Abstract
A field study was conducted along a fluorine gradient of soil pollution in Tunisia from Gabes, the most polluted site, to Smara, the reference site. Variations of fluoride (F) concentrations in soils were detected over 1 year in Gabes, Skhira, and Smara. F concentrations in the aerial part of two native plant species, i.e., Erodium glaucophyllum and Rhanterium suaveolens, were above the usual background concentrations. Bioaccumulation factors ranged from 0.08 to 1.3. With F concentrations in aerial parts up to 355 mg kg−1, both species may be described as F accumulators. Both species showed an earlier vegetative growth in Gabes than in Smara. However, some difference between their strategies could be observed, i.e., E. glaucophyllum shortening the period of its vegetative growth with an escape strategy and R. suaveolens decreasing its ratio of alive/dead parts potentially lowering the F toxicity by storage in dead cells. However, at a tissue level, mechanisms of tolerance were similar. Leaf section micrographs of both species showed a higher calcium accumulation in leaf midveins at Gabes than at Smara, confirming the role of calcium in plant F tolerance strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite the global importance of the phosphate industry and its high economic value for many countries (Haneklaus et al. 2015; Tayibi et al. 2009), by-product discharge and gaseous and particulate emissions, especially fluorides (F), cause severe environmental pollution where phosphate plants are located. The impact of F on the environment, and notably on the health of the flora and fauna, has been previously detailed (Davison and Weinstein 2006; Fornasiero 2001; Weinstein and Davison 2003). However, most of the previous studies on the effects of F on plant species have dealt with F accumulation in plant organs to determine potential toxicity (Álvarez-Ayuso et al. 2011; Ben Abdallah et al. 2006a, 2006b; Pack and Sulzbach 1976; Vike and Håbjørg, 1995). Among previous studies, long-term assays are less numerous (Domingos et al. 2003; Fornasiero 2003) and only a few have provided information on plant health and reproduction (Davison and Weinstein 2006; Kozlov et al. 2007). However, the effects of F on plant seasonal development, corresponding to a sensitive indicator usually affected by pollution (Kozlov et al. 2007; Ryser and Sauder 2006; Zvereva et al. 2010), need to be evaluated especially in the field, most of the studies on this topic being conducted under controlled conditions.
Retarded growth and leaf trait damages have been reported as general and well-known plant responses to F emissions from industrial activities (Franzaring et al. 2007; Mesquita et al. 2011). Ionomics, dealing with mineral nutrients, may be helpful to better understand stress tolerance mechanisms in plants (Singh et al. 2016). In a previous study, we found variations in Ca and Mg leaf content in Atractylis serratuloides due to fluoride pollution; both elements are involved in fluoride tolerance of this plant species (Boukhris et al. 2015b).
Rhanterium suaveolens Desf. (Asteraceae) and Erodium glaucophyllum L. (L’Hér.) (Geraniaceae) have been also identified as F tolerant plant species in polluted ecosystems in SE Tunisia (Boukhris et al. 2015a). Our hypothesis is that all the gypsophytes spontaneously growing in the surroundings of industrial sites emitting F share a common mechanism of enhancing F tolerance using Ca and Mg. A 1-year monitoring survey of plant vegetative growth of these two native plant species along a gradient of F pollution in the Gulf of Gabes (East of Tunisia) was therefore carried out. Moreover, scanning electron microscopy (SEM) coupled to X microanalysis (EDAX) was used to determine whether F pollution impacts major nutrient distribution in leaf and root of both plant species.
Materials and methods
Study area
The study area is located along a F gradient on the southeast coast of Tunisia from Gabes to Smara (60 km south of Sfax city; Fig. 1). The two sites of Gabes and Skhira are located close to phosphate fertilizer factories, and Smara, distant from any industrial activity, was used as a reference site, as previously reported by Boukhris et al. (2015a 2015b). The soils of the three sites are calcic-magnesic soils containing gypsum and limestone (Mtimet 2001).
