Abstract
The liver is one of the vital and sensitive organs which are usually exposed against the toxicity of mercury (Hg) and cadmium (Cd). The main objective of the current study was to evaluate the potential toxicological effects of both Cd and Hg as individual and combined. Hepatotoxicity was evaluated by monitoring the biochemical parameters of the liver and their accumulation in the liver as well as therapeutic role of vitamin C in said toxicity in rabbits (Oryctolagus cuniculus). In this research, cadmium chloride (1.5 mg/kg), mercuric chloride (1.2 mg/kg), and vitamin C (150 mg/kg of body weight) were orally administered to treatment groups of the rabbits for 28 alternative days. Various biochemical parameters of the liver such as lactate dehydrogenase (LDH), aspartate aminotransferase (ASAT), bilirubin, alanine aminotransferase (ALAT), total protein, and gamma glutamyl transferase (GGT) were estimated using blood samples. Some biochemical parameters like ASAT, ALAT, LDH, GGT, and bilirubin were significantly elevated (P ≤ 0.001) in individual Cd and Hg treatment groups, while the level of total protein was found to be significantly declined. The effects of Cd and Hg in the presence of vitamin C on these biochemical parameters were low as compared to metals-treated groups. Similar results were found when rabbits were treated with co-administration of both metals and vitamin C. Accumulation of Cd and Hg found to be higher in the liver. However, chemoprevention and chemotreatment with vitamin C significantly (P ≤ 0.01) minimized the toxicological effects of both metals but not regained the accumulation similar to that of the control group. The findings of this study provide awareness on accumulation of metals in the liver in rabbits and their toxicity tested through biochemical parameters as well as the therapeutic role of vitamin C in such alterations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industrialization in developing countries brought an increment in metals contamination. Consumption and exposure of xenobiotic materials or human-made compounds such as heavy metallic complexes cause pollution (Jagadeesan and Pillai 2007). Heavy metals are non-biodegradable and produce probable effects even at low dosages (Tay et al. 2010). Cadmium (Cd), a heavy metal, is an ecological and industrial pollutant. This enters into the body mostly by ingestion as well as inhalation then cause prolonged illness (Bernard 2008; Mutlu et al. 2012).
Hepatic damage is elevated globally due to introduction of contaminated factors. The Cd is one of the well-known atmospheric pollutants that are harmful to hepatic tissues (Nithya et al. 2012).When Cd binds with metallothionein (MT), which is cysteine-rich protein, its absorption increases about 3000-fold. In the liver, complexes of cysteine metallothionein cause hepatotoxicity and then it goes into the kidney producing nephrotoxicity by a massed in the renal tissue (Castagnetto et al. 2002). Transportation of Cd into the hepatic system takes place via dual processes concerning the attachment to the cell membrane (Delraso et al. 2003). The second-phase process by membrane transporters that are existing in the liver sinusoidal cell membrane (Fujishiro et al. 2009).
Accumulation of Cd is the source of destruction to numerous organs such as the brain, liver, nephron, testes, lungs, constituents of blood, and cartilage (Ercal et al. 2001). It is categorized as type 1 carcinoma (human carcinoma) due to its features as a pulmonary carcinogen (Waisberg et al. 2003). After severe consumption, indications like burning sensation, stomach soreness, sickness, heaving, muscle spasm, loss of awareness, salivation, dizziness, astonishment, and seizures frequently express in 15 to 30 min (Baselt and Cravey 2000). In vitro studies in human or mouse hepatocytes revealed that cell death plays a major character in hepatic toxicity by Cd (Yu et al. 2011).
Mercury (Hg) is a silver liquid heavy metal that exists in numerous preparations containing organic, inorganic, and elemental mercury. All forms of Hg have a changed pathway for exposure as well as different toxicity (Chibunda and Janssen 2009; Kampalath and Jay 2015).
The elevated levels of Hg can harm the kidneys and nervous system as well as developing infant (Sakamoto et al. 2012). Introduction of Hg at high level modifies the tasks of the lungs and brain damage leading to nervousness, loss of consciousness, vomiting, nausea, prickliness, skin rashes, and fluctuations in visualization, hair loss, increased heart rate, or blood pressure. These symptoms are common; then, it is tough to analyze the problem (Martin and Griswold 2009). Mercury causes various complaints including epilepsy (Caito and Aschner 2015) and myocardial infarction (Genchi et al. 2017) as well as respiratory disorders in offspring (Heinrich et al. 2017). It is stored into the bile then partially absorbed in hepatic via hepatic vein. The unusual amount of Hg moves in tissue due to binding of mercury with cysteine complex in the gall bladder as well as the bile duct (Birch et al. 2014).
Furthermore, Hg changed the biological then hematological factors in animals (Huq et al. 2013). Toxicity of Hg in the liver cause increased ALT in serum, serum bilirubin levels, ornithine carbonyl transferase, enlargement of the liver, and fatty liver as well as decline in the production of hepatic blood clotting components (Joshi et al. 2012). Exposure of Hg occurs as a result of ingestion and inhalation as well as skin interaction. Both Cd and Hg mostly accumulated in the liver (Yannai et al. 1991). The toxicity of these two heavy metals causes disruption in the functions of certain plasmatic enzymes such as LDH, ALAT, ASAT, and ALP (Koyu et al. 2006).
