Abstract
In order to understand the effect of submergence on nutrient release of the reforested tree leaves and assess the environmental risk of leaf decomposition under submergence, the mass loss and nutrient release rates of three reforestation tree species, Taxodium ascendens Brongn, Taxodium distichum (L.) Rich., and Salix matsudana Koidz., at different elevation in the hydro-fluctuation zone of the Three Gorges Dam Reservoir (TGDR) region were tested in situ. Results showed that the initial macroelement contents of the leaves of the three tree species varied among different elevations due to different submergence stresses. All foliar mass loss rates of the three tree species at 165 m a.s.l. were significantly higher than that at 175 m a.s.l. (except that of S. matsudana at 165 m a.s.l.), after 179 days of incubation commenced September 20. After 138 days of incubation commenced October 5, the foliar mass loss rates of the three tree species at 170 m a.s.l. were significantly higher than that at 175 m a.s.l. Moreover, the leaf mass loss rates of S. matsudana were higher than the other two species when compared at the same elevation of the same incubation period. In addition, foliar release rates of N and Ca in T. ascendens, C, N, and Ca in T. distichum as well as Ca in S. matsudana at 165 m a.s.l. after 179 days of incubation and at 170 m a.s.l. after 138 days of incubation were significantly higher than that at 175 m a.s.l., respectively. Leaf mass loss rates of T. ascendens were significantly correlated with its initial leaf K, Ca, and Mg contents. In contrast, leaf mass loss rates of T. distichum had significant correlations with the initial leaf P and K contents, as well as C/P and N/P ratios. However, the mass loss rates of S. matsudana significantly correlated with initial leaf N, P, and Ca contents and C/N, C/P, and N/P ratios. Foliar nutrient release rates, especially the foliar release of C, N, and P of the three tree species, had significant correlations with initial leaf C/P and N/P ratios. Our results suggested that submergence facilitated the decomposition and nutrient release rates of the leaves of the three woody species, especially the broad leaves of S. matsudana, which may potentially cause secondary pollution to the water body of the TGDR. Thus, we suggested that a sustainable harvest of leaves of the reforested forest stands prior to submergence should be considered in the hydro-fluctuation zone so as to protect the water quality of the TGDR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Riparian plants are important component of river ecosystems, which can affect water quality by intercepting and absorbing nutrients of the surface runoff during exposure (Jordan et al. 1993; Daniels and Gilliam 1996; Dosskey et al. 2010; Peng et al. 2014) and by releasing nutrients of the decomposed plants during submergence (Wang et al. 2012a). The alterations of exposure and submergence of the hydro-fluctuation zone often make the riparian plants become the sink or source of the nutrients (e.g., carbon, nitrogen, and phosphorus), which may cause water quality degradation or even eutrophication of the reservoir.
This is especially the case in the Three Gorges Dam Reservoir (TGDR). The great water level change of the TGDR has formed a large area of hydro-fluctuation zone (Ye et al. 2011; Ma et al. 2012), which has an annual 30-meter of water level fluctuation from 145 m above sea level (a.s.l.) to 175 m a.s.l. and a 350 km2 drawdown zone in summer (Willison et al. 2013). This artificial water regime has brought about a significant change in eco-environment of the TGDR (Zhang and Lou 2011). Research has shown that the number of intense algal blooms increased significantly in its tributary backwaters (Liu et al. 2012). Eutrophication in the tributary backwater of the TGDR increased more than two times and became a prominent environmental threat during the time period from 2007 to 2010 (Xu et al. 2013) when compared to that during early years of the impoundment. Moreover, because the artificial hydrological regime of the TGDR also leads to a long-term periodic deep submergence to this hydro-fluctuation zone, most riparian plants cannot endure these great water level changes and die gradually (Wang et al. 2012b). Consequently, death of the intolerant riparian plants of the TGDR causes the eco-environment degradation of this region seriously and thus the function decline of the riparian ecosystem correspondingly (Langer et al. 2008), which significantly threatens the safety operation of the TGDR. Former studies have shown that riparian vegetation restoration is an important measure in improving and protecting the eco-environment of the hydro-fluctuation zone of the TGDR (Ye et al. 2012; Peng et al. 2014). Currently, there is quite some promising revegetation available in the hydro-fluctuation zone of the TGDR (Willison et al. 2013; Ye et al. 2012). However, when more and more restored vegetation is being established, little attention has been paid to leaf decomposition and nutrient releasing of those restored vegetation during submergence (Kröger et al. 2007), which may both directly and indirectly lead to water eutrophication of the TGDR.
