Abstract
Polychlorinated diphenyl ethers (PCDEs) are a class of potential persistent organic contaminants, which have been widely detected in aquatic environment. In the present study, the effects of 3,4,4′-tri-CDE and its two possible metabolites (2-MeO-3′,4,4′-tri-CDE and 2-HO-3′,4,4′-tri-CDE) on oxidative stress biomarkers in liver of Carassius auratus were evaluated. The fish were treated with these three compounds at different doses (0.1, 1, and 10 μg/L) via semi-static water exposure. The liver samples were individually taken at 3, 7, and 21 days for analysis of oxidative stress indicators, including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), reduced glutathione (GSH), and malondialdehyde (MDA). Compare to the control group, the hepatic antioxidant enzyme activity and GSH contents showed significant decreases (p < 0.05) at high-dose treatment (10 μg/L) and prolonged exposure time (21 days) in most of the toxicant-treated groups, indicating the occurrence of oxidative stress in fish liver. However, no consistent trend of the variations of antioxidant parameters was observed at low doses (0.1 and 1 μg/L). Meanwhile, the lipid peroxidation was significantly induced with extending exposure time and increasing dose. In addition, the toxicity order of three compounds was discussed using the integrated biomarker response (IBR) index. Notably, 2-HO-3′,4,4′-tri-CDE was indicated to cause the most severe hepatic oxidative stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a series of typical organic pollutants, polychlorinated diphenyl ethers (PCDEs) are structurally similar to polybrominated diphenyl ethers and polychlorinated biphenyls. There are 209 kinds of PCDE congeners, which may present different physicochemical properties and toxic effects. In addition, PCDEs have been widely used as electric insulating oil, fire retardants, and plastic additives in numerous products (Kruz and Ballschmiter 1995; Koistinen 2000). Due to the inevitable release of PCDEs from practical applications, they have been ubiquitously detected in a variety of environmental matrices, including sediments, surface water, birds, fish, and even human adipose tissues (Koistinen et al. 1995; Villeneuve et al. 1999). For instance, Koistinen et al. (1995) found that the concentration of PCDEs in the sediment of the Kymijoki River in Finland reached 130–550 ng/g dry weight (d.w.). Domingo et al. (2006) estimated the levels of PCDEs in 14 types of seafood in Catalonia, Spain, which were in the range of 1.031–7.088 ng/g d.w. and also concluded that the maximum exposure of PCDEs to adults in this region could reach up to 39 ng/day. Recently, we detected 15 kinds of PCDEs in sediments and surface water from the Yangtze River (Nanjing section) and Chaohu Lake, with their concentrations ranging from 0.73 to 1.80 μg/L and 1.24 to 3.98 μg/kg d.w., respectively (Qin et al. 2015; Zhang et al. 2018).
Because of their dioxin-like structures, the toxic effects of PCDEs in organisms have attracted growing attention worldwide. Iverson et al. (1987) found that the hepatic cytochrome P-450 and monooxygenase could be significantly induced by PCDEs in Sprague-Dawley rats. Our recent studies demonstrated that 4,4′-di-CDE induced oxidative stress in mice liver and green algae (Zhang et al. 2014; Fang et al. 2018). However, to date, the knowledge regarding the aquatic toxicity of PCDEs is still insufficient. Qin et al. (2014) suggested that 4,4′-di-CDE could exert some adverse effects on zebrafish and inhibited the embryo incubation. Moreover, PCDEs have also been reported to undergo biological metabolism or other environmental processes to generate some other byproducts like hydroxyl derivatives (OH-PCDEs) and methoxyl derivatives (MeO-PCDEs) (Rosiaka et al. 1997; Zeng et al. 2007). For example, Koistinen et al. (2010) reported that the concentrations of OH-PCDEs and MeO-PCDEs in the mussels incubated in the Kymijoki River and Vuoksi River reached 80.9–97.9 ng/g and 89.4–111 ng/g lipid weight (l.w.), respectively. However, no information is currently available regarding the environmental levels of OH-PCDEs and MeO-PCDEs. Although some toxicity data have been reported for PCDEs, toxicological studies of these two derivatives of PCDEs are still limited.
The metabolism of xenobiotics in organisms could produce a mass of reactive oxygen species (ROS) (Mittal et al. 2014). Normally, superfluous ROS could be cleared by antioxidant defense system in organisms, including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and non-enzyme antioxidants (e.g., reduced glutathione (GSH)) (Van de Oost et al. 2003). However, oxidative damages emerged when the concentration of ROS is overwhelmingly increased, which could disrupt cell metabolism and destroy cell components (Almroth et al. 2008; Lushchak 2011). The polyunsaturated fatty acids on the surface of cell membrane can also be attacked by ROS, producing lipid peroxides and finally causing cell failure and releasing malondialdehyde (MDA) (Chen et al. 2006). Consequently, these biomarkers associated with ROS have been widely used to evaluate and compare the adverse effects in living organisms after aqueous exposure to environmental pollutants (Kodavanti et al. 2011; Zhang et al. 2014).
Crucian carp (Carassius auratus) has been widely used in the aquatic toxicity tests for its features of easy to culture under laboratory conditions and sensitive to pollutants (Samanta et al. 2018; Jabeen et al. 2018). Husak et al. (2016) indicated that metribuzin-containing herbicide Sencor free affected radical generation in gills of fish. PBDEs have also been shown to induce or inhibit the activity of antioxidant enzymes in C. auratus (Xie et al. 2014). In our previous study, the occurrence of hepatic oxidative stress in fish exposed to six brominated flame retardants was demonstrated, from which the comparative toxicity was proposed (Feng et al. 2013).
