Abstract
Pesticide-induced oxidative stress, as one of mechanism of toxicity, has been a focus of toxicological research. However, there is a lack of data for certain pesticides-oxidative stress effects especially on terrestrial amphibians. This study evaluates the prooxidative effects of orally administered insecticide deltamethrin (DM) in some tissues of the terrestrial toad Bufotes viridis. Toads were randomly divided and assigned to a control group and a test group that was orally exposed to the pesticide (5 mg/kg of body weight/daily) for 21 days. Animals were euthanized from each group on days 7, 14, and 21, and the liver, leg muscle, ventral skin, and gastrointestinal tissue (GIT) were dissected and used for analysis. From battery of investigated antioxidant components, superoxide dismutase (SOD) was the most differentiate parameter in all examined tissues. For the period of prolonged exposure to pesticide, antioxidative strategy of Bufotes viridis was based on SOD utilization in attempt to maintain the oxidative disbalance at acceptable level. The integrated biomarker response (IBR) as the measure of the overall biochemical response to DM exposure revealed that the group exposed for 21 days had the highest response. Our work has offered valuable data ensuring evidence that toads exposed to deltamethrin developed adaptive reactions that were tissue-specific and that DM-generated systemic toxicity was time-dependent. The present work showed that oxidative stress has significant role in pesticide-induced toxicity and contributes to better understanding of ecotoxicological risk in the terrestrial amphibians exposed to DM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pyrethroid class of insecticides is extensively used in many countries because of their short biodegradation period, low toxicity, and low affinity to accumulate in organisms (Laskowski 2002). Deltamethrin (DM) belongs to the type II pyrethroid insecticide. In Serbia, it is utilized for regulating various pests in stored products (Kljajić and Perić 2009), as well as an insecticide to control mosquitoes and for ectoparasite treatment. The main mechanism of DM neurotoxicity is based on the modification of sodium channel kinetics. In general, DM renders the channels open for a prolonged time so that the membrane potential is eventually depolarized and action potential initiation is impossible (Narahashi 1992).
Amphibians, as secondary consumers in many food chains, assume an important position in the ecosystem, and therefore, the regression or destruction of their population has a major influence on other organisms. Amphibians are extremely vulnerable to pesticide toxicity (Paškova et al. 2011). Aside from the first line of contact through the semi-permeable skin, the animals can be contaminated with pesticide via nutrition: as insects are the basic food for adult amphibians, they can be exposed to DM by ingesting insects exposed to pesticides (Çakıcı 2015).
Although the half-life of DM is relatively short (about 33 days under aerobic conditions) (California 2000), recent findings point to the bioaccumulation of DM and its byproducts in insects (Gutiérrez et al. 2016). Exposure evaluation of terrestrial organisms is complicated because of different routes of pesticide entry and the relative deficiency of information required for its proper evaluation. Stewart and Seesink (1996) performed a comprehensive study to establish the risk to reptiles fed with brown locusts contaminated with DM. They calculated that the maximum daily dose of DM a lizard could receive by eating contaminated locusts is about 34 mg/kg body weight. There is some basis for serious concerns if we consider that 460-fold lower doses (< 0.074 mg/kg) of DM resulted in toxicity with symptoms such as hyperactivity and ataxia in lizards and snakes (Mutschmann 1991). The above indicates that the concentration of DM that can be ingested is not negligible, but there are no data on the possible DM concentration which could be ingested via food in amphibians.
For the present study, we have chosen the green toad Bufotes viridis (formerly known as Pseudepidalea or Bufo viridis), one of the most widespread old-world amphibians. In some cases, their abundance in anthropogenic localities is much higher than in the natural environment. Their broad distribution, ease of catch, and rapid adjustment to laboratory conditions are some of the ecotoxicological features that make them a suitable test species for laboratory studies. The causality between pesticide mechanisms of toxicity and a general disturbance of the organism’s antioxidant potential has been examined; however, the precise mechanism has not yet been established (Sayeed et al. 2003; Hernández-Moreno et al. 2010). Our previous results have shown that acute administration of DM causes oxidative stress in B. viridis (Radovanović et al. 2017). The antioxidant defense system (AOS) is comprised of several biochemical defense processes that are established in all aerobic organisms to avoid damage caused by reactive oxygen species (ROS) (Chromcova et al. 2015; Faggio et al. 2015). Both enzymatic and nonenzymatic antioxidants counteract the deleterious effects of ROS and protect cells from oxidative damage (Livingstone 2001; Faggio et al. 2016; Messina et al. 2014). Establishing biomarkers of oxidative stress is essential for a reliable interpretation of oxidative stress disorders in a research setting (Fazio et al. 2014). The integrated biomarker response (IBR) represents a method for merging all the evaluated biomarker responses under one universal “stress index” (Broeg and Lehtonen 2006). IBR is increasingly used in toxicological studies as a suitable tool for providing a comprehensive assessment of the health status of species exposed to sublethal concentrations of different pollutants (Devin et al. 2017; Dey et al. 2016; Freitas et al. 2017; Felício et al. 2018).
