternative days decreased the level of MCHC. Level of MCHC in Cd (20.4 ± 0.9%) and Hg (15.6 ± 0.6%) showed the highest significant difference as compared with the control group, whereas co-administration of Cd + Hg (13.6 ± 0.7%) showed a higher significant difference compared with the control group (31.1 ± 0.9%) at the 14th day. Prevention of Cd + Vit C (26.4 ± 1.0%) that of Hg + Vit C (21.8 ± 1.3%) showed a higher significant difference as compared with Cd (20.4 ± 0.9%) and Hg (15.6 ± 0.6%) respectively. Cd + Hg + Vit C (20.3 ± 1.4%) showed a higher significant difference as compared with the combination of Cd + Hg (13.6 ± 0.7%) at the 14th day (Fig. 7a).
Analysis of serum mean corpuscular hemoglobin in the treatment groups on day 14 (a) and day 28 (b) of experiments. For abbreviation and keys, see Fig. 2
At the 28th day, the level of MCHC in the Cd (20.0 ± 1.2%), Hg (14.6 ± 1.1%), and Cd + Hg group (16.4 ± 0.9 nmol/l) showed the highest significant decrease as compared with the control group (30.0 ± 0.9%). Chemo-prevention with cadmium, i.e., Cd + Vit C (28.2 ± 0.9%), showed the highest significant difference while chemo-treatment with cadmium, i.e., Cd + Vit C (27.7 ± 1.8%), showed a higher significant difference as compared with Cd (20.0 ± 1.2%). Chemo-prevention of mercury, i.e., Hg + Vit C (24.1 ± 1.2%), showed a higher significant difference and chemo-treatment of mercury, i.e., Hg + Vit C (22.7 ± 1.1%), showed merely a significant difference as compared with Hg (14.6 ± 1.1%) at the 28th day. Chemo-prevention and chemo-treatment of metal combination, i.e., Cd + Hg + Vit C (23.1 ± 1.1%), showed the highest significant difference and Cd + Hg + Vit C (22.9 ± 1.0%) showed simply a significant difference in comparison with the combination of Cd + Hg (16.4 ± 0.9%) on the 28th day(Fig. 7b).
Effect on packed cell volume
Oral administration of cadmium chloride, mercury chloride, and their co-administration for 28 alternative days declined the level of PCV. The level of PCV in Cd (19.4 ± 1.2%) and Hg (16.2 ± 0.7%) showed the highest significant difference compared with the control group, whereas co-administration of Cd + Hg (20.8 ± 1.0%) showed a higher significant difference compared with the control group (29.1 ± 1.1%) at the 14th day. Prevention of Cd + Vit C (26.6 ± 0.8%) and that of Hg + Vit C (23.3 ± 1.0%) showed a higher significant difference as compared with Cd (19.4 ± 1.2%) and Hg (16.2 ± 0.7%) respectively. Cd + Hg + Vit C (26.6 ± 0.9%) showed a higher significant difference as compared with the combination of Cd + Hg (20.8 ± 1.0%) at the 14th day (Fig. 8a).
Analysis of serum packed cell volume in the treatment groups on day 14 (a) and day 28 (b) of experiments. For abbreviation and keys, see Fig. 2
At the 28th day, the level of PCV in the Cd (19.6 ± 2.3%), Hg (19.6 ± 2.3%), and Cd + Hg group (19.1 ± 0.9%) showed the highest significant decrease as compared with the control group (30.0 ± 0.9%). Chemo-prevention with cadmium, i.e., Cd + Vit C (28.2 ± 0.9%), showed the highest significant difference while chemo-treatment with cadmium, i.e., Cd + Vit C (27.1 ± 0.8%), showed a higher significant difference as compared with Cd (19.6 ± 2.3%). Chemo-prevention of mercury, i.e., Hg + Vit C (24.2 ± 0.8%), showed a higher significant difference and chemo-treatment of mercury, i.e., Hg + Vit C (23.6 ± 1.4%), showed merely a significant difference as compared with Hg (19.6 ± 2.3%) at the 28th day. Chemo-prevention and chemo-treatment of metal combination, i.e., Cd + Hg + Vit C (31.0 ± 1.4%), showed the highest significant difference and Cd + Hg + Vit C (25.6 ± 0.7%) showed simply a significant difference in comparison with the combination of Cd + Hg (19.1 ± 0.9%) on the 28th day (Fig. 8b).