This research aimed to investigate and compare the antioxidant status in C. auratus after individual exposure to a representative PCDE (3,4,4′-tri-CDE) and its two possible metabolites (2-MeO-3′,4,4′-tri-CDE and 2-HO-3′,4,4′-tri-CDE). Changes of four typical antioxidant biomarkers (SOD, CAT, GPx, and GSH) and the degree of lipid peroxidation indicator (MDA) that related to ROS in fish liver were determined. In addition, the toxicity order of these three compounds on aquatic species was evaluated based on the integrated biomarker response (IBR) index.
Materials and methods
Reagents and materials
2-MeO-3′,4,4′-tri-CDE, 2-HO-3′,4,4′-tri-CDE, and 3,4,4′-tri-CDE were synthesized and purified according to our previous methods (Zhang et al. 2013, 2015). Their structures were confirmed by gas chromatography–mass spectrometry (GC-MS) (Trace DSQII, Thermo Scientific, USA) and characterized by their 1H NMR spectra. The purities of these three chemicals (> 99%) were measured using high-performance liquid chromatography (HPLC) (1200, Agilent, USA). Table 1 shows the structural formulas and environmental-related properties of these three test chemicals.
Silica gel (100–200 mesh), magnesium sulfate anhydrous, dimethylsulfoxide (DMSO), and sodium sulfate anhydrous of analytical reagent (AR) grade were supplied by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Methylbenzene, petroleum ether, acetone, and other solvents of AR grade were obtained from Nanjing Chemical Reagent Co., Ltd. (Nanjing, China). The kits for oxidative stress biomarkers were supplied by Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Deionized water was produced by a Millipore Purification System (Elix 20, Millipore, USA).
Fish culture and experimental design
All of the experimental procedures were complied with the laboratory animal welfare regulations of China and ratified by Animal Care and Use Committee of the Anhui University. Juvenile C. auratus weighting 25.2 ± 2.6 g (mean ± standard deviation) were supplied by Hefei Yufeng aquatic products market (Anhui, China). Fish were originally cultured in an aquaria containing aerated and dechlorinated freshwater for 7 days. The fish was fed twice a day and the food residues were removed during domestication period. Before the anatomy and formal experiments, no food was offered for 24 h to eliminate the effects of feeding. The water characteristics throughout the whole exposure are listed in Table 2.
Ten acclimated fish were randomly selected and cultured in several aquaria as a treatment and control group, in which they were treated individually with 3,4,4′-tri-CDE, 2-MeO-3′,4,4′-tri-CDE, and 2-HO-3′,4,4′-tri-CDE at different doses (0, 0.1, 1, and 10 μg/L) via single water exposure for 3, 7, and 21 days. The volume of aquaria for keeping 10 fish was 25 L, and 20 L of the exposure medium (2 L per fish) was added. The experiments were performed in semi-static exposure mode, and half-volume (10 L) of expose medium in aquaria was replaced every day.
The dose and time selection was set according to the toxicity studies of alike compounds (Li et al. 2012, 2016). The minimum dose of this experiment is based on our previous study reporting the concentrations of 3′,4,4′-tri-CDE in natural water sampled from the Yangtze River (Nanjing section) up to 0.10–0.14 μg/L (Qin et al. 2015). Additionally, two relatively high concentrations were selected according to the LD50 values calculated by ECOSAR 2.0 (Ecosar Application, USA), i.e., no more than one fifth of the values of LD50. Consider to the lower solubility of tested compound in water, DMSO was used as co-solvent in this work. The stock solutions of 3,4,4′-tri-CDE, 2-MeO-3′,4,4′-tri-CDE, and 2-HO-3′,4,4′-tri-CDE were prepared in DMSO and stored at room temperature in dark. Final DMSO concentrations in the exposure medium were less than 0.01% in all groups, including the solvent control.
The concentrations of three test compounds were monitored during the whole exposure. The nominal concentrations of three test compounds in the exposure solutions were analyzed on an HPLC instrument armed with a diode-array detector (DAD). The HPLC analysis conditions were set based on the descriptions reported previously (Zhang et al. 2015). The detailed analysis procedures of target compounds in expose medium can be found in the Supporting Information (SI).
Sample preparation and biochemical analysis
The liver was chosen as the target tissue due to its importance during metabolic function and cellular detoxification (Xu et al. 2018). At the end of each exposure, five fish in each group were killed by striking and dissected and sampled on the ice sheet. Of the NaCl solution, 0.9% was used to clean the extraneous tissue, and the samples were kept at − 80 °C before biochemical analysis. Afterward, the liver was homogenized in cold physiological saline (1:10, w/v) by an Ultra Turrax homogenizer (Vortex 2, IKA, Germany), followed by 4 °C centrifuge at 4000×g for 15 min (8504R, Eppendorf, Germany), and the supernatants were taken for biochemical determination.
The supernatants obtained were diluted 100-fold to measure the activities of SOD, CAT, and GPx, while 20-fold dilution was used to determine the levels of GSH and MDA. All dilutions were completed on the ice. These oxidative stress indicators were measured following the instructions of the Diagnostic Reagent Kits. Briefly, SOD activity was determined at 550 nm following the method reported by Xu et al. (2011), which depended on the inhibitory effect of superoxide anion radicals on the reduction degree of cytochrome C. Activity of CAT was measured based on the residual amount of H2O2 at 405 nm following the method that reported by Góth (1991). GPx activity was tested in the light of the method described by Lawrence and Burk (1976). GSH level was evaluated at 412 nm according to the method of Jollow et al. (1974). MDA content was determined at 532 nm by reacting with thiobarbituric acid (TBA) on the basis of the method reported by Devasagayam (1986). Protein was assayed by the Coomassie Brilliant Blue staining technology according to the method of Bradford (1976).