As the literature data about the chronic impact of DM on amphibians is scarce and focused only on aquatic specimens and tadpoles, we instated the experimental model of our study with adult terrestrial toads. The focus was on the oral route of exposure as an important pathway in the terrestrial habitat. We wanted to contribute to the knowledge about the ecotoxicological risk of DM on adult toads as non-target organisms by examining its effect as a redox stressor. To that end, we did assessment of antioxidant parameters in adult B. viridis toads that were orally exposed to sublethal DM concentrations. The antioxidant profile and efficiency were characterized in order to define the relationship between the antioxidant response and time-dependent pesticide-induced oxidative stress.
Materials and methods
Animal collection and pesticide toxicity test
Adult individuals of the green toad, Bufotes viridis, were collected by hand net in the national park Fruška Gora, a protected area that spreads along the eponymous 80-km-long mountain range lying between the Danube and Sava rivers. Since the metabolic activity of the animals changes with age and weight and affects the parameters to be measured (Canli and Furness 1993), individuals of similar size were used. The toads were transported to the laboratory in a covered basket. A total of 42 animals were randomly assigned to the experimental groups: control and test, 21 individual each.
Acclimation to laboratory conditions in 300-L glass terrariums (120x60x50cm) lasted for 2 weeks before the start of the experiment (Goulet and Hontella 2003). The natural habitat was enabled in the variety of soil substrate in terrariums that were cleaned twice a week. The toads were subjected to a natural 12:12 light/dark photoperiod at a laboratory temperature of 20 ± 2 °C. Once a day, the terrarium walls were sprayed with dechlorinated tap water to ensure adequate humidity. Twice a week, the toads were fed with earthworms (Ezemonye and Tongo 2010).
The trade name of the insecticide used in this experiment is “K-Othrine SC25” (2.5 g/100 mL), a product of Bayer. We tested the prepared formulated product because amphibians come into contact with an insecticide formulation instead of pure active ingredients. The insecticide, containing DM as the active ingredient was purchased from an agricultural pharmacy. For this study, the insecticide was dissolved in dechlorinated tap water to provide more realistic approach (Rendon-von Osten et al. 2005). The first group of toads was maintained as a control, while the test group was chronically exposed to a sublethal concentration of pesticide (5 mg/kg body weight/daily) for 7, 14, and 21 days. The concentration used for chronic exposure was selected according to our previous results (Radovanović et al. 2017).
In agreement with national and institutional strategy for the safety of animal well-being during experiments, the ethic regulations have been proceeding. Animal capture was approved by the Serbian Ministry of Agriculture and Environmental Protection (Permissions No. 353-01-146/2016-17). The Animal Ethical Committee of the Institute for Biological Research “Siniša Stanković,” University of Belgrade, approved the experiment.
Determination of biometric parameters
Before the animals were euthanized, the average body mass (BM) and snout-vent length (SVL) were recorded. The BM and SVL were in the following order: 7-day control 37.46 ± 2.18 g, 77.86 ± 2.09 mm; 7-day exposure 36.38 ± 4.14 g, 81.43 ± 3.56 mm; 14-day control 28.59 ± 3.39 g, 77.00 ± 2.33 mm; 14-day exposure 31.92 ± 2.26 g, 78.14 ± 1.96 mm; 21-day control 36.41 ± 4.01 g, 80.29 ± 1.91 mm; 21-day exposure 39.17 ± 3.93 g, 81.29 ± 2.05 mm. No mortality outcome was detected during the experiment. Seven individuals from the control and test groups each were chosen randomly on 7, 14, and 21 days of exposure. The toads were euthanized by decapitation. Four different tissues were isolated for the present study: the liver, leg muscle, ventral skin, and gastrointestinal tissue. The samples were stored at − 80 °C until further biochemical analysis.
Tissue processing
The tissues were chopped and homogenized with an Ultra-Turrax homogenizer (Janke and Kunkel, IKA-Werk, Staufen, Germany) in sucrose (5 vol of 25 mM) (Lionetto et al. 2003) containing 10 mM Tris-HCl, pH 7.5 (Rossi et al. 1983). Formed homogenates were then sonicated at 40 kHz for periods of 30 s, and portions of the resulting sonicates were currently used for measuring the total concentration of glutathione (GSH). The remaining sonicated material was centrifuged at 100,000×g at 4 °C for 90 min (Takada et al. 1982) and the formed supernatants were used for measuring enzyme activities.
Biochemical analyses
Superoxide dismutase (SOD) activity was measured and monitored at 480 nm according to the method of Misra and Fridovich (1972) which is based on the autoxidation of adrenaline to adrenochrome. Catalase (CAT) activity was measured according to the procedure of Claiborne (1984) and read at 240 nm. Determination of GSH-Px activity is based on the nicotinamide adenine dinucleotide phosphate (NADPH) oxidation with t-Butyl hydroperoxide as a substrate and read at 340 nm (Tamura et al. 1982). Glutathione reductase (GR) activity was assessed by the method of Glatzle et al. (1974), which includes the reduction of glutathione disulfide (GSSG) to reduced GSH using NADPH as a substrate. Glutathione S-transferase (GST) activity determination was based on the reaction of the -SH group of GSH with 1-chloro-2,4-dinitrobenzene (CDNB) (Habig et al. 1974). All enzyme activities were expressed in U/mg protein.
