Abstract
Biofouling is a serious issue in membrane-based water and wastewater treatment as it critically compromises the efficacy of the water treatment processes. This investigation demonstrates the antimicrobial and antifouling properties of a nanocomposite membrane system composed of carboxyl-functionalized graphene oxide (COOH-GO) and polyphenylsulfone (PPSU). The PPSU/COOH-GO nanocomposite membrane exhibited excellent antimicrobial properties, achieving maximum bacteriostasis rates of 74.2% and 81.1% against the representative Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa, respectively) and 41.9% against the representative Gram-positive bacterium (Staphylococcus aureus). The PPSU/COOH-GO nanocomposite membrane inhibited the attachment, colonization, and the biofilm formation of three species. Antifouling was assessed through filtration experiments using a model foulant bovine serum albumin (BSA). The fouling mechanisms were investigated by Hermia’s models (complete blocking, intermediate blocking, standard blocking, and cake formation), and the analysis involved fitting the volumetric flux decline experimental data to models. The fouling study revealed a less irreversible fouling and increased flux recovery ratio for the PPSU/COOH-GO nanocomposite membrane. Complete blocking of pores and cake formation were the major fouling mechanisms for the membrane.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Membrane technology is a green, energy-efficient, and cost-efficient separation and purification technology with diverse potential applications in food processing, protein separation, biotechnology, pharmaceutical manufacture, reduction of microorganisms, and wastewater treatments (Shannon et al. 2008; Bogdanović et al. 2015; Lee et al. 2016; Koh and Lee 2017; Morelos-Gomez et al. 2017; Xu et al. 2017). However, practical application of membrane technology is hampered by membrane fouling, which reduces membrane performance stability and water flux, shortens the service life, and increases the maintenance and operational costs. Fouling is generally initiated by the growth and deposition of the foulants on membrane surface. Foulants include contaminating microorganisms, inorganic or organic compounds, and colloids (Wang and Tang 2011; Hu et al. 2016; Perreault et al. 2016; Emadzadeh et al. 2017; Hou et al. 2017; Ma et al. 2017; Shockravi et al. 2017; You et al. 2017; Corbatón-Báguena et al. 2018; Zhang et al. 2018b). Biofouling associated with microorganisms of microbial proteins is common and can lead to a biofilm formation on the surface of membranes. Biofilms result from the attachment and proliferation of bacteria. This colonization, along with the accumulation of secreted extracellular polysaccharides and proteins, can block the membrane pores, degrade the membrane, and reduce membrane flux (Ahmed et al. 2012; Perreault et al. 2016; Gao et al. 2017a; Hou et al. 2017; Liu et al. 2017; Srinivasan et al. 2018).
Elimination of membrane biofouling is challenging, since even low residual numbers of microorganisms can regenerate the biofilm. The ideal approach is to restrict the attachment and surface proliferation of Gram-positive bacteria and Gram-negative bacteria, with the goals of reducing the decomposition of essential metabolites, preserving cell membrane structure, and maintaining the activity of enzymes used for protein denaturation (Yu et al. 2013a; Koh and Lee 2017; Qiu et al. 2017; Zhang et al. 2018a). Membrane hydrophilicity, surface charge, and surface roughness also influence membrane fouling, and recent research efforts have focused on enhancing membrane surface hydrophilicity and charge through membrane modification (Yu et al. 2013a; Ben-Sasson et al. 2014; Emadzadeh et al. 2017; Zhu et al. 2017; Hu et al. 2018; Shukla et al. 2018). The numerous modification methods that have been applied include coating, grafting, and, germane to this study, mixed matrix membranes (Ho et al. 2017; Abdel-Karim et al. 2018; Moradi et al. 2018; Nasrollahi et al. 2018). Mixed matrix membranes can incorporate inorganic nanoparticles including carbon nanotubes, zinc oxide, titanium dioxide, aluminum oxide, silicon dioxide, nanogold, and graphene oxide (GO) (Safarpour et al. 2016; Chung et al. 2017; Shukla et al. 2017; Hu et al. 2018; Wang et al. 2018).
GO nanoparticles have been widely researched concerning membrane applications due to their superior physico-chemical properties caused by different functional groups, which render them bactericidal yet comparatively safe for humans (Akhavan and Ghaderi 2010; Benelli 2018). The carboxyl groups (COOH) on GO makes it easy to obtain hydrophilic and negatively charged membranes. GO forms strong complexes with water molecules, is outstandingly suited for membrane formation, and enhances antibacterial and antifouling properties of membranes (Luyts et al. 2013; Yu et al. 2015; Xu et al. 2016; Eng et al. 2017). GO also has reactive groups that can strongly interact with bacterial cells and easily cover cell surfaces. As a result, the surface affinity for bacteria is increased. GO has also been implicated as a possible antibiotic for bacteria because it can permanently damage bacteria by disrupting membrane potential (Gao et al. 2017b). The sharpened edges of GO can physically damage to bacterial walls. These collective advantages of GO may be useful in new antibacterial and antifouling technologies.
In pursuing our previous study on the nanocomposite membranes, comprising polyphenylsulfone (PPSU) and carboxylic-functionalized GO (COOH-GO), it was observed that carboxylated-GO produced a membrane with enhanced properties (such as surface charge, hydrophilicity and membrane morphology) that exhibited superior performance in terms of heavy metals removal. Here, we discuss the extraordinary potential of COOH-GO in developing a nanocomposite membrane with antimicrobial and antifouling properties. The use of the COOH-GO nanofiller increased COOH capture through the formation of a strong complex with the PPSU matrix and produced an electrostatic interaction between negatively charged membrane and the foulants. The fabricated PPSU/COOH-GO nanocomposite membrane demonstrated the effective and long-lasting reduction of biofouling. The antibacterial performance of the membrane was examined using Escherichia coli and Pseudomonas aeruginosa as representative Gram-negative bacteria and Staphylococcus aureus as a representative Gram-positive species. The antifouling mechanisms were investigated using 1.0 g/l BSA as the model protein in the feed solutions using Hermia’s models (complete blocking, intermediate blocking, standard blocking, and cake formation). The experimental data obtained during the ultrafiltration was compared with the predictions based on the cake formation, complete blocking of the membrane surfaces, and fouling mechanisms.