Taxodium distichum, Taxodium ascendens, and Salix matsudana, three deciduous tree species with strong flooding resistance ability (Li et al. 2010; Yang et al. 2014; Zhang et al. 2015), have already been extensively used in vegetation restoration of the upper portion (165 to 175 m a.s.l.) of the hydro-fluctuation zone of the TGDR and survived very well in this region (Wang et al. 2016a). Although these plants have high flooding endurance abilities, their leaves will shed and rot inevitably under long-term deep submergence. As investigation on water environment of the TGDR showed that water quality degradation has been of the main environmental concern in the TGDR currently (Fu et al. 2010; Huang et al. 2016; Ren et al. 2016), massive nutrients, such as N and P, released by decomposition of the leaves of reforested trees in the hydro-fluctuation zone of the TGDR area, may have become a crucial source to deteriorate its water quality (Wang et al. 2012a; Yuan et al. 2013). Thereby, if these tree species are continuously planted in the upper portion (all of which performing not well if planted under 165 m a.s.l.) of the hydro-fluctuation zone of the TGDR, large amount of nutrients potentially released by their leaf decomposition may aggravate the deterioration of water quality. Therefore, estimating the nutrient release of the three dominant tree species will have a great influence on prediction of water quality deterioration in the TGDR. However, the nutrient release characteristics of these tree species are still not known.
Previous studies have observed that chemical nature and nutritional quality of leaves are important factors controlling breakdown rates of leaves in streams (Alvim et al. 2015). The leaves with low contents of structural macromolecules (lignin and cellulose) and defense compounds (polyphenols and tannins) as well as with high concentrations of nitrogen and phosphorus are more susceptible to microbial colonization, thus leading to a higher decomposition rate (Kalburtji et al. 1999; Das et al. 2008; Zhang et al. 2014; Alvim et al. 2015; van Diepen et al. 2016). In addition, broad, soft, or green leaves have faster breakdown rate than coniferous, tough, or senescence leaves (Fonte and Schowalter 2004; Li et al. 2009; Wang et al. 2016b) usually through enhancing microbial activity, which may exert more stress on water quality. Besides, the hydrological regimes also have a vital effect on leaf decomposition rate (Neckles and Neill 1994; Sun et al. 2012). Different water depth often results in various physicochemical conditions, which in turn regulates the leaf decomposition (Xie et al. 2017).
Studies on decomposition of some herb plants in the hydro-fluctuation zone of the TGDR have showed that submergence could accelerate the decomposition rate (Chen and Jiang 2006; Wang et al. 2012a; Zhang et al. 2010) and increase the nutrient release of these herb species (Wang et al. 2012a; Xiao et al. 2017). However, significant differences in decomposition and nutrient release were found among different species (Albariño and Balseiro 2002; Xiao et al. 2017). Due to the variation in elevation and initial leaf nutrient content between herbs and trees (Xiao et al. 2017), decomposition and nutrient release of the leaves of the trees may be changed as compared to that of the herbs (Rincón and Covich 2014). More importantly, as the overall biomasses of the trees are usually higher than that of the herbs under the same growing conditions in the riparian zone, the leaves of the trees will be expected to release more nutrients into the water during submergence. Thus, our research studied the foliar decomposition of the three reforestation tree species at different elevation of the upper portion of the hydro-fluctuation zone of the TGDR. The main objectives of this study were to (1) investigate leaf decomposition rate of the three tree species in the hydro-fluctuation zone of the TGDR and (2) evaluate foliar nutrient release from the three tree species.
Methods
Site description
The experiment was conducted in an artificial revegetation site with an area of 5.7 ha in Ruxi River basin of the TGDR in Zhong County, Chongqing Municipality of China (30°24′ 16″~30° 24′ 56″ N, 108° 08′ 03″~108° 08′ 21″ E). This region belongs to subtropical southeast monsoonal climate. The annual mean temperature is 18.2 °C, and precipitation is 1200 mm. The relative humidity of this region is about 80%. The soil is classified as purple soil (Regosols in FAO Taxonomy or Entisols in USDA Taxonomy). The water level of the reservoir in Zhong County begins to rise from 145 to 175 m a.s.l. in late autumn (September–October) each year and keeps the highest water level for about 2 months (November–December). After that period, the water level begins to drop gradually from 175 to 145 m a.s.l. in a longer time period (January–May) and then keeps the lowest water level until the end of September.