Estimation of the concentration of GSH is based on GSH oxidation by 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) and NADPH reduction in the presence of GR (Griffith 1980). The concentration was expressed as nmol/g tissue and measured at 412 nm. The -SH group concentration was obtained at 412 nm by the Ellman method (Ellman 1959) with DTNB and was expressed in μmol/g tissue.
Lipid peroxidation was measured and monitored at 532 nm according to the method of Ohkawa et al. (1979), which is based on the reaction of thiobarbituric acid (TBA) with malondialdehyde (MDA) whereby a red-colored product is formed. The results were expressed as nmol TBARS/mg tissue.
All samples were measured at the same time in a Nicolet Evolution 600 UV-Vis spectrophotometer in triplicate using and a temperature-controlled cuvette holder. All chemicals used in present study were obtained from Sigma-Aldrich (St Louis, MO, USA).
Integrated biomarker response
The approach for joining all of the evaluated biomarker responses in one universal “stress index” or IBR was utilized for the estimation of the potential toxicity of DM depending on the application time in different treated groups of toads. The IBR calculation was based on the procedure described by Devin et al. (2014).
Statistical analysis
All data were log transformed in order to improve the normality of variables and were expressed as the mean ± standard error (SE). To establish variations between the means of the treated and control groups, we performed one-way ANOVA and the post hoc Fisher LSD test. A significance level of p < 0.05 was established for all cases. We performed canonical discriminant analysis that revealed differentiation among the treated groups based on all measured antioxidant components for all examined tissues (Darlington et al. 1973). All statistical analyses were performed with the STATISTICA 8.0 software, except the IBR value, which was calculated in R 3.4.1. using the script provided by Devin et al. (2014).
Results
In the course of the study, no lethality or visual modifications in toad activities were noticed in any of the examined groups.
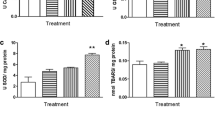
In Table 1 and Fig. 1, we present the results of the analysis of all investigated parameters in the liver. The concentration of total proteins increased significantly at day 21 in exposed toads compared to the control group, but decreased compared to the group exposed to the insecticide for 14 days (Table 1). SOD activity was significantly decreased at days 14 and 21 as compared to the control groups (Fig. 1a). CAT activity and TBARS concentration displayed an increasing trend at day 14, which was opposite to GR activity that showed a declining trend on day 14 as compared to day 7 of the tested group. GSH-Px activity displayed a decreasing trend on day 21 as compared to the groups exposed for 7 and 14 days (Fig. 1d). A downward trend in the concentration of -SH groups as compared to the group exposed for 7 days was noted (Table 1).
Effects of DM on a SOD, b CAT, c GSH, d GSH-Px, e GST, and f GR in the liver of Bufotes viridis. Data are log transformed and presented as the means ± SE (standard error). The parametric one-way ANOVA was used to establish significant differences between control (white column) and treated (black column) group. The minimum significant level was p < 0.05. The asterisk indicates significant differences with respect to the control; the number sign indicates significant differences between treated groups. SOD, superoxide dismutase; CAT, catalase; GSH, glutathione; GSH-Px, glutathione peroxidase; GST, glutathione-S-transferase; GR, glutathione reductase
The results of all investigated parameters in the muscle are presented in Table 1 and Fig. 2. The concentration of the total proteins showed a significantly decreased trend on day 14 of exposure as compared to the other treated groups (Table 1). On the 21st day, significant inductions in SOD and GST activities as compared to the control were observed (Fig. 2a, e). GSH-Px activity and the concentration of -SH groups decreased on the 14th day of exposure with respect to the control and the 7-day exposure group (Fig. 2d and Table 1).
Effects of DM on a SOD, b CAT, c GSH, d GSH-Px, e GST, and f GR in the muscle of Bufotes viridis. Data are log transformed and presented as the means ± SE (standard error). The parametric one-way ANOVA was used to establish significant differences between control (white column) and treated (black column) group. The minimum significant level was p < 0.05. The asterisk indicates significant differences with respect to the control; the number sign indicates significant differences between treated groups. SOD, superoxide dismutase; CAT, catalase; GSH, glutathione; GSH-Px, glutathione peroxidase; GST, glutathione-S-transferase; GR, glutathione reductase
Table 1 and Fig. 3 show the results of the examination of the parameters in the GIT. The concentration of total proteins was higher on day 21 in exposed toads as compared to the group exposed for 7 days (Table 1). SOD activity exhibited a decrease on the 7th and14th days of exposure relative to the control (Fig. 3a). The activities of CAT, GSH-Px, GST, and the -SH group concentration were induced on day 7 in exposed toads as compared to the control group (Fig. 3b, d, e and Table 1). The concentration of TBARS was increased on the 7th day in comparison to the control group of animals (Table 1).
