Abstract
Lichens are useful biomonitors for atmospheric polycyclic aromatic hydrocarbons (PAHs). Different sample preparation techniques were explored in this regard, including ultrasound-assisted solvent extraction, microwave-assisted extraction, Soxhlet, and the quick, easy, cheap, effective, rugged, and safe (QuEChERS) technique. It was found that a QuEChERS technique using hexane:acetone (1:1, v/v), never reported before for application to lichens, provided the best recoveries of internal standards, the highest total peak area for all PAHs of interest, and %RSDs comparable with the other preparation techniques tested. The optimized sample preparation technique was found to be a comparatively fast method (45 min), with good recoveries (96%), using less solvents and minimal energy consumption. Strong matrix effects were found: both strong enhancement (for the lighter PAHs) and strong suppression (for the heavier PAHs). The use of matrix-matched standards is thus imperative for the accurate determination of PAH concentrations in the lichen samples.

“Note: This data is mandatory. Please provide.”
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are analytes of interest in environmental and food analyses as a result of their toxic and carcinogenic properties, according to the International Agency for Research on Cancer (IARC) (Clapp et al. 2008). Benzo[a]pyrene (BaP) was the first PAH classified as carcinogenic and since then, benzo[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[j]fluoranthene, benzo[k]fluoranthene, indeno[1,2,3-cd]pyrene, and dibenzo[ah]anthracene have also been listed as carcinogenic and teratogenic chemicals (Ravindra et al. 2001).
Lichens are symbiotic organisms that accumulate hydrophobic and hydrophilic compounds via volatilization of compounds from the soil, wind impaction, or wash-off from rainwater (Jones and Duarte-Davidson 1997; Trapp and Matthies 1997). Lichens have been used as biomonitors for sulfur dioxide, metals (including cobalt, zinc, mercury, zinc, cesium, lead), fluoride, and radionuclides (Hawksworth and Rose 1970; Garty et al. 1977; Garty 2001; Sloof and Wolterbeek 1991, 1992, 1995; Gombert et al. 2003; Forbes et al. 2009; Zhao et al. 2019). More recently, the use of lichens as biomonitors has focused on organic pollutants, particularly PAHs as well as dioxins and furans, as reviewed by Van der Wat and Forbes (2015).
Historically, Soxhlet has been applied to the extraction of organic analytes from lichens (Augusto et al. 2004), including the extraction of PAHs from this matrix (Augusto et al. 2010, 2012; Shukla and Upreti 2009; Shukla et al. 2012). Soxhlet has been successfully used for the extraction of PAHs from soils, bark, and pine needles (Dean et al. 1995; Di Lella et al. 2006; Ratola et al. 2006; Orecchio et al. 2008; Augusto et al. 2010). In addition, ultrasound-assisted extraction (USAE) has been applied to the lichen matrix (Guidotti et al. 2003), with improvements to the technique suggested by Domeño et al. (2006).
The use of microwave energy to extract PAHs from lichens is uncommon; however, microwave-assisted extraction (MAE) has been performed to extract PAHs from numerous matrices such as soils, sediments, and even smoked meat samples (Chee et al. 1996; Bartolomé et al. 2005; Srogi 2006; Purcaro et al. 2009). A study on PAHs in spruce needles and pollen (Tomaniova et al. 1998) found MAE to be superior over USAE, using a solvent scheme of n-hexane:acetone (3:2, v/v). A study by Ratola et al. (2009) on PAHs in pine needles found better recoveries for the higher molecular weight PAHs using MAE, compared with USAE, where a solvent scheme of hexane:dichloromethane (1:1) was used. Similar findings were seen in the study by Purcaro et al. (2009) on smoked meats, where extracts obtained by MAE were found to have higher extraction efficiencies for PAHs than USAE extracts from the same sample.
A more recent sample extraction technique was designed for the extraction of pesticides in food products. The features of this technique developed by Anastassiades et al. (2003), named QuEChERS, are that it is quick, easy, cheap, effective, rugged, and safe. As a result of the commercialization of sample preparation packs, this effective technique has risen in popularity and is now commonly applied to pesticide analyses in foodstuffs (EN 15662:2008) (European Commitee for Standardization 2009). The method is presented as an alternative to traditional liquid-liquid extraction and solid-phase extraction techniques, since it minimizes solvent use, extraction time, and the total number of steps in the extraction process (Wilkowska and Biziuk 2011).
QuEChERS has been used for extracting PAHs from foodstuffs, with seafood being the first samples reported (João Ramalhosa et al. 2009; Forsberg et al. 2011; Kalachova et al. 2011). QuEChERS has also been applied to PAHs in teas, where a study by Drabova et al. (2012) found that ethyl acetate was the preferred extraction solvent. Cvetkovic et al. (2016) investigated PAHs in soil using QuEChERS and found that acetonitrile:water (2:1, v/v) was the preferred extraction solvent although their extraction procedure included a sonication step. There are no publications to date that have used the extraction of PAHs from lichens using the QuEChERS technique.