The concentration of cadmium in body organs
In comparison with the control group (0 ± 0 mg/kg body weight), the kidney, heart, liver, and lungs treated with Cd (164.40 ± 8.25 mg/kg, 14.00 ± 1.15 mg/kg, 42.33 ± 1.86 mg/kg, 10.00 ± 0.58 mg/kg) and Cd + Hg (146.07 ± 8.05 mg/kg, 11.67 ± 1.20 mg/kg, 30.33 ± 2.03 mg/kg, and 11.33 ± 0.88 mg/kg body weight) showed the highest significant difference. Comparing the Cd group (164.40 ± 8.25 mg/kg, 14.00 ± 1.15 mg/kg, 42.33 ± 1.86 mg/kg, 10.00 ± 0.58 mg/kg) with the prevention group of Cd + Vit C (158.20 ± 5.95 mg/kg, 5.67 ± 0.88 mg/kg, 18.00 ± 1.15 mg/kg, 5.00 ± 0.58 mg/kg) showed the highest significant difference while the treatment group of Cd + Vit C showed a higher significant difference in the heart (8.00 ± 1.15 mg/kg) and kidney (169.10 ± 5.92 mg/kg) while a significant difference in the lungs (6.67 ± 0.33 mg/kg) and the highest significant difference in the liver (27.00 ± 1.73 mg/kg body weight). In the kidney and liver, the prevention group Cd + Vit C (158.20 ± 5.95 mg/kg and 18.00 ± 1.15 mg/kg body weight respectively) showed a significant difference as compared with the treatment group Cd + Vit C (27.00 ± 1.73 mg/kg and 169.10 ± 5.92 mg/kg body weight). The comparison of the Cd + Hg group (146.07 ± 8.05 mg/kg, 11.67 ± 1.20 mg/kg, 30.33 ± 2.03 mg/kg, 11.33 ± 0.88 mg/kg) with the prevention group Cd + Hg + Vit C showed the highest significant difference in the kidney (119.17 ± 4.40 mg/kg), liver (11.33 ± 1.45 mg/kg), and lungs (4.33 ± 0.33 mg/kg) and a higher significant difference in the heart (4.33 ± 0.33 mg/kg) while the treatment group Cd + Hg + Vit C showed a significant difference in the kidney (131.27 ± 7.65 mg/kg) and heart (6.67 ± 0.88 mg/kg body weight) while a higher significant difference in the liver (19.33 ± 1.20 mg/kg) and lungs (6.67 ± 0.88 mg/kg body weight). In the liver, the prevention group Cd + Hg + Vit C (11.33 ± 1.45 mg/kg) showed a significant difference as compared with the treatment group Cd + Hg + Vit C (19.33 ± 1.20 mg/kg body weight) shown in (Tables 1 and 2).
