Abstract
Potentially toxic elements (PTEs) are hazardous contaminants with great global environmental/ecological concerns due to their toxic, persistence, and bio-accumulative nature. This study investigates the concentrations of PTEs (Cd, Co, Cu, Fe, Ni, Mn, Pb, and Zn) in drinking water sources and consumers’ biomarkers such as hair, nails, urine, and blood. For this purpose, drinking water (n = 190) and consumer biomarker (n = 60) samples were collected from five districts of the Southern Khyber Pakhtunkhwa, Pakistan. Samples were extracted and analyzed for selected PTEs concentrations using an inductively coupled plasma mass spectrometer (ICP-MS, PerkinElmer Optima 7000 DV, USA). The concentrations of PTEs were observed within the drinking water guidelines set by the World Health Organization (WHO), except for Fe, Mn, and Pb. The determined concentrations of PTEs were used to evaluate the health risk through exposure, particularly hazard quotient (HQ) and hazard index (HI). The PTEs contamination of drinking water has led to the highest mean ADI values (39.0 and 91.8 μg/kg/day) and HQ values (0.306 and 0.130) for Zn in adults and children, respectively. The mean values of HQ and HI for selected PTEs were observed within the safe health limits (< 1). Among studied biomarkers, hair showed the highest concentrations for Mn, Zn, Cd, and Pb, plasma for Co and Cu, nails for Ni, and red blood cells (RBCs) for Fe only. This study concluded that chronic exposure of PTEs through drinking water consumption has led to their bioaccumulation in human biomarkers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The contamination of environment with potentially toxic elements (PTEs) is a great global environmental concern due to their toxic nature for all living organisms. These PTEs are highly persistent in nature and easily bio-accumulated in ecological resources such as plants and animals, and subsequently find their way to reach into the human body (Antoniadis et al. 2017b; Ishtiaq et al. 2018; Sun et al. 2016). These PTEs are released from both natural (weathering and erosion of bedrocks and ore deposits) and anthropogenic (mining, agricultural, and industrial) activities (Antoniadis et al. 2017a; Shah et al. 2012) and eventually contaminate the water sources (Khan et al. 2018; Tripathee et al. 2016). The contamination of water with PTEs not only impairs its quality but also poses threats to the exposed population (Khan et al. 2016). Ingestion of PTE contaminated water (Kumar et al. 2016) and food (Riaz et al. 2018) are the major pathways for human exposure. Among PTEs, iron (Fe), zinc (Zn), copper (Cu), and manganese (Mn) are essential elements and required for normal human body functions. However, these essential elements may cause health hazards if exceeded their safe threshold limits of exposure (Ullah et al. 2017). Other PTEs including lead (Pb), cadmium (Cd), nickel (Ni), cobalt (Co), arsenic (As), and mercury (Hg) are extremely hazardous even in minute concentrations (Saddique et al. 2018; Shakoor et al. 2017). Low-dose exposure to PTEs in non-occupational environment could become a serious problem, especially to the most sensitive subgroups of population, including pregnant women and children (Rodríguez-Barranco et al. 2013).
The PTEs, after their intake via different pathways, are partially metabolized by human body and eliminate them in saliva, sweat, urine, and feces (Gil et al. 2011; Omokhodion and Crockford 1991). Therefore, various studies have focused on human blood, urine, nails, and scalp hair samples as biomarkers of PTE exposure (Molina-Villalba et al. 2015; Sheng et al. 2016; Song and Li 2015). Each of these human biomarkers has advantages and limitations over others. For example, PTEs concentrations in blood and urine reflect recent exposure, while hair levels reflect the past exposure, providing an average of the growth over period (weeks to years) depending on length of hair, easy collection, transport, and storage (Bermejo-Barrera et al. 1998; Molina-Villalba et al. 2015; Rahman et al. 2015).
In this study, we quantified the concentrations of PTEs including Mn, Fe, Co, Ni, Cu, Pb, and Cd in drinking water samples obtained from different sources present in southern Khyber Pakhtunkhwa, Pakistan. The potential health risk of these PTEs was also evaluated using the hazard index (HI) via drinking water consumption. In addition, the selected PTE concentrations were measured in human biomarkers including blood (plasma and RBCs), urine, nails, and scalp hair collected from the residents within the study area. Furthermore, this study also tested the correlations between PTEs concentrations in drinking water and human biomarkers.