This work compares the use of Soxhlet, USAE, MAE, and a novel QuEChERS method as sample preparation techniques with extract PAHs from a bulk lichen sample collected along an urban road in Pretoria, South Africa. A comparison of the results from the sample preparation techniques is made, using ANOVA, in order to establish whether a faster, more effective, and greener sample preparation technique, compared with the traditional Soxhlet method, can be identified for the extraction of PAHs from the lichen matrix.
Materials and method
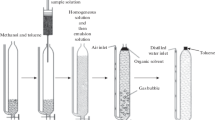
The general experimental approach employed to establish the best method of extracting PAHs from lichen samples is shown in Fig. 1. Four distinct sample preparation techniques were investigated followed by an identical sample cleanup procedure prior to instrumental analysis.
Solvents and standards
All solvents used were purchased from Merck (Gauteng, South Africa) and were of analytical grade, except for the acetonitrile, which was from the LiChrosolv range from Merck (Darmstadt, Germany) and was liquid chromatography grade. All water was purified using a Millipore system (MA, USA). High purity nitrogen gas for the blown down of extracts was obtained from Afrox (Gauteng, South Africa). Standards were obtained from Sigma Aldrich (St Louis, USA). A PAH standard mix of nominal concentration 2000 μg ml−1 for each PAH in methylene chloride was obtained from Supelco (St Louis, USA) containing 15 PAHs, as listed in Table 1 with their associated abbreviations. The surrogate standard was a mix of both phenanthrene-d10 and pyrene-d10, prepared gravimetrically from the pure solids dissolved in toluene, resulting in a final standard of concentration 500 ng μl−1 for each PAH.
Sampling area and procedure
Parmotrema austrosinense (Zahlbr.) Hale lichens were sampled midmorning during a 1-day sampling campaign in the dry season so that any variations caused by changes in humidity, wind, and temperature as well as seasonal and mesoclimatic effects were eliminated. The choice of location was based on the high density of lichen-bearing Jacaranda mimosifolia trees in the area. The urban road had medium traffic density for most hours of the day; thus, the lichens were expected to contain PAHs at suitable levels for method development. Lichens were exclusively identified and thus sampled, on the southern side of the tree trunks, which is facing towards the road. Lichens were removed from the trunk and low branches of the trees 1–2 m from the road at heights 1–1.7 m above ground level to avoid any bias from adsorption of PAHs volatilized from contaminated soil. Lichens were carefully pried from the bark with stainless steel tweezers and placed directly into one large amber glass bottle and sealed tightly as suggested by Szulejko et al. (2014). This bulk lichen sample (> 100 g) was then transported to the laboratory, placed inside a resealable plastic bag, and stored at 4 °C for 1 day. The exogenous matter was then removed from the lichens using tweezers. The lichen samples were placed in an oven (1.60 kW, Binder, Germany) at 35 °C for 4–5 days to constant mass. The lichens were then ground to a powder using a marble pestle and mortar, transferred to a glass bottle, wrapped in aluminum foil, placed in a resealable plastic bag, and stored at − 18 °C until required.
Sample preparation
Soxhlet extraction
A 0.2-g dried and ground bulk lichen sample was weighed into a Whatman glass microfiber thimble (25 mm ID × 90 mm in length, tapered, high purity), spiked with 10 μl surrogate standard, and extracted with 100 ml dichloromethane using the traditional Soxhlet apparatus for 6 h. The extraction was performed in triplicate. The same procedure was followed, using acetonitrile. Both types of extracts were cooled prior to blow down to 2 ml under N2, followed by the cleanup step (the “Sample extract clean-up procedure” section), after which all extracts were made up to a final volume of 500 μl in toluene. Blank extractions were also performed.
Ultrasound-assisted solvent extraction
A 0.2-g portion of dried and ground bulk lichen sample was placed into a 15-ml amber vial (Supelco, St Louis, MO) and spiked with 10 μl surrogate standard, and 12 ml dichloromethane or hexane:acetone (1:1, v/v) extraction solvent was added. After sonication for 15 min in an ultrasonic bath, the contents were transferred to a centrifugation tube and centrifuged at 6000 U min−1 for 10 min. The solvent was decanted, and the lichen solids were placed back into the original glass vial and the extraction was repeated with fresh extraction solvent, followed by centrifugation. A total of three sequential extractions were thus performed and the extracts combined. The temperature of the water bath was maintained under 30 °C. Each extraction procedure was prepared in triplicate. The combined extraction solvent for each sample was then blown down under nitrogen to 2 ml. The extracts were cleaned up (the “Sample extract clean-up procedure” section) and all extracts were made up to a final volume of 500 μl in toluene. Blank extractions were also performed.
Microwave-assisted extraction
Three portions of the bulk lichen sample of mass 0.2 g each were weighed out into 100-ml quartz microwave tubes with Teflon® lined caps. The lichen portions were spiked with 10 μl of the surrogate standard and 12 ml hexane:acetone (1:1, v/v) was added. They were then digested in an Anton Paar Synthos 3000 microwave system using the heating program: 2-min ramp from 0 to 150 W, held at 150 W for either 5, 10, or 20 min, then cooled at 0 W for 10 min as shown in Table 2. The pressure was increased at 2.0 bar s−1. An extraction using dichloromethane as solvent was also performed in triplicate, as shown in Table 2. The extracts were then removed, decanted into amber vials, and concentrated to 2 ml under N2 before the same cleanup procedure was conducted (the "Sample extract clean-up procedure" section) after which all extracts were made up to a final volume of 500 μl in toluene. Blanks were similarly prepared.