Vitamin C (ascorbic acid) is a hydrophilic antioxidant found in green vegetables such as spinach, tomatoes, and fruits like lemons and strawberries (Padayatty et al. 2004). It defends all growing animals such as rats, guinea pigs, turkeys, chickens, mice, and fish from toxicity of heavy metals (Gajawat et al. 2005). Due to the presence of its free sulfhydryl group, it has capability to bind with heavy metals, which result in decrease the antioxidant level from various organs and re-establish the level of enzymes (Rana et al. 2010). It has a defensive character against fluctuations persuaded by metals in the kidney, liver, lungs, and testis and cytotoxicity in bone marrow in rats (Chang et al. 2009). Vitamin C is non-essentialism suppressors which have ability to decrease lipid peroxidation then forage free radicals (El-Sokkary and Awadalla 2011). Vitamin C plays a role in steroid production in metal-exposed rats that decline physiological strain-associated injury in sperm formation (Acharya et al. 2008).
The main objective of this study was to assess the toxicological effects of both metals Cd and Hg as individual and combined. Hepatotoxicity was evaluated by monitoring the biochemical parameters of the liver and their accumulation in the liver as well as therapeutic role of vitamin C in said toxicity in rabbits.
Materials and methods
Ethical statement
All animal trials were executed according to the local and worldwide protocols. The nearby direction is the Wet op de dierproeven (article 9) of Dutch law (international) and a similar law regulated by the Bureau of Animal Experiment Licensing, Local University.
Chemicals used
The tested chemicals and their prepared solutions used in this study were similar as described in our previous published study (Ali et al. 2019). The stock solutions were prepared in distilled water. All the working solutions were prepared freshly from stock solutions. All other chemicals and solutions used were of pro-analysis quality and obtained from regular commercial sources
Experimental animals
During research, the European rabbits (Oryctolagus cuniculus) of age 1 month were selected as the animal model. Rabbits were acclimatized for 2 weeks before the start of the experiment. During the acclimatization and experiments, appropriate food containing green fodder, vegetables, fruits, and dry feed (grains, i.e., wheat) as well as free access to water was provided. The experimental animals’ details were extensively explained in our recently published study (Ali et al. 2019).
Administration of cadmium and mercury
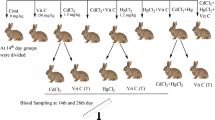
Sixty-six rabbits of both genders were arbitrarily classified into eight groups, i.e., one control and seven treatment groups. The administration of cadmium chloride, mercuric chloride, and vitamin C was carried out according to our recent published study (Ali et al. 2019). The experimental scheme for this study was shown in Fig. 1. The freshly prepared solutions (2 ml for each rabbit of each respective group) of mercuric chloride, cadmium chloride, and mixture of both metals and vitamin C at the dose rate of 1.2 mg/kg (6% of LD50), 1.5 mg/kg (10% of LD50), and 150 mg/kg body weight respectively were orally administered through gavages (Ghosh and Bhattacharya 1992).
Biochemical parameters
The blood sample was collected according to our recent published study (Ali et al. 2019). For the analysis of biochemical parameters, blood samples were centrifuged at 2000 rpm for 10 min at room temperature for the separation of serum. The aliquots were kept at − 20 °C until analysis (Naito and David 1984). Total protein (Henry 1964), alanine aminotransferase (ALAT), aspartate aminotransferase (ASAT) and lactate dehydrogenase (LDH) (Reitman and Frankel 1957), bilirubin (Malloy and Evelyn 1937), and gamma glutamyl transferase (GGT) (Rosalki et al. 1970) were determined either by already prescribed methods or by using commercially available kits
Estimation of metal concentration in the liver
At the end of the experiment, the same rabbits from which blood was collected were euthanized by intravenous administration of an over dose of sodium pentobarbital and livers were removed surgically and weighed on a digital weigh balance and stored at − 20C until further study. Each frozen liver sample was thawed, rinsed in distilled water, blotted followed by shifting into pre-weighed labeled glass flasks, and kept in oven at 105 °C until consistent weight. Dried weight of the liver was measured after cooling the flask in desiccators. For acid digestion following the protocol of Du Preez and Steyn (1992) with slight modification according to Yousafzai and Shakoori (2008), 1 ml perchloric acid and 5 ml nitric acid were added in each flask and samples were incubated for 12 hours at 30 °C. After incubation, perchloric acid (4 ml) and nitric acid (5 ml) were introduced in each flask and placed on a hot plate at 250 °C to digest and evaporate the mixture. Then, residual mixture (about 0.5 ml) diluted with distilled water up to 25 ml and filtered. The filtrate used as a sample to determine the metals contents by atomic absorption spectrophotometer. The liver sample of the rabbit from the control group was considered as blank. The standard solutions of different concentration were run as samples repeatedly after running the five liver samples to check the accuracy of the machine. Metal concentrations were expressed in milligrams per kilogram for the liver sample.
Data analysis
Data were presented as mean ± SEM. Normality of the data was assessed by Kolmogorov–Smirnov test and then analyzed statistically by one-way ANOVA, with “Dunnett’s multiple comparison test,” to identify any significant differences among the group means. GraphPad Prism version 5.0 for windows (GraphPad Software, San Diego, CA, USA) was used for analyses. Values of P ≤ 0.05 were considered as significant.