Materials and methods
Materials and chemicals
PPSU (Ultrason P 3010) was purchased from BASF (Germany). COOH-GO was supplied by Grafen Chemical Industries (Turkey). N-methyl-pyrrolidone (NMP; Loba Chemie, India) was used as a solvent. Polyethylene glycol (PEG, MW = 600), sodium lauryl sulfate, sodium azide, sodium chloride, disodium hydrogen phosphate dehydrate, and potassium dihydrogen phosphate were all purchased from Merck (Germany). BSA was procured from Sigma-Aldrich (USA). Deionized water prepared using the Milli-Q system (Millipore, USA) was consistently used.
Fabrication of membrane
A fabrication method of the resulting membranes was similar to our previously published paper (Shukla et al. 2018). According these methods, PPSU and PPSU/COOH-GO nanocomposite membranes were prepared through phase inversion technique. COOH-GO (0.5 wt%) was mixed to N-methyl-pyrrolidone solvent and sonicated for 1 h using a sonifier (Branson Ultrasonics Corporation, USA) to disperse the solvent and reduce aggregation. Vacuum-dried PPSU polymer (17.0 wt%) and polyethylene glycol additive (10 wt%) were then added to the solution mixture and stirred at 70 ± 5 °C for 24 h to produce a homogeneous solution. For membrane casting, the solution was poured onto a cleaned glass plate and spread out using a casting blade with a gap of 78 ± 3 μm and immersed into a non-solvent coagulation bath (water) at ambient temperature. Finally, the obtained membranes were washed a number of times using a distilled water and preserved in 0.2% sodium azide solution until further study.
Preparation of microbial suspension
The antimicrobial and antiadhesion properties of prepared membranes were determined using pure cultures of E. coli (ATCC-25922) and P. aeruginosa (ATCC-PAO1) as model Gram-negative bacteria and S. aureus (ATCC-9144) as the model Gram-positive bacterium. The bacteria were cultured in Nutrient Broth in a shaking incubator (200 rpm) at 37 °C for 24 h. The bacterial biomass of each culture was washed several times with phosphate buffered saline (PBS) to remove media. The final suspension of each culture was diluted in sterile 0.9% NaCl solution to a concentration of approximately 107 colony-forming units (CFU/ml).
Antimicrobial activity of membrane
The antimicrobial properties of the uncoated PPSU and PPSU/COOH-GO membranes were examined by standard plate count protocols to determine the viable number of each tested bacterium remaining in the suspensions. Bacteriostasis rates were used to quantitatively examine the antibacterial activities of PPSU/COOH-GO membranes. The PPSU and PPSU/COOH-GO membranes (6 cm2) were placed in 6-well flat bottom polystyrene plates (Sigma-Aldrich) containing 3 × 105 CFU/ml E. coli, P. aeruginosa, or S. aureus suspension for 6 h at 37 °C and 150 rpm. The membranes were removed from each bacterial suspension and were serially diluted 10-fold, with 100 μl of each diluted bacterial suspension spread onto agar plates and incubated for 24 h at 35 °C. The number of colonies that developed was analyzed using the standard plate count technique. The bacteriostasis rate (Br) was calculated using Eq. (1):
where A is a number of the colonies of PPSU (control) membrane and B is a number of the colonies of PPSU/COOH-GO membrane.
Adhesion test
For adhesion testing, 6 cm2 of each PPSU and PPSU/COOH-GO membrane was immersed in E. coli, P. aeruginosa, or S. aureus suspensions in 6-well flat bottom polystyrene plates (Sigma-Aldrich). The plates were incubated at 150 rpm and 37 °C for 6 h. Each membrane was removed and washed to eliminate planktonic bacteria and loosely attached bacteria. The membranes were fixed with 2.5% glutaraldehyde at 4 °C for 6 h; dehydration in a sequential series of 25, 50, 75, and 100% ethanol (10 min each); and dried at 30 °C in a desiccator. Samples were then mounted on stubs, coated with gold, and viewed using scanning electron microscopy (SEM) at an accelerating voltage of 20 kV (Ansari et al. 2014).
Antifouling properties of membrane
The volumetric flux of pure water (Jv) was measured at 2 bar TMP. BSA (1.0 g/l in 0.1 mol/l phosphate buffer, pH 7.0) as a model fouling protein was used as the feed solution at room temperature. The foulant volumetric flux (Jvp) data were collected at 15 min intervals during the filtration process using a filtration CF042 cell (Sterlitech, USA). An effective membrane area was approximately 42 cm2. Subsequently, the fouled membrane samples were washed using deionized water and the final water volumetric flux (Jv1) was determined. The volumetric flux variation was used to evaluate the membrane fouling property, which was defined by the flux recovery ratio (JvRR) using Eq. (2):
To study the fouling behavior of the membranes in further detail, several parameters including the total fouling ratio (Rt), reversible fouling ratio (Rr), and irreversible fouling ratio (Rir) were defined using Eqs. (3)–(5), respectively (Huang et al. 2018):
Statistical data analysis
The one-way analysis of variance (abbreviated one-way ANOVA) was used to determine the antimicrobial and antifouling properties of pure PPSU and PPSU/COOH-GO nanocomposite membranes whether there were any statistically significant differences between the means of two or more independent duration of interaction by against Gram-positive and Gram-negative bacteria and protein. ANOVA was performed with IBM SPSS® statistics software version 25. Tukey’s HSD test was applied as post hoc test to compare the multiple treatments. All statistical data were checked for normality and equality of residual error variances assumptions of ANOVA.