QuEChERS extraction
The QuEChERS extraction involved a 30-min agitation time using a vortex mixer (Heidolph REAX) and the use of ice to cool down the extracts in order to control the extraction temperature, as shown in Fig. 2. Hexane:acetone (1:1, v/v), hexane:dichloromethane (1:1, v/v), and dichloromethane only were investigated as extraction solvents. The solvent volumes, spike volumes, and water volumes were kept constant in all extractions (as shown in Fig. 2), and blank extracts were performed alongside each extraction.
Sample extract cleanup procedure
The sample extract cleanup procedure was taken directly from a lichen optimization study by Blasco et al. (2007). Briefly, Strata–NH2 solid-phase extraction (SPE) cartridges (500 mg, bed volume 6 ml) were used. 0.05 g of both florisil and Na2SO4 was added to the column, and hexane:dichloromethane (65:35, v/v) was used as the elution solvent. The eluent was evaporated to dryness, made up in 500 μl toluene, vortexed for 2 min, and stored at − 18 °C until analysis.
Instrumental analysis
All analyses were performed on an Agilent 6890 Series GC (Palo Alto, CA, USA) coupled to an Agilent 5975C MSD (Palo Alto, CA, USA) operated in both scan and selected ion monitoring (SIM) modes. The ions monitored were m/z 128, 154, 156, 166, 178, 202, 228, 252, 276, and 278. Helium was used as the carrier gas at a flow rate of 1.0 ml min−1 in constant flow mode. A 1-μl (manual injection, splitless, purge flow 40 ml min−1) volume of extract was introduced onto a Restek Rxi®-PAH column (60 m, 0.25 mm ID, 0.25 μm df), with an oven program at 80 °C (1 min), 30 °C/min to 180 °C, and 2 °C/min to 320 °C. The solvent delay was set to 6.5 min, with an inlet temperature of 275 °C and transfer line temperature set to 300 °C. The total run time was 74 min. All PAHs were quantified using the SIM ion peak areas. The statistical programs StatPlus:mac v5 and JMP 10 software were used for data analysis.
Matrix-matched standards
Five matrix-matched standards were prepared using the modified QuEChERS extraction using 12 ml hexane:acetone (1:1, v/v) with a 30-min shaking step followed by the given cleanup procedure (the “Sample extract clean-up procedure” section). The five extracts were then spiked, with an increase in concentration of the standard PAH mix from 0.01–0.5 ng μl−1. An unspiked lichen extract was also prepared and the entire matrix-matched standard set was analyzed by gas chromatography–mass selective detector (GC–MSD). The matrix effect of each PAH was then calculated using the equation:
A negative % matrix effect implied a suppression of the signal, and a positive % matrix effect implied signal enhancement. The extent to which the matrix was interfering with the instrumental response of the analyte was then classified as strong, medium, or soft according to the work by Rajski et al. (2013).
Fluorescence spectroscopy
Fluorescence spectroscopy was used to determine the relative concentration of chlorophyll in the bulk sample extract and that of a sample of the same lichen species collected 20 km away. Both chlorophyll a (410 nm excitation) and chlorophyll b (452 nm excitation) were monitored using a FluoroMax4 Spectrofluorometer (Horiba Scientific, Jobin Yvon Technology, Edison, NJ). QuEChERS extracts using hexane:acetone (1:1) were analyzed at both excitation wavelengths by placing 30 μl of each sample extract in a clean quartz cuvette to which 3000 μl toluene was added with aspiration. Toluene was used as the blank. Summing the peak heights of both chlorophyll a and chlorophyll b for each sample of interest gave a comparative indication of the total chlorophyll content of the extracts.
Results and discussion
Comparison of extraction methods
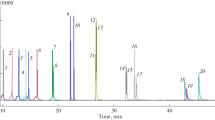
A comparison between the extraction efficiencies of the different sample extraction techniques was made in order to establish which sample preparation technique was most suited to the extraction of PAHs from Parmotrema austrosinense (Zahlbr.) Hale lichens. The profile of PAHs extracted from the native lichen matrix would provide an indication of the ability of the method to quantitatively and qualitatively extract the analytes of interest. Figure 3 includes the results from the traditional sample preparation techniques, namely Soxhlet and USAE previously used by other groups in PAH biomonitoring studies (Domeño et al. 2006; Augusto et al. 2010; Shukla and Upreti 2013), as well as MAE and QuEChERS.
It was found that Soxhlet with dichloromethane outperformed Soxhlet using acetonitrile by extracting a greater number of PAHs as seen in Fig. 4, producing a larger total PAH concentration. Soxhlet did not efficiently extract the PAHs under investigation, despite its application in other lichen studies (Augusto et al. 2010; Bajpai et al. 2013) and recoveries of > 80% having been reported (Shukla and Upreti 2013). The principles of Soxhlet extraction also did not conform to the desired outcomes of this study, since it uses large solvent quantities and has long extraction times, rendering it unsuitable as a fast, environmentally friendly extraction procedure.