Materials and methods
Study area
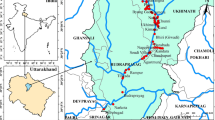
The study area, comprised of districts Dera Ismail Khan (DI Khan), Bannu, Karak, Lakki Marwat and Tank in Southern Khyber Pakhtunkhwa Province (Pakistan), has a total population of 4.95 million (Fig. 1). The selected area has hot summer (48.9 °C) and cold winter (2.8 °C) and mostly drained and irrigated by the rivers Indus, Kuram, and Gambila. The people within the study area are mainly associated with the agriculture sector, and the major crops include wheat, maize, rice, sugarcane, grams, barley, and vegetables.
Water sampling and preparation
Water samples (n = 190) were collected from various drinking water sources with replicates (n = from 5 to 18) from tube wells, bore wells, dug wells, hand pumps, springs, and streams/ponds throughout the selected districts (n = 5), as mentioned earlier. Before collection of each sample, the water from tube wells, bore wells, and hand pumps were allowed to flow for 5–10 min, and then, the bottle (250 ml) was washed three times and filled with sampling water. Each sample was filtered and 400 μl of concentrated nitric acid (65% HNO3, Suprapur, Merck, Germany) was added, marked, and transferred to the laboratory of the Department of Environmental Sciences, University of Peshawar, and stored in the freezer (− 20 °C) according to procedure adopted from Kippler et al. (2016). Latitude and longitude coordinates were also noted for each sampling point using Global positioning system (GPS).
Human biomarkers sampling and preparation
The biomarkers including blood, urine, nails, and scalp hair were used to estimate the internal dose of PTEs exposure (Davis et al. 2017; Rahman et al. 2015). Biomarkers’ samples (n = 60) including mid-stream spot urine (early morning time), blood, nails, and scalp hair were collected from individuals in the study area. This study was performed after taking the approval from the ethical committee. Adult participants and parents of children for this study were asked for their consents before taking the samples from them.
Blood and urine
Fasting blood and mid-stream spot urine samples were collected in K2 EDTA tubes (5 ml) using new plastic bottles (100 ml). Blood samples were centrifuged and separated to plasma and red blood cells (RBCs). After collection, samples were properly marked, acidified with HNO3 (65% Suprapur, Merck, Germany) and stored in cooling blocks (− 20 °C) and transported to laboratory for further analyses.
Blood (5 ml) and urine (25 ml) samples were put in 25 and 100 ml polypropylene tubes and mixed with 1 ml of 1% HNO3 (65% Suprapur, Merck, Germany) and kept overnight as the procedure adopted from Ettinger et al. (2017).
Nails and scalp hair
Nails and scalp hair samples were collected using stainless steel nail clippers and scissors and stored in zip lock polyethylene bags, labeled and transported to the laboratory. Nails and scalp hair were washed, clean, and dried according to procedure adopted from the Gault et al. (2008).
Nails and scalp hair were digested according to the method adopted from Rahman et al. (2015). Briefly, (100-mg) samples were mixed with 2 ml of concentrated HNO3 (65% Suprapur, Merck, Germany) and 1 ml of H2O2 in polypropylene tubes and kept overnight. Samples were treated for 15 min each at 70 and 115 °C in the microwave accelerated reaction system (CEM-Mars.V.194A05). After cooling, samples were diluted with 1% of HNO3.
Acidified water and digested blood, urine, nails, and scalp hair samples were analyzed within 2-month period using an inductively coupled plasma mass spectrometer (ICP-MS) in the Institute of Urban Environment, Chinese Academy of Sciences, 1799, Jimmie Road, Xiamen 361021 China.