Results
Effect on alanine aminotransferase concentration
Orally administration of cadmium chloride (1.5 mg/kg body weight), mercury chloride (1.2 mg/kg body weight), and co-administration of CdCl2 + HgCl2 in rabbits for 28 alternative days showed a significant difference in alanine aminotransferase concentration. At the 14th day, the highest significant elevation from 34.0 ± 1.9 to 49.7 ± 2.5 IU/l and 49.7 ± 2.5 IU/l in the level of ALAT was found in Hg and Cd + Hg treatment groups as compared to control, respectively (Table 1).
At the 28th day, the highest significant elevation 34.4 ± 2.0 to 51.0 ± 1.6 IU/l, 53.8 ± 1.7 IU/l, and 49.6 ± 1.2 IU/l in the level of ALAT of Cd-, Hg-, and combined Cd + Hg–exposed groups was observed as compared to the control group, respectively. The highest significant decline varied from 49.0 ± 1.2 to 38.4 ± 1.6 IU/l was observed in the Cd + Hg (P)–treated group. Chemotreatment with Vit C showed high significant decrease from 51.0 ± 1.6 to 43.3 ± 1.0 IU/l and 49.0 ± 1.2 to 41.1 ± 1.6 IU/l in Cd- and Cd + Hg–treated groups with respect to control, respectively. Prevention with Vit C in Hg + Vit C (P) showed higher significant decline (53.8 ± 1.7 to 45.1 ± 1.3 IU/l) (Table 2).
Effect on total protein concentration
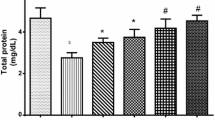
Total protein was found significantly decreased from 7.5 ± 0.4 to 6.0 ± 0.4 g/dl in combined CdCl2- and HgCl2-treated rabbits with respect to the control group whereas this decline was more significantly (7.5 ± 0.4 to 5.4 ± 0.2 g/dl, 5.7 ± 0.3 g/dl) observed in Cd- and Hg-treated groups, respectively. At the 14th day, prevention with vitamin C causes a significant elevation in total protein (Cd, 5.4 ± 0.2 g/dl; Cd + Vit C (P), 6.8 + 0.1 g/dl; Hg, 5.7 ± 0.3 g/dl; Hg + Vit C, 7.1 ± 0.2 g/dl). Non-significant difference was observed in the Cd + Hg + Vit C (P)–treated group (Table 1). At the 28th day, there was a highest significant decline (P ≤ 0.001) in the level of protein was observed with respect to the control group. Significant elevation in the level of total protein was (Cd + Hg, 4.8 + 0.3 g/dl; Cd + Hg + Vit C (P), 6.5 ± 0.3 g/dl) observed in the cadmium and mercury prevention group. While higher significant increase was (Cd, 4.4 ± 0.1 g/dl; Cd + Vit C (P), 6.3 + 0.3 g/dl; Hg, 3.7 ± 0.2 g/dl; Hg + Vit C, 5.7 ± 0.3 g/dl) observed in the level of total protein in cadmium and mercury prevention groups with usage of vitamin C at the 28th day of treatment. Treatment with vitamin C showed significant increase (4.4 ± 0.1 to 6.0 ± 0.3 g/dl) (3.7 ± 0.2 to 5.4 ± 0.4 g/dl) in the level of total protein in Cd + Vit C (T) and Hg + Vit C (T) when compared to cadmium- and mercury-treated groups, respectively (Table 2).
Effect on bilirubin concentration
Administration of Cd, Hg, and Cd + Hg caused the highest significant increase in the level of bilirubin (control, 1.7 ± 0.3 μmol/l; Cd, 4.5 ± 0.2 μmol/l; Hg, 4.0 ± 0.2 μmol/l; Cd + Hg, 3.7 ± 0.1 μmol/l). Pretreatment with vitamin C at the 14th day of treatment showed that level of bilirubin was the highest significantly decreased (Cd, 4.5 ± 0.2 μmol/l; Cd + Vit C, 3.0 ± 0.2 μmol/l) in the Cd + Vit C–treated group. Whereas significant decline was Hg, 4.0 ± 0.2; Hg ± Vit C, 3.1 ± 0.1 μmol/l (Cd + Hg, 3.7 ± 0.1 μmol/l; Cd + Hg + Vit C, 2.8 ± 0.1 μmol/l) (Table 1). At the 28th day of treatment, the highest significant elevation in the level of bilirubin (1.8 ± 0.1 to 4.8.3 ± 0.1 μmol/l, 4.4 ± 0.1 μmol/l, 4.2 ± 0.2 μmol/l) was observed in Cd-, Hg-, and Cd + Hg–exposed groups was observed as compared to the control group, respectively. Prevention with Vit C showed the highest significant decline (Cd, 4.8.3 ± 0.1 μmol/l; Cd + Vit C (P), 3.2 ± 0.1 μmol/l) (Hg, 4.4 ± 0.1 μmol/l; Hg + Vit C (P), 3.3 ± 0.1 μmol/l) (Cd + Hg, 4.2 ± 0.2 μmol/l; Cd + Hg + Vit C (P), 3.1 ± 0.1 μmol/l). Chemotreatment with Vit C showed significant decrease in the level of bilirubin in the Hg treatment group. Whereas higher significant decline in the level of bilirubin was (Cd, 4.8.3 ± 0.1 μmol/l; Cd + Vit C (T), 3.7 ± 0.2 μmol/l) (Cd + Hg, 4.2 ± 0.2 μmol/l; Cd + Hg + Vit C (T), 3.3 ± 0.1 μmol/l) investigated (Table 2).