Experimental design
The foliar decomposition experiment was conducted in situ in a mixed riparian forest at elevations of 165, 170, and 175 m a.s.l., dominated by 5-year-old saplings of Taxodium ascendens, Taxodium distichum, and Salix matsudana.
In September 2015 when the 165 m a.s.l. of the hydro-fluctuation zone of the TGDR was submerged, the first batch of leaf samples at elevations of 175, 170, and 165 m a.s.l. was prepared. Ten trees of T. ascendens of the same growth condition were selected at each of three elevations of the study site and their fresh leaves were collected and mixed together for further weighting. Meanwhile, the leaf samples of the other two species were also prepared following the same method as above. For each species, 10.00 g fresh leaves were weighed and put into a litter bag measuring 20 cm × 20 cm with a mesh size of 0.25 mm. There were in total 20 litter bags per species prepared for each elevation, of which 10 bags were put in envelops and brought to the lab to test the initial dry weight and macroelement content while the other 10 bags were fastened in situ on the soil surface of each elevation with long stainless steel nails. These litter bags at each elevation were taken back to the lab when 165 m a.s.l. was exposed.
In early October, when the 170 m a.s.l. of the hydro-fluctuation zone of the TGDR was submerged, the second batch of fresh leaf samples was prepared. The same former method was used for preparing fresh leaf samples and litter bags and fixing leaf litter bags of these three species at elevations of 175 and 170 m a.s.l. These litter bags at the two elevations were taken back to the lab for further test when 170 m a.s.l. was exposed.
In late October, when the 175 m a.s.l. of the hydro-fluctuation zone of the TGDR was submerged, the third batch of fresh leaf samples was prepared. Fresh leaf collecting, weighting and litter bag fixing of these three species at elevation of 175 m a.s.l. were again conducted using the same method as before. These litter bags were taken back to the lab for further analysis when 175 m a.s.l. was exposed.
During the whole incubation period, the temperature (Fig. 1) and water level change were recorded. The incubation and submergence time of each batch in each elevation were shown in Table 1. After the litter bags were taken back to the lab, they were quickly cleaned with tap water to remove the soil and other impurities adhering outside the litter bag. Then the litter was dried to constant weight in drying oven at 80 °C for 72 h after cleaned carefully with ultrapure water.
Litter chemical analysis
The dry leaf samples were ground to powder, and the initial (the commencement of the treatment) and final (end of the treatment) macroelement contents of C, N, P, potassium (K), calcium (Ca), and magnesium (Mg) were tested. The content of C and N were determined using a CHNS-O elemental analyzer (CHNS-O-Vario EL cube, Heraeus Elementar, Hanau, Germany). The contents of other elements were tested using inductively coupled plasma mass spectrometry (ICP-OSE) after heat digestion with a nitric acid and hydrogen peroxide mixture in a microwave digestion system (SpeedWave MWS-4).
Data computation
The mass loss rate of leaf samples was calculated by the following formula:
Mass loss rate = (M0 − Mt) / M0 × 100
In which, M0 is the initial dry weight of the fresh leaf, and Mt is the final dry weight of the fresh leaf.
The macroelement release rate of leaf samples was calculated using the following formula:
Macroelement release rate = (M0C0 − MtCt) / M0C0 × 100
In which, C0 is the initial element content of the fresh leaf, and Ct is the final element content of the fresh leaf.
Statistical analysis
One-way ANOVA was performed to reveal the variation on mass loss and nutrient release rate among elevations of leaf samples of the first batch. Independent t test was used to reveal the variation on mass loss and nutrient release rate between elevations of leaf samples of the second batch. Pearson’s correlation analysis was used to test the relationships among the initial nutrient content, mass loss and nutrient release rate of each species. All the processes were performed using SPSS 19.0 (IBM, Chicago, USA) for windows. Figures were conducted by Origin 8.0. Data in the text were shown as mean ± standard error (SE).