Located in the Mediterranean arid bioclimate (Emberger 1954), these three sites are exposed to low rainfall and elevated annual average temperatures, ca. 159 mm and 21 °C, respectively, over the 30 last years (Chaieb and Boukhris 1998; Floret and Pontanier 1978; Zahran 2010). In 2011, annual average temperature was 21 °C, ranging from 10 °C in January to 28 °C in from July to September. This same year, rainfall ranged from 0 to 88 mm between July and October (Online Resource).
Plant material and growth patterns
Rhanterium suaveolens Def., a chamaephyte, and Erodium glaucophyllum L. (L’Hér.), a hemicryptophyte, were selected since both plant species were identified as fluoride-tolerant species and are abundant at the three sites (Boukhris et al. 2015a). Seasonal vegetative growth monitoring was based on estimations of percentages of green tissues (photosynthetically active sensu Smith et al. 1997). Concerning the aboveground phytomass of each tuft, the ratio of alive/dead shoots was visually estimated as described by Bokhorst et al. (2011). A shoot was considered dead when all its leaves had died and considered as alive when at least one green leaf was still present. This ratio was monitored for both species during 1 year (2011–2012), and a comparison was made following the gradient of F pollution of the different sites. Observations in triplicate were undertaken in May 2011, June 2011, January 2012, March 2012, and May 2012. For each species at each site, three mature individuals were randomly selected and monitored during the growth survey. In June, the phytovolumes (PV) of aboveground parts of five individuals of E. glaucophyllum and R. suaveolens per site were determined following the equation—PV = 2/3 π (½ (½D1 + ½D2)2)½ H—as described in Mighri et al. (2011) for Artemisia herba-alba with D1 and D2 the two orthogonal diameters of the crown and H the height in centimeters. Since the vegetation physiognomy present in the three areas was sparse, containing plant patches and bare ground areas, we also did a density monitoring in a 1-ha surface area per site for each site using the method of point quadrat (Gounot 1969) in June. A total of 20 quadrats were randomly placed in each three sites. Within each quadrat, we numbered individuals of E. glaucophyllum and R. suaveolens and expressed the results as density of tuft per square meter for each species.
Soil and plant fluoride analyses
During the period of the study and simultaneously with the ratio of alive/dead shoot monitoring, soil and plant samples were collected for F analyses. Three soil samples (0–20 cm depth) near each individual per species per site were collected and air dried. Likewise, leaves and stems of the middle of the shoots of three plants per species were also collected. Plant samples were washed with tap water several times and subsequently dipped into 0.01 M HCl for 5 min followed by thorough washing with distilled water. Then, the collected samples were dried at 80 °C during 72 h in an oven (Memmert) and stored until analysis. Fluoride analyses were performed on each soil and shoot sample at each of the five monitoring times.
Soil and plant samples were analyzed in triplicate. Soil samples were sieved (2 mm mesh), and then an extraction was performed using 5 g of soil sample mixed with 20 ml HCl (1 N) during 90 min and mixed with total ionic strength adjustment buffer (TISAB) for analysis using a fluoride-specific ion electrode (inlab/Model WTW) coupled to a pH-meter (pH ION R503) at ambient temperature (Mezghani et al. 2005). In addition, dried ground (< 1.0 mm) plant samples (0.5 g) were mixed with potassium carbonate (4 g) and sodium carbonate (4 g) and heated in an electric oven at 700 °C during 3 h. Then, hydrochloric acid wet process mineralization was performed for all samples followed by filtration and adjustment with distilled water for determination of F content by potentiometry as described by Mezghani et al. (2005).
Bioaccumulation factors (BAF), i.e., F concentration in aerial part/F concentration in surrounding soil, were calculated for each soil/plant pair and monthly average BAF were given for each site.
Scanning electron microscopy and elemental analysis
According to the results of F analyses, we selected plant samples with the highest F concentrations (i.e., in June 2011) for microanalysis X. Fresh samples of leaves, stems, and roots of both plant species were prepared, as described by Rabier et al. (2008).