Effect on lactate dehydrogenase concentration
Oral administration of cadmium chloride, mercury chloride, and CdCl2 + HgCl2 for 28 alternative days elevated the level of serum lactate dehydrogenase. At the 14th day of treatment, the higher and highest significant increase (Cont, 182.2 ± 8.5 IU/l; Cd, 251.2 ± 9.9 IU/l; Hg, 268.7 ± 7.0 IU/l; Cd + Hg, 234.8 ± 10.7 IU/l) was observed in the level of LDH. Prevention with vitamin C showed that level of LDH in serum was significantly decrease (Cd, 251.2 ± 9.9 IU/l; Cd + Vit C (P), 203.2 ± 7.2 IU/l; Hg, 268.7 ± 7.0 IU/l; Hg + Vit C (P), 218.5 ± 7.9 IU/l; Cd + Hg, 234.8 ± 10.7 IU/l; Cd + Hg + Vit C (P), 190.0 ± 8.6 IU/l) as shown in Table 1. At the 28th day, the highest significant elevation in the level of LDH of Cd-, Hg-, and Cd + Hg–exposed groups was (200.3 ± 4.9 to 276.0 ± 10.3 IU/l, 291.7 ± 6.5 IU/l, 270.5 ± 7.9 IU/l) observed as compared to the control group, respectively. Administration of Vit C through gavage in Cd-, Hg-, and Cd + Hg–treated groups caused a highest significant decline in the level of LDH was 276.0 ± 10.3 to 215.0 ± 8.3 IU/l, 291.7 ± 6.5 to 233.2 ± 6.6 IU/l, and 270.5 ± 7.9 to 203.8 ± 8.5 IU/l, respectively. Chemotreatment with Vit C showed high and higher significant decrease (Hg, 291.7 ± 6.5 IU/l; Hg + Vit C (T), 246.5 ± 10.2 IU/l) (Cd + Hg, 270.5 ± 7.9 IU/l; Cd + Hg + Vit C (T), 41.1 ± 1.6 IU/l) in mercury and Cd + Hg treatment groups respectively, while the highest significant decline was Cd, 276.0 ± 10.3 IU/l; Cd + Vit C (T), 218.8 ± 7.7 IU/l (Table 2).
Effect on aspartate aminotransferase concentration
The highest significant elevation in the level of ASAT was Cont, 6.8 ± 0.3 IU/l; Cd, 9.4 ± 0.3 IU/l; Hg, 9.5 ± 0.5 IU/l. Prevention with Vit C showed that level of ASAT in serum was significant and higher significantly decrease (Cd, 9.4 ± 0.3 IU/l; Cd + Vit C (P), 7.2 ± 0.4 IU/l; Cd + Hg, 9.0 ± 0.3 IU/l; Cd + Hg + Vit C (P), 7.0 ± 0.4 IU/l) in Cd + Vit C (P) and Cd + Hg + Vit C (P) treatment groups, respectively (Table 1). At the 28th day, the highest significant elevation in the level of ASAT was (6.5 ± 0.4 to 11.2 ± 0.4 IU/l, 11.5 ± 0.3 IU/l, 10.6 ± 0.2 IU/l) observed with respect to the untreated group. The highest significantly decline in the level of ASAT was (11.2 ± 0.4 to 8.3 ± 0.7 IU/l) (11.5 ± 0.3 to 9.4 ± 0.6 IU/l) (10.6 ± 0.2 to 7.5 ± 0.4 IU/l) observed in Cd, Hg, and Cd + Hg prevention groups, respectively. Chemotreatment with Vit C showed that significant decrease in ASAT was Cd + Hg, 10.6 ± 0.2 IU/l; Cd + Hg + Vit C (T), 7.9 ± 0.4 IU/l as shown in Table 2.
Effects on gamma glutamyl transferase concentration
At the 14th day of treatment, the highest significant increase (Con, 6.26 ± 0.2 IU/l; Cd, 13.5 ± 0.3 IU/l; Hg, 13.7 ± 0.6 IU/l; Cd + Hg, 15.3 ± 0.5 IU/l) was observed in the level of GGT. Significantly decrease in the level of GGT (Cd, 13.5 ± 0.3 IU/l; Cd + Vit C (P), 9.0 ± 0.4 IU/l; Hg, 13.7 ± 0.6 IU/l; Hg + Vit C (P), 8.4 ± 0.4 IU/l; Cd + Hg, 15.3 ± 0.5 IU/l; Cd + Hg + Vit C (P),11.6 ± 0.6 IU/l) was found in Cd, Hg, and Cd + Hg prevention groups, respectively (Table 1). At the 28th day, the highest significant elevation in the level of GGT of Cd-, Hg-, and Cd + Hg–exposed groups was (7.15 ± 0.37 to 15.10 ± 0.45 IU/l, 13.70 ± 0.61 IU/l, 16.53 ± 0.35 IU/l) observed as compared to the control group, respectively. Administration of Vit C through gavage in Cd-, Hg-, and Cd + Hg–treated groups caused a highest significant decline in the level of GGT was (15.10 ± 0.45 to 9.50 ± 0.39 IU/l) (13.70 ± 0.61 to 8.80 ± 0.35 IU/l) (16.53 ± 0.35 to 12.23 ± 0.63 IU/l), respectively. Chemotreatment with Vit C showed significant decrease (Hg, 13.70 ± 0.61 IU/l; Hg + Vit C (T), 11.02 ± 0.75 IU/l) in the Hg-treated group. While higher significant decrease was (Cd + Hg, 16.53 ± 0.35 IU/l; Cd + Hg + Vit C (T), 13.40 ± 0.34 IU/l) (Cd, 15.10 ± 0.45 IU/l; Cd + Vit C (T), 11.72 ± 0.75 IU/l) observed in Cd + Hg and Cd treatment groups, respectively (Table 2).