The concentration of mercury in body organs
In comparison with the control group (0 ± 0 mg/kg), the kidney, heart, liver, and lungs treated with Hg (114.73 ± 7.02 mg/kg, 10.33 ± 0.88 mg/kg, 27.33 ± 2.33 mg/kg, 8.33 ± 0.33 mg/kg) and Cd + Hg (94.10 ± 7.16 mg/kg, 9.67 ± 1.45 mg/kg, 24.33 ± 1.45 mg/kg, 8.00 ± 0.58 mg/kg body weight) showed the highest significant difference. Comparing the Hg group (114.73 ± 7.02 mg/kg, 10.33 ± 0.88 mg/kg, 27.33 ± 2.33 mg/kg, 8.33 ± 0.33 mg/kg body weight) with the prevention group, Cd + Vit C showed the highest significant difference in the kidney (118.87 ± 6.40 mg/kg), heart (4.00 ± 0.58 mg/kg), and lungs (4.00 ± 0.58 mg/kg) and a higher significant difference (14.00 ± 2.89 mg/kg) in the liver, while the treatment group Cd + Vit C showed a significant difference in the kidney, heart, liver, and lungs (128.03 ± 7.25 mg/kg, 5.67 ± 0.33 mg/kg, 17.67 ± 1.20 mg/kg, 5.67 ± 0.33 mg/kg body weight respectively). In the kidney, the prevention group Cd + Vit C (118.87 ± 6.40 mg/kg body weight) showed a significant difference as compared with the treatment group Cd + Vit C (128.03 ± 7.25 mg/kg body weight). The comparison of the Cd + Hg group (94.10 ± 7.16 mg/kg, 9.67 ± 1.45 mg/kg, 24.33 ± 1.45 mg/kg, 8.00 ± 0.58 mg/kg) with the prevention group Cd + Hg + Vit C showed a higher significant difference in the kidney (69.03 ± 4.33 mg/kg) and heart (3.67 ± 0.33 mg/kg), and the highest significant difference in the liver (8.33 ± 0.67 mg/kg) and lungs (3.67 ± 0.33 mg/kg body weight), while the treatment group Cd + Hg + Vit C showed a significant difference in the kidney (80.50 ± 8.33 mg/kg), heart (5.00 ± 0.88 mg/kg body weight), and liver (14.33 ± 1.48 mg/kg body weight) with no significant difference in the lungs (5.67 ± 0.88 mg/kg body weight) as shown in Tables 1 and 2.
Discussion
An increased use of metals in anthropogenic activities has increased the exposure to several environmental contaminants and their role has been identified in disrupting many physiologic functions in the body. In the present study, it is demonstrated that a chronic exposure of toxic metals had adverse effects on thyroid function parameters in exposed rabbits. As indicated by serum level endpoints, thyroid homeostasis was significantly altered with the exposure of both toxic metals either alone or in combination. The results from thyroid function tests revealed that cadmium, mercury, and their co-administration had significantly decreased the average values of T3 and T4 as compared with that of control, whereas TSH increased in both cadmium- and mercury-treated rabbits. However, the chemo-prevention with vitamin C had showed a significant elevation in the level of both T3 and T4 that draws persuasive support from the work of Gupta and Kar (1999) that the administration of Cd to Swiss male mice induced thyroid malfunctioning and lipid peroxidation leading to a change in T3, T4, and hepatic type I iodothyronine 5-Monpdeiodinase (5 D-I) activity in the serum. As far as we know, the effect of chronic exposure to CdCl2 and HgCl2 on thyroid has not been reported yet. Many studies showed that the mechanisms interfering with liver thyroid hormone metabolism via diminished “outer ring de-iodine enzyme ORD” activity that converts thyroid hormone T4 in biologically active form (T3); defects in signaling pathway of T3 also result in decreased T4 conversion. In addition, cadmium and mercury cause thyroid iodine uptake to fall by disrupting the follicular cells of the thyroid (Gupta and Kar 1999). Long-term exposure to cadmium and mercury can induce activity in liver microsomal enzymes, especially Uridine DiPhosphate Glucournyl Tranferase (UDP-GT) (Wade et al. 2003) and phenol Sulftransferase that result in T3 and T4 evacuation. Cadmium accumulation might depress oxidative phosphorylation in mitochondrial thyroid follicle epithelial cells with consistent energy loss that inhibits synthesis and release of thyroid hormones (Yoshizuko et al. 1991). Cadmium treatment can lead to histological changes, initiation of the fibrotic process, inflammatory reaction, and over-conversion of the growth factor B thus playing important role in thyroid morphology (Ruze et al. 1999).
The metal reduced hepatic 5D-I activity but the serum T3 level was touch up by supplementation of ascorbic acid. The thyroid may accumulate mercury in follicular cells at exposure (WHO 1991; Falnoga et al. 2000). It has been shown that moderate occupational exposure affects a seleno-enzyme called deiodinase that is accountable for the deiodination of T4 to T3 (Barregård et al. 1994; Ellingsen et al. 2000). This causes T4 level to increase by reversing T3 levels and raise the T4/T3 ratio (Barregård et al. 1994; Ellingsen et al. 2000).