Precision and accuracy
Before reading the actual water and biomarkers samples, a mix of standard was run on the ICP-MS. After 10 samples, another separate standard was run to check the accuracy of machine and method. The accuracies of PTEs measurements were verified with standard reference materials of urine (Clinchek-control; RECIPE, Munich, Germany) and human hair (GBW09101, Shanghai Institute of Nuclear Research Academia Sinica, China). During analyses, each sample was measured in triplicate and the mean values were used for result interpretation. Recoveries of PTEs were found in the confidence level of 94 ± 8% for standard reference materials and 95 ± 6% for sample triplicate. All glassware and new plastic wares were washed with 2% HNO3 (65% Suprapur, Merck, Germany) and deionized water.
Risk assessment
Basic information like drinking water consumption and source, age, gender, body weight, exposure frequency, and health data were collected during the field survey. Respondents (male and female) were included both children (1–14 years) and adults (15–65 years). In the study area, water is pumped or collected from various sources and stored in plastic or cemented container. More than 98% of population uses this fresh or stored water without any treatment for drinking and other domestic purposes. Therefore, fresh or raw water was used to calculate the risk. Oral intake of PTEs through contaminated water is the major pathway for human exposure (Khan et al. 2018). Risk assessment was calculated through exposure such as average daily intake (ADI) and risk index, e.g., hazard quotient (HQ) and HI.
Average daily intake of PTEs through drinking water consumption was calculated using the equation adopted from the US EPA (1998).
where C, IR, EF, ED, BW, and AT are the PTEs concentrations in drinking water (mg/l), ingestion rate (2 l/day), frequency of exposure (365 days/year), exposure duration (30 years), body weight (children 30.6 kg (< 14 years age) and adults 72 kg (> 15 years age), and average time, i.e., 365 days/year × ED for non-carcinogens, respectively (Muhammad et al. 2010).
Hazard quotient values through PTEs exposure were calculated by dividing the ADI values over the RfD of respective element as equation adopted from the US EPA (1998).
where oral toxicity reference dose values (RfD) of PTEs such as Pb, Zn, Co, Ni, Cu, Fe, and Mn were used as 0.036, 0.3, 0.03, 0.02, 0.037, 0.7, and 0.14 mg/kg/day, respectively (USEPA 2005). Exposed population is assumed to be safe if HQ < 1 (Muhammad et al. 2011).
where HI is health index and sum of HQ for all selected PTEs.
Mapping for PTEs
Latitude and longitude of each sampling site was marked with GPS and used for the PTEs concentrations and distribution maps using ArcGIS.
Statistical analysis
Statistical analyses such as Pearson’s correlation of data and graphical presentation were performed using Sigma plot 12.5 and SPSS 21 (SPSS Inc., Chicago, IL, USA).
Results and discussion
Potentially toxic elements concentrations in drinking water
The concentrations and distribution of PTEs in drinking water sources of selected five districts of Khyber Pakhtunkhwa were summarized (Table 1, Fig. 1). The concentrations of PTEs showed great variations in sources and district/location of drinking water. The studied PTEs showed higher concentrations in shallow-water sources (hand pumps) as compared to deep water sources (tube wells) of the study area except Bannu, Lakki Marwat, and Tank districts and surface water sources (ponds) of Karak (Table 1). Higher contamination levels of shallow-water sources (hand pumps) were consistent with the results reported by Khan et al. (2018) for drinking water. Hand pumps water stayed close to earth surface; therefore, higher PTEs concentrations in shallow water could be attributed to ongoing surface agriculture, domestic, and mining activities (Khan et al. 2018). However, PTEs concentrations were highest in the deep tube well water sources (Table 1). Higher Fe, Zn, and Pb contaminations in the tube well water of district DI Khan could be attributed to PTEs enrichment in bedrocks and rusting of old plumbing pipes. Among PTEs, the highest (1405 μg/l) mean concentration was observed for Zn and the lowest not determined (ND) for Co (Table 1). Other PTEs including Mn, Fe, Ni, Cu, and Pb concentrations were observed between the two extremes. The concentrations of PTEs including Mn, Fe, Co, Ni, Cu, Zn, and Pb were observed within the safe drinking water guidelines of respective elements set by WHO (2011). However, these limits were surpassed by Mn and Fe in hand pump’s water of district Tank, Fe in hand pump’s water of Bannu, DI Khan, Tank and Lakki Marwat (bore well also), and Pb in bore wells of district DI Khan. The results revealed that selected PTEs concentrations were observed lower than those reported by Begum et al. (2015) for drinking water in Swat (northern Pakistan) along the mafic and ultramafic rocks which act as a source of these elements in water.
