Abstract
A nitrosation-reduction method had been applied for the modification of DCE-4 h. It was a kind of non-polar hypercrosslinked resin and synthesized by our group. The functional resin, NR-07, exhibited good adsorption performance for NACs (Nitroaromatics). The adsorption capacity of NR-07 for p-nitrobenzoic acid was almost 1.3 times as much as that of H-103 in 24 h. The adsorption rate of NR-07 calculated by the kinetic function was 1.6 times as much as that of DCE-4h. According to the EA analysis and IR spectrum, the amine and carbonyl group were introduced onto the polymer chains of NR-07. These hydrophilic chemical groups of NR-07 contributed to a higher liquid-film diffusion rate than that of DCE-4h. Besides, the pore volume within 0.46~4.3 nm increased after the modification process, which had a positive effect on the intra-particle diffusion process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitroaromatics are important chemical materials, but also the well-known contaminants as a result of high toxicity and resistance of degradation. The adsorption techniques about resins have been tried for the treatment of the nitroaromatics effluent for a long time (He et al. 2010a; He et al. 2010b; Liu et al. 2015), because of high adsorption capacity and good reused ability. However, the adsorption performance could be easily influenced by the properties of adsorbents (Pan et al. 2006; He et al. 2010b), adsorbates (Huang et al. 2008; Yang et al. 2016), and the solution (Huang et al. 2009a; Huang et al. 2012). For example, non-polar resins may have a great advantage for the adsorption of non-polar adsorbates while may be useless for the adsorption of polar adsorbates in aqueous phase (Huang et al. 2009b). During our research, CH series resins synthesized by our group were non-polar, exhibiting a great adsorption capacity and rate for nitrobenzene (Wang et al. 2017). However, they seemed to be not suitable for the adsorption of polar nitroaromatics according to our later experiments. Therefore, functional resins were necessary to make up this weakness.
A review of literatures revealed that most of functional groups were amine groups (Pan et al. 2006; Zhang et al. 2013; Yang et al. 2014), phenolic hydroxyl groups (Huang et al. 2009a; He et al. 2010b), and carbonyl groups (Huang et al. 2012). In most cases, these groups were introduced onto the polymer chains of resins with the replacement of chlorine element. However, the –CH2Cl group was often used for the post-cross-linking reaction for the increasing of BET surface area (Tsyurupa and Davankov 2006; Meng et al. 2007). As a result, part of chlorine element was consumed for the post-cross-linking reaction and the remainder was used for the chemical modification. Many functional resins synthesized in this way exhibited good adsorption capacity for some polar adsorbates (Pan et al. 2002; Pan et al. 2003; Pan et al. 2006). However, few works have been done about chemical modification of hypercrosslinked resin without –CH2Cl group. Besides, the study of the adsorption process was not complete. Most attention was focused on the diffusion process while the effect of the surface properties was ignored.
The modifications of PS (polystyrene), PES (polyether sulfone), and MWCNTs (multiwalled carbon nanotubes) by nitration reagents had been studied a few decades ago (Bajaj and Chattebjee 1966; Bajaj and Chattebjee 1967; Daly et al. 1988; Shen et al. 2009). The nitro group could be successfully introduced onto the benzene ring so that the characters of polymer were changed obviously. Besides, activated carbon modified by nitration reagent for the adsorption of NACs were also studied (Liu and Wang 2011; Goto et al. 2015). The oxidation process and subsequent heat treatment could improve the physicochemical properties of the surface and broaden the mesopore channel, resulting in a higher adsorption capacity for nitrobenzene (Liu and Wang 2011). As for the organic polymers, the great oxidation effect of HNO3 and H2SO4 could take an adverse effect on the polymer molecule weight and framework (Philippides et al. 1992; Botvay et al. 1999).
These researches provided a new idea that the amine group and the O-containing group could be introduced onto the polymer chains simultaneously without –CH2Cl group. A kind of moderate nitrosation reagent was tried for the modification process. The mechanism of the nitrosation reaction has been studied thoroughly. Additionally, effects of this modification method on the adsorption performance of functional resins were evaluated by various static experiments. Different NACs were chosen as the adsorbates for the study of adsorption mechanism.