The USAE results showed that two extractions were not sufficient to extract the PAHs from the lichen matrix, with particular regards to the PAHs of MW 178 g mol−1 and higher. The %RSD of the USAE 2 extracts (two consecutive extractions) was also very high, suggesting that more than two extractions on the same matrix are required in order to reduce the %RSD, thus improving the precision of the preparation technique, as well as increasing the number of PAHs partitioned into the extract and improving the method limit of detection as a result of the greater number of extractions on the sample. Comparing the results of USAE 3 and USAE 4, the peak areas were very similar, and the %RSD values did not improve for all PAHs with the extra extraction. It was therefore decided that three consecutive extractions on the lichen matrix would be sufficient, similar to other studies on PAHs in environmental matrices (Ratola et al. 2006; Drabova et al. 2012). It was also found that dichloromethane extracted PAHs better than n-hexane:acetone under ultrasonic energy; dichloromethane extracted a greater quantity of each PAH, as seen in Fig. 4. Dichloromethane also provided better recoveries of the surrogate standard and greater peak intensities for the identified PAHs, compared with the n-hexane:acetone extracts. However, no heavy PAHs (MW 228 g mol−1 and higher) were detected in any of the USAE extracts.
In terms of the MAE extractions, the n-hexane:acetone (1:1, v/v, abbreviated as MAE Hex:Ace)–based extraction performed better than when using dichloromethane as solvent (abbreviated as MAE DCM), both qualitatively and quantitatively, as can be seen in Fig. 3. The application of n-hexane:acetone as extraction solvent extracted more PAHs and extracted heavier PAHs such as Phe, FluAn, Pyr, and Ant, as opposed to the dichloromethane extraction which extracted only the lightest PAHs: Nap, Acy, and Ace and with lower efficiency. Comparing the recovery of the surrogate standards in the MAE dichloromethane and MAE n-hexane:acetone extracts in Fig. 5, it can be seen that n-hexane:acetone performed better and confirms it as the choice of solvent when using MAE, over dichloromethane. The total number of PAHs extracted from the bulk sample was only determined to be 3, with the 5-min extraction time, as opposed to 7 (10 min) and 8 (20 min) (Fig. 4). It was observed, however, that the recoveries of the surrogate standards as well as the %RSDs were best in the samples that were only extracted for 5 min. This suggested a non-selective degradation of PAHs with longer extraction times (Camel 2000). Despite this, 5 min was not suitable because it was insufficient in extracting PAHs from the matrix, extracting only 3 PAHs, compared with 7 and 8 (for the 10 and 20 min extraction), respectively. Comparing the total peak area of all identified PAHs, the 5-min extractions were dwarfed by the other two extraction times. Overall, for MAE, the 20-min extraction time extracted more PAHs as well as provided the highest total peak area for all identified PAHs.
The QuEChERS extraction using dichloromethane only (Q DCM), as well as the extraction with n-hexane:dichloromethane (1:1, v/v) (Q Hex:DCM), performed well compared with the previously investigated sample preparation techniques, allowing for the identification of 8 PAHs in the bulk lichen sample. However, as seen in Fig. 4, the hexane:acetone extraction (Q Hex:Ace) outperformed both of the other solvent schemes using QuEChERS, extracting 11 of the 16 PAHs of interest from the bulk lichen sample. Of particular interest is the extraction of heavier PAHs: BaP, IcdP, and DahA, which were identified in none of the other extracts of the native bulk lichen sample. Quantitatively, the Q Hex:Ace also performed best, as can be seen in Fig. 3, where the average peak area for most of the identified PAHs is largest in the n-hexane:acetone extract. It is clear from the % recovery of both phenanthrene-d10 and pyrene-d10 that Q Hex:A extracts had the best extraction efficiency, with recoveries of 96% and 178% for the respective surrogate standards. The recoveries for the surrogate standards in the DCM and n-hexane:DCM extracts were comparable, as were the %RSDs for the surrogate standard recoveries. The %RSDs for the surrogate standards were lowest for the Q Hex:A extract (15.2 and 30.6%), confirming that Q Hex:A should be the solvent scheme of choice for QuEChERS extractions of lichens.
The average total peak areas for all identified PAHs, as shown in Fig. 3, afford the conclusion that the QuEChERS Hex:A extraction technique outperformed the other sample extraction techniques both qualitatively and quantitatively. Q Hex:Ace performed better than the conventional sample extraction techniques in terms of surrogate standard recoveries, total targeted PAHs extracted (total PAH peak area), and the largest total number of individual PAHs extracted (11 PAHs in the native bulk sample, compared with 6 using either the Soxhlet dichloromethane or USAE). One-way ANOVA was performed on the results shown in Fig. 3 to identify significant differences between the sample extraction techniques. For the QuEChERS extractions, a significant difference (p = 0.0064, F(2,6) = 13.191, Fcrit = 10.925, 99% confidence level) was observed between the Q Hex:Ace extracts and the Q DCM and Q hexane:DCM extracts. Furthermore, one-way ANOVA revealed that there were no significant differences between the other sample extraction techniques (MAE, USAE, and Soxhlet) where p = 0.0103 and F(2,6) = 10.81 (Fcrit = 10.925) are at 99% confidence level. When one-way ANOVA was performed on the QuEChERS Hex:Ace and MAE, USAE, and Soxhlet results, the calculated p value of 0.00004 (F(3,8) = 71.66, Fcrit 7.591) showed that it can confidently be claimed that QuEChERS outperformed the other sample extraction techniques.