According to the data reported by Nishida et al. (1986), thyroidal secretion of T4 was subdued by mercury while Kawada et al. (1980) suggested that both organic and inorganic Hg disrupted thyroid function by interfering both with the production of thyroidal hormones and the conversion of T4 to T3. Experimental exposure of methyl mercury to monkeys indicated high concentrations of inorganic Hg in the pituitary (Vahter et al. 1995). Thyroid dysfunction in our study can be explained by the probable accretion of Hg in the thyroid gland. Since, T4 synthesis is associated with the thyroid gland, so its decline in the serum level of Hg-treated rabbits could suggest that Hg-induced thyroid dysfunction is due to affected production and/or secretion of T4 by the follicular cells of the thyroid gland. A significant increase in TSH levels in the Hg-treated rabbits could also affect regulatory enzymes associated with the hypothalamic-pituitary-thyroid (HPT) axis. A significant increase in serum TSH level in both heavy metal exposed rabbits may be explained because of feedback control mechanisms as previously reported by different researchers (Hammouda et al. 2008; Mohamed et al. 2015). Metabolism of thyroid hormones in the peripheral tissues by deiodination, conjugation, deamination, and decarboxylation enzymatic reactions may influence thyroid role at the cellular level (Badiei et al. 2010; Paier et al. 1993; Chaurasia et al. 1996; Kelly 2000; Pilat-Marcinkiewicz et al. 2003).
The present study revealed that oral administration of CdCl2, HgCl2, and their combined usage significantly decreases the level of Hb and PCV in all the cadmium chloride– and mercury chloride–exposed groups with respect to the control group. The supplementation of vitamin C significantly restored the level of Hb and hematocrit at the 28th day of treatment. These results can be interpreted from the observations of Hounkpatin et al. (2012) who debated about the causes that affect the common stipulations of the animals such as fluid balance and nutritional condition influence hematocrit pathology.
Previous studies showed that individual stimulation of CdCl2 in chicks causes the elevation of MCHC level at the beginning; however, due to increased concentration of dose, MCHC levels were declined (Gabol et al. 2014). Current study showed that the level of MCHC significantly declined at the 28th day of treatment in cadmium chloride and mercury chloride groups, however. Chemo-treatment with vitamin C stabilizes the level of MCHC in intoxicated rabbits.
The endocrine system like thyroid hormones plays an important role in homeostasis. Thyroid hormone is involved in hemoglobin synthesis. Hypothyroidism also causes anemia probably through decreasing the oxygen, resultantly also perturbs the RBCs indices like PCV, MCH, and MCHC (Dorgalaleh et al. 2013; Iddah et al. 2013). These findings suggest exploring the molecular mechanisms altering hematopoiesis that may provide the targets for therapeutic interventions.
Our findings are in line with the Grosicki (2004) and Jiraungkoorskul et al. (2007). They demonstrated that vitamin C reduced the metal-induced toxicity. In current research, it was observed that co-administration of both metals with vitamin C exhibited more protection than the group supplemented with vitamin C separately. The restoration of liver functions from CdCl2 and HgCl2 toxicity was observed in the prevention groups due to vitamin C which indicated the free radical scavenging mechanism and detoxification effect (Suzuki 1990).
The metals tend to accumulate in different organs such as the lungs, kidney, liver, and heart (Siraj et al. 2016). The amount of accumulation depends on stage, prolonged, and short-term exposures both revealed the diverse outcomes (Ali et al. 2016). The liver is a target organ for the accumulation of CdCl2 in rabbits, afterwards it accumulates in renal system and finally in tissues as well as in skeletal muscles (Josthna et al. 2012). The present study revealed that vital organs such as the liver and kidneys had significant accumulation of toxic metals in all metals’ exposed groups. However, accumulation was less in the CdCl2 + HgCl2–treated group than individual cadmium chloride– and mercury chloride–exposed group. Pretreatment and chemo-treatment with vitamin C significantly decline the concentration in studied vital organs.