Effects of DM on a SOD, b CAT, c GSH, d GSH-Px, e GST, and f GR in the gastrointestinal tissue of Bufotes viridis. Data are log transformed and presented as the means ± SE (standard error). The parametric one-way ANOVA was used to establish significant differences between control (white column) and treated (black column) group. The minimum significant level was p < 0.05. The asterisk indicates significant differences with respect to the control; the number sign indicates significant differences between treated groups. SOD, superoxide dismutase; CAT, catalase; GSH, glutathione; GSH-Px, glutathione peroxidase; GST, glutathione-S-transferase; GR, glutathione reductase
The results of all investigated parameters in skin tissue are presented in Table 1 and Fig. 4. The concentration of total proteins was lower at on day 14 in exposed toads when compared to animals exposed for 7 days (Table 1). SOD activity exhibited a declining trend on the 21st day when compared to the group exposed for 14 days (Fig. 4a). The activity of CAT was induced and GR activity was decreased on day 14 in exposed toads as compared to the control group (Fig. 4b, f). On day 14 of exposure, the concentration of GSH displayed a decreasing trend with respect to animals treated for 7 and 21 days (Fig. 4c).
Effects of DM on a SOD, b CAT, c GSH, d GSH-Px, e GST, and f GR in the skin of Bufotes viridis. Data are log transformed and presented as the means ± SE (standard error). The parametric one-way ANOVA was used to establish significant differences between control (white column) and treated (black column) group. The minimum significant level was p < 0.05. The asterisk indicates significant differences with respect to the control; the number sign indicates significant differences between treated groups. SOD, superoxide dismutase; CAT, catalase; GSH, glutathione; GSH-Px, glutathione peroxidase; GST, glutathione-S-transferase; GR, glutathione reductase
Canonical discriminant analysis revealed differences among the treated groups, based on all measured antioxidant components for all examined tissues (Table 2, Fig. 5). The first canonical function (Root 1) in the analysis accounted for 80.44% of the total heterogeneity in toad liver. Fourteen and 21 days exposed group were separated along the first canonical function and they were mostly differentiated with regard to SOD and GR activities. The second canonical function (Root 2) in the analysis accounted for 12.99% of the total heterogeneity in toad liver. GST activity as well as -SH groups and GSH concentration were the main factors that contributed to differentiation of the groups of toads exposed for 7 and 14 days along the second canonical function.
In the muscle tissue, Root 1 accounted for 48.99% and Root 2 for 33.06% of the total heterogeneity. The main factors in Root 1 that contributed to the differentiation between the treated groups of toads were SOD and GSH-Px activities and in Root 2 GST and GR activities. The group exposed for 21 days was separated along the first canonical function from the 7-day group.
In the GIT, the first canonical function accounted for 75.90% and Root 2 for 19.40% of the total heterogeneity. The main factors in Root 1 that contributed to the separation of the 7- and 14-day groups of toads from control group were SOD and GSH-Px activities and in Root 2, the concentrations of GSH and TBARS. The 14-day group was separated along the second canonical function.
Root 1 in the analysis accounted for 76.22% of the total heterogeneity in the toad skin. The 21-day group was separated from 14-day group along the first canonical function, and it was mostly differentiated by SOD activity and GSH concentration. Root 2 accounted for 13.41% of the total heterogeneity in toad skin, and GR activity and -SH group concentration were the main factors that contributed to the differentiation.
The liver IBR values for each treated group were, respectively, as follows: control, 0.678; day 7, 0.696; day 14, 3.964; and day 21, 7.260. The results showed that the group of toads exposed for 21 days to the pesticide had the highest biomarker response with an IBR value of 7.260, and the control group had the lowest value (0.678) according to the evaluation of all of the biomarker sets. The calculated scores for IBR were specific for each group and were used to create the star plot (Fig. 6). The lines in each axis are determined by the biomarker response to pesticide exposure and correlate with the relative biomarker response inside that particular exposure group. The obtained star plot was created to provide a comparison between the exposure groups for every set of biomarkers.
Discussion
Pesticides can affect the balance between oxidants and antioxidants as well as normal cell functioning. Thus, the examination of oxidative stress markers has an important place in studies of pesticide toxic mechanisms (Radovanović et al. 2017; Mariel et al. 2014). In this regard, in the present study, we examined the biochemical responses in different toad tissues with a focus on the changes in the examined parameters in the context of the time elapsed after initiation of pesticide application. The present study reveals post-exposure changes in the oxidative stress profile of the liver, muscle, skin, and GIT after 21 days of treatment with the pyrethroid insecticide DM.