Results
Initial macroelement content of leaf samples
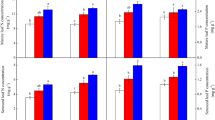
Initial foliar macroelement contents of the three tree species were shown in Fig. 2. The leaf macroelement contents of each species varied among the three elevations. In the first batch, leaf samples of T. ascendens and T. distichum from 165 and 170 m a.s.l. had significantly higher C content than that from 175 m a.s.l. (all p < 0.001, Fig. 2 A1 and B1). However, the first batch leaf samples of S. matsudana from 165 and 170 m a.s.l. displayed significantly lower C content when compared to that from 175 m a.s.l. (both p < 0.001, Fig. 2 C1). Leaf samples of T. ascendens in the first batch also had significantly higher N and Mg contents at both lower elevations of 165 and 170 m a.s.l. (except the Mg content at 170 m a.s.l.), as compared to that at 175 m a.s.l., respectively (both p < 0.001 for N, p < 0.05 for Mg, Fig. 2 A2 and A6). In contrast, the leaf samples of both T. distichum and S. matsudana in the first batch exhibited significantly lower N content at 170 m a.s.l. but comparable N content at 165 m a.s.l., when compared to that at 175 m a.s.l., respectively (170 m a.s.l., both p < 0.001; 165 m a.s.l., both p > 0.05; Fig. 2 B2 and C2). However, the leaf Mg content of the first batch decreased significantly in T. distichum in contrast to a significant increase in S. matsudana at both 165 and 170 m a.s.l., when compared to that of 175 m a.s.l., respectively (both p < 0.01 for T. distichum, p < 0.001 for S. matsudana, Fig. 2 B6 and C6). Moreover, leaf samples of the three species at both 165 and 170 m a.s.l. never demonstrated significantly higher P, K, and Ca contents as compared to that at 175 m a.s.l., respectively (Fig. 2 A3, B3, C3, A4, B4, C4, A5, B5, and C5). In the first batch, the foliar C/N ratio of T. ascendens decreased significantly with elevation decline (p < 0.001, Fig. 2 A7), which was contrary to its C/P and N/P ratios change. But, leaf samples of both T. distichum and S. matsudana in the first batch always displayed their highest foliar C/N, C/P, and N/P ratios at the elevation of 170 m a.s.l. (all p < 0.001, Fig. 2 B7, B8, B9, C7, C8, and C9).
In the second batch, leaf samples of the three species from 170 m a.s.l. showed significantly higher C content than that from 175 m a.s.l. (all p < 0.001, Fig. 2 A1, B1, and C1). Foliar N content of T. ascendens in the second batch was not significantly changed between 170 and 175 m a.s.l. (p > 0.05, Fig. 2 A2), in contrast to a 47.12% significantly higher foliar Mg content at 170 m a.s.l. as compared to that at 175 m a.s.l. (p < 0.001, Fig. 2 A6). On the contrary, leaf samples of T. distichum and S. matsudana in the second batch had significantly lower N and Mg contents at 170 m a.s.l. when compared to that at 175 m a.s.l. (both p < 0.05 for T. distichum, both p < 0.01 for S. matsudana, Fig. 2 B2, C2, B6, and C6). Leaf K content of T. ascendens and S. matsudana at 170 m a.s.l. was significantly higher than that at 175 m a.s.l. (p < 0.01 for T. ascendens and p < 0.001 for S. matsudana, Fig. 2 A4 and C4), whereas there was no significant difference in foliar K content between the two elevations in T. distichum (p > 0.05, Fig. 2 B4) in the second batch. Furthermore, leaf samples of T. ascendens had comparable P content between both 170 and 175 m a.s.l. (p > 0.05, Fig. 2 A3), in contrast to a 134.69% significantly higher foliar P content in T. distichum and 42.16% significantly lower foliar P content in S. matsudana at 170 m a.s.l. compared to that at 175 m a.s.l., respectively (both p < 0.001, Fig. 2 B3 and C3). In the second batch, foliar Ca content at 170 m a.s.l. was significantly lower in T. ascendens (p < 0.001, Fig. 2 A5) but being significantly higher in T. distichum (p < 0.05, Fig. 2 B5), which was in contrast to having no significant difference in S. matsudana (p > 0.05, Fig. 2 C5), when compared to that at 175 m a.s.l., respectively. The second batch foliar C/N, C/P, and N/P ratios of T. ascendens at the elevation of 170 m a.s.l. were comparable to that at 175 m a.s.l. (all p > 0.05, Fig. 2 A7, A8, and A9). However, the foliar C/N, C/P and N/P ratios of S. matsudana at the elevation of 170 m a.s.l. were significantly higher than that at 175 m a.s.l. in the second batch (all p < 0.001, Fig. 2 C7, C8, and C9). Although the second batch foliar C/N ratio of T. distichum at the elevation of 170 m a.s.l. was significantly higher, the foliar C/P and N/P ratios were significantly lower at the elevation of 170 m a.s.l., when compared to that at 175 m a.s.l., respectively (all p < 0.05, Fig. 2 B7, B8, and B9).