In the set-up of our experiment, more cadmium and mercury compounds are accumulated in the organs of rabbits and had altered the biochemical profile of serum when administered orally. Changed levels of these biomarkers resulted in thyroid injury. However, the intensity of these alterations of biochemical profile was encountered in the presence of vitamin C co-administration. These findings may indicate a protective mechanism of ascorbic acid in thyroid toxicity in toxic metals-intoxicated rabbits. Ameliorating potential of vitamin C in cadmium- and mercury-administered rabbits was observed on the 14th and 28th day of experiment. Vitamin C treatment exhibit a reduction in the adverse effects of cadmium compounds by restoring hematological and biochemical changes as reported earlier (Abdelaziz et al. 2013). Co-administration of ascorbic acid has significantly decreased the entry of chromium into the cells (Lin et al. 2018; Rana et al. 2018). In present research, more concentration of cadmium and mercury was found to be in the thyroid gland of rabbit, when exposed to these metals, which depicts that the thyroid gland might be capable of accumulating these metals in higher concentration. While supplementation of vitamin C can prevent the production of free radicals and maintain the hematological parameters, these findings may indicate the therapeutic mechanism of vitamin C against toxic metal–induced thyroid toxicity and biochemical alterations in intoxicated rabbits.
Referencess
Akinhanmi TF, Ademuyiwa O, Odukoya OO, Babayemi DO, Ogunlesi AO, Adamson I, Akinhanmi AO (2016) Amelioration of lead toxicity in an occupationally exposed population with ascorbic acid. J Chem Soc Nigeria 41(1):69–75
Ali TH, Abed AA, Abdul Ellah A (2016) Cadmium accumulation in some organs of mosquito fish Gambusia holbrooki. TJPS 21(1):16–18
Ali S, Hussain S, Khan R, Mumtaz S, Ashraf N, Andleeb S, Shakir HA, Tahir HM, Khan MKA, Ulhaq M (2019) Renal toxicity of heavy metals (cadmium and mercury) and their amelioration with ascorbic acid in rabbits. Environ Sci Pollut Res Int 26(4):3909–3920
Badiei K, Mostaghni K, Nikghadam P, Paurjafar M (2010) The effect of mercury on thyroid function of sheep. Intern J Vet Res 4(4):277–281
Barregård L, Lindstedt G, Schütz A, Sällsten G (1994) Endocrine function in mercury exposed chloralkali workers. Occup Environ Med 51(8):536–540
Bhattacharjee CR, Deys S, Goswami P (2003) Protective role of ascorbic acid against lead toxicity in blood of albino mice as revealed by metal uptake, lipid profiles, and ultrastructural features of erythrocytes. Bull Environ Contam Toxicol 70:1189–1196
Buha A, Wallace D, Matovic V, Schweitzer A, Oluic B, Micic D, Djordjevic V (2017) Cadmium exposure as a putative risk factor for the development of pancreatic cancer: three different lines of evidence. Biomed Res Int 2017:1–8
Buha A, Matovic V, Antonijevic B, Bulat Z, Curcic M, Renieri EA, Tsatsakis AM, Schweitzer A, Wallace D (2018) Overview of cadmium thyroid disrupting effects and mechanisms. Int J Mol Sci 19(5):1501
Chaurasia SS, Gupta P, Kar A, Maiti PK (1996) Free radical mediated membrane perturbation and inhibition of type-I iodothyronine 5′-monodeiodinase activity by lead and cadmium in rat liver homogenate. Biochem Mol Biol Int 39:765–770