Risk assessment
Human exposure to the PTEs could occur through main routes, e.g., ingestion or intake of contaminated drinking water (Muhammad et al. 2011) and food (Khan et al. 2013). Hence, in order to understand that how much of PTEs were transferred into the human body, it will be very essential to investigate the ADI of these elements ingested via drinking water.
Table 2 summarizes the values of ADI through PTEs consumption in various drinking water sources of five districts of Khyber Pakhtunkhwa. The ADI values revealed great variation in different drinking water sources and locations. The majority of shallow drinking water sources (hand pumps) were observed with higher ADI values for PTEs consumption as compared to deep (tube wells) sources. Higher ADI values through consumption of shallow-water were due to their higher contamination levels. The DI Khan district showed higher ADI values for majority of PTEs consumption as compared to other districts (Table 2).
Average daily intakes of PTEs were highest for Zn, followed by Fe, while the lowest for Co (Table 2). Higher ADI values for Zn and Fe were attributed to their higher contamination levels as compared to other PTEs in drinking water sources of the study area. The ADI values for PTEs consumption through same drinking water were observed higher for children (91.8 μg/kg/day) as compared to adults (39.0 μg/kg/day) (Table 2). Higher ADI values through PTEs consumption in drinking water may be attributed to their low body weight. Higher ADI values through PTEs consumption for children were consistent with those reported by Saddique et al. (2018).
The hazards of PTEs on human health through water consumption have been well documented (Muhammad et al. 2011; Tripathee et al. 2016). Potential health risk such as HQ values of exposed population posed by the PTEs were estimated from ADI values. The highest mean HQ values through consumption of drinking water were observed for Zn, followed by Pb in the DI Khan, while the lowest in district Karak (Table 3). Higher HQ value of Zn and Pb could be attributed to their concentrations, toxicity, and respective RfD values (Khan 2013). The HQ values depend upon the concentrations of PTEs, RfD values, and toxicity of each the element. Results of this study revealed that HQ values were within the safe limits (< 1). The HQ values were found lower than those reported by Gul et al. (2015) in the drinking water of district Mardan, Khyber Pakhtunkhwa. The HI values are sum of HQ of all studied PTEs. Our results revealed that the highest HI values were observed through consumption of drinking water from hand pumps in district Tank, followed by tube well water in district DI Khan (Fig. 2). Higher risk values through hand pump water in district Tank were due to higher HQ values of individual PTEs that were attributed to higher contamination level of hand pump’s water. Similarly, the higher HI values of children were attributed to their higher sensitivity and low body weight as compared to adults.
Hazard index values through consumption of PTEs present in various drinking water sources of the study area, while BT, BB, BH, LT, LB, LH, DT, DB, DH, TT, TB, TH, KT, KB, KH, KD, and KP stands for tube wells, bore wells, hand pumps, dug wells and ponds present in district Bannu, Lakki Marwat, DI Khan, Tank, and Karak, respectively
Potentially toxic elements in biomarkers
The monitoring of PTEs concentrations in the environmental samples, including water (this study), soil, and food (Rehman et al. 2016) revealed the presence of contamination in the study area. The ingestion of contaminated water could induce PTEs health burden in the exposed human population. The current study on the human body burden of PTEs has included five types of human biomarkers including nails, urine, hair, plasma, and RBCs along with other reported studies (Table 4).
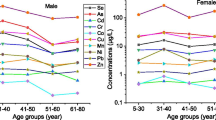
Human population, especially children and infants, may be very vulnerable to neurotoxic effects of Mn exposure. Exposure to low concentration of Mn has previously been reported with toxic effects on neurodevelopmental outcomes in children by Riojas-Rodríguez et al. (2010). The concentrations of Mn in hair, nails, urine, plasma, and RBCs ranged from 21.5, 10.9, 9.78, 2.85, and 4.85 μg/g, respectively. The concentrations of Mn in the studied population hair and urine were higher than those reported by Huang et al. (2014) and Wang et al. (2011), while those of nails and blood samples were lower than Samanta et al. (2004) and Zheng et al. (2013). Fe is one of the human essential elements and requires for normal function of hemoglobin, myoglobin, and a number of enzymatic activities. Fe concentrations lower than the required concentration could cause deficiency effects, while at higher concentration characterizes for toxic effects including diarrhea, vomiting, liver, kidney, and blood problems (Muhammad et al. 2011). The concentrations of Fe in the blood of studied population were higher than those reported by Samanta et al. (2004).