Experiments
Materials
Nitrobenzene, 1,2-dichloroethane(DCE), aluminum chloride, sodium nitrite, nitric acid (65 wt%), sulfuric acid (98 wt%), concentrated hydrochloric acid (37 wt%), stannous chloride, p-nitrobenzoic acid, p-nitrophenol, and magnesium sulfate in AR grade were purchased from Sinopharm Chemical Reagent Co., Ltd. Cl-St-DVB resin (7% crosslinking degree, 17 wt% of chlorine element), and the commercial resins, H-103, were purchased from Nankai University in Tanjing. Organic solvents were used without any further treatment.
Preparation of hypercrosslinked resin
The preparation process was the same as that of CH series in reference 10, but the type of Cl-St-DVB was changed. Dry Cl-St-DVB beads (10 g) were swollen in 50 mL of DCE at room temperature for 12 h, followed by the addition of aluminum chloride (2×g). The mixture was stirred uniformly before being heated to 353 K gradually in 20 min, and the reaction was maintained at 353 K for 4 h. The hypercrosslinked resin was washed by ethanol and deionized water under the ultrasonic condition. Then, the resin was dried in air dry oven at 343 K. This hypercrosslinked resin was named as DCE-4 h.
Preparation of amine-modification resin
Nitroso-modified resin
Two grams of DCE-4h resin was swelled into 50 mL of nitrobenzene at room temperature for 2 h. Sodium nitrite (0.14 g) was added into the mixture, and the temperature was controlled at 295 K. Nitric acid (0.67 mL) (65 wt%) was added into the system drop wise in 10 min. The nitrosation reaction was conducted at 295 K for 1 h. After that, the nitrosation resin was filtrated and washed by alcohol and deionized water under ultrasonic condition. The nitrosation resin, called N-07, was dried in air dry oven at 343 K.
Amine-modified resin
One gram of nitrosation resin and 5 g of stannous chloride were added into 60 mL solvent (30 mL of concentrated hydrochloric acid and 30 mL of ethanol). The reduction reaction was conducted at the reflux state for 10 h. The reduction resin was washed by sulfuric acid solution with a concentration of 0.05 mol/L until the filtrate was clean. Then, it was washed by deionized water until the filtrate was neutral. Finally, the reduction resin, named NR-07, was dried in air dry oven at 343 K.
Static adsorption
The adsorption of nitrobenzene: 100 mg of various resins and 100 mL of nitrobenzene solution at 1000 mg/L were added into the 250-mL conical flask with cover.
The adsorption of p-nitrophenol: 20 mg of various resins and 30 mL of p-nitrophenol solution at 1000 mg/L were added into the 50-mL centrifuge tube.
The adsorption of p-nitrobenzoic acid: 20 mg of various resins and 200 mL of p-nitrobenzoic acid solution at 100 mg/L were added into the 250-mL conical flask with cover.
These flasks were shaken under 180 rpm at 303 K for 24 h to ensure adsorption equilibrium in a QYC 2102C INCUBATOR (Shanghai FuMa laboratory equipment Co. Ltd). The adsorption capacity was calculated by Eq. (1):
The adsorption kinetic tests were carried out under different conditions:
-
1.
Twenty milligrams of dry resins was added into 200 mL of p-nitrobenzoic solution with a concentration of 100 mg/L. The samples were collected under the certain time intervals and detected by UV-Vis method at the λmax = 271 nm in moderate aqueous phase.
-
2.
Twenty milligrams of resins were wetted by water in advance. The remaining operation process was not changed.
The fixed bed adsorption and desorption
Fixed bed adsorption and desorption were investigated in a glass column (10 mm diameter and 360 mm length) packed with 10 mL of resin (wet volume). The fixed bed was equipped with a Langer pump (BT100-2J, China) to ensure a constant flow rate, 30 mL/h. The resin was rinsed with ethanol and water before adsorption, and acetone was introduced as desorption solvent with a rate of 30 mL/h after the adsorption. The dynamic tests were carried out at 293 K at the initial concentration of 100 mg/L for p-nitrobenzoic acid.
Characterization
BET surface area and pore volume were determined by N2 adsorption-desorption with ASAP 2020 (Micromeritics) at 77 K. The concentration of NACs in the aqueous solution was determined by UV-Vis spectrophotometer (Varian). The nitro and amine group were analyzed by FTIR spectroscopy with Nicolet iN10 (Thermo scientific), and the samples were dispersed into KBr to form disks. The content of C, H, and N element was determined by EA (Element Analysis, Vario EL cube), and the content of O element was calculated by subtracting.