In the third batch, foliar C content of T. ascendens at the elevation of 175 m a.s.l. was significantly higher than that in the first batch (p < 0.001), but comparable to that in the second batch (p > 0.05, Fig. 2 A1). In contrast, foliar C content of T. distichum at 175 m a.s.l. in the third batch was significantly higher than that in both the first and second batch (both p < 0.01, Fig. 2 B1), whereas foliar C content of S. matsudana at 175 m a.s.l. in the third batch was significantly lower when compared to that in the first batch but significantly higher when compared to that in the second batch (both p < 0.001, Fig. 2 C1). Likewise, leaf samples of T. ascendens from 175 m a.s.l. in the third batch showed significantly higher N content as compared to that in both the first and second batch (both p < 0.001, Fig. 2 A2). But, in contrast, leaf samples of T. distichum and S. matsudana in the third batch did not exhibit significantly higher N content when compared to that in the first batch and second batch, respectively (Fig. 2 B2 and C2). Furthermore, the leaf Mg content of the three species at 175 m a.s.l. in the third batch had no significant change as compared to that in the second batch (all p > 0.05), which was contrary to a significant decrease in both T. ascendens and T. distichum and a significant increase in S. matsudana in foliar Mg content in the third batch when compared to that in the first batch (all p < 0.001, Fig. 2 A6, B6, and C6). Foliar P content of T. ascendens at 175 a.s.l in the third batch was significantly lower than that in the first batch but significantly higher than that in the second batch (both p < 0.001, Fig. 2 A3). However, no significant difference was detected in foliar K and Ca contents at 175 a.s.l. between the first and third batch as well as between the second and third batch (all p > 0.05, Fig. 2 A4 and A5). Furthermore, leaf samples of T. distichum at 175 a.s.l. in the third batch never exhibited significantly higher P, K, and Ca contents as compared to that in the first and second batches, respectively (Fig. 2 B3, B4, and B5). Although leaf samples of S. matsudana at 175 a.s.l. in the third batch did not display significantly higher P and K contents compared to that in the first and second batch, respectively (Fig. 2 C3 and C4), its foliar Ca content in the third batch was significantly higher than that in the first batch (p < 0.001) but comparable to that in the second batch (p > 0.05, Fig. 2 C5). The foliar C/N ratio in T. ascendens and S. matsudana at the elevation of 175 a.s.l. in the third batch was not significantly changed when compared to that in the second batch (both p > 0.05), but on the contrary, having a significant change when compared to that in the first batch (both p < 0.001, Fig. 2 A7 and C7). Moreover, the foliar C/N ratio in T. distichum at 175 a.s.l. in the third batch was comparable to that in the first batch (p > 0.05), but significantly higher than that in the second batch (p < 0.01 Fig. 2 B7). The foliar C/P and N/P ratios in T. ascendens at 175 a.s.l. in the third batch were significantly higher than that in the first batch but lower than that in the second batch (all p < 0.001, Fig. 2 A8 and A9). In contrast, the foliar C/P and N/P ratios in T. distichum at 175 a.s.l. in the third batch were significantly higher than that in both the first and second batch (all p < 0.001, Fig. 2 B8 and B9). Moreover, the foliar C/P and N/P ratios in S. matsudana at 175 a.s.l. in the third batch were also significantly higher than that in the second batch (both p < 0.001), but when compared to that in the first batch, the third batch foliar C/P ratio had no significant change while the foliar N/P ratio demonstrating a significant decrease (C/P ratio, p > 0.05; N/P ratio, p < 0.001; Fig. 2 C8 and C9).