Membrane fouling mechanism
To ascertain the potential mechanism of membrane fouling, Hermia proposed a mathematical model to define the declined volumetric flux during constant transmembrane pressure (TMP). The fouling mechanism was ascribed to four basic types of fouling: complete blocking, intermediate blocking, standard blocking, and cake formation models. These models are physically meaningful and therefore contribute to the understanding of the membrane fouling mechanisms. Hermia’s models can be written in a common mathematical equation shown in Eq. (6):
The fouling model was characterized by the value of n in Eq. (6). Complete blocking model (n = 2), standard blocking model (n = 1.5), intermediate pore blocking model (n = 1), and cake formation model (n = 0) are presented in Eq. (7)–(10), respectively (Ng et al. 2014):
Results and discussion
Antimicrobial properties of membrane
The antimicrobial, antibiofilm, and antifouling properties of COOH-GO and polymeric membrane functionalized with graphene-based nanomaterials for Gram-negative and Gram-positive bacteria and biofilm forming microorganisms have been amply reported (Akhavan and Ghaderi 2010; Abinaya et al. 2018; Gurunathan et al. 2012; Yu et al. 2013b; Zhang et al. 2015; Ji et al. 2016; Gao et al. 2017b; Lu et al. 2017; Whitehead et al. 2017; Yousefi et al. 2017; Zou et al. 2017). However, the antimicrobial and antiadhesion activities of PPSU/COOH-GO have not been yet explored. The antimicrobial properties of pure PPSU and nanocomposite PPSU/COOH-GO membranes were presently assessed using E. coli, P. aeruginosa, and S. aureus cultures (Fig. 1). Each tested culture (1 × 107 CFU/ml) was exposed to prepared membranes at 37 °C for 6 h. After spreading on agar plates overnight, the number of bacterial colonies was determined. Viable counts of E. coli, P. aeruginosa, and S. aureus were higher on PPSU membranes than on PPSU/COOH-GO nanocomposite membranes (Fig. 2a, c, e). Furthermore, the counts on the PPSU/COOH-GO membranes significantly decreased after 6 h (Fig. 2b, d, f). It has previously been observed that the deposition of COOH-GO on the PPSU surface restricted biofouling and inhibited bacterial growth. As shown in Fig. 2, the viable count from the PPSU/COOH-GO nanocomposite was significantly decreased compared to the membrane prepared of pure PPSU. The bacteriostasis rates of the nanocomposite PPSU/COOH-GO membrane for E. coli and P. aeruginosa were 74.2% and 81.1%, respectively (Fig. 3). These values were similar to that reported for GO-functionalized hyperbranched polyethylenimine (HPEI)/polyethersulfone (PES) membranes (74.8%) against E. coli (Yu et al. 2013b). The 41.9% bacteriostasis rate of the PPSU/COOH-GO nanocomposite membrane against S. aureus (Fig. 3) was similar to the previous report of 53.7% inactivation of S. aureus using GO-functionalized polyvinylidene fluoride membranes (Zeng et al. 2016). The values of F, df, and P were showed 115.253, 2, and 0.001 by using through the statistical analysis and post-hoc letters from ANOVA followed by Tukey’s HSD test. The data clearly indicate that Gram-negative bacteria were affected more by COOH-GO than the Gram-positive species. This superior antimicrobial activity for E. coli and P. aeruginosa is likely attributed to the physical puncture of bacterial cells) and chemical effects, such as oxidative stress, that occur when the bacteria directly contact the PPSU/COOH-GO nanocomposite membrane (Gurunathan et al. 2012; Sanchez et al. 2012; Perreault et al. 2015). These findings implicate the PPSU/COOH-GO nanocomposite membrane as a promising nanomaterial for antifouling systems.
Microbial adhesion
Polymeric membrane surfaces are readily colonized by bacteria that reside in the water that contacts the membrane. The bacteria can attach and proliferate to form biofilms. These biofilms represent a complex community of bacteria embedded in an extracellular polymeric matrix (Zodrow et al. 2009; Sawada et al. 2012). Biofilms develop on water filtration membrane units that are generally used in water treatment systems. This problem may be controlled if the adherence and colonization of bacteria on the membrane could be prevented. Prior studies have demonstrated that GO-functionalized membranes significantly restrict the attachment and biofilm formation of bacterial cells by disrupting the cell integrity and loss of cell viability (Akhavan and Ghaderi 2010; Hu et al. 2010; Mejías Carpio et al. 2012; Shanmuganathan et al. 2018).
In the present study, the attachment of both Gram-negative and Gram-positive bacteria on the surface of PPSU and PPSU/COOH-GO nanocomposite membranes was investigated using E. coli, P. aeruginosa, and S. aureus. After 12 h of incubation of the membranes with each of the bacterial cultures, bacteria that remained attached on the membrane surface were observed by SEM. Significant inhibition of biofilm formation and growth of all three bacteria were evident on PPSU/COOH-GO nanocomposite membranes (Fig. 4). In contrast, the biofilm formed by E. coli, P. aeruginosa, and S. aureus on PPSU membranes covered a larger surface area and had a smooth and intact appearance, indicating that the cells were normal and healthy (Fig. 4a, c, e). The biofilm formed on PPSU/COOH-GO nanocomposite membranes displayed an altered morphology and was scattered over the membrane surface as individual cells rather than as the visual appearance that is typical of a biofilm (Fig. 4b, d, f). Scanning electron micrographs revealed that the PPSU/COOH-GO nanocomposite membrane inhibited attachment, colonization, and biofilm formation by the three tested bacteria (Fig. 4b, d, and f). Furthermore, an obvious increase in size, roughness, indentations, and elongation of E. coli and P. aeruginosa cells suggested that the cell wall and membrane of the Gram-negative bacteria were severely damaged due to direct contact with the PPSU/COOH-GO nanocomposite membrane (Fig. 4b, d). The exact mode of action of the GO-based polymeric membrane is not completely understood. (Liu et al. 2011) reported the irreversible damage and destruction of E. coli due the direct contact with of the graphene-based membrane surface. Oxidative stress and lipid peroxidation have been proposed to play major roles in the killing of bacterial cells when exposed to the GO-based polymeric membrane (Liu et al. 2011; Gurunathan et al. 2012; Krishnamoorthy et al. 2012). The sharp edges of graphene may penetrate the bacteria and disrupting their membrane integrity (Akhavan and Ghaderi 2010; Hu et al. 2010). In the current study, the effect of COOH-GO on bacterial adhesion was more pronounced for the two Gram-negative bacteria than for S. aureus. The reduced adhesion of S. aureus could be attributed due to the thick peptidoglycan layer. Our collective data demonstrating the strong antimicrobial and antiadhesion properties of the PPSU/COOH-GO nanocomposite membrane agreed with the excellent performance reported for other types of GO-coated membranes, such as polyethersulfone, polypropylene, polyvinylidene fluoride, polyamide, and polysulfone (Perreault et al. 2013; Yu et al. 2013b; He et al. 2015; Zhang et al. 2015; Zeng et al. 2016).