Metal accumulation in liver samples
Concentration of Hg and Cd in the liver of rabbits was measured through atomic absorption spectrophotometer. The liver of rabbits of the control group was treated as control/blank. The highest significant Cd concentration up to 42.33 ± 1.86 mg/kg and 30.33 ± 2.03 mg/kg was measured in Cd- and Cd + Hg–treated groups, respectively. Administration of Vit C showed the highest significantly decline in Cd concentration in the liver of rabbits of Cd + Vit C (P) (18.00 ± 1.15 mg/kg)– and Cd + Hg + Vit C (P) (11.33 ± 1.45 mg/kg)–exposed groups. The highest significant declined in concentration of Cd was observed in the Cd + Hg + Vit C (T) group as compared to the individual Cd-treated group. The highest significant concentration of Hg 27.33 ± 2.33 mg/kg was measured in the liver of rabbits exposed to Hg. While the presence of Vit C significantly decreased the concentration of Hg up to 14.00 ± 2.89 mg/kg in the Hg + Vit C (P) group as shown in Fig. 2b.
Concentration of a cadmium (Cd) and b mercury (Hg) (mg/kg) in the liver of control and experimental groups after the 28th day exposure. Asterisk indicates the significance difference between control and Cd, Hg, and Cd + Hg treatment groups. Number sign indicates the significance difference between Cd and Cd + Vit C (P) treatment groups. Tilde indicates the significant difference between Hg and Hg + Vit C (P) treatment groups. Ampersand indicates the significant difference between Cd + Hg and Cd + Hg + Vit C (P) treatment groups. Commercial at indicates the significance difference between Cd and Cd + Vit C (T) treatment groups. Circumflex accent indicates the significant difference between Hg and Hg + Vit C (T) treatment groups. Percent sign indicates the significant difference between Cd + Hg and Cd + Hg + Vit C (T) treatment groups. Each bar represents the mean value of six replicates and SEM. Statistical signs: $, @, %, ^ = p≤ 0.05, %%, ## = p≤ 0.01, ***, ###, @@@, &&&= p≤ 0.001
Discussion
In this study, we presented the toxicological evaluation of cadmium and mercury which was exposed individually and in combination to rabbits. Meanwhile, the remedial effect of vitamin C was also analyzed with respect to rehabilitates the altered hepatic functions by the aforesaid chemicals in experimentally designed exposure and duration in rabbits. Cadmium has been testified to be a hepatotoxic component. It has persuaded histopathological alterations in the liver and diminished biomarkers of liver functions in humans and other animals (Agarwal et al. 2010). After severe and prolonged disclosures to cadmium (Cd), the liver is one of the life-threatening target tissues (Yazihan et al. 2015). Hepatic damage due to introduction of mercury and cadmium is well known by higher intensities of liver enzymes in serum showing the damage of efficient reliability of liver tissue production and outflow of enzymes in blood (Renugadevi and Prabu 2010). According to Hosseini et al. (2018), mercury chloride (HgCl2) produced histopathological and ultrastructure injuries in the hepatic system by cell necrosis and periportal fatty disintegration.
In this study, it was observed that administration of Cd, Hg, and Cd + Hg showed the highest significant elevation (P ≤ 0.001) in the level of ASAT in individual Cd- and Hg-treated rabbits with respect to the control group at the 28th day of the experiment. Elevation of this enzyme indicates the impairment of hepatocytes. Different studies revealed that ASAT is increased in the circulatory system when hepatocytes or other organs are injured (Nathwani et al. 2005). Co-administration of cadmium and mercury in rats causes elevated levels of ASAT and ALAT in the liver as compared to the control group (Dardouri et al. 2016). In the present study, Vit C caused significantly (P < 0.01) decline in the level of ASAT in the Cd + Vit C (P) treatment group. Vitamin C reduces the effects of cadmium chloride and mercury chloride but not regain the values similar to the control group. Administration of CdCl2 and HgCl2 alone produced higher enzymatic disturbances than combine treatment with CdCl2 + HgCl2 due to antagonism among cadmium and mercury (Imed et al. 2008). Elevated level of LDH, ASAT, and ALAT in methyl mercury–exposed batch was improved by post treatment with Vit C. Vitamin C acts as an antioxidant and plays an important role to defend the hepatic system from oxidative impairment due to exposure of methyl mercury (Hounkpatin et al. 2012).