Calculation of IBR index
For assessing the possible toxicity intensity of three test chemicals in fish liver, this study used a calculated method by combining all determined biomarker responses into a general “stress index” named as “IBR” (Beliaeff and Burgeot 2002). The following is a simple description on the calculation of IBR. For each biomarker, the mean and standard deviation of the data were calculated. Data were normalized on the basis of the following equation (Eq. (1)):
in which Y stands for the normalized data, X is the result of each biomarker response, m is the average result of the indicator, while S refers to the standard deviation of the indicator. Z = Y when activated or Z = − Y when inhibited. The minimum value (Min) of the normalized data of the biomarker is obtained in all treatments. S is counted as the following equation (Eq. (2)):
in which S ≥ 0 and the value of |Min| means the absolute value. The star map areas (Ai) were calculated using the following equations (Eqs. (3) and (4)):
where α = 2π/n, which refers to the angle between two adjacent auxiliary lines. The value of Si obtains from each biomarker. The corresponding IBR value was computed using the following equation (Eq. (5)):
where n represents the number of selected biomarkers.
In addition, the IBR values of control group were calculated using the average biomarker values of the control group at different exposure times (3 days, 7 days, and 21 days).
Statistical analysis
Three biological duplications were performed for determination of each biochemical parameter and all data were expressed as the mean ± SD. Statistical analysis was accomplished using the SPSS 17.0 (SPSS Inc., Chicago, USA) for Windows. The normality (Kolmogorov-Smirnov test) and homogeneity of variances (Levene test) of all measured data were performed. The differences of experimental groups as compared to the control were evaluated by a one-way analysis of variance (ANOVA) and the Dunnett test. Also, the significant intergroup differences were explained using post hoc LSD multiple comparison test and Duncan’s test. 0.01 < p < 0.05 was considered significant difference, and p < 0.01 showed highly significant difference. Graphical information was plotted using Origin 8.0 software (Origin Lab, USA).
Results
In this study, neither obvious signs of morbidity nor mortality of fish was recorded during 21 days of exposure. The actual average concentrations of three compounds in three dose groups (0.1, 1, and 10 μg/L) were determined using HPLC throughout the exposure durations (Table 3). Results suggested no obvious differences (within 10%) between the nominal and determined levels of three test compounds. Consequently, the nominal concentrations were selected in the following discussions.
Antioxidant enzymes
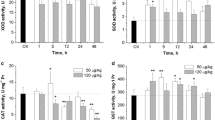
Effects of aqueous exposure to these three chemicals on the activities of SOD, CAT, and GPx in liver of C. auratus are shown in Fig. 1. The activity of SOD was unchanged (p > 0.05) after 3 days, apart from the inductions (p < 0.05 or p < 0.01) in fish exposed with 3,4,4′-tri-CDE (10 μg/L), 2-MeO-3′,4,4′-tri-CDE (10 μg/L), and 2-HO-3′,4,4′-tri-CDE (0.1 μg/L and 1 μg/L), and the overall induction was observed except 2-HO-3′,4,4′-tri-CDE (10 μg/L) (Fig. 1a). For SOD activity after 7 and 21 days, only the high-dose groups (10 μg/L) of these three compounds showed significant inhibition (p < 0.05 or p < 0.01). SOD activity was significantly reduced (p < 0.05 or p < 0.01) with the extending exposure time, except for 3,4,4′-tri-CDE (1 μg/L) and 2-MeO-3′,4,4′-tri-CDE (0.1 μg/L). SOD activity in fish liver treated with high-dose groups of 2-HO-3′,4,4′-tri-CDE showed the greatest decrease, i.e., about three times as less with the control.
Effect of exposure to 3,4,4′-tri-CDE, 2-MeO-3′,4,4′-tri-CDE, and 2-HO-3′,4,4′-tri-CDE on the activity of hepatic antioxidant enzymes (a SOD, b CAT, c GPx) in C. auratus. Data are expressed as the means ± SD, n = 3, for each data point. Superscript letters (a–f) indicate differences among the experimental treatments at the same exposure time for each of the compounds. *Significantly different from controls (p < 0.05), **highly significantly different from controls (p < 0.01)
For the CAT activity, minor variations (p > 0.05) were observed after 3 days, and significant variations (p < 0.05) occurred only in the treatments by 3,4,4′-tri-CDE (1 μg/L) and 2-HO-3′,4,4′-tri-CDE (Fig. 1b). Alteration of CAT activity was also not significant (p > 0.05) when the exposure time was 7 days, except for 3,4,4′-tri-CDE (10 μg/L), 2-MeO-3′,4,4′-tri-CDE (10 μg/L), and 2-HO-3′,4,4′-tri-CDE (1 μg/L). Nevertheless, conspicuous inhibitions (p < 0.05) were detected for almost all treatments after 21 days. CAT activity in the treatments of 3,4,4′-tri-CDE and 2-MeO-3′,4,4′-tri-CDE showed the highest inducement after 7 days. Howbeit, the change in CAT activity in 2-HO-3′,4,4′-tri-CDE and the high-dose groups of 3,4,4′-tri-CDE and 2-MeO-3′,4,4′-tri-CDE revealed a gradual decrease over time. Similar to the changes of SOD activity, CAT activity in fish liver treated with high-dose groups of 2-HO-3′,4,4′-tri-CDE presented the most significant changes.
During the whole exposure period, the activity of GPx showed minor variations in most experimental treatments. Also, it was noted that GPx activity was remarkably inhibited (p < 0.05) in several high-dose groups of 3,4,4′-tri-CDE and 2-HO-3′,4,4′-tri-CDE, whereas the significantly induced GPx activity (p < 0.05) was detected in the medium-dose groups (1 μg/L) of 2-HO-3′,4,4′-tri-CDE (Fig. 1c).