Antifouling properties of the nanocomposite membrane
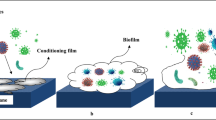
The antifouling properties of a membrane can be ascribed to several mechanisms, which include the foulants that are adsorbed or deposited into or on the membrane, cake layer formation on the membrane surface, and morphological and physical changes during the prolonged use of the membrane for filtration. PPSU/COOH-GO nanocomposite membrane incorporating the COOH-GO nanomaterial effectively improved the membrane antifouling properties, as it was shown in surface and cross-section morphology (Fig. 5). We performed a long-term filtration experiment using BSA to explore the antifouling properties. The flux recovery ratio (JvRR) was evaluated, and the results are shown in Fig. 6. The PPSU/COOH-GO nanocomposite membrane displayed a higher JvRR value of 95.3% than the pure PPSU membrane (61.7%) as shown in Fig. 6a, b. The outstanding antifouling property of the nanocomposite membrane was attributed to the nanoparticles embedded in the PPSU matrix. The markedly higher JvRR value indicated that the deposition of the BSA on the membrane surface could be directly prevented by hydraulic cleaning owing to the surface charge and surface hydrophilicity of the nanocomposite membrane. The excellent antifouling properties of the PPSU/COOH-GO nanocomposite membrane reflected the presence of numerous carboxylic groups of the GO nanoparticles on the membrane surface. The carboxylic groups increased the negative charge of the membrane and enhanced its hydrophilicity (Shukla et al. 2018). The negative charge and hydrophilicity of the surface effectively decreased the fouling via the adsorption of water molecules and the subsequent increase in the electrostatic repulsion between the surfaces of membrane and protein, which hindered cake formation and adsorption of foulants onto the membrane surface also shown the model in Fig. 1 (Mo et al. 2012; Kaleekkal et al. 2016). To investigate the antifouling properties of the prepared membranes in more detail, during BSA filtration, Rt, Rr, and Rir were determined. As revealed in Fig. 6a, Rt and Rir for the pure PPSU membrane were 64.0% and 34.5%, respectively. These ratios were less for the nanocomposite membrane (42% and 5.5%, respectively). The reduced Rt of the PPSU/COOH-GO nanocomposite membrane was indicative of the reduced deposition of foulants and reduced adsorption to the membrane surface. These reductions would contribute to a very low flux decline. On the other hand, the Rir values indicate that the foulants could not be removed by physical cleaning due to the strong attachment of foulants molecules to the surface or their plugging of the membrane pores. The Rr of the PPSU and nanocomposite membranes (30% and 36.5%, respectively) indicated that the foulant molecules were easily eliminated by simple backwashing through hydraulic cleaning and recovery of water flux for loose deposition of the cake layer on the PPSU/COOH-GO nanocomposite membrane surface. The F, df, and P values were showed for pure PPSU membrane 53.315, 3, and 0.001 and for PPSU/COOH-GO nanocomposite membrane 203.922, 3, and 0.000 against the protein fouling. For comparison, pure PPSU membrane and PPSU/COOH-GO nanocomposite membrane incorporating the COOH-GO nanomaterial and their antifouling properties were compared by using through the statistical analysis. Foregoing explanations support the idea that, for the nanocomposite membrane, adhesion forces, such as Van der Waals force, electrostatic force, hydrogen bonding force, and hydrophilic force, are responsible for the elimination of the BSA foulant at the membrane surface because the surface of membrane harbors many carboxylic functional groups and provides good clamping of the membranes (Ayyaru and Ahn 2017; Chen et al. 2018). The above results markedly demonstrate the superior antifouling character of PPSU/COOH-GO nanocomposite membrane.
Fouling mechanisms
Continuing the evaluation, Hermia’s models were applied to understand the fouling mechanisms occurring throughout membrane filtration with BSA. The experimental fouling data were fit with the models to predict the flux decline of pure PPSU and nanocomposite PPSU/COOH-GO membranes (Fig. 7). The correlation coefficients (R2) were used to assess the fitted model parameters and identify the type of pore blockings. The findings are summarized in Table 1. The R2 values that were obtained were the highest, indicating the suitability of the theoretical Hermia models to describe membrane fouling (Ng et al. 2014). The obtained experimental and model-predicted data provided an indication of the predominant factor of the gradual temporal decline of the flux. The cake formation model was the dominant mechanism for a sample containing COOH-GO nanomaterials (Fig. 7). Thus, cake formation was implicated as the primary fouling mechanism, followed by intermediate pore blocking. For pure PPSU membranes, the best modeling of the data occurred with the complete blocking mechanism. Data from the combined fouling models as well as experimental data for the membranes are depicted in Fig. 7. Hermia’s models displayed a lack of fit for the experimental flux decline data over the entire time interval for PPSU membranes and indicated the involvement of membrane fouling in several simultaneous mechanisms (Torkamanzadeh et al. 2016). The findings can be interested as indicating that the fouling of PPSU membranes very likely is due to the membrane’s lower surface charge and hydrophobic characteristic. The BSA flux of the membranes depended on the membrane hydrophilicity as well as pore structure. The results of the combined fouling mechanisms confirm the positive influence of the embedded COOH-GO in the membrane surface in the resistance to membrane fouling.