In this study, the highest significant elevation (P ≤ 0.001) in the level of LDH was observed in intoxicated rabbits with CdCl2 and HgCl2. Fewer modifications were observed in the combined CdCl2 + HgCl2–treated group with the passage of time. These results showed that elevation may occur due to damage of the plasma membrane of hepatocytes which result in leakage of LDH in blood. Prevention with vitamin C at the highest significantly declines the level of LDH in Cd-, Hg-, and Cd + Hg–treated groups. When cells are damaged, the level of LDH is increased in various parts of body containing the liver (Thapa and Walia 2007). When mallard ducks were treated with methyl mercury, the level of LDH was increased in plasma which shows that biological injuries occur in the heart and liver (El-Demerdash et al. 2001). According to Dardouri et al. (2016), co-exposure of CdCl2 + HgCl2 in rats showed that the level of LDH was higher as compared to when rats treated with individual cadmium and mercury chloride.
In this study, it was noted that administration of CdCl2, HgCl2, and co-administration of Cd + Hg had declined the level of total protein. The highest significant decline in the level of total protein was observed in all CdCl2- and HgCl2-exposed groups during treatment. Prevention and post treatment with vitamin C significantly increased the level of total protein. Exposure of mercury (Hg) causes decline the level of total protein. Depletion may occur due to cirrhosis or ability of protein required to the plasma toxicants elevated as well as synthesis of protein is declined (Sweety et al. 2008). It was reported that the normal level of total protein in serum of mice was increased. Administration of vitamin C prohibited antagonistic effects of heavy metals on hepatic parameters (Musa et al. 2012). These studies co-relate with our results.
Results of our study showed that the level of GGT was the highest significantly increased in all cadmium- and mercury-exposed groups with respect to the control group. Prevention with vitamin C the highest significance (P ≤ 0.001) declined the level of GGT in all cadmium- and mercury-exposed groups of rabbits. In all individual and combined Cd and Hg chemotreatment groups, the level of GGT was also decline but the level of decline was lower than prevention groups. The level of GGT significantly elevated in cadmium-exposed rats (Kim et al. 2001). Exposure of combination of heavy metals such as Cd, Hg, and Pb significantly increases the level of GGT in plasma (Bashandy et al. 2011). In this study, when liver samples were processed by using atomic absorption spectrophotometer, it was observed that cadmium chloride and mercury chloride had significant accumulation in the liver with the dose concentration of 1.5 mg/kg and 1.2 mg/kg body weight respectively in all intoxicated groups. Hence, findings indicate that the liver possesses the capacity to accumulate cadmium and mercury in higher concentrations when exposed to these metals. Josthna et al. (2012) studied that the liver is a sensitive organ for accumulation of Cd and Hg in mice; afterwards, it accumulates in the renal system and finally in tissues as well as in skeletal muscles.
In the present research, more Hg and Cd are accumulated in the liver of rabbits and had changed the biochemical profile of serum when administered orally. Increased levels of these biomarkers resulted into the hepatic injury. However, the intensity of these alterations of biochemical profile was encountered in the presence of vitamin C co-administration. According to Kaur and Sharma (2017), vitamin C (ascorbic acid) defends all growing animals such as rats, guinea pigs, turkeys, chickens, mice, and fish from toxicity of heavy metals (Cd, Hg, and Pb) as well as might be helpful in repairing the oxidative inhibitor (antioxidant) in hepatic structure. These findings may depict a protective mechanism of ascorbic acid in hepatic toxicity in heavy metals–intoxicated rabbits.
Conclusion
These results showed that the liver is a delicate organ exaggerated by exposure of CdCl2 and HgCl2. Cadmium and mercury can induce the hepatocellular impairment by elevation of oxidative stress in the liver. Results of the recent study show that individual exposure of CdCl2 and HgCl2 and co-administration of cadmium and mercury alters the various biochemical parameters (ALAT, ASAT, LDH, GGT, and bilirubin) of the animal model which can be ameliorated through supplementation of vitamin C. Ameliorating potential of vitamin C in metals-administered rabbits was observed on the 14th and 28th days of the experiment. Vitamin C acts as an antioxidant agent which can alleviate the hepatic toxins like Cd and Hg by inhibiting the lipid peroxidation, preventing the formation of reactive oxygen species, and maintaining the activity of biochemical and hematological parameters. Vitamin C can stabilize the cell membrane in hepatic impairment stimulated by mercury and cadmium. Based on these findings, vitamin C seems to have protective mechanism in hepatic toxicity.