Leaf mass loss of three tree species
In the first batch, the leaf mass loss rates of T. ascendens, T. distichum, and S. matsudana were significantly different among the three elevations (p < 0.001, 0.001, and 0.01, respectively, Fig. 3 A, B, and C). The first batch mass loss rate of T. ascendens and T. distichum at 165 m a.s.l. were significantly higher than that at 170 and 175 m a.s.l. (p < 0.01 and 0.05 for T. ascendens, both p < 0.001 for T. distichum, respectively), with a mass loss rate of 52.21 ± 2.79% at 165 m a.s.l. in T. ascendens and 59.83 ± 2.57% at 165 m a.s.l. in T. distichum. In the meantime, the leaf mass loss rate of T. ascendens at 170 m a.s.l. in the first batch was significantly lower than that at 175 m a.s.l. (p < 0.01). In contrast, no significant difference was found in leaf mass loss rate of T. distichum between 170 and 175 m a.s.l. in the first batch (p > 0.05, Fig. 3 B). However, the leaf mass loss rate of S. matsudana at 170 m a.s.l. in the first batch was significantly increased (p < 0.01), in contrast with its insignificant change at 165 m a.s.l (p > 0.05, Fig. 3 C), when compared to that at 175 m a.s.l.
In the second batch, the leaf mass loss rates across the three species were significantly higher at 170 m a.s.l. than that at 175 m a.s.l. (both p < 0.001 for T. ascendens and T. distichum, p < 0.01 for S. matsudana).
In the third batch, the mass loss rate of T. distichum at 175 m a.s.l. was the smallest among all groups, with a mass loss rate of 27.05 ± 2.2%. However, the leaf mass loss rate of S. matsudana at 175 m a.s.l. in the third batch was significantly higher than that of the other two species, with a mass loss rate about 62.4 ± 2.39% (both p < 0.001, Fig. 3 A, B, and C).
Leaf nutrient release of three tree species
In the first batch, the foliar C release rate of T. ascendens was not significantly different among the three elevations (p > 0.05, Fig. 4 A1). However, the foliar release rates of N, K, Ca, and Mg of T. ascendens at 165 m a.s.l were significantly higher than that at both 170 and 175 m a.s.l., respectively (N, p < 0.01; K and Ca, p < 0.001; Mg, p < 0.05; Fig. 4 A2, A4, A5, and A6). On the contrary, the foliar P release rate of T. ascendens at 165 m a.s.l. was significantly lower than that at 170 and 175 m a.s.l. (both p < 0.001, Fig. 4 A3). Very interestingly, there was no significant difference between 170 and 175 m a.s.l. in the foliar release rates of N, P, Ca, and Mg in T. ascendens in the first batch (all p > 0.05, Fig. 4 A2, A3, A5, and A6). The foliar release rates of C, N, Ca, and Mg of T. distichum at 165 m a.s.l. in the first batch were also significantly higher than that at both 170 and 175 m a.s.l. (except Mg at 170 m a.s.l.), respectively (C, p < 0.05; N and Ca, all p < 0.001; Mg, p < 0.01), in which no significant difference was detected between 170 and 175 m a.s.l. in the foliar release rates of these macroelements (all p > 0.05, Fig. 4 B1, B2, B5, and B6). Although the foliar P and K release rates of T. distichum at 165 m a.s.l. in the first batch were not significantly changed, significantly lower foliar P and K release rates of this species at 170 m a.s.l. were found, when compared to that at 175 m a.s.l. (165 m a.s.l., both p > 0.05; 170 m a.s.l., p < 0.001, 0.05, respectively; Fig. 4 B3 and B4). As leaf samples of S. matsudana showed almost the same change trend as that of T. distichum among the three elevations in foliar P and K release rates in the first batch, other foliar release rates of C, N, Ca, and Mg of this species were to some extent different to that of T. distichum. Leaf samples of S. matsudana had the highest C release rate at 170 m a.s.l. (p < 0.01, Fig. 4 C1) among the three elevations. Although leaf samples of S. matsudana had almost identical foliar N release rate among the three elevations (p > 0.05, Fig. 4 C2), foliar Ca and Mg release rates of this species at both 165 and 170 m a.s.l. were significantly augmented when compared to that at 175 m a.s.l., respectively, in the first batch (165 m a.s.l., p < 0.001; 170 m a.s.l., p < 0.01; Fig. 4 C5 and C6).
Leaf nutrient release rate of C (1), N (2), P (3), K (4), Ca (5), and Mg (6) of T. ascendens (A), T. distichum (B), and S. matsudana (C) at different elevation of the hydro-fluctuation zone of the TGDR. Different letters indicate significant differences between or among elevations in each batch (α = 0.05)
In the second batch, foliar C, N, and Ca release rates of the three species at 170 m a.s.l. were significantly increased as compared to that at 175 m a.s.l., respectively (all p < 0.001, Fig. 4 A1, A2, A5, B1, B2, B5, C1, C2, and C5). However, there were no significant difference in foliar P, K, and Mg release rates between the two elevations respectively in T. ascendens and S. matsudana in the second batch (except Mg of S. matsudana) (all p > 0.05, Fig. 4 A3, A4, A6, C3, C4, and C6), whereas leaf samples of T. distichum showed significantly higher foliar P and K as well as identical foliar Mg release rates at 170 m a.s.l., as compared to that at 175 m a.s.l., respectively (P, p < 0.001; K, p < 0.05; Mg, p > 0.05; Fig. 4 B3, B4, and B6).