Cadmium is one of the non-essential PTEs and has well-known for acute and chronic toxicity including kidney problems and potential developmental and other harmful health effects in children (ATSDR 2008). Contaminated food and water account for the major source of Cd exposure. The concentrations of Cd in the hair of studied population were higher than those reported by Huang et al. (2014), while those of urine, nails, and blood were lower than those reposted by Samanta et al. (2004), Sheng et al. (2016), and Molina-Villalba et al. (2015). The Pb stays in contaminated environment and considers as highly toxic element that may result in memory loss and reduced growth in children. A study conducted by Watanabe et al. (2000) observed that over 60% of total Pb intake could be attributed to dietary exposure. Toxic effects of Pb include neurologic and developmental effects in children (ATSDR 2008). The concentrations of Pb in the studied population’s hair, urine, nails, and blood were observed lower than the studies conducted by the Wang et al. (2009) and Wongsasuluk et al. (2017). Zn is also one of the essential elements and needs for normal function of living beings. The deficiency of Zn could lead to poor healing of wounds, muscles’ weakness, and hair loss, while high concentration may cause anemia (Muhammad et al. 2011). The concentrations of Zn in the studied population’s hair, nails, and urine were higher than those reported by the Dongarrà et al. (2011), Samanta et al. (2004), and Sheng et al. (2016), while its concentrations in blood were found lower than reported by Sheng et al. (2016).
Nickel is required in a specific amount for cell membrane metabolism, lipid, and hormone. However, its higher concentration may cause burning and redness of skin, itching, and asthma in human beings (Knight et al. 1997). The concentrations of Ni in the studied population nails were higher than those reported by Sukumar and Subramanian (2007), while that of hair, urine, and blood were lower than Sheng et al. (2016) and Huang et al. (2014). Like Ni, Co is also needed in minute quantity for normal body functions. However, higher concentration of Co may cause polycythemia, over-production of RBCs and abnormal thyroid artery (Robert and Mari 2003). The concentrations of Co in the biomarkers of studied population were found higher than those reported by Sheng et al. (2016). Cu is an essential element; however, its higher intake through drinking water can lead to several health problems (Kidd 2003). The concentrations of Cu in the studied population urine were higher than those reported by Wang et al. (2011), while those of hair, nails, and blood were lower than reported by Wang et al. (2009), Samanta et al. (2004) and Sheng et al. (2016).
Statistical analyses (Pearson correlation of PTEs and human biomarkers)
Table 5 summarizes the findings about of correlation between PTEs concentrations in drinking water and human biomarkers. For example, the concentrations of Cu in drinking water showed significant correlation with that of nails, RBCs, and urine. Similarly, Fe in drinking water showed the correlation with that of plasma and RBCs. The Mn in drinking water showed the correlation with that of plasma only (Table 5). Results of this study have found a correlation between drinking water and nails, plasma, RBCs, and urine for the studied PTEs concentrations such as Mn, Fe, and Cu concentrations. Results of this study suggest that nails could be a good biomarker for Cu, plasma for Mn and Fe, RBCs for Fe and Cu, and urine for Cu only. The correlation between PTEs concentrations in biomarkers including urine, nails, and blood has been previously documented to health burden for the assessment of environmental exposures (Gil et al. 2011). Higher correlations of PTEs concentrations in human biomarkers are consistent with those reported by Molina-Villalba et al. (2015), Sheng et al. (2016), and Xing et al. (2016).