Conclusions
To develop a cost-effective membrane technology for the biofouling, such as that caused by microorganisms or protein in an aqueous environment, the antimicrobial and antifouling properties of a PPSU/COOH-GO nanocomposite membrane fabricated through an immersion precipitation phase inversion process were evaluated. The nanocomposite had excellent antibacterial properties, with higher bacteriostasis rate for two Gram-negative bacteria (74.2% for E. coli and 81.1% for P. aeruginosa) compared to the 41.9% rate for the Gram-positive bacterium S. aureus. E. coli and P. aeruginosa were more susceptible than S. aureus. In addition, the nanocomposite membrane inhibited attachment, colonization, and biofilm formation by these bacteria. The cell wall and outer membrane of E. coli and P. aeruginosa were susceptible to physical damage upon direct contact with the PPSU/COOH-GO nanocomposite membrane. Furthermore, the surface of the nanocomposite membrane significantly enhanced antifouling, as demonstrated by a less irreversible fouling and higher flux recovery ratio. The cake formation and complete blocking were the main fouling mechanisms for the membranes. Hence, the prepared PPSU/COOH-GO nanocomposite membrane demonstrated excellent antimicrobial and antifouling properties when tested against biofoulant; thus, the developed GO-based nanocomposite membrane is an optimum choice for water treatment.
Abbreviations
- PPSU:
-
polyphenylsulfone
- COOH-GO:
-
carboxyl-functionalized graphene oxide
- E. coli :
-
Escherichia coli
- P. aeruginosa :
-
Pseudomonas aeruginosa
- S. aureus :
-
Staphylococcus aureus
- BSA:
-
bovine serum albumin
- CFU:
-
colony-forming unit
- TMP:
-
transmembrane pressure
- J v :
-
pure water volumetric flux
- J vp :
-
foulant volumetric flux
- J v1 :
-
final water volumetric flux
- J v RR :
-
flux recovery ratio
- R t :
-
total fouling ratio
- R r :
-
reversible fouling ratio
- R ir :
-
irreversible fouling ratio
- J vp0 :
-
initial volume flow rate
- J vpt :
-
volume flow at a particular time
- K c :
-
membrane surface area blocked per unit of permeate volume
- t :
-
time
- K s :
-
constant of the standard blocking model
- K i :
-
constant of the intermediate blocking model
- K cf :
-
constant of the cake formation model
References
Abdel-Karim A, Leaper S, Alberto M, Vijayaraghavan A, Fan X, Holmes SM, Souaya ER, Badawy MI, Gorgojo P (2018) High flux and fouling resistant flat sheet polyethersulfone membranes incorporated with graphene oxide for ultrafiltration applications. Chem Eng J 334:789–799. https://doi.org/10.1016/j.cej.2017.10.069
Abinaya M, Vaseeharan B, Divya M, Vijayakumar S, Govindarajan M, Alharbi NS, Khaled JM, al-anbr MN, Benelli G (2018) Structural characterization of Bacillus licheniformis Dahb1 exopolysaccharide—antimicrobial potential and larvicidal activity on malaria and Zika virus mosquito vectors. Environ Sci Pollut Res 25:18604–18619. https://doi.org/10.1007/s11356-018-2002-6
Ahmed F, Santos CM, Vergara RAMV, Tria MCR, Advincula R, Rodrigues DF (2012) Antimicrobial applications of electroactive PVK-SWNT nanocomposites. Environ Sci Technol 46:1804–1810. https://doi.org/10.1021/es202374e
Akhavan O, Ghaderi E (2010) Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 4:5731–5736. https://doi.org/10.1021/nn101390x
Ansari MA, Khan HM, Khan AA, Ahmad MK, Mahdi AA, Pal R, Cameotra SS (2014) Interaction of silver nanoparticles with Escherichia coli and their cell envelope biomolecules. J Basic Microbiol 54:905–915. https://doi.org/10.1002/jobm.201300457
Ayyaru S, Ahn YH (2017) Application of sulfonic acid group functionalized graphene oxide to improve hydrophilicity, permeability, and antifouling of PVDF nanocomposite ultrafiltration membranes. J Memb Sci 525:210–219. https://doi.org/10.1016/j.memsci.2016.10.048
Benelli G (2018) Plant-borne compounds and nanoparticles: challenges for medicine, parasitology and entomology. Environ Sci Pollut Res 25:10149–10150. https://doi.org/10.1007/s11356-017-9960-y
Ben-Sasson M, Zodrow KR, Genggeng Q, Kang Y, Giannelis EP, Elimelech M (2014) Surface functionalization of thin-film composite membranes with copper nanoparticles for antimicrobial surface properties. Environ Sci Technol 48:384–393. https://doi.org/10.1021/es404232s
Bogdanović U, Vodnik V, Mitrić M, Dimitrijević S, Škapin SD, Žunič V, Budimir M, Stoiljković M (2015) Nanomaterial with high antimicrobial efficacy copper/polyaniline nanocomposite. ACS Appl Mater Interfaces 7:1955–1966. https://doi.org/10.1021/am507746m
Chen F, Shi X, Chen X, Chen W (2018) Preparation and characterization of amphiphilic copolymer PVDF-g-PMABS and its application in improving hydrophilicity and protein fouling resistance of PVDF membrane. Appl Surf Sci 427:787–797. https://doi.org/10.1016/j.apsusc.2017.08.096
Chung YT, Mahmoudi E, Mohammad AW, Benamor A, Johnson D, Hilal N (2017) Development of polysulfone-nanohybrid membranes using ZnO-GO composite for enhanced antifouling and antibacterial control. Desalination 402:123–132. https://doi.org/10.1016/j.desal.2016.09.030