References
Acharya UR, Mishra M, Patro J, Panda MK (2008) Effect of vitamins C and E on spermatogenesis in mice exposed to cadmium. Reprod Toxicol 25(1):84–88
Agarwal R, Raisuddin S, Tewari S, Goel SK, Raizada RB, Behari JR (2010) Evaluation of comparative effect of pre- and posttreatment of selenium on mercury-induced oxidative stress, histological alterations, and metallothionein mRNA expression in rats. J Biochem Mol Toxicol 24(2):123–135
Ali S, Hussain S, Khan R, Mumtaz S, Ashraf N, Andleeb S, Shakir HA, Tahir HM, MKA K, Ulhaq M (2019) Renal toxicity of heavy metals (cadmium and mercury) and their amelioration with ascorbic acid in rabbits. Environ Sci Pollut Res Int 26:3909–3920
Baselt RC, Cravey RH (2000) Disposition of toxic drugs and chemicals in man, 4th edn. Year Book Medical Publishers, Chicago, IL, pp 105–107
Bashandy SA, Alhazza IM, El-Desoky GE, Al-Othman ZA (2011) Hepatoprotective and hypolipidemic effects of Spirulina platensis in rats administered mercuric chloride. Afr J Pharm Pharmacol 5(2):175–182
Bernard A (2008) Cadmium & its adverse effects on human health. Indian J Med Res 128(4):557–564
Birch RJ, Bigler J, Rogers JW, Zhuang Y, Clickner RP (2014) Trends in blood mercury concentrations and fish consumption among US women of reproductive age, NHANES, 1999–2010. Environ Res 133:431–438
Caito S, Aschner M (2015) Neurotoxicity of metals. ClinNeurol 131(2):169–189
Castagnetto JM, Hennessy SW, Roberts VA, Getzoff ED, Tainer JA, Pique ME (2002) MDB: the metalloprotein database and browser at the Scripps Research Institute. Nucleic Acids Res 30(1):379–382
Chang HJ, Park JS, Lee EK, Kim MH, Baek MK, Kim HR, Jeong HG, Choi SY, Jung DY (2009) Ascorbic acid suppresses the 2, 3, 7, 8-tetrachloridibenxo-p-dioxin (TCDD)-induced CYP1A1 expression in human HepG2 cells. Toxicol In Vitro 23(4):622–626
Chibunda R, Janssen C (2009) Mercury residues in free-grazing cattle and domestic fowl form the artisanal gold mining area of Geita district, Tanzania. Food Addit Contam 26(11):1482–1487
Dardouri K, Haouem S, Gharbi I, Sriha B, Haouas Z, El Hani A, Hammami M (2016) Combined effects of Cd and Hg on liver and kidney histology and function in wistar rats. J Agr Chem Environ 5(04):159
Delraso NJ, Foy B, Gearhart J, Frazier J (2003) Cadmium uptake kinetics in rat hepatocytes: correction for albumin binding. Toxicol Sci 72(1):19–30
Du Preez H, Steyn G (1992) A preliminary investigation of the concentration of selected metals in the tissues and organs of the tigerfish (Hydrocynus Vit Ctatus) from the Olifants River, Kruger National Park, South Africa. Water SA 18(2):131–136
El-Demerdash F, Yousef M, Elagamy E (2001) Influence of paraquat, glyphosate, and cadmium on the activity of some serum enzymes and protein electrophoretic behavior (in vitro). J Environ Sci Health Part B 36(1):29–42
El-Sokkary GH, Awadalla EA (2011) The protective role of vitamin C against cerebral and pulmonary damage induced by cadmium chloride in male adult albino rat. The Neuroendocrinol J 4(1):20–30
Ercal N, Gurer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 1(6):529–539
Fujishiro H, Okugaki S, Kubota K, Fujiyama T, Miyataka H, Himeno S (2009) The role of ZIP8 down-regulation in cadmium-resistant metallothionein-null cells. J Appl Toxicol 29(5):367–373
Gajawat S, Sancheti G, Goyal P (2005) Vitamin C against concomitant exposure to heavy metal and radiation: a study on variations in hepatic cellular counts. Asian J Exp Sci 19(2):53–58
Genchi G, Sinicropi MS, Carocci A, Lauria G, Catalano A (2017) Mercury exposure and heart diseases. Int J Environ Res Public Health 14(1):74
Ghosh N, Bhattacharya S (1992) Thyrotoxicity of the chlorides of cadmium and mercury in rabbit. Biomed Environ Sci 5(3):236–240
Heinrich J, Guo F, Trepka MJ (2017) Brief report: low-level mercury exposure and risk of asthma in school-age children. Epidemiology 28(1):116–118
Henry RJ (1964) Colorimetric determination of total protein: clinical Chemistry. Harper and Row, New York, pp 181–183
Hosseini A, Rajabian A, Fanoudi S, Farzadnia M, Boroushaki MT (2018) Protective effect of Rheum turkestanicum root against mercuric chloride-induced hepatorenal toxicity in rats. J Phytomed 8(6):488–491
Hounkpatin A, Johnson R, Guedenon P, Domingo E, Alimba C, Boko M, Edorh P (2012) Protective effects of vitamin C on haematological parameters in intoxicated wistar rats with cadmium, mercury and combined cadmium and mercury. Int Res J Biol Sci 1(8):76–81
Huq M, Awal M, Mostofa M, Ghosh A, Das A (2013) Effects of vitamin E and vitamin C on Mercury induced toxicity in mice. Progress Agric 19(2):93–100
Imed M, Fatima H, Abdelhamid K (2008) Protective effects of selenium (Se) and zinc (Zn) on cadmium (Cd) toxicity in the liver and kidney of the rat: histology and Cd accumulation. Food Chem Toxicol 46(11):3522–3527
Jagadeesan G, Pillai SS (2007) Hepatoprotective effects of taurine against mercury induced toxicity in rat. J Environ Biol 28(4):753–756