Conclusion
This study concluded that highest levels of selected PTEs contamination were observed for shallow-water (hand pump) in districts Bannu, Lakki Marwat, Tank, and Karak. The mean concentrations of selected PTEs including Mn, Co, Ni, Cu, Zn, and Pb were found within the safe drinking water guidelines of respective elements as set by (WHO). However, Fe mean concentration surpassed these limits in all shallow water sources of the study area except district Karak. These limits are also surpassed by Fe in tube well water and Pb in bore well water of DI Khan. Higher contamination levels of shallow drinking water have led to higher ADI values for Zn, followed by Fe as compared to other PTEs in drinking water. The highest HQ value was observed for Zn, followed by Pb. The intake of contaminated drinking water has led to accumulation of PTEs in human biomarkers which was confirmed by the statistical analyses such as Pearson’s correlation that revealed strong positive correlation.
References
Afridi H et al (2009) Evaluation of arsenic, cobalt, Copper and manganese in biological samples of steel mill workers by electrothermal atomic absorption spectrometry. Toxicol Ind Health 25:59–69. https://doi.org/10.1177/0748233709103036
Antoniadis V, Golia EE, Shaheen SM, Rinklebe J (2017a) Bioavailability and health risk assessment of potentially toxic elements in Thriasio plain, near Athens, Greece. Environ Geochem Health 39:319–330
Antoniadis V, Shaheen SM, Boersch J, Frohne T, Du Laing G, Rinklebe J (2017b) Bioavailability and risk assessment of potentially toxic elements in garden edible vegetables and soils around a highly contaminated former mining area in Germany. J Environ Manag 186(Part 2):192–200. https://doi.org/10.1016/j.jenvman.2016.04.036
ATSDR (2008) (Agency of Toxic substances and Disease Registry), 1999, 2007, 2008, 2012. Draft Toxicological Profile for Lead, Cadmium, Manganese and Mercury. Separate Profile Per Metal
Begum S, Shah M, Muhammad S, Khan S (2015) Role of mafic and ultramafic rocks in drinking water quality and its potential health risk assessment, northern Pakistan. J Water Health 13:1130–1142
Bermejo-Barrera P, Moreda-Pineiro A, Moreda-Pineiro J, Bermejo-Barrera A (1998) Determination of aluminium and manganese in human scalp hair by electrothermal atomic absorption spectrometry using slurry sampling. Talanta 45:1147–1154
Davis MA et al (2017) Assessment of human dietary exposure to arsenic through rice. Sci Total Environ 586:1237–1244
Dongarrà G, Lombardo M, Tamburo E, Varrica D, Cibella F, Cuttitta G (2011) Concentration and reference interval of trace elements in human hair from students living in Palermo, Sicily (Italy). Environ Toxicol Pharmacol 32:27–34
Ettinger AS et al (2017) Arsenic levels among pregnant women and newborns in Canada: results from the maternal-infant research on environmental chemicals (MIREC) cohort. Environ Res 153:8–16
Gault AG et al (2008) Arsenic in hair and nails of individuals exposed to arsenic-rich groundwaters in Kandal province, Cambodia. Sci Total Environ 393:168–176
Gil F, Hernández AF, Márquez C, Femia P, Olmedo P, López-Guarnido O, Pla A (2011) Biomonitorization of cadmium, chromium, manganese, nickel and lead in whole blood, urine, axillary hair and saliva in an occupationally exposed population. Sci Total Environ 409:1172–1180
Gul N, Shah M, Khan S, Khattak N, Muhammad S (2015) Arsenic and heavy metals contamination, risk assessment and their source in drinking water of the Mardan district, Khyber Pakhtunkhwa, Pakistan. J Water Health 13:1073–1084
Huang M, Chen X, Shao D, Zhao Y, Wang W, Wong MH (2014) Risk assessment of arsenic and other metals via atmospheric particles, and effects of atmospheric exposure and other demographic factors on their accumulations in human scalp hair in urban area of Guangzhou, China. Ecotoxicol Environ Saf 102:84–92
Ishtiaq M, Jehan N, Khan SA, Muhammad S, Saddique U, Iftikhar B, Zahidullah (2018) Potential harmful elements in coal dust and human health risk assessment near the mining areas in Cherat, Pakistan. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-018-1655-5