Corbatón-Báguena M-J, Álvarez-Blanco S, Vincent-Vela M-C (2018) Evaluation of fouling resistances during the ultrafiltration of whey model solutions. J Clean Prod 172:358–367. https://doi.org/10.1016/j.jclepro.2017.10.149
Emadzadeh D, Ghanbari M, Lau WJ, Rahbari-Sisakht M, Rana D, Matsuura T, Kruczek B, Ismail AF (2017) Surface modification of thin film composite membrane by nanoporous titanate nanoparticles for improving combined organic and inorganic antifouling properties. Mater Sci Eng C 75:463–470. https://doi.org/10.1016/j.msec.2017.02.079
Eng AYS, Sofer Z, Sedmidubský D, Pumera M (2017) Synthesis of carboxylated-graphenes by the Kolbe-Schmitt process. ACS Nano 11:1789–1797. https://doi.org/10.1021/acsnano.6b07746
Gao Q, Yu M, Su Y, Xie M, Zhao X, Li P, Ma PX (2017a) Rationally designed dual functional block copolymers for bottlebrush-like coatings: in vitro and in vivo antimicrobial, antibiofilm, and antifouling properties. Acta Biomater 51:112–124. https://doi.org/10.1016/j.actbio.2017.01.061
Gao Y, Wu J, Ren X, Tan X, Hayat T, Alsaedi A, Cheng C, Chen C (2017b) Impact of graphene oxide on the antibacterial activity of antibiotics against bacteria. Environ Sci Nano 4:1016–1024. https://doi.org/10.1039/C7EN00052A
Gurunathan S, Han JW, Dayem AA et al (2012) Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int J Nanomedicine 7:5901–5914. https://doi.org/10.2147/IJN.S37397
He L, Dumée LF, Feng C, Velleman L, Reis R, She F, Gao W, Kong L (2015) Promoted water transport across graphene oxide–poly(amide) thin film composite membranes and their antibacterial activity. Desalination 365:126–135. https://doi.org/10.1016/j.desal.2015.02.032
Ho KC, Teow YH, Ang WL, Mohammad AW (2017) Novel GO/OMWCNTs mixed-matrix membrane with enhanced antifouling property for palm oil mill effluent treatment. Sep Purif Technol 177:337–349. https://doi.org/10.1016/j.seppur.2017.01.014
Hou S, Xing J, Dong X, Zheng J, Li S (2017) Integrated antimicrobial and antifouling ultrafiltration membrane by surface grafting PEO and N-chloramine functional groups. J Colloid Interface Sci 500:333–340. https://doi.org/10.1016/j.jcis.2017.04.028
Hu W, Peng C, Luo W, Lv M, Li X, Li D, Huang Q, Fan C (2010) Graphene-based antibacterial paper. ACS Nano 4:4317–4323. https://doi.org/10.1021/nn101097v
Hu M, Zheng S, Mi B (2016) Organic fouling of graphene oxide membranes and its implications for membrane fouling control in engineered osmosis. Environ Sci Technol 50:685–693. https://doi.org/10.1021/acs.est.5b03916
Hu Y, Lü Z, Wei C, Yu S, Liu M, Gao C (2018) Separation and antifouling properties of hydrolyzed PAN hybrid membranes prepared via in-situ sol-gel SiO2nanoparticles growth. J Membr Sci 545:250–258. https://doi.org/10.1016/j.memsci.2017.09.081
Huang H, Yu J, Guo H, Shen Y, Yang F, Wang H, Liu R, Liu Y (2018) Improved antifouling performance of ultrafiltration membrane via preparing novel zwitterionic polyimide. Appl Surf Sci 427:38–47. https://doi.org/10.1016/j.apsusc.2017.08.004
Ji H, Sun H, Qu X (2016) Antibacterial applications of graphene-based nanomaterials: recent achievements and challenges. Adv Drug Deliv Rev 105:176–189. https://doi.org/10.1016/j.addr.2016.04.009
Kaleekkal NJ, Thanigaivelan A, Rana D, Mohan D (2016) Studies on carboxylated graphene oxide incorporated polyetherimide mixed matrix ultrafiltration membranes. Mater Chem Phys 186:146–158. https://doi.org/10.1016/j.matchemphys.2016.10.040
Koh E, Lee YT (2017) Antimicrobial activity and fouling resistance of a polyvinylidene fluoride (PVDF) hollow-fiber membrane. J Ind Eng Chem 47:260–271. https://doi.org/10.1016/j.jiec.2016.11.042
Krishnamoorthy K, Veerapandian M, Zhang LH, Yun K, Kim SJ (2012) Antibacterial efficiency of graphene nanosheets against pathogenic bacteria via lipid peroxidation. J Phys Chem C 116:17280–17287. https://doi.org/10.1021/jp3047054
Lee A, Elam JW, Darling SB (2016) Membrane materials for water purification: design, development, and application. Environ Sci Water Res Technol 2:17–42. https://doi.org/10.1039/C5EW00159E
Liu S, Zeng TH, Hofmann M, Burcombe E, Wei J, Jiang R, Kong J, Chen Y (2011) Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS Nano 5:6971–6980. https://doi.org/10.1021/nn202451x
Liu C, Faria AF, Ma J, Elimelech M (2017) Mitigation of biofilm development on thin-film composite membranes functionalized with Zwitterionic polymers and silver nanoparticles. Environ Sci Technol 51:182–191. https://doi.org/10.1021/acs.est.6b03795
Lu X, Feng X, Werber JR, Chu C, Zucker I, Kim JH, Osuji CO, Elimelech M (2017) Enhanced antibacterial activity through the controlled alignment of graphene oxide nanosheets. Proc Natl Acad Sci 114:E9793–E9801. https://doi.org/10.1073/pnas.1710996114
Luyts K, Napierska D, Nemery B, Hoet PHM (2013) How physico-chemical characteristics of nanoparticles cause their toxicity: complex and unresolved interrelations. Environ Sci Impacts 15:23–38. https://doi.org/10.1039/c2em30237c
Ma R, Ji YL, Guo YS, Mi YF, An QF, Gao CJ (2017) Fabrication of antifouling reverse osmosis membranes by incorporating zwitterionic colloids nanoparticles for brackish water desalination. Desalination 416:35–44. https://doi.org/10.1016/j.desal.2017.04.016