Joshi D, Mittal DK, Shukla S, Srivastav AK (2012) Therapeutic potential of N-acetyl cysteine with antioxidants (Zn and Se) supplementation against dimethyl mercury toxicity in male albino rats. Exp Toxicol Pathol 64(1):103–108
Josthna P, Geetharathan T, Sujatha P, Deepika G (2012) Accumulation of lead and cadmium in the organs and tissues of albino rat. Int J Pharm Sci 3(12):114–128
Kampalath RA, Jay JA (2015) Sources of mercury exposure to children in low-and middle-income countries. J Health Pollu 5(8):33–51
Kaur S, Sharma S (2017) A histometric study to assess preventive action of ascorbic acid and garlic on cadmium induced hepatotoxicity in albino mice. Int J Pharm Phytopharmacol Res 23:5(3):8–14
Kim KA, Lee WK, Kim JK, Seo MS, Lim Y, Lee Y (2001) Mechanism of refractory ceramic bre and rock wool induced cytotoxicity in alveolar macrophages. Int. Arch. Occup Environ Health 74:9–15
Koyu A, Gokcimen A, Ozguner F, Bayram DS, Kocak A (2006) Evaluation of the effects of cadmium on rat liver. Mol Cell Biochem 284(1):81–85
Malloy HT, Evelyn KA (1937) The determination of bilirubin with the photometric colorimeter. J Biol Chem 119:481–490
Martin S, Griswold W (2009) Human health effects of heavy metals. Sci Technol Brief Cit 15:1–6
Musa SA, Omoniye IM, Hamman WO, Ibegbu AO, Umana UE (2012) Preventive activity of ascorbic acid on lead acetate induced cerebellar damaged in adult wistar rats. Med Health Sci J 13:99–104
Mutlu A, Lee BK, Park GH, Yu BG, Lee CH (2012) Long-term concentrations of airborne cadmium in metropolitan cities in Korea and potential health risks. Atmospheric Environ 47:164–173
Naito HK, David JA (1984) Laboratory considerations: determination of cholesterol, triglyceride, phospholipid, and other lipids in blood and tissues. Lab Res Methods Biol Med 10(2):1–7
Nathwani RA, Pais S, Reynolds TB, Kaplowitz N (2005) Serum alanine aminotransferase in skeletal muscle diseases. Hepatol 41(2):380–382
Nithya NK, Chandrakumar VG, Senthilkumar S (2012) Efficacy of Momordica charantia in attenuating hepatic abnormalities in cyclophosphamide intoxicated rats. J Pharmacol Toxicol 7(1):38–45
Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, Wesley RA, Levine M (2004) Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med 140(7):533–537
Rana T, Bera AK, Das S, Pan D, Bandyopadhyay S, Bhattacharya D, De S, Sikdar S, Das SK (2010) Effect of ascorbic acid on blood oxidative stress in experimental chronic arsenicosis in rodents. Food Chem Toxicol 48(4):1072–1077
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28(1):56–63
Renugadevi J, Prabu SM (2010) Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp Toxicol Pathol 62(2):171–181
Rosalki SB, Rav D, Lchman D, Prentice M (1970) Determination of serum gamma-glutamyl transpeptidase activity and its clinical applications. Ann Clin Biochem 7:143–147
Sakamoto M, Man Chan H, Domingo JL, Kubota M, Murata K (2012) Changes in body burden of mercury, lead, arsenic, cadmium and selenium in infants during early lactation in comparison with placental transfer. Ecotoxicol Environ Saf 84:179–184
Sweety R, Mathan RR, Kenneth S, Sajwan K (2008) Influence of zinc on cadmium induced haematological and biochemical responses in a freshwater teleost fish Catlacatla. Fish Physiol Biochem 34:169–174
Tay CK, Asmah R, Biney CA (2010) Trace metal levels in water and sediment from the Sakumo II and Muni Lagoons, Ghana. West Afr J App Ecol 16(1):75–94
Thapa B, Walia A (2007) Liver function tests and their interpretation. Indian J Pediatr 74(7):663–671
Waisberg M, Joseph P, Hale B, Beyersmann D (2003) Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicol 192(2):95–117
Yannai S, Berdicevsky I, Duek L (1991) Transformations of inorganic mercury by Candida albicans and Saccharomyces cerevisiae. Appl Environ Microbiol 57(1):245–247
Yazihan N, Kocak MK, Akcil E, Billur D, Ermis E, Cesaretli Y, Erdem O, Uzunalimoglu O (2015) Chronic cadmium toxicity induces inflammation and galectin-3 expression whereas suppresses the hypoxia inducible factor mRNA expression in the liver. Trace Elem 32:1–10
Yousafzai AM, Shakoori AR (2008) Heavy metal accumulation in gills of an endangered South Asian freshwater fish as an indicator of aquatic pollution. Pak J Zoo 40(6):423–430
Yu X, Sidhu JS, Hong SW, Robinson JF, Ponce RA, Faustman EM (2011) Cadmium induced p53 dependent activation of stress signaling, accumulation of ubiquitinated proteins and apoptosis in mouse embryonic fibroblast cells. Toxicol Sci 3(2):1–10
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mumtaz, S., Ali, S., Khan, R. et al. The protective role of ascorbic acid in the hepatotoxicity of cadmium and mercury in rabbits. Environ Sci Pollut Res 26, 14087–14096 (2019). https://doi.org/10.1007/s11356-019-04620-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04620-5