Khan MU, Malik RN, Muhammad S (2013) Human health risk from heavy metal via food crops consumption with wastewater irrigation practices in Pakistan. Chemosphere 93:2230–2238
Khan S, Rauf R, Muhammad S, Qasim M, Din I (2016) Arsenic and heavy metals health risk assessment through drinking water consumption in the Peshawar District, Pakistan. Hum Ecol Risk Assess 22:581–596. https://doi.org/10.1080/10807039.2015.1083845
Khan SA, Khan H, Ishtiaq M, Saddique U, Muhammad S, Farooq M (2018) Shallow water proved higher levels of potentially harmful elements and human health risk along the Sadkal oil exploration and production. Desalin Water Treat https://doi.org/10.5004/dwt.2018.22385
Kidd P (2003) Colloid and clay minerals; latest nutrition fad. Total Health 19:1–41
Kippler M, Skröder H, Rahman SM, Tofail F, Vahter M (2016) Elevated childhood exposure to arsenic despite reduced drinking water concentrations—a longitudinal cohort study in rural Bangladesh. Environ Int 86:119–125
Knight C, Kaiser J, Lalor G, Robotham H, Witter J (1997) Heavy metals in surface water and stream sediments in Jamaica. Environ Geochem Health 19:63–66
Kumar M, Rahman MM, Ramanathan A, Naidu R (2016) Arsenic and other elements in drinking water and dietary components from the middle Gangetic plain of Bihar, India: health risk index. Sci Total Environ 539:125–134
Molina-Villalba I, Lacasaña M, Rodríguez-Barranco M, Hernández AF, Gonzalez-Alzaga B, Aguilar-Garduño C, Gil F (2015) Biomonitoring of arsenic, cadmium, lead, manganese and mercury in urine and hair of children living near mining and industrial areas. Chemosphere 124:83–91
Muhammad S, Shah MT, Khan S (2010) Arsenic health risk assessment in drinking water and source apportionment using multivariate statistical techniques in Kohistan region, northern Pakistan. Food Chem Toxicol 48:2855–2864
Muhammad S, Shah MT, Khan S (2011) Health risk assessment of heavy metals and their source apportionment in drinking water of Kohistan region, northern Pakistan. Microchem J 98:334–343
Omokhodion F, Crockford G (1991) Lead in sweat and its relationship to salivary and urinary levels in normal healthy subjects. Sci Total Environ 103:113–122
Rahman M et al (2015) Associations of total arsenic in drinking water, hair and nails with serum vascular endothelial growth factor in arsenic-endemic individuals in Bangladesh. Chemosphere 120:336–342
Rehman ZU, Khan S, Qin K, Brusseau ML, Shah MT, Din I (2016) Quantification of inorganic arsenic exposure and cancer risk via consumption of vegetables in southern selected districts of Pakistan. Sci Total Environ 550:321–329
Riaz A, Khan S, Muhammad S, Liu C, Shah MT, Tariq M (2018) Mercury contamination in selected foodstuffs and potential health risk assessment along the artisanal gold mining, Gilgit-Baltistan, Pakistan. Environ Geochem Health 40:625–635
Riojas-Rodríguez H, Solís-Vivanco R, Schilmann A, Montes S, Rodríguez S, Ríos C, Rodríguez-Agudelo Y (2010) Intellectual function in Mexican children living in a mining area and environmentally exposed to manganese. Environ Health Perspect 118:1465–1470
Robert G, Mari G (2003) Human health effects of metals. US Environmental Protection Agency Risk Assessment Forum, Washington
Rodríguez-Barranco M, Lacasaña M, Aguilar-Garduño C, Alguacil J, Gil F, González-Alzaga B, Rojas-García A (2013) Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. Sci Total Environ 454:562–577
Saddique U, Muhammad S, Tariq M, Zhang H, Arif M, Jadoon IA, Khattak NU (2018) Potentially toxic elements in soil of the Khyber Pakhtunkhwa province and tribal areas, Pakistan: evaluation for human and ecological risk assessment. Environ Geochem Health 1–14
Samanta G, Sharma R, Roychowdhury T, Chakraborti D (2004) Arsenic and other elements in hair, nails, and skin-scales of arsenic victims in West Bengal, India. Sci Total Environ 326:33–47. https://doi.org/10.1016/j.scitotenv.2003.12.006