Mejías Carpio IE, Santos CM, Wei X, Rodrigues DF (2012) Toxicity of a polymer–graphene oxide composite against bacterial planktonic cells, biofilms, and mammalian cells. Nanoscale 4:4746–4756. https://doi.org/10.1039/c2nr30774j
Mo Y, Tiraferri A, Yip NY, Adout A, Huang X, Elimelech M (2012) Improved antifouling properties of polyamide nanofiltration membranes by reducing the density of surface carboxyl groups. Environ Sci Technol 46:13253–13261. https://doi.org/10.1021/es303673p
Moradi G, Zinadini S, Rajabi L, Dadari S (2018) Fabrication of high flux and antifouling mixed matrix fumarate-alumoxane/PAN membranes via electrospinning for application in membrane bioreactors. Appl Surf Sci 427:830–842. https://doi.org/10.1016/j.apsusc.2017.09.039
Morelos-Gomez A, Cruz-Silva R, Muramatsu H, Ortiz-Medina J, Araki T, Fukuyo T, Tejima S, Takeuchi K, Hayashi T, Terrones M, Endo M (2017) Effective NaCl and dye rejection of hybrid graphene oxide/graphene layered membranes. Nat Nanotechnol 12:1083–1088. https://doi.org/10.1038/nnano.2017.160
Nasrollahi N, Vatanpour V, Aber S, Mahmoodi NM (2018) Preparation and characterization of a novel polyethersulfone (PES) ultrafiltration membrane modified with a CuO/ZnO nanocomposite to improve permeability and antifouling properties. Sep Purif Technol 192:369–382. https://doi.org/10.1016/j.seppur.2017.10.034
Ng CY, Mohammad AW, Ng LY, Jahim JM (2014) Membrane fouling mechanisms during ultrafiltration of skimmed coconut milk. J Food Eng 142:190–200. https://doi.org/10.1016/j.jfoodeng.2014.06.005
Perreault F, Tousley ME, Elimelech M (2013) Thin-film composite polyamide membranes functionalized with biocidal graphene oxide nanosheets. Environ Sci Technol Lett 1:71–76. https://doi.org/10.1021/ez4001356
Perreault F, Fonseca de Faria A, Elimelech M (2015) Environmental applications of graphene-based nanomaterials. Chem Soc Rev 44:5861–5896. https://doi.org/10.1039/C5CS00021A
Perreault F, Jaramillo H, Xie M, Ude M, Nghiem LD, Elimelech M (2016) Biofouling mitigation in forward osmosis using graphene oxide functionalized thin-film composite membranes. Environ Sci Technol 50:5840–5848. https://doi.org/10.1021/acs.est.5b06364
Qiu WZ, Zhao ZS, Du Y et al (2017) Antimicrobial membrane surfaces via efficient polyethyleneimine immobilization and cationization. Appl Surf Sci 426:972–979. https://doi.org/10.1016/j.apsusc.2017.07.217
Safarpour M, Vatanpour V, Khataee A (2016) Preparation and characterization of graphene oxide/TiO2 blended PES nanofiltration membrane with improved antifouling and separation performance. Desalination 393:65–78. https://doi.org/10.1016/j.desal.2015.07.003
Sanchez VC, Jachak A, Hurt RH, Kane AB (2012) Biological interactions of graphene-family nanomaterials: an interdisciplinary review. Chem Res Toxicol 25:15–34. https://doi.org/10.1021/tx200339h
Sawada I, Fachrul R, Ito T, Ohmukai Y, Maruyama T, Matsuyama H (2012) Development of a hydrophilic polymer membrane containing silver nanoparticles with both organic antifouling and antibacterial properties. J Membr Sci 387–388:1–6. https://doi.org/10.1016/j.memsci.2011.06.020
Shanmuganathan R, MubarakAli D, Prabakar D, Muthukumar H, Thajuddin N, Kumar SS, Pugazhendhi A (2018) An enhancement of antimicrobial efficacy of biogenic and ceftriaxone-conjugated silver nanoparticles: green approach. Environ Sci Pollut Res 25:10362–10370. https://doi.org/10.1007/s11356-017-9367-9
Shannon MA, Bohn PW, Elimelech M, Georgiadis JG, Mariñas BJ, Mayes AM (2008) Science and technology for water purification in the coming decades. Nature 452:301–310. https://doi.org/10.1038/nature06599
Shockravi A, Vatanpour V, Najjar Z, Bahadori S, Javadi A (2017) A new high performance polyamide as an effective additive for modification of antifouling properties and morphology of asymmetric PES blend ultrafiltration membranes. Microporous Mesoporous Mater 246:24–36. https://doi.org/10.1016/j.micromeso.2017.03.013
Shukla AK, Alam J, Alhoshan M, Dass LA, Muthumareeswaran MR (2017) Development of a nanocomposite ultrafiltration membrane based on polyphenylsulfone blended with graphene oxide. Sci Rep 7:41976–41987. https://doi.org/10.1038/srep41976
Shukla AK, Alam J, Alhoshan M, Arockiasamy Dass L, Ali FAA, M. R M, Mishra U, Ansari MA (2018) Removal of heavy metal ions using a carboxylated graphene oxide-incorporated polyphenylsulfone nanofiltration membrane. Environ Sci Water Res Technol 4:438–448. https://doi.org/10.1039/C7EW00506G
Srinivasan R, Vigneshwari L, Rajavel T, Durgadevi R, Kannappan A, Balamurugan K, Pandima Devi K, Veera Ravi A (2018) Biogenic synthesis of silver nanoparticles using Piper betle aqueous extract and evaluation of its anti-quorum sensing and antibiofilm potential against uropathogens with cytotoxic effects: an in vitro and in vivo approach. Environ Sci Pollut Res 25:10538–10554. https://doi.org/10.1007/s11356-017-1049-0
Torkamanzadeh M, Jahanshahi M, Peyravi M, Rad AS (2016) Comparative experimental study on fouling mechanisms in nano-porous membrane: cheese whey ultrafiltration as a case study. Water Sci Technol 74:2737–2750. https://doi.org/10.2166/wst.2016.352