GSH levels
The effects of three test chemicals on GSH level in fish liver are shown in Fig. 2a. No obvious alteration (p > 0.05) in GSH level was found after 3 days of chemical exposure other than 3,4,4′-tri-CDE (10 μg/L) and 2-HO-3′,4,4′-tri-CDE (1 μg/L). GSH level showed conspicuous alterations (p < 0.05 or p < 0.01) except for 3,4,4′-tri-CDE (0.1 μg/L) and 2-MeO-3′,4,4′-tri-CDE (0.1 μg/L) after 7 days of exposure. Also, GSH levels of 3,4,4′-tri-CDE (10 μg/L), 2-MeO-3′,4,4′-tri-CDE (10 μg/L), and 2-HO-3′,4,4′-tri-CDE (1 μg/L and 10 μg/L) was significantly decreased (p < 0.05 or p < 0.01) after 21 days. Especially, the most obvious alteration were noted in the high-dose groups with the prolonged exposure time, in which 2-HO-3′,4,4′-tri-CDE was particularly significant.
Effect of exposure to 3,4,4′-tri-CDE, 2-MeO-3′,4,4′-tri-CDE, and 2-HO-3′,4,4′-tri-CDE on GSH level (a) and MDA content (b) in Carassius auratus. Data were expressed as the means ± SD, n = 3, for each data point. Superscript letters (a–f) indicate differences among the experimental treatments at the same exposure time for each of the compounds. *Significantly different from controls (p < 0.05), **highly significantly different from controls (p < 0.01)
MDA contents
As shown in Fig. 2b, MDA content was remarkably enhanced (p < 0.05 or p < 0.01) and presented the time-dependent pattern in the majority of the treatments, except for all compounds at low-dose group (0.1 μg/L) after 21 days. Particularly, the level of MDA was highly induced (p < 0.05 or p < 0.01) in the high-dose treatments of 2-HO-3′,4,4′-tri-CDE after 21 days.
IBR index
In this study, the responses of all biochemical parameters after different experimental treatments were standardized and depicted as the star plots for each treatment (Fig. 3). The ranges of IBR values for three chemical treatments were from 7.95 in 2-MeO-3′,4,4′-tri-CDE after 7 days (2.66 for 0.1 μg/L, 1.63 for 1 μg/L, and 3.66 for 10 μg/L) to 48.08 in 2-HO-3′,4,4′-tri-CDE after 21 days (11.53 for 0.1 μg/L, 12.29 for 1 μg/L, and 24.26 for 10 μg/L; Fig. 4). Hence, the rank of the most affected treatment could be arranged in the following order: 2-HO-3′,4,4′-tri-CDE-21 days > 2-HO-3′,4,4′-tri-CDE-7 days > 2-MeO-3′,4,4′-tri-CDE-21 days > 3,4,4′-tri-CDE-21 days > 2-HO-3′,4,4′-tri-CDE-3 days > 2-MeO-3′,4,4′-tri-CDE-3 days > 3,4,4′-tri-CDE-7 days > 3,4,4′-tri-CDE-3 days > 2-MeO-3′,4,4′-tri-CDE-7 days.
Discussion
It was proved that PCDEs have the potential to cause the toxicity in the early growth stage of fish (Qin et al. 2014), and also exert the significant disruption on the hepatic enzyme activities in living organisms (Carlson et al. 1980; Chui et al. 1985; Metcalfe et al. 1997). The current study demonstrated that 3,4,4′-tri-CDE and its two derivatives (MeO-3′,4,4′-tri-CDE and 2-HO-3′,4,4′-tri-CDE) have the ability to trigger hepatic oxidative stress even under environmentally relevant concentrations, and the treated fish exhibited a series of biochemical changes.
Antioxidative responses
Normally, the free radicals and their scavenging rates by the living organisms are in a state of dynamic equilibrium. However, the xenobiotic pollutants could induce the increasing production of intracellular ROS and thus break the balance, causing cell damage in aquatic species (Van de Oost et al. 2003; Zhang et al. 2014). The enzymatic system and non-enzymatic system form an important antioxidant defense, while its synthesis and reduction are controlled and influenced by external factors. Both of them function together to protect the organisms against the oxidative damage induced by exogenous substances (Wang et al. 2006; Luo et al. 2006). This work showed the significantly changed oxidative stress biomarkers following with different exposure concentrations of three test compounds and the extending exposure durations.
Changes in antioxidant enzyme activities can reflect the oxidative stress caused by ROS. SOD catalyzes superoxide anion radicals (O2−) to hydrogen peroxide (H2O2) and O2, and CAT catalyzes H2O2 to H2O and O2. They constitute the first-line to defense excess reactive oxygen radicals in cells. CAT and GPx serve jointly on the scavenging agents of resulted H2O2 and other hydroperoxides, which reduce the concentration of H2O2 in the organism (Sun et al. 2006; Zhang et al. 2014). In the present research, the activity of SOD and CAT was significantly increased in most treatments after 3 days of exposure to three chemicals, indicating that antioxidation mechanisms may act as the adaptive responses to clean ROS (Sun et al. 2006). In general, exposure to these three chemicals triggered an initial induction of SOD activity, followed by inhibition. CAT activity also showed a decreasing trend over time in the high-dose treatment. SOD and CAT activities of 2-HO-3′,4,4′-tri-CDE exhibited the similar pattern throughout the exposure, whereas the treatments by the other two compounds at low to medium concentrations were the most induced after 7 days of exposure. These results suggested that 2-HO-3′,4,4′-tri-CDE-treated groups may yield the most severe oxidative stress intensity. The inhibitive response of these antioxidant enzymes may reflect their failure to adequately scavenge the oxidative substances accumulated under chemical exposure. Similar findings were also reported on 4,4′-tri-CDE in freshwater algae Scenedesmus obliquus with different concentrations (Fang et al. 2018). It presumably illustrated that slight oxidative stress was caused by lower concentrations and severe oxidative stress by higher concentrations (Xie et al. 2011). In addition, the SOD and CAT activities in liver of C. auratus exposed to phenanthrene were similar to that of 3,4,4′-tri-CDE and 2-MeO-3′,4,4′-tri-CDE (Yin et al. 2007).