Das K, Siebert U, Gillet A, Dupont A, Di-Poď C, Fonfara S, Mazzucchelli G, De Pauw E, De Pauw-Gillet MC (2008) Mercury immune toxicity in harbour seals: links to in vitro toxicity. Environ Health 7:52–60
Dorgalaleh A, Mahmoodi M, Varmaghani B, Kiani NF, Saeeidi KO, Alizadeh SH, Tabibian SH, Bamedi T, Momeni M, Abbasian S, KashaniKhatib Z (2013) Effect of thyroid dysfunctions on blood cell count and red blood cell indice. Iran J Ped Hematol Oncol 3(2):73–77
Edwards J, Ackerman C (2016) A review of diabetes mellitus and exposure to the environmental toxicant cadmium with an emphasis on likely mechanisms of action. Curr Diabetes Rev 12:252–258
Ellingsen DG, Efskind J, Haug E, Thomassen Y, Martinsen I, Gaarder PI (2000) Effects of low mercury vapour exposure on the thyroid function in chloralkali workers. J Appl Toxicol 20:483–489
Falnoga I, Tusek-Znidaric M, Horvat M, Stegnar P (2000) Mercury, selenium, and cadmium in human autopsy samples from Idrija residents and mercury mine workers. Environ Res 84:211–218
Feki-Tounsi M, Hamza-Chaffai A (2014) Cadmium as a possible cause of bladder cancer: a review of accumulated evidence. Environ Sci Pollut Res 21:10561–10573
Gabol K, Khan MZ, Khan MUA, Khan P, Fatima F, Siddiqui S, Jabeen T, Baig N, Iqbal MA, Usman M, Hashmi A, Tabish M (2014) Induced effects of lead, chromium and cadmium on Gallus domesticus. Can J Pure App Sci:30–35
Grosicki A (2004) Influence of vitamin C on cadmium absorption and distribution in rats. J Trace Elem Med Biol 18(2):183–187
Gupta P, Kar A (1999) Cadmium induced thyroid dysfunction in chicken; hepatic type I iodothyronine 5-D-I activity and role of lipid peroxidation. Biochm Physiolo Pharmacol Endocrinol 123(1):39–44
Hartwig A (2013) Cadmium and cancer. Met Ions Life Sci 11:491–507
Herbaczynska CK, Ktoseiewicz WB, Cedro K, Wasek BW (1995) Supplementation with vitamins C and E suppresses leukocyte oxygen free radical production in patients with myocardial infarction. Eur Heart J 16:1044–1049
Hounkpatin ASY, Johnson RC, Guedenon P, Domingo E, Alimba CG, Boko M, Edorh PA (2012) Protective effects of vitamin C on haematological parameters in intoxicated wistar rats with cadmium, mercury and combined cadmium and mercury. Int Res J Biol Sci 1(8):76–81
IARC (2012) Personal habits and indoor combustions, volume 100. Lyon, France
Ibraheem AS, Seleem AA, El-Sayed MF, Hamad BH (2016) Single or combined cadmium and aluminum intoxication of mice liver and kidney with possible effect of zinc. J Basic Appl Zoo 77:91–101
Iddah MA, Macharia BN, Ng’wena AG, Keter A, Ofulla AV (2013) Thyroid hormones and hematological indices levels in thyroid disorders patients at moi teaching and referral hospital, Western Kenya. ISRN Endocrinol, 2013, 385940. https://doi.org/10.1155/2013/385940
Jancic SA, Stosic BZ (2014) Cadmium effects on the thyroid gland. Vitam Horm 94:391–425
Javed M, Usmani N (2012) Toxic effects of heavy metals (Cu, Ni, Fe Co, Mn, Cr, Zn) to the haematology of Mastacembelus armatus thriving in Harduaganj Reservoir, Aligarh, India. Glob J Med Res 12:59–64
Javed M, Usmani N (2015) Impact of heavy metal toxicity on hematology and glycogen status of fish: a review. Proc Natl Acad Sci India Sect B Biol Sci 85:889–900
Jiraungkoorskul W, Sahaphong S, Kosai P, Kim MH (2007) The effect of ascorbic acid on cadmium exposure in the gills of Puntius altus. Int J Zool Res 3:77–85
Josthna P, Geetharathan T, Sujatha P, Deepika G (2012) Accumulation of lead and cadmium in the organs and tissues of albino rat. Int J Pharm Life Sci 3(12):2186–2189