Shah M, Ara J, Muhammad S, Khan S, Tariq S (2012) Health risk assessment via surface water and sub-surface water consumption in the mafic and ultramafic terrain, Mohmand agency, northern Pakistan. J Geochem Explor 118:60–67
Shakoor MB et al (2017) Human health implications, risk assessment and remediation of as-contaminated water: a critical review. Sci Total Environ 601–602:756–769. https://doi.org/10.1016/j.scitotenv.2017.05.223
Sheng J, Qiu W, Xu B, Xu H, Tang C (2016) Monitoring of heavy metal levels in the major rivers and in residents’ blood in Zhenjiang City, China, and assessment of heavy metal elimination via urine and sweat in humans. Environ Sci Pollut Res 23:11034–11045
Song Q, Li J (2015) A review on human health consequences of metals exposure to e-waste in China. Environ Pollut 196:450–461
Sukumar A, Subramanian R (2007) Relative element levels in the paired samples of scalp hair and fingernails of patients from New Delhi. Sci Total Environ 372:474–479. https://doi.org/10.1016/j.scitotenv.2006.10.020
Sun L, Wu Q, Liao K, Yu P, Cui Q, Rui Q, Wang D (2016) Contribution of heavy metals to toxicity of coal combustion related fine particulate matter (PM 2.5) in Caenorhabditis elegans with wild-type or susceptible genetic background. Chemosphere 144:2392–2400
Tripathee L, Kang S, Sharma CM, Rupakheti D, Paudyal R, Huang J, Sillanpää M (2016) Preliminary health risk assessment of potentially toxic metals in surface water of the Himalayan rivers, Nepal. Bull Environ Contam Toxicol 97:855–862
Ullah Z, Naz A, Saddique U, Khan A, Shah W, Muhammad S (2017) Potentially toxic elements concentrations and human health risk assessment of food crops in Bajaur agency, Pakistan. Environ Earth Sci 76:482
US EPA (1998) United states environmental protection agency. Integrated risk information system (IRIS)
USEPA (2005) (US Environmental Protection Agency), 2005. Guidelines for carcinogen risk assessment. Risk assessment forum, Washington, DC. EPA/630/P-03/001F
Wang T, Fu J, Wang Y, Liao C, Tao Y, Jiang G (2009) Use of scalp hair as indicator of human exposure to heavy metals in an electronic waste recycling area. Environ Pollut 157:2445–2451
Wang H, Han M, Yang S, Chen Y, Liu Q, Ke S (2011) Urinary heavy metal levels and relevant factors among people exposed to e-waste dismantling. Environ Int 37:80–85. https://doi.org/10.1016/j.envint.2010.07.005
Watanabe T et al (2000) Background lead and cadmium exposure of adult women in Xian City and two farming villages in Shaanxi Province, China. Sci Total Environ 247:1–13
WHO (2011) World Health Organization guidelines for drinking water quality, 4th edit. Recommendations, vol 1. Geneva
Wongsasuluk P, Chotpantarat S, Siriwong W, Robson M (2017) Using urine as a biomarker in human exposure risk associated with arsenic and other heavy metals contaminating drinking groundwater in intensively agricultural areas of Thailand. Environ Geochem Health 1–26
Xing R, Li Y, Zhang B, Li H, Liao X (2016) Indicative and complementary effects of human biological indicators for heavy metal exposure assessment. Environ Geochem Health 1–13
Zheng G, Xu X, Li B, Wu K, Yekeen TA, Huo X (2013) Association between lung function in school children and exposure to three transition metals from an e-waste recycling area. J Expo Sci Environ Epidemiol 23:67–72
Funding
Financial support was provided by the Higher Education Commission (HEC), Islamabad, Pakistan, technical support of University of Peshawar, Pakistan, Institute of Urban Environment, Chinese Academy of Sciences and anonymous reviewers for their time and inputs for quality improvement of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Rehman, U.u., Khan, S. & Muhammad, S. Associations of potentially toxic elements (PTEs) in drinking water and human biomarkers: a case study from five districts of Pakistan. Environ Sci Pollut Res 25, 27912–27923 (2018). https://doi.org/10.1007/s11356-018-2755-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2755-y