ROS, which are generated as a consequence of pesticide-induced toxicity, react with macromolecules and disturbing their synthesis. In many organisms during stress, proteins will be utilized as a source of energy through the process of amino acid oxidation (Begum and Vijayaraghavan 1996). In the present study, an increase in the total protein concentration was observed after 21 days of exposure when compared to the control liver tissue. However, when the general trend is observed during 3 weeks of exposure, the reduction in total protein concentration was detected in the liver, muscle, and skin. Immobilization of proteins can be the consequence of an organism’s attempt to deal with longer exposure to a toxic substance (Jenkins and Smith 2003). Prolonged exposure may decrease the total protein concentration as the result of increased proteolysis and potential consumption of the resulting products in metabolism (Ravinder and Suryanarayana 1988), as well as the repercussion of increased energy requirements for homeostasis maintenance and tissue repair (Neff 1985). This has led some authors to recommend changes in the total protein concentrations as markers of oxidative stress in treated toads (Tongo et al. 2012). Khan et al. (2003) noted a total protein decrease in the liver and kidneys of the frog Rana tigrina after treatment with cypermethrin and permethrin while Tongo et al. (2012) reported a reduction in total protein concentration in tissues of the African toad Bufo regularis after exposure to diazinon for 28 days. The authors assumed that the extended and sustained exposure to the pesticide leads to a progressive decline in the total protein concentration in tissues. The present study is consistent with the previous reports.
Canonical discriminant analysis clearly distinguished SOD from the battery of AOS enzymes. This enzyme contributed most to the separation in the liver, muscle and skin of the 21-day group, as well as the GIT in the 7- and14-day groups.
SOD activity was decreased in comparison to the control on days 14 and 21 in the liver and on days 7 and 14 in the GIT of treated toads, suggesting a direct causal relation between enzyme activity and time-dependent environmental stress. Clearly, the enzyme was inhibited, as the toxicant was applied for a longer period. Peixoto et al. (2004) observed that pesticide can induce mitochondrial O2− production and if SOD was inhibited, the O2− concentration could reach hazardous levels. DM caused ROS formation and a switch of the first protection provided by SOD in the liver, which was displayed through a decline in activity. GSH-Px activity in the liver was also decreased on the 21st day of exposure. This enzyme is primarily restricted to liver cytosol and mitochondria, with the liver being a source of this biomarker (Duzguner and Erdogan 2010). Decreased liver GSH-Px activity can be the result of O2− and H2O2 overproduction or final outcome of enzyme oxidative inactivation as the concentration of insecticide increases in the tissue (El-Tawil and Abdel-Rahman 2001; Bartoskova et al. 2013; Sehonova et al. 2017). In the present study, oxidative damage was also confirmed by the increased concentration of TBARS in the liver on the 14th day of the experiment. The decreased liver SOD activity of treated toads as compared to the control, along with the simultaneous declining trend in the GSH-Px activity and the increase in TBARS concentration, allowed us to conclude that the liver’s AOS was severely affected by DM-induced free radicals after prolonged exposure. Some authors confirmed a decreased activity of antioxidant enzymes and increased TBARS levels in the liver of animals exposed to DM for 15 and 30 days (Rehman et al. 2006; Manna et al. 2005).
Despite the importance of GSH-dependent enzymes in pesticide toxicity, we did not observe statistically significant changes in GST activity and GSH levels in the liver, although GR exhibited a declining trend on the 14th day of exposure. Several investigations that tested the herbicide Roundup effects on GST and GR did not observe any decreases or changes in enzyme activities, which suggests that the compound was probably metabolized in other biotransformation processes (Langiano and Martinez 2008; Lushchak et al. 2009).
In contrast to the liver, increased GST activity in the muscle and the GIT of exposed toads was coupled with a decline in GSH-Px activity in the muscle and an increase in GSH-Px activity in the GIT. The observed different trends in activity could be the result of tissue differences and of variable rates of free radical generation. Furthermore, the pesticide itself may be present in dissimilar amounts in the tissues, thus producing differential inhibition, or the inhibitor may be metabolized at different rates. The observed increase in GST activity in the muscle and the GIT of treated toads is comparable to the reports of Ezemonye and Tongo (2010) who studied the influence of endosulfan and diazinon pesticide on GST activity Bufo regularis for 28 days and detected significant increases in activity. The same authors (2013) recorded an increase in GST activity in B. regularis which they explained by the fact that pesticides initiate detoxification reactions that are based on the addition of the GSH group to pollutants, rendering them more hydrosoluble and more easily excreted (Moorhouse and Casida 1992). It can be deduced that glutathione-dependent enzymes possess the potential to limit the damage caused by the reactive oxygen species that is generated during pesticide-detoxification processes (Van der Oost et al. 2003).
Sulfhydryl groups are important determinants of the conformational and functional features of protein molecules, and protein exposure to free radicals results in numerous modifications that can lead to the structural changes resulting in loss of enzymatic activity and hence altered physiological function (Headlam and Davies 2004). The profiles of thiol groups were considerably changed after chronic application of DM to B. viridis, although the concentrations of -SH groups did not exhibit any consistencies in different tissues. The decreased content of -SH groups in the liver and muscle after 14 days of application confirmed the influence of the pesticide on the proteins.