The most crucial detoxification function of GPx is to terminate the spread of the radical chain reactions, and thus protect the membranes from oxidative damage. As the second-line defense enzyme for oxidative stress, GPx also participates in the decomposition process of H2O2 and lipid peroxide. The change of GPx activity in our study was not very obvious, which may be related to the sequence of antioxidant enzymes for protection against oxidative damage (Pandey et al. 2003). Notably, the GPx activity only showed significant inhibition in the first 21 days of the high-dose group of 3,4,4′-tri-CDE and 2-HO-3,4,4-tri-CDE, suggesting that the hydroperoxide products may overwhelm the antioxidant defense and thus impair GPx synthesis. The dose-dependent trend reported herein was recently reported by Xing et al. (2017) in Polymesoda erosa exposed to short-chain chlorinated paraffins (SCCPs). As an oxyradical scavenger, GSH also serves as the important reagent in conjugation with electrophilic substances, and plays a critical role in scavenging free radicals and detoxification of electrophilic compounds (Cheung et al. 2001; Feng et al. 2013). GSH not only contributes to eliminate some reactive molecules from the cells but also has some direct interactions with certain ROS for cellular detoxification (Zhang et al. 2008). It is demonstrated that exposure to organic pollutants could lead to the time-dependent and/or dose-dependent variations of GSH levels in different fish species (Majeed et al. 2014). The present work revealed that GSH level in fish liver was notably induced in the medium-dose group of 3,4,4′-tri-CDE and 2-MeO-3′,4,4′-tri-CDE, suggesting that synthesis of GSH was activated due to the compensatory responses. On the other hand, the inhibition of hepatic GSH levels in fish was remarkably suppressed in a time-dependent pattern after long-term exposure to high-dose groups of these three compounds. It is noteworthy that GSH levels at the highest-exposure concentration after 21 days were only about 43% of the control, indicating that the liver has no ability to remove the excessive oxyradicals (Pandey et al. 2008). In other words, the rates of GSH synthesis may be overwhelmed by its oxidation rates (Zhang et al. 2008), leading to its exhaustion and the resultant transformation of the cell from the redox balance to other oxidative status (Feng et al. 2015).
Overall, our results demonstrated that PCDEs and its two derivatives could result in the excessive generation of cellular ROS, which may greatly disrupt the homeostasis of antioxidant defense system. Under certain exposure conditions, the activity of three antioxidant enzymes and level of GSH only presented some insignificant alterations, reflecting the protective processes of antioxidant defenses, i.e., radical-scavenging performance. In other words, these antioxidants worked jointly to clear the ROS and protect the organism against the harmful damage caused by the free radicals (Li et al. 2016). Moreover, the observed severe OS in fish liver even after lower dose exposure of toxicants might be due to the high bioaccumulation potentials of three compounds.
Estimation of lipid peroxidation
The degree of lipid peroxidation was shown by MDA content, which could reflect the production of reactive radicals in living species. Bebianno et al. (2005) confirmed that high content of MDA was partly attributed to the failure of antioxidant defense system. The growth of MDA content has a strong destructive effect on the cell membrane, and has been considered as the decisive factor for oxidative stress in organisms exposed to various pollutants. In this study, MDA content was positively correlated with the exposure time, presenting the time-dependent effect of these three chemicals on MDA content in fish liver. The significantly elevated MDA content was mostly noted after 21 days, indicating that the antioxidant system was greatly damaged under the chemical stress. This could result in more rapid accumulation of ROS to attack the cell membrane and then aggravate the lipid peroxidation. A previous study also confirmed the lipid peroxidation in fish livers and gills exposed to hexabromobenzene based on the dramatically provoked MDA contents in the high-dose groups with longer exposure period (Feng et al. 2014). Comparatively, exposure to 2-HO-3′,4,4′-tri-CDE showed a more obvious growth trend of MDA content, implying that its interaction with fish liver could induce ROS more easily and thereby cause the cell membrane to be attacked. Our more recent study suggested that exposure to 4,4′-di-CDE can also lead to the significantly enhanced MDA levels in S. obliquus (Fang et al. 2018).
Comparative intensity of the oxidative stress-inducing potentials of three test pollutants
In this work, the calculated IBR index was employed to analyze and compare the toxicity intensity of 3,4,4′-tri-CDE, 2-MeO-3′,4,4′-tri-CDE, and 2-HO-3′,4,4′-tri-CDE more visually and effectively. IBR index has been demonstrated as the valuable parameter for quantitative evaluation of the toxicological effects of different organic compounds (Kim et al. 2010). In general, the higher IBR value indicates the greater OS stress (Yan et al. 2016). The IBR results of the present study showed that 2-HO-3′,4,4′-tri-CDE-21 days can cause the most significant changes in biochemical responses. As the exposure time of these three chemicals was prolonged, the IBR values were prone to increase, while the time-dependent tendency might be attributed to the high accumulation of cellular ROS triggered by the test compounds in fish liver. Similar results were also found in C. auratus exposed to hexabromobenzene (Feng et al. 2014). Notably, 2-HO-3′,4,4′-tri-CDE-21 days induced the most severe stress on fish liver than two other compounds, possibly indicating its highest toxicity. The reason may be that hydroxyl groups combined with water molecule to form hydrogen bond, thus promoting the water solubility of chemicals and the permeation into the cell (Kodavanti et al. 2003; Zhang et al. 2015).