Result and discussion
The IR spectrum
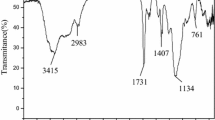
The IR spectra of the nitrosation and reduction resin are displayed in Fig. 1. Obvious changes were observed after the nitrosation and reduction process compared to the IR spectrum of DCE-4h. Unexpectedly, new vibration bands appeared at 1560 and 1384 cm−1 which should be assigned to the –NO group rather than –NO2 group (Daly et al. 1988) (1527 and 1347 cm−1), suggesting the occurrence of nitrosation during the nitration process. The employment of NaNO2 in the nitration process promoted the production of NO+ which afforded the –NO in the resin. After the reduction reaction, the C–N vibration band (1312 cm−1) emerged on the reduction resin and the –NO vibration band disappeared indicating the successful and complete conversion of –NO group into –NH2 group by reduction reagent SnCl2. Besides, the peak of 1610 cm−1 became boarder in the nitrosation resin and reduction resin than that in DCE-4h and showed an obvious overlap with newly generated vibration at 1667 cm−1. That may be due to the generation of C=O (1667 cm−1)(Chen et al. 2004) because of the oxidation of the nitration reagent during the nitration and nitrosation process, which was further verified by the element analysis. The mechanism of the nitrosation and reduction process is described in Figs. S1 and S2.
The comparison of NR-07, DCE-4h, and H-103
As shown in Table 1, after the nitrosation reaction, the physicochemical properties of DCE-4h had been changed obviously. First, the BET surface area of NR-07 was 1065.5 m2/g decreasing from 1143.4 m2/g of DCE-4h. However, the volume with pore diameter in 0.46~50 nm of NR-07 followed the sequence: NR-07 > DCE-4h > H-103, as shown in Table 1 and Fig. S3. Second, the N and O contents of NR-07 were obviously higher than those of DCE-4h, indicating the chemical modification process had introduced the amine group and carbonyl group onto the polymer chains of NR-07.
These changes may take a great effect on the adsorption performance of DCE-4h. Firstly, the decreasing of BET surface area could cause the decreasing of adsorption capacity, but the adding of amine group and carbonyl group was helpful for a higher adsorption capacity for polar NACs. This would be verified by the static adsorption experiments. Secondly, the polar groups were introduced onto polymer chains meaning NR-07 was more hydrophilic than DCE-4h. The hydrophilia could increase the affinity between the adsorbent and the aqueous phase (Huang et al. 2009b). NR-07 may have an advantage to DCE-4h in the liquid-film diffusion stage which would be tested by different kinetic adsorption experiments. Thirdly, the small mesopore volume was enlarged by the nitrosation reagent which would contribute to a better intra-particle diffusion process in NR-07 than that in DCE-4h. This would be tested by the intra-particle diffusion model.
Static adsorption for NACs
The static adsorption capacity of DCE-4h, NR-07, and H-103 is displayed in Fig. 2. An interesting thing could be found that the adsorption capacity for p-nitrophenol and p-nitrobenzoic acid of NR-07 were higher than those of DCE-4h while it is different for the adsorption of nitrobenzene.
The adsorption capacity of DCE-4h, NR-07, and H-103 for NACs. (Note: The adsorption conditions of nitrobenzene: C0 = 1 g/L, the dosage of resin was 0.1 g, V = 100 mL, T = 303 K, t = 24 h; the adsorption conditions of p-nitrophenol: C0 = 1 g/L, the dosage of resin was 20 mg, V = 30 mL, pH = 3.5, T = 303 K, t = 24 h; the adsorption conditions of p-nitrobenzoic acid: C0 = 0.1 g/L, the dosage of resin was 20 mg, V = 200 mL, pH = 7, T = 303 K, t = 24 h)
As mentioned above, the BET surface area of NR-07 was lower than that of DCE-4h, while the N and O elements content were higher than those of DCE-4h. The amine and carbonyl group could work as the adsorption sites for the p-nitrophenol and p-nitrobenzoic acid because of the hydrogen bond or the acid-base effect between the amine group and the phenol/carboxyl group. Therefore, the generation of these new adsorption sites contributed to a higher adsorption capacity for NR-07. When nitrobenzene was chosen as the adsorbate, those interactions could be ignored. As a result, the decreasing of BET surface area was the main factor to the lower adsorption capacity of NR-07 for nitrobenzene. Therefore, NR-07, a kind functional resin, was more suitable for the adsorption of those polar NACs.