In the third batch, foliar macroelement release rates of S. matsudana at 175 m were significantly higher than that of the other two species at the same elevation (all p < 0.001, Fig. 4). However, there had no significant difference in foliar C, N, K, and Mg release rates at 175 m a.s.l. between T. ascendens and T. distichum in the third batch (all p > 0.05, Fig. 4 A1, A2, A4, A6, B1, B2, B4, and B6). On the contrary, the P release rate of T. ascendens was significantly lower than that of T. distichum (p < 0.05, Fig. 4 A3 and B3), while the Ca release rate of T. ascendens was significantly higher than T. distichum in the third batch (p < 0.001, Fig. 4 A5 and B5).
Overall, in the first batch, the leaves of the three tree species released an average of 267.46 ± 4.48 mg/g of C, 7.69 ± 0.51 mg/g of N, 1.02 ± 0.05 mg/g of P, 7.08 ± 0.35 mg/g of K, 11.96 ± 0.55 mg/g of Ca, and 0.54 ± 0.04 mg/g of Mg. In the second batch, the leaves of the three tree species released an average of 291.67 ± 9.48 mg/g of C, 7.55 ± 0.63 mg/g of N, 1.61 ± 0.24 mg/g of P, 5.52 ± 0.18 mg/g of K, 12.72 ± 0.68 mg/g of Ca, and 0.56 ± 0.06 mg/g of Mg. But, in the third batch, the leaves of the three tree species released an average of 252.99 ± 6.86 mg/g of C, 4.25 ± 0.74 mg/g of N, 0.57 ± 0.07 mg/g of P, 3.59 ± 0.29 mg/g of K, 11.66 ± 1.2 mg/g of Ca, and 0.49 ± 0.11 mg/g of Mg.
Correlations
The leaf mass loss rate of T. ascendens had no significant correlation with initial C, N, and P contents as well as C/N, C/P, and N/P ratios (Table 2). In contrast, it was significantly correlated with foliar initial contents of K, Ca, and Mg. Unlike in T. ascendens, the leaf mass loss rate of T. distichum had significant correlation with initial P and K contents as well as C/P and N/P ratios of the leaves. In contrast to the former two conifer species, the leaf mass loss rate of S. matsudana had a significantly negative correlation with foliar initial N, P, and Ca contents and significantly positive correlation with C/N, C/P, and N/P ratios.
Furthermore, the foliar macroelement release rates of the three species (except for the foliar K of S. matsudana) had significant correlations with their mass loss rates (Table 3).
The leaf release rates of C, N, and P of T. ascendens had significant correlations with its initial P, Ca, and Mg contents as well as C/P and N/P ratios. Meanwhile, the leaf release rates of K, Ca, and Mg of this species significantly correlated with the initial Mg content. However, the release rates of C, N, and P of the leaves of T. distichum had significant correlations with its initial P content and C/P and N/P ratios. In contrast with the former two conifer tree species, the foliar release rates of C, N, and P S. matsudana had significant correlations with its initial P and Ca contents as well as C/P and N/P ratios. Yet, the release rates of K, Ca, and Mg of this species significantly correlated with initial K and Mg contents of the leaves (Table 4).