Thirty-micrometer-thick transversal sections were cut at − 25 °C using a cryomicrotome (Cryo-cut II microtome Reichert-Jung) and then immediately placed on SEM specimen holders and carbon metallized (10–15 nm) for observation under an ESEM (environmental scanning electron microscope) Philips XL 30 microscope with detector EDAX SDD Apollo 10. X-ray mapping was performed for 20 min to give the elemental distribution for each selected element (Si, Mg, F, Ca, S, Cu, Cl, Fe, K, Na, Mn, P, Ni, Al, and P). In all cases, the voltage was 20 kV. SEM images were obtained with back-scattered electron (BSE) or secondary electron (SE) imaging. BSE imaging was used to study the micromorphology of the plant organs. To help identification of the different tissues of the analyzed organs, transversal histological sections were stained with carmino-green staining and observed with an optical microscope.
Statistical analysis
Statistical analyses were performed for all data using JMP 10 statistical software (SAS Institute, Cary, NC, USA). Fluoride concentrations in the aerial parts and in soils were compared over the monitoring period for each plant species at p ≤ 0.05. Densities of plants and phytovolumes were compared between sites in June for each plant species at p ≤ 0.05. The non-parametric tests Dunn (all pairs for joint ranks) or Wilcoxon (each pair) were used due to non-normal distributions of data.
Results
Fluoride contamination in soils and plants
The gradient of F soil pollution, i.e., Gabes > Skhira > Smara is maintained over time (Table 1). The site at Gabes was the most polluted, with an average concentration of F ca. 1300 mg kg−1; Skhira was ca. ten times less polluted, with an average of 140 mg kg−1; and the Smara site considered as reference had only ca. 40 mg kg−1. A lower concentration in F in soils from Gabes was observed in January 2012 compared to March and May 2012 but cannot be attributed to a dilution effect after a period of rainfall and less particulate F deposit (Online Resource).
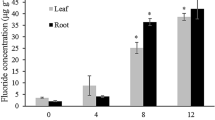
Average F concentrations in plant aerial parts followed the same gradient as soil contamination, with ca. 190, 91 and 36 mg kg−1 F in Gabes, Skhira, and Smara, respectively. F concentrations in plants and BAF in Skhira were constant throughout the observation period, although they were higher in May 2011 in Gabes and Smara compared to January 2012. However, BAF were < 1 at all sites and in all periods except in the reference site of Smara in May 2011.
Plant traits
E. glaucophyllum showed a maximal ratio of alive/dead shoots between January and March 2012 (Fig. 2a), representing the period of favorable climatic conditions for vegetative growth of this Mediterranean species. However, a lag time before the maximal level of this ratio was observed between the sites, i.e., this maximal occurred in January in Gabes and later in the other sites, particularly in the control (Smara). Full foliage (ratio = 90) of this species was concomitant with flowering phenophases (March 2012). During the dry period (July to September 2011), the aboveground parts of each tuft were totally dried.
In contrast, for R. suaveolens, the ratio of alive/dead shoots appeared proportionally reduced following the pollution gradient (Gabes > Skhira > Smara), from May 2011 to June 2011. Moreover, the maximal level of this ratio for this perennial was observed in Gabes since March, although it was the highest only in May in Smara and Skhira (Fig. 2b), this lag time mostly not being linked with high rainfall (Online Resource).
Thus, different ratios of alive/dead shoots patterns were observed in both plant species in Gabes compared to Smara.
The phytovolumes of both species decreased following the increasing F gradient in June (Table 2). The chamaephyte R. suaveolens had significantly higher phytovolumes than the hemicryptophyte E. glaucophyllum.
In June, the density of individuals forming tufts was significantly higher in Gabes than in Smara whatever the plant species (Table 2). The density of E. glaucophyllum was also higher in Gabes than in Skhira, illustrating a gradient of density of this species following the gradient of F concentration in soils. For R. suaveolens, the density was low and no significant differences in density were observed between Gabes and Skhira sites.
Nutrient localization in organ sections
EDAX cartography was performed on leaf, stem, and root sections of E. glaucophyllum and R. suaveolens for all the detected peaks of the X-ray spectra to determine element localization in plant tissues. Only maps of Si, Mg, F, Ca, and S in leaf and root sections (Figs. 3, 4, 5, and 6) are shown since all elements in stem maps as well as Cu, Cl, Fe, K, Na, and P maps in roots and leaves had identical distribution patterns between the Gabes and Smara samples. Even with high total F content in the organs from Gabes, F concentrations in both species were below the threshold of detection of the equipment (≥ 1%) at the level of a 30-μm-thick transversal section mapping with the selected electron acceleration voltage.