According to Fig. 2, the relative adsorption advantage of NR-07 was the most obvious compared to H-103 when p-nitrobenzoic acid was chosen as the adsorbate. Therefore, the following researches were carried out for the adsorption of p-nitrobenzoic acid.
The adsorption kinetics
The adsorption kinetic equations were displayed as follows:
The pseudo-first-order and the pseudo-second-order model are given by Eqs. (2) and (3) respectively (Lorencgrabowska and Gryglewicz 2007):
where t (min) and Qt (mg/g) are, respectively, time and the amount of nitrobenzene adsorbed by the resin at time t. Qe exp. (mg/g) and Qe(mg/g) are the amount of nitrobenzene adsorbed at equilibrium-experimental data and equilibrium-calculated data. k1 (1/min) and k2 (g/mg min) are the first- and second-order-rate constant of adsorption.
The experimental data fitted the pseudo-second-order model better than the pseudo-first-order model according to the value of R2 in Table 2. The value of k2 of these three resins followed the sequence H-103 > NR-07 > DCE-4h in Table 2.
As shown in Fig. 3, an interesting thing was found that the adsorption capacity of three resins for p-nitrobenzoic acid followed the sequence: NR-07 > H-103 > DCE-4h while it turned to be NR-07 > DCE-4h > H-103 after 6 h. This may have resulted from different advantages of these resins in different adsorption stages. Therefore, it is necessary to analyze the adsorption process in detail.
Liquid-film diffusion
Commonly, the adsorption diffusion process could be divided into two stages: (1) the adsorbates cross the external boundary layer from the aqueous phase, and (2) the adsorbates diffuse in the pore channel of resins (Zhang et al. 2013) as shown in Fig. 4.
As mentioned above, H-103 and NR-07 were more hydrophilic than DCE-4h because of the higher content of N and O element. These hydrophilic groups increased the affinity to the bulk aqueous phase, which may facilitate the interaction between the surface of the resin and the adsorbate molecule and contribute to a higher liquid-film diffusion rate (Yu et al. 2004).
The process of the adsorbates cross the interface between the surface of the resin and the bulk aqueous phase is described by Eq. (4) (Huang et al. 2008):
where F is the fractional attainment of equilibrium (F = Qt/Qe) and kf (min−1) is the liquid-film diffusion rate parameter.
According to the fitting of liquid-film diffusion model, the kf of these three resins followed the sequence H-103 > NR-07 > DCE-4h in the whole adsorption process (as shown in Fig. 4) which was consistent with the assumption.
Intra-particle diffusion
The process of the adsorbates diffuse in the pore channel in the resin is described by Eq. (4) (Huang et al. 2008):
where Qt(mg/g) and kp(mg/g min1/2) are the amount of adsorbates at time t (min) and intra-particle rate constant respectively.
The relationship between the molecular size of adsorbates and the diameter of the pore channel has a great influence on the intra-particle process. According to the pore diameter control mechanism, the pore with a size that was 2~6 times as much as that of the adsorbate would play a key role in the intra-particle diffusion process (Huang et al. 2008). The molecular size of p-nitrobenzoic acid in optimal state was calculated based on the bond length and the bond angle by the ChemBioOffice 2010 software as shown in Table 3. The detail information of the bond length and the bond angle of p-nitrobenzoic acid in optimal conformation is displayed in Fig. S4. Therefore, the key pore diameter seemed to be 0.46 ~4.3 nm which could have a significant effect on the intra-particle diffusion rate of p-nitrobenzoic acid in NR-07, DCE-4h, and H-103. Fig 5.
According to the fitting of intra-particle diffusion model, the adsorption rate followed the sequence: NR-07 > DCE-4h > H-103(as shown in Fig. 6). The pore volume of these three resins within 0.46~4.3 nm of pore diameter followed the sequence: NR-07 > DCE-4h > H-103, meaning that more channels were accessible for p-nitrobenzoic acid to get into the micropore in NR-07.
As a result of the chemical modification, the adsorption advantage of DCE-4h to H-103 was maintained by NR-07 and the kinetic behavior of NR-07 was also improved obviously compared to DCE-4h.
The effect of hydrophilia on the adsorption process
During the research of the intra-particle model, a different state in the adsorption process of DCE-4h is found in Fig. 6, compared with that of NR-07 in the initial stage. It seemed the adsorption process in that part was restrained by some factors.