Matrix-matched standards
In making these comparisons between the different sample extraction techniques and conditions, it was assumed that the matrix effects were similar for all the extracts. Once the most suitable sample preparation technique had been determined, matrix-matched standard curves were created (for which r2 values ranged from 0.93 for DahA to 0.99 for Phe), and the matrix effects were calculated. Table 3 shows the results from the calculation of the % matrix effect for each of the PAHs of interest. The observed matrix effects ranged from strong suppression (IcdP) to strong enhancement (Nap, Acy, Flu, Ant, Phe, FluAn, Pyr, BaA, Chr, and BbF). The LODs for individual PAHs derived from the matrix-matched standards ranged between 0.1 and 7.4 ng g−1 dried weight.
The PAHs experiencing the most severe matrix effects were Ant and Pyr, with % matrix effects above 300%, indicating a very strong enhancement. These results heavily impact the interpretation of the analysis, since any diagnostic ratio or toxic equivalence quotient could be severely changed due to matrix effects. This would lead to misleading conclusions about the main sources of PAH contamination in the atmosphere. The observed matrix suppression for Ace, IcdP, BghiP, and DahA is also problematic since the suppression of analytes, already present at low levels, means that they might not be detected in a sample, despite their presence in the atmosphere. This type of matrix effect is most difficult to overcome since a corrected calibration is not able to correct for the interference, if the analyte is being suppressed to the extent that it is not detected in a sample extract. It should be noted that no non-impacted lichen standard reference material is available which can be used as a PAH-free matrix, where even lichens sampled in remote valleys in the Alps have been found to contain PAHs (Nascimbene et al. 2014). The practical implications of the severity of the matrix effects include regular liner replacements and the removal of the head of the column (or the employment of a guard column) impacting both on the cost and time of analysis.
Potential matrix effects arising from lichen chlorophyll content
All the extracts prepared in this study ranged in color from murky, olive green to a clear, bright emerald color after cleanup, suggesting that chlorophyll was still present in the samples and that matrix effects could influence the results. The dispersive SPE cleanup step in the conventional QuEChERS technique was not effective at cleaning up the lichen extracts either since the graphitized carbon black (GCB) formulated to remove pigments has been found to result in losses of PAHs (Sadowska-Rociek et al. 2013) and was therefore omitted from the sample preparation method. The type of matrix effects ranged from strong enhancement to strong suppression. Benzo[a]pyrene (BaP), known to be carcinogenic (Nisbet and Lagoy 1992; Clapp et al. 2008), experienced only a soft enhancement (% matrix effect of 6%) which suggested that the BaP concentration calculations would be the least affected by any matrix effects, thus providing a more accurate representation of the presence of this particularly harmful PAH in the atmospheric environment in which the lichen was sampled. Its ubiquitous presence is of concern towards human and ecological health (Ravindra et al. 2001) and the correct quantitation of BaP is paramount to understanding and interpreting the impact it may have on the environment.
The species used in the cleanup optimization study by Blasco et al. (2007) was lichens of the Parmelia sulcata type, which differ from the Parmotrema austrosinense (Zahlbr.) Hale used in this study, which may lead to inter-species differences. In order to establish whether the different sample extracts in this study had similar chlorophyll content after the cleanup procedure, the chlorophyll content in each extract was semi-quantified. The results for the relative total chlorophyll content (the sum of chlorophyll a and chlorophyll b, fluorescence intensity expressed in counts per second) were compared between the lichen sample investigated in this study and a lichen sample of identical species, sampled 20 km away. The contributions of chlorophyll a and chlorophyll b were not consistent between sampling points, suggesting that the chlorophyll content was not consistent within our lichen species. A similar result was observed by Beekley and Hoffman (1981) and Beltman et al. (1980) who found that chlorophyll content varies within the same species of lichen; moreover, a link between variation in chlorophyll content and exposure of lichens to pollutants, specifically lead, has been shown (Gurbanov and Unal 2019). There is a common lack of reporting of the age (and by implication, the size) of lichens when sampling and this means that variations in chlorophyll content introduced by aging effects cannot be accounted for. The lack of reporting the age of lichens means that comparing chlorophyll contents of different lichen species becomes complicated as a result of possible and undefined degradation effects occurring with time, as well as possible increases in chlorophyll content after exposure to pollutants (Canas et al. 1997). Our results suggest that pollutant assimilation rates based on chlorophyll content in combination with the dry weight of lichens should be further investigated, in agreement with Tretiach and Carpanelli (1992) and that a single sample preparation procedure may not sufficiently cleanup all extracts.