Jurczuk M, Moniuszko-Jakoniuk J, Brzoska MM, Roszczenko A (2005) Vitamins E and C concentrations in the liver and kidney of rats exposed to cadmium and ethanol. Pol J Environ Stud 14(5):599
Kawada J, Nishida M, Yoshimura Y, Mitani K (1980) Effects of organic and inorganic mercurials on thyroidal functions. Aust J Pharm 3(3):149–159
Kelly GS (2000) Peripheral metabolism of thyroid hormones: a review. Altern Med Rev 5:306–333
Klaassen CD, Liu J, Diwan BA (2009) Metallothionein protection of cadmium toxicity. Toxicol Appl Pharmacol 238:215–220
Lange IG, Daxenberger A, Schiffer B, Witters H, Ibarreta D, Meyer HHD (2002) Sex hormones originating from different livestock production systems: fate and potential disrupting activity in the environment. Anal Chim Acta 473:27–37
Larsson SC, Orsini N, Wolk A (2015) Urinary cadmium concentration and risk of breast cancer: a systematic review and dose-response meta-analysis. Am J Epidemiol 182:375–380
Lin TJ, Huang YL, Chang JS, Liu KT, Yen MC, Chen FW, Shih YL, Jao JC, Huang PC, Yeh IJ (2018) Optimal dosage and early intervention of l-ascorbic acid inhibiting K2Cr2O7-induced renal tubular cell damage. J Trace Elem Med Biol 48:1–7
Linne JJ. Ringsrud KM (1999) Clinical laboratory science, the basic and routine techniques. 4th ed., Saint Louis, Moseby. pp: 279–295.
Maffini MV, Rubin BS, Sonnensschein C, Soto AM (2006) Endocrine disruptors and reproductive health: the case of bisphenol-A. Mol Cell Endocrinol 254–255:179–186
Matović V, Buha A, Bulat Z, Đukić-Ćosić D (2011) Cadmium toxicity revisited: focus on oxidative stress induction and interactions with zinc and magnesium. Arch Ind Hyg Toxicol 62:65–76
Matović V, Buha A, Dukić-Ćosić D, Bulat Z (2015) Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem Toxicol 78:130–140
Meyer KJ, Reif JS, Veeramachaneni DN, Luben TJ, Mosley BS, Nuckols JR (2006) Agricultural pesticide use and hypospadias in eastern Arkansas. Environ Health Perspect 114:1589–1595
Mezynska M, Brzóska MM (2018) Environmental exposure to cadmium—a risk for health of the general population in industrialized countries and preventive strategies. Environ Sci Pollut Res 25:3211–3232
Naito HK (1984) High-density lipoprotein (HDL) cholesterol. Kaplan A et al. Clin Chem The C.V. Mosby Co. St Louis, Toronto, Princeton, pp 1207–1213 and 437
Nishida M, Yamamoto T, Yoshimura Y, Kawada J (1986) Subacute toxicity of methylmercuric chloride and mercuric chloride on mouse thyroid. Aust J Pharm 9:331–338
Okediran BS, Kasali OB, Omotainse SO, Akinloye OA (2016) Haemato-biochemical alterations as biomarkers of lead induced toxicity in male wistar rats. Bangl J Vet Med 14(2):227–232
Pacyna JM, Travnikov O, De Simone F, Hedgecock IM, Sundseth K, Pacyna EG, Steenhuisen F, Pirrone N, Munthe J, Kindbom K (2016) Current and future levels of mercury atmospheric pollution on a global scale. Atmos Chem Phys 16(19):12495–12511
Paier B, Hagmüller K, Noli MI, Gonzalez PM, Stiegler C, Zaninovich AA (1993) Changes induced by cadmium administration on thyroxine deiodination and sulfhydryl groups in rat liver. J Endocrinol 138:219–224
Pal D, Suman S, Kolluru V, Sears S, Das TP, Alatassi H, Ankem MK, Freedman JH, Damodaran C (2017) Inhibition of autophagy prevents cadmium-induced prostate carcinogenesis. Br J Cancer 117(1):56–64