Concerning E. glaucophyllum, more Ca and less S were accumulated in the midvein of leaf sections and in the cortex of the root sections from Gabes than from Smara (Figs. 3g, h, i, k and 4g, h, i, k). For R. suaveolens, more Ca was deposited in the leaf midvein and in the cortex of the root in Gabes compared to Smara (Figs. 5g, h, i, k and 6g, h, i, k).
Neither structural difference nor alterations were detected comparing SEM micrographs of plant sections (leaf and root) from Gabes to Smara for both species (Figs. 3a, b, 4a, b, 5a, b, and 6a, b).
R. suaveolens leaf lamina was thinner (Fig. 5) than E. glaucophyllum leaf lamina (Fig. 3), with a thick-walled epidermis and thick cuticle.
Bright crystals in the midvein were clearly visible in BSE observations of E. glaucophyllum leaf section (Fig. 3a) and point analysis of these crystals indicated high Ca contents.
Discussion
Fluoride soil contamination and bioaccumulation factors of the two native plant species
Fluoride concentrations ranged from 33 to 1683 mg kg−1 in the analyzed soils from the Gulf of Gabes. However, the soil F concentrations in Smara, representing a maximum of 51 mg kg−1 F, can be considered as geochemical background level compared to F average concentration in many soils of the world ranging from 100 to 600 mg kg−1 F, as reviewed by Davison and Weinstein (2006). Variations of F concentrations in soils were detected throughout the year in Gabes and Smara. In Gabes, the lowest concentration in January 2012 was concomitant with a period of rainfall. However, as reviewed by Kabata-Pendias and Szteke (2015), a high percentage of F input is firmly retained by the soil, mainly as CaF2 in alkaline soils, and leaching removes only a small amount of F from soils. Moreover, in Skhira, no seasonal variation of F in soils was observed. A possible link between these variations and a reduction of the industrial superphosphate production in Gabes during the “Jasmine revolution” may be borne in mind (Taib 2013). As previously described, the same three soils have a pH ca 7.5 with a conductivity ranging from 1.5 to 2.3 mS cm−1 along with Cr concentrations ranging from 41 to 120 mg kg−1 (Boukhris et al. 2015a). It has been demonstrated that Cr may increase F toxicity in plants due to enhanced F absorption when F and Cr concentrations are high (Zhou and Sun 2002). In these author’s study, F and Cr concentrations in soils range from 219 to 823 mg kg−1 and 73 to 88 mg kg−1, respectively. Soil concentrations up to 1683 and 120 mg kg−1, for F and Cr respectively, were previously reported in the area of our study (Boukhris et al. 2015a) with significantly higher concentrations in Gabes than in Skhira. Accordingly, a higher phytotoxicity of F in Gabes than in Skhira is observed.
F concentrations in the aboveground part of both plant species were above the background contents of F in plants, usually lower than 10 mg kg−1 (Davison and Weinstein 2006). Bioaccumulation factors (BAF) ranged from 0.08 to 1.3. Due to F contents in aerial parts ranging from 26 to 355 mg kg−1, E. glaucophyllum and R. suaveolens may be described as F accumulators. Similar results were previously obtained for Atractylis serratuloides, another native plant species growing in the steppes of SE Tunisia (Boukhris et al. 2015b). Previous papers described plant species able to accumulate up to thousand milligrams per kilogram of F in their aerial parts (Saini et al. 2013; Singh et al. 2018), but most of these studies were conducted in laboratory conditions and some of them under hydroponic conditions far away from realistic conditions. However, our results may not drive to a phytoextraction strategy, biomass, and F accumulation of E. glaucophyllum and R. suaveolens being together not efficient. However, from an ecological point of view, the occurrence of these plant species nearby sources of F pollution may play a role that needs to be reinforced by limiting wind and water erosion processes.