As mentioned above, the toxicity effects of three test compounds in fish were different, which may be partly due to the metabolism of the compounds, leading to the possible activation or inactivation. The n-octanol/water partition coefficient (logKow) is an important parameter characterizing the environmental behaviors of persistent organic pollutants (Varanka et al. 2001). Generally, the pollutants tend to be enriched in organisms when the logKow values are greater than 4.0 (Kelly et al. 2007). Thus, the balance between ROS generation and the antioxidant defense system could be destroyed by the bioaccumulation of xenobiotics, which caused oxidative stress. Due to the hydrophilicity of hydroxyl groups, the logKow value decreased, which may be another reason why hepatic antioxidant status was the most affected in fish exposed to 2-HO-3′,4,4′-tri-CDE. In addition, the effect of 2-HO-3′,4,4′-tri-CDE on single biomarker of fish liver was also stronger, which was in good accordance with the calculated IBR index. These results demonstrated that this parameter may be served as an effective supplement to predict the compound toxicity.
In view of the widespread existence of PCDEs in water, their potential metabolism, and the uncertainty of aquatic toxicity of their metabolites, further studies should be focused on the evaluation of chronic toxicity of different PCDE congeners and their biotransformation derivatives on various tissues of aquatic organisms, which could fill information gap on the risk assessments of some related contaminants in the water environment.
Conclusions
This study suggested that semi-static water exposure (3 to 21 days) of 3,4,4′-tri-CDE, 2-MeO-3′,4,4′-tri-CDE, and 2-HO-3′,4,4′-tri-CDE at different doses (0.1–10 μg/L) could induce ROS formation and trigger obviously oxidative stress in freshwater fish liver. These treatments caused the significant inductions or inhibitions at various degrees on the activity of SOD and CAT as well as GSH level in fish livers, indicating that these parameters can be used as sensitive biomarkers for assessing the hepatic OS induced by PCDEs. Meanwhile, the GPx activity was not sensitive throughout the whole exposure. The lipid peroxidation was observed based on the significantly induced MDA content following with the increasing exposure time and dose. Moreover, IBR analysis showed the toxicity intensity of these three chemicals, from which exposure to 2-HO-3′,4,4′-tri-CDE for 21 days presented the most severe aquatic toxicity.
References
Almroth BC, Albertsson E, Sturve J, Förlin L (2008) Oxidative stress, evident in antioxidant defenses and damage products, in rainbow trout caged outside a sewage treatment plant. Ecotoxicol Environ Saf 70:370–378
Bebianno MJ, Company R, Serafim A, Camus L, Cosson RP, Fiala-Médoni A (2005) Antioxidant systems and lipid peroxidation in Bathymodiolus azoricus from mid-Atlantic ridge hydrothermal vent field. Aquat Toxicol 75:354–373
Beliaeff B, Burgeot T (2002) Integrated biomarker response: a useful tool for ecological risk assessment. Environ Toxicol Chem 21:1316–1322
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Carlson GP, Smith EN, Johnson KM (1980) Induction of xenobiotic metabolism in rat liver by chlorinated biphenyl ether isomers. Drug Chem Toxicol 3:293–303
Chen HL, Hsu CH, Hung DZ, Hu ML (2006) Lipid peroxidation and antioxidant status in workers exposed to PCDD/fs of metal recovery plants. Sci Total Environ 372:12–19
Cheung CCC, Zheng GJ, Li AMY, Richardson BJ, Lam PKS (2001) Relationships between tissue concentrations of polycyclic aromatic hydrocarbons and antioxidative responses of marine mussels. Perna viridis. Aquat Toxicol 52:189–203
Chui YC, Hansell MM, Addison RF, Law FC (1985) Effects of chlorinated diphenyl ethers on the mixed-function oxidases and ultrastructure of rat and trout liver. Toxicol Appl Pharmacol 81:287–294
Devasagayam TPA (1986) Lipid peroxidation in rat uterus. BBA-Mol Cell Biol L 876:507–514
Domingo JL, Bocio A, Falcoa G, Llobet JM (2006) Exposure to PBDEs and PCDEs associated with the consumption of edible marine species. Environ Sci Technol 40:4394–4399
Fang BX, Shi JQ, Qin L, Feng MB, Cheng DR, Wang TT, Zhang XS (2018) Toxicity evaluation of 4,4′-di-CDPS and 4,4′-di-CDE on green algae Scenedesmus obliquus: growth inhibition, change in pigment content, and oxidative stress. Environ Sci Pollut Res 25:15630–15640
Feng MB, Qu RJ, Wang C, Wang LS, Wang ZY (2013) Comparative antioxidant status in freshwater fish Carassius auratus exposed to six current-use brominated flame retardants: a combined experimental and theoretical study. Aquat Toxicol 140–141:314–323
Feng MB, Qu RJ, Li Y, Wei ZB, Wang ZY (2014) Biochemical biomarkers in liver and gill tissues of freshwater fish Carassius auratus following in vivo exposure to hexabromobenzene. Environ Toxicol 10:1460–1470