The majority of the examined parameters in the GIT exhibited significant increases on the 7th day of application when we detected increases in CAT, GST, and GSH-Px activities and concentrations of -SH groups and TBARS. This trend could be related to the fact that the pyrethroid was orally ingested and that the GIT was the first route of contact with the toxicant.
Multi-biomarker studies like this are frequently difficult to interpret, and the generated results are not easy to incorporate in the ecotoxicological framework. IBR merges various biomarkers to a single value, which can be applied to express the stress level induced by toxicants. We decided to determine the IBR index for liver tissue because it is the main metabolic organ with a strong antioxidant capacity. It was evident that the group of toads that were exposed to the pesticide for 21 days had the highest biomarker response among the tested groups. The group that was exposed longer to the pesticide and the groups exposed for shorter periods were clearly distinguished in the liver tissue, and a causal relationship for the estimated biomarkers and time-dependent exposure to the pesticide was observed. IBR exhibited a satisfactory agreement with the exposure time for the different groups because the IBR values increased from the control to the 21-day exposure group.
Conclusions
The experimental data presented herein provide evidence that DM-induced systemic toxicity was time-dependent and that toads subjected to this pyrethroid develop tissue-specific adaptive reactions in protecting cells from to the resulting oxidative stress. According to our results, SOD was the most distinguishing component among the battery of AOS enzymes in all examined tissues, and the antioxidative strategy of Bufotes viridis during prolonged exposure to the pesticide was based on the utilization of SOD to maintain the oxidative imbalance at an acceptable level during prolonged DM exposure. The present study reinforces the idea that oxidative stress is a significant mechanism in pesticide-induced toxicity but additional analyses are desirable. Ecologically significant and well-conducted investigations will increase our understanding of the adverse effects on non-target organisms and provide information with regard to the preservation of amphibian populations. For that cause, results of the present study will be useful course for other toxicological studies related to amphibians.
References
Bartoskova M, Dobsikova R, Stancova V, Zivna D, Blahova J, Marsalek P, Zelníckova L, Bartos M, Di Tocco FC, Faggio C (2013) Evaluation of ibuprofen toxicity for zebrafish (Danio rerio) targeting on selected biomarkers of oxidative stress. Neuro Endocrinol Lett 34:102–108
Begum G, Vijayaraghavan S (1996) Alterations in protein metabolism of muscle tissue in the fish Clarias batrachus Linn by commercial grade dimethoate. Bull Environ Contam Toxicol 57:223–228
Broeg K, Lehtonen KK (2006) Indices for the assessment of environmental pollution of the Baltic Sea coasts: integrated assessment of a multi-biomarker approach. Mar Pollut Bull 53:508–522
Çakıcı Ö (2015) Histopathologic changes in liver and kidney tissues induced by carbaryl in Bufotes variabilis (Anura: Bufonidae). Exp Toxicol Pathol 67:237–243
California EPA (2000) Deltamethrin risk characterization document—volume 1. California Environmental Protection Agency, Health Assessment Section, Medical Toxicology Branch, Department of Pesticide Regulation, Sacramento
Canli M, Furness RW (1993) Toxicity of heavy metals dissolved in seawater and influences of sex and size on metal accumulation and tissue distribution in the Norway lobster Nephrops norvegicus. Mar Environ Res 36:217–223
Chromcova L, Blahova J, Zivna D, Plhalova L, Casuscelli Di Tocco F, Divisoval PM, Faggio C, Tichy F, Svobodova Z (2015) NeemAzal T/S - toxicity to early-life stages of common carp (Cyprinus carpio L.). Vet Med 60:23–30
Claiborne A (1984) Catalase activity. In: Greenwald RA (ed) Handbook of methods for oxygen radical research. CRC Press Inc, Boca Raton, pp 283–284
Darlington RB, Weinberg S, Walberg H (1973) Canonical variate analysis and related techniques. Rev Educ Res 43:433–454
Devin S, Burgeot T, Giambérini L, Minguez L, Pain-Devin S (2014) The integrated biomarker response revisited: optimization to avoid misuse. Environ Sci Pollut Res 21:2448–2454
Devin S, Buffet PE, Châtel A, Perrein-Ettajani H, Valsami-Jones E, Mouneyrac C (2017) The integrated biomarker response: a suitable tool to evaluate toxicity of metal-based nanoparticles. Nanotoxicology 11(1):1–6