Both species have an important ecological interest in plant communities of Tunisian arid ecosystems (Le Houérou 2008). They are characteristic of the two most dominant soil types of southern Tunisia. R. suaveolens is the main species of the Jeffara sandy coastal plain, and its natural distribution is from central Tunisia to North of Tripolitania in Libya. The species E. glaucophyllum, a gypsophyte, is characteristic of gypsum substrate of south Tunisia, which constitutes a great ecological originality of the region (Floret 1981). Floret and Pontanier (1982) estimated the density of R. suaveolens around 27.000 tufts ha−1, corresponding to ca. 2–3 tufts m−2. Their observations are consistent with the current results of our field observation (Table 2), indicating that the density of plants is a stable criterion in such abiotic conditions.
Plant tolerance strategies under fluoride pollution
Vegetative growth of E. glaucophyllum and R. suaveolens was affected by F pollution. E. glaucophyllum and R. suaveolens are both known as stress tolerant species, but the first one is known as more adapted to disturbed areas and, the latter, more competitive following Grime’s theory (Tarhouni et al. 2010). E. glaucophyllum showed an earlier leaf flushing, corresponding to a maximal ratio of alive/dead shoots, in Skhira and Gabes than in Smara. Consequently, the faster life cycle may shorten the period of exposure to F. One hypothesis may be that this plant species has an escape strategy (that differs from the excluder strategy preventing F absorption) following Franks (2011). This is in agreement with some life traits of arid hemicryptophytes described as “drought-escapers,” sensu Shantz (1927), or “drought-evaders,” sensu Small (1973). Accordingly, Zvereva et al. (2010) have concluded that Raunkiaer’s classification of life forms appeared to be the best predictor of species’ responses to pollution.
R. suaveolens had a higher ratio of alive/dead shoots in Gabes than at the other sites during favorable period and a lower during dry season. Due to a vegetative growth period limited by the drought episodes in the year, it also suggests a faster vegetative growth of this species under F pollution and may be part of the plant response to F pollution as it was demonstrated by Zvereva et al. (2010). However, this decreasing ratio of alive/dead shoots in June was linked to a lower phytovolume leading to a bioconcentration effect of F in plant tissues. It has been proven that dead cells could not only sorb metals in the cell wall but also potentially retain metals within the cells themselves (Ebbs et al. 2016). In the same way but for a non-metal element, the hypothesis of a high F tolerance of this plant species due to a F storage in the dead parts is emitted.
These different growth patterns under F pollution between the two observed plant species may reflect their different biological types, i.e., hemicryptophyte (E. glaucophyllum) and chamaephyte (R. suaveolens), and provide information regarding the long-term effect of F exposure on their ecosystems since vegetative traits are the key characteristics for predicting the response of future communities and ecosystems (Jablonski et al. 2002).
To reduce a long-term impact on their plant community, it is necessary to better understand the mechanisms of their fluoride tolerance.
Corroborating the role of calcium in fluoride tolerance of plants
Leaf section micrographs of both species showed a thick cuticle, especially in R. suaveolens, with narrow lamina and sclerenchymatous cell bundle, revealing their xerophytic characteristics enabling reduction of leaf tissue pollutant diffusion and water loss through stomata, as previously described in industrialized arid lands (Haworth and McElwain 2008; Thévenard et al. 2005). Neither necrosis nor leaf injuries were observed on spontaneous populations of both species in the field, even in Gabes. In both species, Ca distribution in leaf midveins was higher in Gabes than in Smara, and this may be considered as a physiological response to F. The role of Ca is primordial in lowering F phytotoxicity. The complex CaF2 reduces F translocation and its phytotoxic effects on the aboveground parts of plants (Álvarez-Ayuso et al. 2011; Ben Abdallah et al. 2006b; Weinstein and Davison 2003). Moreover, in leaf sections from both plant species from Gabes, the Ca distribution pattern in maps was superimposable with the detected bright crystals in the mesophyll and the midvein, which suggests the formation of Ca crystals. Calcium crystals are involved in plant detoxification and defense according to Nakata and McConn (2000). This is also in agreement with the important source of Ca in the calci-magnesic soils from the Gulf of Gabes (Boukhris et al. 2015b). This highlights the involvement of calcium ion in E. glaucophyllum and R. suaveolens F tolerance strategy, as was recently reported in our previous work on Atractylis serratuloides under the same ecological conditions (Boukhris et al. 2015b). These results are in agreement with the recognized role of calcium in plant abiotic-stress resistance (Song et al. 2008).