Total PAH concentration
The concentration of individual PAHs obtained with matrix-matched standards and the total PAHs identified in the bulk lichen sample are given in Table 4. The total targeted PAHs quantified in the lichen sample of 633 ng g−1 dw, although reflecting the concentration of a bulk sample at a single location, were similar to those reported in studies sampling lichens in industrial areas, such as Augusto et al. (2013), who reported a highest total PAH concentration of 556 ng g−1 dw in a highly industrialized region in Portugal. They were also similar to the values found by Nascimbene et al. (2014) of 785 ng g−1 in lichens collected next to a road in the Eastern Italian Alps, as well as the results from Blasco et al. (2007) who found concentrations of 352–1654 ng g−1 dw next to a national highway in Spain. These results were expected, considering that the lichen samples in this study were taken next to a road, carrying mainly light motor vehicles and a limited number of small trucks. A total PAH isomer index was calculated according to Mannino and Orecchio (2008) and Orecchio (2010):
An index of 5 was thus obtained for the bulk lichen sample which confirmed high temperature (i.e., combustion) sources. Emissions from a few domestic fires and household emissions may also be expected to have contributed to the PAH profile in the study area.
Conclusion
The new sample extraction techniques explored in this study (QuEChERS and MAE) performed better in terms of the quantities of PAHs extracted than the traditional techniques (USAE and Soxhlet). ANOVA confirmed that the new QuEChERS technique developed to extract PAHs from lichens using hexane and acetone (1:1, v/v) can confidently be claimed to have outperformed the other sample extraction techniques in this study. Since heavier PAHs were extracted using the Q Hex:Ace technique, it was concluded that the lichen species chosen in this study was a good choice for the biomonitoring of PAHs, since it accumulates both lighter and heavier PAHs, reflecting the presence of both gas-phase and particulate-phase PAHs in the atmosphere.
The observed matrix suppression for Ace, IcdP, BghiP, and DahA highlighted the importance of quantifying and, where necessary, correcting the calculated concentration values of various PAHs extracted from lichen samples. Attention should particularly be paid to PAHs reported to be undergoing matrix suppression, since these PAHs may be underrepresented in studies leading to biased PAH profiles and misunderstood exposure risks.
Considering the differing concentrations of chlorophyll in two different lichens (of the same species) sampled in this study, it was concluded that a single sample cleanup procedure may not be effective for all sample extracts. It is furthermore recommended that the impact of chlorophyll on analytical results should be further investigated to better allow for comparison between future studies.
The observed matrix effects necessitate a revision of the sample cleanup methods typically employed for lichen extracts. Due to the importance of sample preparation in delivering accurate and reliable analytical results, the extraction methodology as well as sample cleanup techniques employed should be continually revised and optimized as new techniques become available (such as new PAH-specific sorbents), to enhance recoveries of analytes and reproducibility of results.
Furthermore, the use of standard additions (ideally analyzing replicates of the matrix-matched standards starting with separate lichen samples) is considered paramount to the accurate determination of the concentration of PAHs incorporated into the lichen thallus, in order to account for the variations induced by the mesoclimate and pollution levels of the sampling area, the age of the lichens sampled, and the differences in chlorophyll content amongst extracts.
References
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86:412–431
Augusto S, Pinho P, Branquinho C, Pereira MJ, Soares A, Catarino F (2004) Atmospheric dioxin and furan deposition in relation to land-use and other pollutants: a survey with lichens. J Atmos Chem 49:53–65
Augusto S, Máguas C, Matos J, Pereira MJ, Branquinho C (2010) Lichens as an integrating tool for monitoring PAH atmospheric deposition: a comparison with soil, air and pine needles. Environ Pollut 158:483–489
Augusto S, Pereira MJ, Máguas C, Branquinho C (2012) Assessing human exposure to PAHs in a petrochemical region based on data from environmental biomonitors. J Toxicol Environ Health 75:1–10
Augusto S, Pereira MJ, Máguas C, Branquinho C (2013) A step towards the use of biomonitors as estimators of atmospheric PAHs for regulatory purposes. Chemosphere 92:626–632
Bajpai R, Karakoti N, Upreti DK (2013) Performance of a naturally growing Parmelioid lichen Remototrachyna awasthii against organic and inorganic pollutants. Environ Sci Pollut Res 20:5577–5592