Dey S, Samanta P, Pal S, Mukherjee AK, Kole D, Ghosh AR (2016) Integrative assessment of biomarker responses in teleostean fishes exposed to glyphosate-based herbicide (Excel Mera 71). Emerg Contaminants 2(4):191–203
Duzguner V, Erdogan S (2010) Acute oxidant and inflammatory effects of imidacloprid on the mammalian central nervous system and liver in rats. Pestic Biochem Physiol 97:13–18
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
El-Tawil OS, Abdel-Rahman MS (2001) The role of enzyme induction and inhibition on cypermethrin hepatotoxicity. Pharmacol Res 44:33–40
Ezemonye L, Tongo I (2010) Sublethal effects of endosulfan and diazinon pesticides on glutathione-S-transferase (GST) in various tissues of adult amphibians (Bufo regularis). Chemosphere 81:214–217
Faggio C, Fazio F, Marafioti S, Arfuso F, Piccione G (2015) Oral administration of gum arabic: effects on haematological parameters and oxidative stress markers in Mugil cephalus. Iran J Fish Sci 14(1):60–72
Faggio C, Pagano M, Alampi R, Vazzana I, Felice MR (2016) Cytotoxicity, haemolymphatic parameters, and oxidative stress following exposure to sub-lethal concentrations of quaternium-15 in Mytilus galloprovincialis. Aquat Toxicol 180:258–265
Fazio F, Cecchini S, Faggio C, Caputo AR, Piccione G (2014) Stability of oxidative stress biomarkers in flathead mullet, Mugil cephalus, serum during short-term storage. Ecol Indic 46:188–192
Felício AA, Freitas JS, Scarin JB, de Souza Ondei L, Teresa FB, Schlenk D, de Almeida EA (2018) Isolated and mixed effects of diuron and its metabolites on biotransformation enzymes and oxidative stress response of Nile tilapia (Oreochromis niloticus). Ecotoxicol Environ Saf 149: 248–256
Freitas JS, Felício AA, Teresa FB, de Almeida EA (2017) Combined effects of temperature and clomazone (Gamit®) on oxidative stress responses and B-esterase activity of Physalaemus nattereri (Leiuperidae) and Rhinella schneideri (Bufonidae) tadpoles. Chemosphere 185:548–562
Glatzle D, Vuilleumier JP, Weber F, Decker K (1974) Glutathione reductase test with whole blood, a convenient procedure for the assessment of the riboflavin status in humans. Experientia 30:665–667
Goulet NB, Hontella A (2003) Toxicity of cadmium, endosulfan and atrazine in adrenal steriodogenic cells of two amphibian species; Xenopus laevis and Rana catesbeiana. Environ Toxicol Chem 22:2106–2113
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106:207–212
Gutiérrez Y, Santos HP, Serrão JE, Oliveira EE (2016) Deltamethrin-mediated toxicity and cytomorphological changes in the midgut and nervous system of the mayfly Callibaetis radiatus. PLoS One. https://doi.org/10.1371/journal.pone.0152383
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Headlam HA, Davies MJ (2004) Markers of protein oxidation: different oxidants give rise to variable yields of bound and released carbonyl products. Free Radic Biol Med 36:1175–1184
Hernández-Moreno D, Soler F, Miguez MP, Perez-Lopez M (2010) Brain acetylcholinesterase, malondialdehyde and reduced glutathione as biomarkers of continuous exposure of tench, Tinca tinca, to carbofuran or deltamethrin. Sci Total Environ 408:4976–4983
Jenkins F, Smith J (2003) Effects of sublethal concentration of endosulfan on haematological and serum biochemical parameters in the carp, Cyprinus carpio. Bull Environ Contam Toxicol 70:993–997
Khan MZ, Nazia M, Fatima F, Rahilla T, Gabol K (2003) Comparison in the effect of lambda cyhalothrin with permethrin on cholinesterase activity in wildlife species Rana cyanophlyctis and Rana tigrina Ranidae: Amphibia Bull Pure. Appl Sci 2:43–49
Kljajić P, Perić I (2009) Residual effects of deltamethrin and malathion on different populations of Sitophilus granarius (L.) on treated wheat grains. J Stored Prod Res 45:45–48
Langiano C, Martinez CB (2008) Toxicity and effects of a glyphosate-based herbicide on the neotropical fish Prochilodus lineatus. Comp Biochem Physiol C 147:222–231
Laskowski DA (2002) Physical and chemical properties of pyrethroids. Rev Environ Contam Toxicol 174:149–170
Lionetto MG, Caricato R, Giordano ME, Pascariello MF, Marinosci L, Schettino T (2003) Integrated use of biomarkers (acetylcholineesterase and antioxidant enzyme activities) in Mytilus galloprovincialis and Mullus barbatus in an Italian coastal marine area. Mar Pollut Bull 46:324–330
Livingstone DR (2001) Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull 42:656–666
Lushchak OV, Kubrak OI, Storey JM, Storey KB, Lushchak VI (2009) Low toxic herbicide Roundup induces mild oxidative stress in goldfish tissues. Chemosphere 76:932–937
Manna S, Bhattacharyya D, Mandal TK, Das S (2005) Repeated dose toxicity of deltamethrin in rats. Indian J Pharmacol 37:161–164