Conclusion
E. glaucophyllum and R. suaveolens, two native gypsophytes spontaneously growing nearby superphosphate factories in Tunisia, may be considered as F accumulators with F contents in their aerial parts following the gradient of F soil pollution as the following: Gabes > Skhira > Smara. Higher accumulation of Ca at a tissue level in the aerial parts of both species in Gabes suggests a protection mechanism of Ca against F phytotoxicity potentially related to Ca-F interactions as previously demonstrated in other gypsophytes. Phenological adaptations of these species that may lower their F exposure in the most F polluted sites were also observed. However, shortening the vegetative growth period or reducing the alive aerial plants may raise questions regarding the long-term impact of this pollution on the sustainability of the plant cover and its potential consequence on F dispersion in the environment with a reduced vegetation barrier.
References
Álvarez-Ayuso E, Giménez A, Ballesteros JC (2011) Fluoride accumulation by plants grown in acid soils amended with flue gas desulphurisation gypsum. J Hazard Mater 192:1659–1666
Ben Abdallah F, Elloumi N, Mezghani I, Boukhris M, Garrec J-P (2006a) Survival strategies of pomegranate and almond trees in a fluoride polluted area. Comptes Rendus Biologies 329:200–207
Ben Abdallah F, Elloumi N, Mezghani I, Garrec J-P, Boukhris M (2006b) Industrial fluoride pollution of Jerbi grape leaves and the distribution of F, Ca, Mg, and P in them. Fluoride 39:43–48 http://www.fluorideresearch.org/391/files/39143-48.pdf. Accessed 4 July 2018
Bokhorst S, Bjerkew JW, Streetz LE, Callaghan TV, Phoenix GK (2011) Impacts of multiple extreme winter warming events on sub-Arctic heathland: phenology, reproduction, growth, and CO2 flux responses. Glob Chang Biol 17:2817–2830. https://doi.org/10.1111/j.1365-2486.2011.02424.x Accessed 4 July 2018
Boukhris A, Laffont-Schwob I, Mezghani I, El Kadri L, Prudent P, Pricop A, Tatoni T, Chaieb M (2015a) Screening biological traits and fluoride contents of native vegetations in arid environments to select efficiently fluoride-tolerant native plant species for in-situ phytoremediation. Chemosphere 119:217–223
Boukhris A, Laffont-Schwob I, Rabier J, Salducci M-D, El Kadri L, Tonetto A, Tatoni T, Chaieb M (2015b) Changes in mesophyll element distribution and phytometabolite contents involved in fluoride tolerance of the arid gypsum-tolerant plant species Atractylis serratuloides Sieber ex Cass. (Asteraceae). Environ Sci Pollut Res 22(10):7918–7929
Chaieb M, Boukhris M (1998) Flore Des Zones Arides Et Sahariennes De Tunisie, Ed. L’Or du Temps, Tunis 290 p (In French)
Davison AW, Weinstein LH (2006) Some problems relating to fluorides in the environment: effects on plants and animals. In: Tressaud A (ed) Fluorine and the environment, atmospheric chemistry, emissions and lithosphere, vol 1. Elsevier, Amsterdam, pp 251–298
Domingos M, Klumpp A, Rinaldi MCS, Modesto IF, Klumpp G, Delitti WBC (2003) Combined effects of air and soil pollution by fluoride emissions on Tibouchina pulchra Cogn., at Cubatão, SE Brazil, and their relations with aluminium. Plant Soil 249:297–308
Ebbs SD, Bradfield SJ, Kumar P, White JC, Musante C, Ma X (2016) Accumulation of zinc, copper, or cerium in carrot (Daucus carota) exposed to metal oxide nanoparticles and metal ions. Environ Sci Nano 3(1):114–126
Emberger L (1954) Une Classification Biogéographique Des Climats. Rec Trav Lab Bot Géol Zool Univ Montpellier Sér Bot 7:3–43 (in French)
Floret C (1981) The effects of protection on steppic vegetation in the Mediterranean arid zone of Southern Tunisia. Vegetatio 46(1):117–129