Bartolomé L, Cortazar E, Raposo JC, Usobiaga A, Zuloaga O, Etxebarria N, Fernández LA (2005) Simultaneous microwave-assisted extraction of polycyclic aromatic hydrocarbons, polychlorinated biphenyls, phthalate esters and nonylphenols in sediments. J Chromatogr A 1068:229–236
Beekley P, Hoffman GR (1981) Effects of sulfur dioxide fumigation on photosynthesis, respiration, and chlorophyll content of selected lichens. Bryologist 84:379–389
Beltman IH, de Kok LJ, Kuiper PJC, van Hasselt PR (1980) Fatty acid composition and chlorophyll content of epiphytic lichens and a possible relation to their sensitivity to air pollution. Oikos 35:321–326
Blasco M, Domeno C, Bentayeb K, Nerín C (2007) Solid-phase extraction clean-up procedure for the analysis of PAHs in lichens. Int J Environ An Chem 87:833–846
Camel V (2000) Microwave-assisted solvent extraction of environmental samples. TrAC Trend Anal Chem 19:229–248
Canas M, Orellana L, Pignata M (1997) Chemical response of the lichens Parmotrema austrosinense and P. Conferendum transplanted to urban and non-polluted environments. Ann Bot Fenn 34:27–42
Chee KK, Wong MK, Lee HK (1996) Optimization of microwave-assisted solvent extraction of polycyclic aromatic hydrocarbons in marine sediments using a microwave extraction system with high-performance liquid chromatography-fluorescence detection and gas chromatography-mass spectrometry. J Chromatogr A 723:259–271
Clapp RW, Jacobs MM, Loechler EL (2008) Environmental and occupational causes of cancer new evidence (2005–2007). Rev Environ Health 23:1–37
Cvetkovic J et al (2016) Optimization of QuEChERS extraction procedure for determination of polycyclic aromatic hydrocarbons in soil by gas chromatography-mass spectrometry. Anal Methods 8:1711–1720
Dean JR, Barnabas IJ, Fowlis IA (1995) Extraction of polycyclic aromatic hydrocarbons from highly contaminated soils: a comparison between Soxhlet, microwave and supercritical fluid extraction techniques. Anal Proc 32(8):305–308
Di Lella LA, Loppi S, Protano G, Riccobono F (2006) Toxic trace elements and organic compounds in the ambient air of Kabul, Afghanistan. Atmos Environ 40:225–237
Domeño C, Blasco M, Sánchez C, Nerín C (2006) A fast extraction technique for extracting polycyclic aromatic hydrocarbons (PAHs) from lichen samples used as biomonitors of air pollution: dynamic sonication versus other methods. Anal Chim Acta 569:103–112
Drabova L, Pulkrabova J, Kalachova K, Tomaniova M, Kocourek V, Hajslova J (2012) Rapid determination of polycyclic aromatic hydrocarbons (PAHs) in tea using two-dimensional gas chromatography coupled with time of flight mass spectrometry. Talanta 100:207–216
European Committee for Standardization (CEN) (2009) EN 15662:2008 - foods of plant origin - determination of pesticide residues using GC-MS and/or LC-MS/MS following acetonitrile extraction/partitioning and clean up by dispersive SPE-QuEChERS method. CEN, Brussels
Forbes PBC et al (2009) Lichens as biomonitors for manganese and lead in Pretoria, South Africa. Fresenius Environ Bull 18(5):609–614
Forsberg ND, Wilson GR, Anderson KA (2011) Determination of parent and substituted polycyclic aromatic hydrocarbons in high-fat salmon using a modified QuEChERS extraction, dispersive SPE and GC–MS. J Agric Food Chem 59:8108–8116
Garty J (2001) Biomonitoring atmospheric heavy metals with lichens: theory and application. Crit Rev Plant Sci 20:309–371
Garty J, Galun M, Fuchs C, Zisapel N (1977) Heavy metals in the lichen Caloplaca aurantia from urban, suburban and rural regions in Israel (a comparative study). Water Air Soil Pollut 8:171–188
Gombert S, Asta J, Seaward MRD (2003) Correlation between the nitrogen concentration of two epiphytic lichens and the traffic density in an urban area. Environ Pollut 123:281–290
Guidotti M et al (2003) Lichens as polycyclic aromatic hydrocarbons bioaccumulators used in atmospheric pollution studies. J Chromatogr A 985:185–190
Gurbanov R, Unal D (2019) The biomolecular alterations in Cladonia convoluta in response to lead exposure. Spectrosc Lett. https://doi.org/10.1080/00387010.2018.1533564
Hawksworth DL, Rose F (1970) Qualitative scale for estimating sulphur dioxide air pollution in England and Wales using epiphytic lichens. Nature 227:145–148
João Ramalhosa M, Paíga P, Morais S, Delerue-Matos C, Prior Pinto Oliveira MB (2009) Analysis of polycyclic aromatic hydrocarbons in fish: evaluation of a quick, easy, cheap, effective, rugged, and safe extraction method. J Sep Sci 32:3529–3538
Jones KC, Duarte-Davidson R (1997) Transfer of airborne PCDD/Fs to bulk deposition collectors and herbage. Environ Sci Technol 31:2937–2943
Kalachova K, Pulkrabova J, Drabova L, Cajka T, Kocourek V, Hajslova J (2011) Simplified and rapid determination of polychlorinated biphenyls, polybrominated diphenyl ethers, and polycyclic aromatic hydrocarbons in fish and shrimps integrated into a single method. Anal Chim Acta 707:84–91
Mannino MR, Orecchio S (2008) Polycyclic aromatic hydrocarbons (PAHs) in indoor dust matter of Palermo (Italy) area: extraction, GC–MS analysis, distribution and sources. Atmos Environ 42:1801–1817