In order to study that strange phenomenon, different adsorption kinetic experiments were carried out under different initial statuses of NR-07 and DCE-4h, as shown in Fig. 7. Some interesting things were found as follows: firstly, in dry initial state, there was no adsorption of p-nitrobenzoic acid onto DCE-4h in the initial 30 min while it is different in wet initial state; secondly, the adsorption capacity of NR-07 and DCE-4h for p-nitrobenzoic acid in the dry initial status was lower than those in wet initial status. Therefore, the wetting process before the adsorption had a great influence on the adsorption process.
When DCE-4h was dry, more time was needed for the water molecule to wet the hydrophobic surface, compared to NR-07. The amine group and the carbonyl group made the surface of NR-07 more hydrophilic than that of DCE-4h. As a result, the adsorption process seemed to be delayed leading to a longer adsorption equilibrium time. Because of the non-polar surface of DCE-4h, it was not suitable for the landing of p-nitrobenzoic acid in initial adsorption stage unless it was wetted. The polarity of resin surface was significant for the adsorption process of polar adsorbates (Li et al. 2002; Goto et al. 2015; Yang et al. 2016).
Therefore, the chemical groups containing N and O element improved the surface hydrophilia which facilitated the wetting process and the liquid-film diffusion process.
The fixed bed adsorption
The fixed bed adsorption and desorption curves of NR-07 for the adsorption of p-nitropenzoic acid are displayed in Fig. S5. The breakthrough point was obtained when the concentration of p-nitrobenzoic acid of the desorption solution reached 100 mg/L. Aqueous solution (440 BV) (BV, the fixed bed volume, mL) of p-nitrobenzoic acid could be effectively treated. After the adsorption, the acetone was selected as the desorption solvent. The max concentration of p-nitrobenzoic acid in the desorption solution reached 12 g/L, and 92% of the p-nitrobenzoic acid could be recycled when 76 BV of acetone was consumed. NR-07 could have a good application for the treatment of NAC effluent.
Conclusion
The HNO3/NB/NaNO2/SnCl2 was a moderate nitration reagent combination. The framework of DCE-4h was maintained relatively after the nitrosation process.
NR-07 exhibited better adsorption performance than DCE-4h and H-103. The adsorption capacity of NR-07 was 1.32 times as much as that of H-103. NR-07 could obviously cut the time to get to the adsorption equilibrium state compared to DCE-4h when p-nitrobenzoic acid was chosen as the adsorbate.
The modification process improved the hydrophilia of the surface and the pore channel of DCE-4h, resulting in a shorter equilibrium time.
References
Bajaj M. I.,Chattebjee S. B. 1966 Nitration of polystyrene part-I: effect of molecular weight of polymer on nitration
Bajaj M. I.,Chattebjee S. B. 1967 Nitration of polystyrene part-II: Effect of nitrating medium on nitration
Botvay A, Mathe A, Poppl L (1999) Nitration of polyethersulfone by ammonium nitrate and trifluoroacetic anhydride. Polym Commun 40:4965–4970
Chen Z.-C., Le Z.-G., Hu Y.,Zheng Q.-G. 2004 Organic reactions in ionic liquids: N-alkylation of phthalimide and several nitrogen heterocycles. Synthesis(2), 208–212
Daly WH, Lee S, Rungaroonthaikul C (1988) Modification of condensation. Polymers 364:4–23
Goto T, Amano Y, Machida M, Imazeki F (2015) Effect of polarity of activated carbon surface, solvent and adsorbate on adsorption of aromatic compounds from liquid phase. Chem Pharm Bull (Tokyo) 63(9):726–730
He C, Huang J, Yan C, Liu J, Deng L, Huang K (2010a) Adsorption behaviors of a novel carbonyl and hydroxyl groups modified hyper-cross-linked poly(styrene-co-divinylbenzene) resin for beta-naphthol from aqueous solution. J Hazard Mater 180(1–3):634–639
He C, Huang K, Huang J (2010b) Surface modification on a hyper-cross-linked polymeric adsorbent by multiple phenolic hydroxyl groups to be used as a specific adsorbent for adsorptive removal of p-nitroaniline from aqueous solution. J Colloid Interface Sci 342(2):462–466