Floret C, Pontanier R (1978) Relations climat-sol-végétation dans quelques formations végétales spontanées du sud Tunisien (production végétale et bilan hydrique des sols). Inst. Rég. Arides–Médenine, Dir. Ress. Eau et Sols Tunis, CEPE/CNRS Montpellier et Orstom—Paris, 96 p (in French)
Floret C, Pontanier R (1982) L’aridité en Tunisie présaharienne: Climat, sol, végétation et aménagement. Trav. et Doc. ORSTOM, n° 150—Paris, 544 p (in French)
Fornasiero RB (2001) Phytotoxic effects of fluorides. Plant Sci 161:979–985
Fornasiero RB (2003) Fluorides effects on Hypericum perforatum plants: first field observations. Plant Sci 165:507–513
Franks SJ (2011) Plasticity and evolution in drought avoidance and escape in the annual plant Brassica rapa. New Phytol 190:249–257
Franzaring J, Klumpp A, Fangmeier A (2007) Active biomonitoring of airborne fluoride near an HF producing factory using standardised grass cultures. Atmos Environ 41:4828–4840
Gounot M (1969) Méthodes d'étude quantitative de la végétation. Masson, Paris 314 p
Haneklaus N, Schnug E, Tulsidas H, Tyobeka B (2015) Using high temperature gas-cooled reactors for greenhouse gas reduction and energy neutral production of phosphate fertilizers. Ann Nucl Energy 75:275–282
Haworth M, McElwain J (2008) Hot, dry, wet, cold or toxic? Revisiting the ecological significance of leaf and cuticular micromorphology. Palaeoecology 262:79–90
Jablonski LM, Wang X, Curtis PS (2002) Plant reproduction under elevated CO2 conditions: a meta-analysis of reports on 79 crop and wild species. New Phytol 156(1):9–26
Kabata-Pendias A, Szteke B (2015) Trace elements in abiotic and biotic environments, chapter 15: Fluorine, CRC Press, pp 109–118
Kozlov MV, Eränen JK, Zverev VE (2007) Budburst phenology of white birch in industrially polluted areas. Environ Pollut 148:125–131
Le Houérou HN (2008) Bioclimatology and biogeography of Africa. Springer-Verlag, Heidelber 240 p
Mesquita GL, Tanaka FAO, Cantarella H, Mattos D Jr (2011) Atmospheric absorption of fluoride by cultivated species. Leaf structural changes and plant growth. Water Air Soil Pollut 219:143–156
Mezghani I, Elloumi N, Ben Abdallah F, Chaieb M, Boukhris M (2005) Fluoride accumulation by vegetation in the vicinity of a phosphate fertiliser plant. Fluoride 38:69–75 https://pdfs.semanticscholar.org/df02/76856028881ac0bc96142195dbc2e06e36d0.pdf. Accessed 4 July 2018
Mighri H, Akrout A, Neffati M (2011) Assessment of essential oil yield of Artemisia herba-alba cultivated in Tunisian arid zone. J Med Plants Res 5(21):5296–5300 https://academicjournals.org/article/article1380533387_Mighri%20et%20al.pdf Accessed 4 July 2018
Mtimet A (2001) Soils of Tunisia. In: Zdruli P, Steduto P, Lacirignola C, Montanarella L (eds) Soil resources of southern and eastern Mediterranean countries CIHEAM, Bari, pp 243–262
Nakata PA, McConn MM (2000) Isolation of Medicago trunculata mutants defective in calcium oxalate formation. Plant Physiol 124:1097–1110
Pack MR, Sulzbach CW (1976) Response of plant fruiting to hydrogen fluoride fumigation. Atmos Environ 10:73–81
Rabier J, Laffont-Schwob I, Notonier R, Fogliani B, Bouraïma-Madjebi S (2008) Anatomical element localization by EDXS in Grevillea exul var. exul under nickel stress. Environ Pollut 156:1156–1116
Ryser P, Sauder WR (2006) Effects of heavy-metal-contaminated soil on growth, phenology and biomass turnover of Hieracium piloselloides. Environ Pollut 140:52–61
Saini P, Khan S, Baunthiyal M, Sharma V (2013) Effects of fluoride on germination, early growth and antioxidant enzyme activities of legume plant species Prosopis juliflora. J Environ Biol 34:205–209
Shantz HL (1927) Drought resistance and soil moisture. Ecology 8(2):145–157