Wang YN, Tang CY (2011) Fouling of nanofiltration, reverse osmosis, and ultrafiltration membranes by protein mixtures: the role of inter-foulant-species interaction. Environ Sci Technol 45:6373–6379. https://doi.org/10.1021/es2013177
Wang W, Zhu L, Shan B, Xie C, Liu C, Cui F, Li G (2018) Preparation and characterization of SLS-CNT/PES ultrafiltration membrane with antifouling and antibacterial properties. J Membr Sci 548:459–469. https://doi.org/10.1016/j.memsci.2017.11.046
Whitehead KA, Vaidya M, Liauw CM, Brownson DAC, Ramalingam P, Kamieniak J, Rowley-Neale SJ, Tetlow LA, Wilson-Nieuwenhuis JST, Brown D, McBain AJ, Kulandaivel J, Banks CE (2017) Antimicrobial activity of graphene oxide-metal hybrids. Int Biodeterior Biodegrad 123:182–190. https://doi.org/10.1016/j.ibiod.2017.06.020
Xu Q, Zeng M, Feng Z, Yin D, Huang Y, Chen Y, Yan C, Li R, Gu Y (2016) Understanding the effects of carboxylated groups of functionalized graphene oxide on the curing behavior and intermolecular interactions of benzoxazine nanocomposites. RSC Adv 6:31484–31496. https://doi.org/10.1039/C5RA28016H
Xu Z, Liao J, Tang H, Li N (2017) Antifouling polysulfone ultrafiltration membranes with pendent sulfonamide groups. J Membr Sci 548:481–489. https://doi.org/10.1016/j.memsci.2017.11.064
You X, Ma T, Su Y, Wu H, Wu M, Cai H, Sun G, Jiang Z (2017) Enhancing the permeation flux and antifouling performance of polyamide nanofiltration membrane by incorporation of PEG-POSS nanoparticles. J Membr Sci 540:454–463. https://doi.org/10.1016/j.memsci.2017.06.084
Yousefi M, Dadashpour M, Hejazi M, Hasanzadeh M, Behnam B, de la Guardia M, Shadjou N, Mokhtarzadeh A (2017) Anti-bacterial activity of graphene oxide as a new weapon nanomaterial to combat multidrug-resistance bacteria. Mater Sci Eng C 74:568–581. https://doi.org/10.1016/j.msec.2016.12.125
Yu H, Zhang X, Zhang Y, Liu J, Zhang H (2013a) Development of a hydrophilic PES ultrafiltration membrane containing SiO2@N-Halamine nanoparticles with both organic antifouling and antibacterial properties. Desalination 326:69–76. https://doi.org/10.1016/j.desal.2013.07.018
Yu L, Zhang Y, Zhang B, Liu J, Zhang H, Song C (2013b) Preparation and characterization of HPEI-GO/PES ultrafiltration membrane with antifouling and antibacterial properties. J Membr Sci 447:452–462. https://doi.org/10.1016/j.memsci.2013.07.042
Yu S, Liu J, Zhu W, Hu ZT, Lim TT, Yan X (2015) Facile room-temperature synthesis of carboxylated graphene oxide-copper sulfide nanocomposite with high photodegradation and disinfection activities under solar light irradiation. Sci Rep 5:16369–16380. https://doi.org/10.1038/srep16369
Zeng Z, Yu D, He Z, Liu J, Xiao FX, Zhang Y, Wang R, Bhattacharyya D, Tan TTY (2016) Graphene oxide quantum dots covalently functionalized PVDF membrane with significantly-enhanced bactericidal and antibiofouling performances. Sci Rep 6:20142–20152. https://doi.org/10.1038/srep20142
Zhang ZB, Wu JJ, Su Y, Zhou J, Gao Y, Yu HY, Gu JS (2015) Layer-by-layer assembly of graphene oxide on polypropylene macroporous membranes via click chemistry to improve antibacterial and antifouling performance. Appl Surf Sci 332:300–307. https://doi.org/10.1016/j.apsusc.2015.01.193
Zhang DY, Hao Q, Liu J, Shi YS, Zhu J, Su L, Wang Y (2018a) Antifouling polyimide membrane with grafted silver nanoparticles and zwitterion. Sep Purif Technol 192:230–239. https://doi.org/10.1016/j.seppur.2017.10.018
Zhang J, Xu Y, Chen S et al (2018b) Enhanced antifouling and antibacterial properties of poly (ether sulfone) membrane modified through blending with sulfonated poly (aryl ether sulfone) and copper nanoparticles. Appl Surf Sci 434:806–815. https://doi.org/10.1016/j.apsusc.2017.11.007
Zhu L, Song H, Zhang D, Wang G, Zeng Z, Xue Q (2017) Negatively charged polysulfone membranes with hydrophilicity and antifouling properties based on in situ cross-linked polymerization. J Colloid Interface Sci 498:136–143. https://doi.org/10.1016/j.jcis.2017.03.055
Zodrow K, Brunet L, Mahendra S, Li D, Zhang A, Li Q, Alvarez PJJ (2009) Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res 43:715–723. https://doi.org/10.1016/j.watres.2008.11.014
Zou F, Zhou H, Jeong DY, Kwon J, Eom SU, Park TJ, Hong SW, Lee J (2017) Wrinkled surface-mediated antibacterial activity of graphene oxide nanosheets. ACS Appl Mater Interfaces 9:1343–1351. https://doi.org/10.1021/acsami.6b15085
Acknowledgments
The authors thank the Deanship of Scientific Research and RSSU at King Saud University for their technical support.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no (RG-1439-85).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Giovanni Benelli
Rights and permissions
About this article
Cite this article
Shukla, A.K., Alam, J., Ansari, M.A. et al. Antimicrobial and antifouling properties of versatile PPSU/carboxylated GO nanocomposite membrane against Gram-positive and Gram-negative bacteria and protein. Environ Sci Pollut Res 25, 34103–34113 (2018). https://doi.org/10.1007/s11356-018-3212-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3212-7