Mariel AC, Alejandra BP, Silvia PC (2014) Developmental toxicity and risk assessment of nonylphenol to the South American toad, Rhinella arenarum. Environ Toxicol Pharmacol 38:634–642
Messina CM, Faggio C, Laudicella AV, Sanfilippo M, Trischitta F, Santulli A (2014) Effect of sodium dodecyl sulfate (SDS) on stress response in the Mediterranean mussel (Mytilus galloprovincialis): regulatory volume decrease (RVD) and modulation of biochemical markers related to oxidative stress. Aquat Toxicol 157:94–100
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Moorhouse KG, Casida JE (1992) Pesticides as activators of mouse liver microsomal glutathione-S-transferase. Pestic Biochem Physiol 44:83–90
Mutschmann F (1991) Ectoparasite control with synthetic pyrethroids in reptiles. In: Gabrisch K, Schildger B, Zwart P (eds) Internationales Kolloquium fur Pathologie und Therapie der Reptilien und Amphibien DVG-4. Deutsche Veterinarmedizinische Gesellschaft, Giessen, pp 95–106
Narahashi T (1992) Nerve membrane Na+ channels as targets of insecticides. Trends Pharmacol Sci 13:236–241
Neff JM (1985) Use of biochemical measurements to detect pollutant-mediated damage to fish. In: Carwel RD, Purdy R, Bahner RC (eds) Aquatic toxicology and hazard assessment. America Society for Testing Material, Philadelphia, pp 155–181
Ohkawa H, Okishi N, Yogi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Paškova V, Hilscherova K, Blaha L (2011) Teratogenicity and embryotoxicity in aquatic organisms after pesticide exposure and the role of oxidative stress. Rev Environ Contam Toxicol 211:25–61
Peixoto F, Vicente J, Madeira VM (2004) A comparative study of plant and animal mitochondria exposed to paraquat reveals that hydrogen peroxide is not related to the observed toxicity. Toxicol in Vitro 18:733–739
Radovanović TB, Nasia M, Krizmanić II, Prokić MD, Gavrić JP, Despotović SG, Gavrilović BR, Borković-Mitić SS, Pavlović SZ, Saičić ZS (2017) Sublethal effects of the pyrethroid insecticide deltamethrin on oxidative stress parameters in green toad (Bufotes viridis L.). Environ Toxicol Chem 36:2814–2822
Ravinder V, Suryanarayana N (1988) Pesticides induced biochemical alterations in a fresh water catfish, Clarias batrachus. Indian J Comp Animal Physiol 6:5–12
Rehman H, Ali M, Atif F, Kaur M, Bhatia K, Raisuddin S (2006) The modulatory effect of deltamethrin on antioxidants in mice. Clin Chim Acta 369:61–65
Rendon-von Osten J, Ortiz-Arana A, Guilhermino L, Soares AM (2005) In vivo evaluation of three biomarkers in the mosquitofish (Gambusia yucatana) exposed to pesticides. Chemosphere 58:627–636
Rossi MA, Cecchini G, Dianzani MM (1983) Glutathione peroxidase, glutathione reductase and glutathione transferase in two different hepatomas and in normal liver. IRCS Med Sci Biochem 11:805
Sayeed I, Parvez S, Pandey S, Bin-Hafeez B, Haque R, Raisuddin S (2003) Oxidative stress biomarkers of exposure to deltamethrin in freshwater fish, Channa punctatus Bloch. Ecotoxicol Environ Saf 56:295–301
Sehonova P, Plhalova L, Blahova J, Doubkova V, Marsalek P, Prokes M, Tichy F, Skladana M, Fiorino E, Mikula P, Vecerek V, Faggio C, Svobodova Z (2017) Effects of selected tricyclic antidepressants on early-life stages of common carp (Cyprinus carpio). Chemosphere 185:1072–1080
Stewart DAB, Seesink LD (1996) Impact of locust control in semi-arid ecosystem in South Africa. In: Brighton Crop Protection Conference—Pests and Diseases. Vol. 3. Leicestershire: British Crop Protection Council, p 1193–1198
Takada Y, Noguchit T, Kayiyama M (1982) Superoxide dismutase in various tissues from rabbits bearing the Vx-2 carcinoma in the maxillary sinus. Cancer Res 42:4233–4235
Tamura M, Oshino N, Chance B (1982) Some characteristics of hydrogen- and alkylhydroperoxides metabolizing systems in cardiac tissue. J Biochem 92:1019–1031
Tongo I, Ezemonye L, Ochei U (2012) Diazinon mediated biochemical changes in the African toad (Bufo regularis). J Xenobiot 2:18–23
Van der Oost R, Beyer J, Vermeulen N (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Acknowledgements
The authors are grateful to Dr. Goran Poznanović for proofreading the manuscript and to Tamara Petrović for the statistical evaluation that significantly enhanced the quality of the paper.
Funding
The present study was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia, Grant No. 173041.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Nasia, M.M., Radovanović, T.B., Krizmanić, I.I. et al. Prooxidant effects of chronic exposure to deltamethrin in green toad Bufotes viridis. Environ Sci Pollut Res 25, 30597–30608 (2018). https://doi.org/10.1007/s11356-018-2979-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2979-x