Feng MB, He Q, Meng LJ, Zhang XL, Sun P, Wang ZY (2015) Evaluation of single and joint toxicity of perfluorooctane sulfonate, perfluorooctanoic acid, and copper to Carassius auratus using oxidative stress biomarkers. Aquat Toxicol 161:108–116
Góth L (1991) A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta 196:143–151
Husak VV, Mosiichuk NM, Maksymiv IV, Storey JM, Storey KB, Lushchak VI (2016) Oxidative stress responses in gills of goldfish, Carassius auratus, exposed to the metribuzin-containing herbicide Sencor. Environ Toxicol Pharmacol 45:163–169
Iverson F, Newsome H, Hierlihy L (1987) Induction of rat hepatic monooxygenase activity by polychlorinated diphenyl ethers. Food Chem Toxicol 25:305–307
Jabeen K, Li BW, Chen QQ, Su L, Wu CX, Hollert H, Shi HH (2018) Effects of virgin microplastics on goldfish (Carassius auratus). Chemosphere 213:323–332
Jollow DJ, Mitchell JR, Zampagilone N, Gilete JR (1974) Bromobenzene-induced liver necrosis: protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–169
Kelly BC, Ikonomou MG, Blair JD, Morin AE, Gobas FAPC (2007) Food web–specific biomagnification of persistent organic pollutants. Science 317:236–239
Kim WK, Lee SK, Jung JH (2010) Integrated assessment of biomarker responses in common carp (Cyprinus carpio) exposed to perfluorinated organic compounds. J Hazard Mater 180:395–400
Kodavanti PR, Ward TR, Derryellin EC, Mckinney JD, Tilson HA (2003) Increased [3H]phorbol ester binding in rat cerebellar granule cells and inhibition of 45Ca2+ buffering in rat cerebellum by hydroxylated polychlorinated biphenyls. Neurotoxicology 24:187–198
Kodavanti PR, Royland JE, Richards JE, Besas J, Macphail RC (2011) Toluene effects on oxidative stress in brain regions of young-adult, middle-age, and senescent brown Norway rats. Toxicol Appl Pharmacol 256(3):386–398
Koistinen J (2000) Polychlorinated diphenyl ethers, the handbook of environmental chemistry, vol 13 Ppart K. Springer Verlag, Berlin, pp 157–201
Koistinen J, Paasivirta J, Suonpera M (1995) Contamination of pike and sediments from Kymijoki River by PCDEs, PCDDs, and PCDFs: contents and patterns compared to pike and sediment from the Bothnian Bay and seals from Lake Saimaa. Environ Sci Technol 29:2541–2547
Koistinen K, Herve S, Ruokojärvi P, Koponen J, Vartiainen T (2010) Persistent organic pollutants in two Finnish watercourses: levels, congener profiles and source estimation by mussel incubation. Chemosphere 80:625–633
Kruz J, Ballschmiter K (1995) Isomer specific determination of 79 polychlorinated diphenyl ethers (PCDEs) in cod liver oil, chlorophenols and a fly ash. Fresenius J Anal Chem 351:98–109
Kurz J, Ballschmiter K (1999) Vapour pressures, aqueous solubilities, Henry’s law constants, partition coefficients between gas/water (K gw), n-octanol/water (K ow) and gas/n-octanol (K go) of 106 polychlorinated diphenyl ethers (PCDE). Chemosphere 38:573–586
Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium deficient rat liver. Biochem Biophys Res Commun 71:952–958
Li Y, Li M, Shi JQ, Yang X, Wang ZY (2012) Hepatic antioxidative responses to PCDPSs and estimated short-term biotoxicity in freshwater fish. Aquat Toxicol 120-121:90–98
Li CG, Qin L, Qu RJ, Sun P, Wang ZY (2016) Responses of antioxidant defense system to polyfluorinated dibenzo-p-dioxins (PFDDs) exposure in liver of freshwater fish Carassius auratus. Ecotoxicol Environ Saf 126:170–176
Luo Y, Su Y, Lin RZ, Shi HH, Wang XR (2006) 2-Chlorophenol induced ROS generation in fish Carassius auratus based on the EPR method. Chemosphere 65:1064–1073
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30
Majeed SA, Nambi KS, Taju G, Vimal S, Venkatesan C, Hameed AS (2014) Cytotoxicity, genotoxicity and oxidative stress of malachite green on the kidney and gill cell lines of freshwater air breathing fish channa striata. Environ Sci Pollut Res 21(23):13539–13550
Metcalfe CD, Metcalfe TL, Cormier JA, Huestis SY, Niimi AJ (1997) Early life-stage mortalities of Japanese medaka (Oryzias Latipes) exposed to polychlorinated diphenyl ethers. Environ Toxicol Chem 16:1749–1754
Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB (2014) Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20:1126–1167
Pandey S, Parvez S, Sayeed L, Haque R, Bin-Hafeez B, Raisuddin S (2003) Biomarkers of oxidative stress: a comparative study of river yamuna fish wallago Attu (B1.&Schn.). Sci Total Environ 309:105–115
Pandey S, Parvez S, Ansari RA, Ali M, Kaur M, Hayat F, Ahmad F, Raisuddin S (2008) Effects of exposure to multiple trace metals on biochemical, histological and ultrastructural features of gills of a freshwater fish, Channa punctate Bloch. Chem Biol Interact 174:183–192
Qin L, Liu F, Liu H, Wei ZB, Sun P, Wang ZY (2014) Evaluation of HODE-15, FDE-15, CDE-15, and BDE-15 toxicity on adult and embryonic zebrafish (Danio rerio). Environ Sci Pollut Res 21:14047–14057