Person RJ1, Tokar EJ, Xu Y, Orihuela R, Ngalame NN, Waalkes MP (2013) Chronic cadmium exposure in vitro induces cancer cell characteristics in human lung cells. Toxicol Appl Pharmacol 273(2):281–288
Pollack AZ, Schisterman EF, Goldman LR, Mumford SL, Albert PS, Jones RL, Wactawski-Wende J (2011) Cadmium, lead, and mercury in relation to reproductive hormones and anovulation in premenopausal women. Environ Health Perspect 119:1156–1161
Pratima G, Anand K (1998) Role of ascorbic acid in cadmium-induced thyroid dysfunction and lipid peroxidation. J Appl Toxicol 18(5):317–320
Rana MN, Tangpong J, Rahman MM (2018) T oxicodynamics of lead, cadmium, mercury and arsenic-induced kidney toxicity and treatment strategy: a mini review. Toxicol Rep 5:704–713
Ruze M, Juna C, Jose G (1999) Single and multiple selenium-zinc-iodine deficiencies affect rat thyroid metabolism and ultra-structure. J Nutr 129:174–180
Sarwar N, Imran M, Shaheen MR, Ishaque W, Kamran MA, Matloob A, Rehim A, Hussain S (2017) Phytoremediation strategies for soils contaminated with heavy metals: modifications and future perspectives. Chemosphere 171:710–721
Silva N, Peiris-John R, Wickremasinghe R, Senanayake H, Sathiakumar N (2012) Cadmium a metalloestrogen: are we convinced? J Appl Toxicol 32:318–332
Siraj M, Khisroon M, Khan A (2016) Bioaccumulation of heavy metals in different organs of Wallago attu from River Kabul Khyber Pakhtunkhwa, Pakistan. Biol Trace Elem Res 172(1):242–250
Smirnoff N, Wheeler GL (2000) Ascorbic acid in plants: biosynthesis and function. Crit Rev Biochem Mol Biol 35(4):291–314
Sundseth K, Pacyna JM, Pacyna EG, Pirrone N, Thorne RJ (2017) Global sources and pathways of mercury in the context of human health. Int J Environ Res Public Health 14(1):105–112
Suzuki Y (1990) Synergism of ascorbic acid and glutathione in reduction of hexavalent chromium in vitro. Ind Health 28:9–19
Tay CK, Asmah R, Biney CA (2009) Trace metal levels in water and sediment from the sakumoii and muni lagoons, Ghana. West. Afric J Appl Ecol 16:75–94
Truby P (2003) Impact of heavy metals on forest trees from mining areas. International conference on mining and the environment III, Sudbury, Ontario, pp 25–36
Uetani M, Kobayashi E, Suwazono Y, Honda R, Nishijo M, Nakagawa H, Kido T and Nogawa K (2006) Tissue cadmium (Cd) concentrations of people living in a Cd polluted area, Japan. BioMetals 19(5):521–525
Vahter ME, Mottet NK, Friberg LT, Lind SB, Charleston JS, Burbacher TM (1995) Demethylation of methyl mercury in different brain sites of Macaca fascicularis monkeys during long-term subdinical methyl mercury exposure. Toxicol Appl Pharmacol 134:273–284
Wade MG, Sophi P, Kenneth W, Edward Y (2003) Thyroid toxicity due to subchronic exposure to a complex mixture of 16 organochlorines, lead, and cadmium. Toxicol Sci 67:207–218
Wilson JX (2002) The physiological role of dehydroascorbic acid. FEBS Lett 527:5–9
Yoshizuko M, Mori N, Hamasaki K (1991) Cadmium toxicity in thyroid gland of pregnant rats. Exp Mol Pathol 55(1):97–104
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, R., Ali, S., Mumtaz, S. et al. Toxicological effects of toxic metals (cadmium and mercury) on blood and the thyroid gland and pharmacological intervention by vitamin C in rabbits. Environ Sci Pollut Res 26, 16727–16741 (2019). https://doi.org/10.1007/s11356-019-04886-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04886-9