Singh S, Parihar P, Singh R, Singh VP, Prasad SM (2016) Heavy metal tolerance in plants: role of transcriptomics, proteomics, metabolomics, and ionomics. Front Plant Sci 6(article 1143):1–36
Singh G, Kumari B, Sinam G, Kumar KN, Shekhar M (2018) Fluoride distribution and contamination in the water, soil and plants continuum and its remedial technologies, an Indian perspective—a review. Environ Pollut 239:95–108
Small E (1973) Xeromorphy in plants as a possible basis for migration between arid and nutritionally-deficient environments. Bot Notiser 126:534–539
Smith SD, Monson RK, Anderson JE (1997) Physiological ecology of North American desert plants. Springer-Verlag, Berlin 286 p
Song W-Y, Zhang Z-B, Shao H-B, Guo X-L, Cao H-X, Zhao H-B, Fu Z-Y, Hu X-J (2008) Relationship between calcium decoding elements and plant abiotic-stress resistance. Int J Biol Sci 4(2):116–125
Taib M (2013) The mineral industry of Tunisia. In: USGS (eds), United States Geological Survey 2011 minerals yearbook, pp 41.1-41.7
Tarhouni M, Ben Salem F, Ouled Belgacem A, Neffati M (2010) Acceptability of plant species along grazing gradients around watering points in Tunisian arid zone. Flora 205:454–461
Tayibi H, Choura M, López FA, Alguacil FJ, López-Delgado A (2009) Environmental impact and management of phosphogypsum. J Environ Manag 90:2377–2386
Thévenard F, Gomez B, Daviero-Gomez V (2005) Xeromorphic adaptations of some Mesozoic gymnosperms. A review with palaeoclimatological implications. Comptes Rendus Palevol 4:67–77
Vike E, Håbjørg A (1995) Variation in fluoride content and leaf injury on plants associated with three aluminium smelters in Norway. Sci Total Environ 163:25–34
Weinstein LH, Davison A (2003) Native plant species suitable as bioindicators and biomonitors for airborne fluoride. Environ Pollut 125:3–11
Zahran MA (2010) Climate vegetation. Afro-Asian Mediterranean and Red Sea coastal lands. Plant and Vegetation. Springer Dordrecht Heidelberg, London New York 344 p
Zhou Q, Sun T (2002) Effects of chromium (VI) on extractability and plant uptake of fluorine in agricultural soils of Zhejiang Province, China. Water Air Soil Pollut 133: 145 p:145–160
Zvereva EL, Roitto M, Kozlov MV (2010) Growth and reproduction of vascular plants in polluted environments: a synthesis of existing knowledge. Environ Rev 18:355–367
Acknowledgments
The authors thank Lefi El Kadri for his help in harvesting and sampling in the field and are grateful to Alain Tonetto for his help in the use of SEM coupled with EDAX. We thank the Groupe Chimique Tunisien, in particular the Direction Centrale de la Recherche, for its assistance and logistical support in carrying out this study. The authors would also like to thank Michael Paul for revising the English of this text and are grateful to the two anonymous reviewers for their suggestions that helped to improve the quality of the manuscript.
Funding
This study was partly funded by the Action Intégrée Franco-Tunisienne of the French Ministère des Affaires Etrangères et Européennes (EGIDE UTIQUE 2012–2014) and the Tunisian Ministère de l’Enseignement Supérieur, de la Recherche Scientifique (UR 11ES71) entitled: “Réponse écophysiologique de la végétation naturelle vis-à-vis de la pollution atmosphérique fluorée, Tunisie.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Gangrong Shi
Electronic supplementary material
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Boukhris, A., Laffont-Schwob, I., Folzer, H. et al. Tolerance strategies of two Mediterranean native xerophytes under fluoride pollution in Tunisia. Environ Sci Pollut Res 25, 34753–34764 (2018). https://doi.org/10.1007/s11356-018-3431-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3431-y