Qin L, Feng MB, Zhang XS, Wang LS, Wang ZY (2015) Occurrence of polychlorinated diphenyl ethers in Nanjing section of the Yangtze River: level and distribution pattern. Environ Sci Pollut Res 22:9224–9232
Rosiaka KL, Seo BW, Chu I, Francis BM (1997) Effects of maternal exposure to chlorinated diphenyl ethers on thyroid hormone concentrations in maternal and juvenile rats. J Environ Sci Health B 32:377–393
Samanta P, Im H, Na J, Jung J (2018) Ecological risk assessment of a contaminated stream using multi-level integrated biomarker response in Carassius auratus. Environ Pollut 233:429–438
Sun YY, Yu HX, Zhang JF (2006) Bioaccumulation, depuration and oxidative stress in fish Carassius auratus under phenanthrene exposure. Chemosphere 63:1319–1327
Van de Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Varanka Z, Rojik I, Varanka I, Nemcsok J, Abraham M (2001) Biochemical and morphological changes in carp (Cyprinus carpio L.) liver following exposure to copper sulfate and tannic acid. Comp Biochem Physiol C 128: 467–477
Villeneuve JY, Niimi AJ, Metcalfe CD (1999) Distribution and bioaccumulation of chlorinated diphenyl ethers in a contaminated embayment of Lake Ontario. J Great Lakes Res 25:760–771
Wang XR, Luo Y, Shi HH, Zhang JF (2006) Application of molecular biomarkers in early diagnosis and ecological risk assessment for water and soil. Environ Chem 25:320–325
Xie XC, Wu YX, Zhu MY, Zhang YK, Wang XR (2011) Hydroxyl radical generation and oxidative stress in earthworms (Eisenia fetida) exposed to decabromodiphenyl ether (BDE-209). Ecotoxicology 20:993–999
Xie ZX, Lu GH, Qi PD (2014) Effects of BDE-209 and its mixtures with BDE-47 and BDE-99 on multiple biomarkers in Carassius auratus. Environ Toxicol Pharmacol 38:554–561
Xing YZ, Nong Y, Lu YZ, Yang ML, Yan B (2017) Response characteristics of oxidative stress biomarkers of Polymesoda erosa to exposure of SCCPs. China Environ Sci 37:3962–3971
Xu D, Zhao J, Hang HC, Wen J (2011) Determination of SOD specific activity in animal and plant tissues by improved xanthine oxidase method. Food Sci 32:237–241
Xu X, Cui Z, Wang X, Wang X, Zhang S (2018) Toxicological responses on cytochrome P450 and metabolic transferases in liver of goldfish (Carassius auratus) exposed to lead and paraquat. Ecotoxicol Environ Saf 151:161–169
Yan LQ, Feng MB, Liu JQ, Wang LS, Wang ZY (2016) Antioxidant defenses and histological changes in Carassius auratus after combined exposure to zinc and three multi-walled carbon nanotubes. Ecotoxicol Environ Saf 125:61–71
Yin Y, Jia HX, Sun YY, Yu HX, Wang XR, Wu JC, Xue YQ (2007) Bioaccumulation and ROS generation in liver of Carassius auratus, exposed to phenanthrene. Comp Biochem Physiol C 145:288–293
Zeng XL, Wang ZY, Ge ZG, Liu HX (2007) Quantitative structure-property relationships for predicting subcooled liquid vapor pressure (P L) of 209 polychlorinated diphenyl ethers (PCDEs) by DFT and the position of cl substitution (PCS) methods. Atmos Environ 41:3590–3603
Zhang X, Yang FX, Zhang XL, Xu Y, Liao T, Song SB, Wang JW (2008) Induction of hepatic enzymes and oxidative stress in Chinese rare minnow (Gobiocypris rarus) exposed to waterborne hexabromocyclododecane (HBCDD). Aquat Toxicol 86:4–11
Zhang XS, Liu F, Wei ZB, Wang ZY (2013) Synthesis of diaryl ethers by CuIcatalyed C-O bond formation via Ullman coupling: assessing the reactivity of aryl halides. Lett Org Chem 10:31–36
Zhang XS, Feng MB, Liu F, Qin L, Qu RJ, Li DL, Wang ZY (2014) Subacute oral toxicity of BDE-15, CDE-15, and HODE-15 in ICR male mice: assessing effects on hepatic oxidative stress and metals status and ascertaining the protective role of vitamin E. Environ Sci Pollut Res 21:1924–1935
Zhang XS, Zeng XL, Qin L, Qu RJ, Shi JQ, Wei ZB, Yang SG, Wang ZY (2015) Experimental investigation on the soil sorption properties and hydrophobicity of polymethoxylated, polyhydroxylated diphenyl ethers and methoxylated-, hydroxylated-polychlorinated diphenyl ethers. Chemosphere 134:84–90
Zhang XS, Wang TT, Gao L, Feng MB, Qin L, Shi JQ, Cheng DR (2018) Polychlorinated diphenyl ethers (PCDEs) in surface sediments, suspended particulate matter (SPM) and surface water of Chaohu Lake, China. Environ Pollut 241:441–450
Funding
This research was financially supported by the National Natural Science Foundation of China (No. 21607001 and 21607058), the Anhui Provincial Natural Science Foundation (No. 1608085QB45), and the Science Research Project of Anhui Education Department (No. KJ2015A090).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 35 kb)
Rights and permissions
About this article
Cite this article
Cheng, D., Cao, K., Wang, T. et al. Evaluation of the oxidative stress in liver of crucian carp (Carassius auratus) exposed to 3,4,4′-tri-CDE, 2-MeO-3′,4,4′-tri-CDE, and 2-HO-3′,4,4′-tri-CDE. Environ Sci Pollut Res 26, 5164–5175 (2019). https://doi.org/10.1007/s11356-018-3938-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3938-2