Huang J-H, Huang K-L, Liu S-Q, Wang AT, Yan C (2008) Adsorption of rhodamine B and methyl orange on a hypercrosslinked polymeric adsorbent in aqueous solution. Colloids Surf A Physicochem Eng Asp 330(1):55–61
Huang J, Wang X, Deng X (2009a) Synthesis, characterization, and adsorption properties of phenolic hydroxyl group modified hyper-cross-linked polymeric adsorbent. J Colloid Interface Sci 337(1):19–23
Huang J, Yan C, Huang K (2009b) Removal of p-nitrophenol by a water-compatible hypercrosslinked resin functionalized with formaldehyde carbonyl groups and XAD-4 in aqueous solution: a comparative study. J Colloid Interface Sci 332(1):60–64
Huang J, Jin X, Deng S (2012) Phenol adsorption on an N-methylacetamide-modified hypercrosslinked resin from aqueous solutions. Chem Eng J 192:192–200
Li A, Zhang Q, Zhang G, Chen J, Fei Z, Liu F (2002) Adsorption of phenolic compounds from aqueous solutions by a water-compatible hypercrosslinked polymeric adsorbent. Chemosphere 47:981–989
Liu S, Wang R (2011) Modified activated carbon with an enhanced nitrobenzene adsorption capacity. J Porous Mater 18(1):99–106
Liu J, Cao J, Chen H, Zhou D (2015) Adsorptive removal of humic acid from aqueous solution by micro- and mesoporous covalent triazine-based framework. Colloids Surf A Physicochem Eng Asp 481:276–282
Lorencgrabowska E, Gryglewicz G (2007) Adsorption characteristics of Congo red on coal-based mesoporous activated carbon. Dyes Pigments 74(1):34–40
Meng G, Li A, Yang W, Liu F, Yang X, Zhang Q (2007) Mechanism of oxidative reaction in the post crosslinking of hypercrosslinked polymers. Eur Polym J 43(6):2732–2737
Pan BC, Xiong Y, Li AM, Chen JL, Zhang QX, Jin XY (2002) Adsorption of aromatic acids on an aminated hypercrosslinked macroporous polymer. React Funct Polym 53:63–72
Pan BC, Xiong Y, Su Q, Li AM, Chen JL, Zhang QX (2003) Role of amination of a polymeric adsorbent on phenol adsorption from aqueous solution. Chemosphere 51(9):953–962
Pan B, Chen X, Pan B, Zhang W, Zhang X, Zhang Q (2006) Preparation of an aminated macroreticular resin adsorbent and its adsorption of p-nitrophenol from water. J Hazard Mater 137(2):1236–1240
Philippides A, Budd PM, Price C (1992) The nitration of polystyrene. Polymer 34(16):3509–3513
Shen X-E, Shan X-Q, Dong D-M, Hua X-Y, Owens G (2009) Kinetics and thermodynamics of sorption of nitroaromatic compounds to as-grown and oxidized multiwalled carbon nanotubes. J Colloid Interface Sci 330(1):1–8
Tsyurupa MP, Davankov VA (2006) Porous structure of hypercrosslinked polystyrene: state-of-the-art mini-review. React Funct Polym 66(7):768–779
Wang C, Xu C, Sun W, Liu F, Yu S, Xian M (2017) Effective adsorption of nitroaromatics at the low concentration by a newly synthesized hypercrosslinked resin. Water Sci Technol 76(9):2328–2336
Yang G, Chen H, Qin H, Feng Y (2014) Amination of activated carbon for enhancing phenol adsorption: effect of nitrogen-containing functional groups. Appl Surf Sci 293:299–305
Yang K, Qi L, Wei W, Wu W, Lin D (2016) Prediction of the sorption capacities and affinities of organic chemicals by XAD-7. Environ Sci Pollut Res Int 23(2):1060–1070
Yu Y, Zhuang Y-Y, Wang Z-H, Qiu M-Q (2004) Adsorption of water-soluble dyes onto modified resin. Chemosphere 54(3):425–430
Zhang H, Li A, Sun J, Li P (2013) Adsorption of amphoteric aromatic compounds by hyper-cross-linked resins with amino groups and sulfonic groups. Chem Eng J 217:354–362
Funding
The research was supported by The Taishan Scholars Projects of Shandong (no. ts501511033) and Natural Science Foundation of Shandong Province (ZR2016BQ39).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Angeles Blanco
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 243 kb)
Rights and permissions
About this article
Cite this article
Xu, C., Wang, C., Sun, W. et al. Preparation of hypercrosslinked amine modification resin and its adsorption properties for nitroaromatics. Environ Sci Pollut Res 26, 10767–10775 (2019). https://doi.org/10.1007/s11356-019-04370-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04370-4