Nascimbene J, Tretiach M, Corana F, Lo Schiavo F, Kodnik D, Dainese M, Mannucci B (2014) Patterns of traffic polycyclic aromatic hydrocarbon pollution in mountain areas can be revealed by lichen biomonitoring: a case study in the Dolomites (Eastern Italian Alps). Sci Total Environ 475:90–96
Nisbet ICT, Lagoy PK (1992) Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul Toxicol Pharmacol 16:290–300
Orecchio S (2010) Assessment of polycyclic aromatic hydrocarbons (PAHs) in soil of a natural reserve (Isola delle Femmine) (Italy) located in front of a plant for the production of cement. J Hazard Mater 173:358–368
Orecchio S, Gianguzza A, Culotta L (2008) Absorption of polycyclic aromatic hydrocarbons by Pinus bark: analytical method and use for environmental pollution monitoring in the Palermo area (Sicily, Italy). Environ Res 107:371–379
Purcaro G, Moret S, Conte LS (2009) Optimisation of microwave assisted extraction (MAE) for polycyclic aromatic hydrocarbon (PAH) determination in smoked meat. Meat Sci 81:275–280
Rajski Ł, Lozano A, Uclés A, Ferrer C, Fernández-Alba AR (2013) Determination of pesticide residues in high oil vegetal commodities by using various multi-residue methods and clean-ups followed by liquid chromatography tandem mass spectrometry. J Chromatogr A 1304:109–120
Ratola N, Lacorte S, Alves A, Barceló D (2006) Analysis of polycyclic aromatic hydrocarbons in pine needles by gas chromatography–mass spectrometry: comparison of different extraction and clean-up procedures. J Chromatogr A 1114:198–204
Ratola N, Lacorte S, Barceló D, Alves A (2009) Microwave-assisted extraction and ultrasonic extraction to determine polycyclic aromatic hydrocarbons in needles and bark of Pinus pinaster Ait. and Pinus pinea L. by GC–MS. Talanta 77:1120–1128
Ravindra K, Mittal AK, Van Grieken R (2001) Health risk assessment of urban suspended particulate matter with special reference to polycyclic aromatic hydrocarbons: a review. Rev Environ Health 16:169–189
Sadowska-Rociek A, Surma M, Cieślik E (2013) Application of QuEChERS method for simultaneous determination of pesticide residues and PAHs in fresh herbs. Bull Environ Contam Toxicol 90:508–513
Shukla V, Upreti DK (2009) Polycyclic aromatic hydrocarbon (PAH) accumulation in lichen Phaeophyscia hispidula of DehraDun City, Garhwal Himalayas. Environ Monit Assess 149:1–7
Shukla V, Upreti D (2013) Lichens reveal air PAH fractionation in the Himalaya. Eviron Chem Lett 11:19–23
Shukla V, Patel DK, Upreti DK, Yunus M (2012) Lichens to distinguish urban from industrial PAHs. Environ Chem Lett 10:159–164
Sloof JE (1995) Lichens as quantitative biomonitors for atmospheric trace-element deposition, using transplants. Atmos Environ 29:11–20
Sloof JE, Wolterbeek H (1991) National trace element air pollution monitoring survey using epiphytic lichens. Lichenologist 23(2):139–165
Sloof JE, Wolterbeek H (1992) Lichens as biomonitors for radiocesium following the Chernobyl accident. J Environ Radioact 16:229–242
Srogi K (2006) A review: application of microwave techniques for environmental analytical chemistry. Anal Lett 39:1261–1288
Szulejko JE, Kim KH, Brown RJ, Bae MS (2014) Review of progress in solvent-extraction techniques for the determination of polyaromatic hydrocarbons as airborne pollutants. TrAC Trend Anal Chem 61:40–48
Tomaniova M et al (1998) Microwave-assisted solvent extraction—a new method for isolation of polynuclear aromatic hydrocarbons from plants. J Chromatogr A 827:21–29
Trapp S, Matthies M (1997) Modeling volatilization of PCDD/F from soil and uptake into vegetation. Environ Sci Technol 31:71–74
Tretiach M, Carpanelli A (1992) Chlorophyll content and morphology as factors influencing the photo synthetic rate of Parmelia Caperata. Lichenologist 24:81–90
Van der Wat L, Forbes PBC (2015) Lichens as biomonitors for organic air pollutants. TrAC Trend Anal Chem 64:165–172
Wilkowska A, Biziuk M (2011) Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chem 125:803–812
Zhao L, Zhang C, Jia S, Liu Q, Chen Q, Li X, Liu X, Wu Q, Zhao L, Liu H (2019) Element bioaccumulation in lichens transplanted along two roads: the source and integration time of elements. Ecol Indic 99:101–107
Acknowledgments
Restek Corporation and Wirsam Scientific are thanked for their support of this research.
Funding
This work was financially supported by the National Research Foundation (NRF grants 90720 and 93394).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclaimer
Opinions expressed and conclusions arrived at are those of the authors and are not necessarily to be attributed to the NRF.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Ester Heath
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Van der Wat, L., Forbes, P.B.C. Comparison of extraction techniques for polycyclic aromatic hydrocarbons from lichen biomonitors. Environ Sci Pollut Res 26, 11179–11190 (2019). https://doi.org/10.1007/s11356-019-04587-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04587-3