Discussion
Mass loss of tree leaves under water
Previous studies have found that leaf traits (e.g., chemical composition and texture), environmental factors (e.g., water flow velocity, dissolved oxygen and water temperature), and aquatic organisms (e.g., detritivorous fresh water macroinvertebrates, bacteria, and fungi) exert a strong influence on plant decomposition in many aquatic ecosystems (Bruder et al. 2014; Fernandes et al. 2014; Zhu et al. 2016). Of all the above-mentioned influence factors, leaf chemical composition plays a vital role in controlling leaf decomposition (Zanne et al. 2015; Liu et al. 2017). In this study, the foliar initial nutrient contents of the three tree species among the three elevations were to some extent different (Fig. 2), which might be mainly attributed to different submergence conditions induced by the periodic impoundment of the TGDR across different elevations (Jie et al. 2012; Ma et al. 2017). Such different foliar initial nutrient contents directly reflected the variance of leaf quality and thus affected the leaf palatability to invertebrates (Hisabae et al. 2011; Tonin et al. 2014), which could then further influence the leaf decomposition. For example, high N and P concentration in leaf could increase the leaf palatability to invertebrates (Graça and Cressa 2010; Hisabae et al. 2011; Tonin et al. 2014), increasing the leaf decomposition rate and resulting in higher pollution (e.g., water eutrophication). Moreover, different foliar initial nutrient contents also played a vital role in affecting the microbial assemblage and activity related to leaf decomposition (Zhang et al. 2008). Such different foliar initial nutrient contents may thus exert differing influence on leaf decomposition of these tree species in the hydro-fluctuation zone of the TGDR.
In terrestrial conditions, plant decomposition is also controlled by both biotic and abiotic factors (Berg 2000), such as leaf quality, temperature change, water availability, and types of decomposers, and so on (Wieder et al. 2009; Fan et al. 2014). For instance, leaf with higher N and P contents and lower C content showed a fast decomposition under terrestrial conditions (Fonte and Schowalter 2004). Besides, higher temperature and precipitation could also cause a fast decomposition of the leaves in terrestrial conditions (Fan et al. 2014). When compared to the terrestrial conditions, a number of different factors under aquatic conditions such as temperature range, water availability, physical abrasion, and dominant detritivores, may impose stronger effects on leaf decomposition both directly and indirectly (Graça et al. 2015; Yue et al. 2016). For example, stronger leaching and flushing effects of the lotic stream led to a higher physical fragmentation of the litter than that under terrestrial conditions, which could thus contribute to litter decomposition rate (Wallis and Raulings 2011). Therefore, the reason about why the decomposition rate of leaf litter under aquatic conditions is higher than that of leaf litter under terrestrial conditions (Vitousek et al. 1994; Kelley and Jack 2002; Chen and Jiang 2006; Yue et al. 2016) may be mainly due to lack of adequate water in terrestrial conditions. Inadequate water supply in terrestrial conditions can limit the growth and development of decomposers while restricting the leaching of soluble chemical compounds during the leaf decomposition phase (Austin and Vitousek 2000; Taylor et al. 2004).
In our study, the ratios of non-submergence days to submergence days during the leaf decomposition period of the first batch were 1.56 (109/70) for 175 m a.s.l., 0.30 (41/138) for 170 m a.s.l., and 0 (0/179) for 165 m a.s.l., respectively (Table 1). The ratios of non-submergence days to submergence days of the second batch were 0.97 (68/70) for 175 m a.s.l. and 0 (0/138) for 170 m a.s.l., respectively (Table 1). The foliar mass loss rates of each of the three tree species both at 165 m a.s.l. in the first batch (except that of S. matsudana) and at 170 m a.s.l. in the second batch were significantly higher than that at 175 m a.s.l. (Fig. 3 A, B, and C), indicating that a deeper and longer submergence at the lower elevation could facilitate the leaf decomposition in the hydro-fluctuation zone of the TGDR. Previous studies also found that mass and carbon declined more rapidly in the litter at fully submerged sites than at dry sites in an ephemeral karst lake in south-central Kentucky (Kelley and Jack 2002). In general, soil water content was the decisive factor determining the activity of decomposer invertebrates as well as the composition and abundance of the soil fauna in fields (Schmidt et al. 2016). In the TGDR, the submergence depth and days increased with elevation decrease. That means the depth and duration of submergence of the leaves at the lower elevation were deeper and longer than that of the leaves at the higher elevation (Table 1). As different flooding usually imposes distinct effects on litter decomposition (Sun et al. 2012), longer and deeper submergence may thus be one of the main reasons to have caused the higher mass loss of the leaves of the three tree species at lower elevations in our study. Besides, the water temperature varied when the leaf samples from each elevation were submerged, with water temperature at lower elevation being relatively higher than that at the upper elevation in each batch (Fig. 1). Higher water temperature could increase the activity of fungal decomposers (Fernandes et al. 2014; Martínez et al. 2014). Because this stimulation effect on fungal biomass and sporulation occurred in both high and low nutrient level (Ferreira and Chauvet 2015), relatively higher water temperature may be another vital factor facilitating the mass loss of leaves from lower elevation.
Some documents showed that water velocity has an effect on leaf decomposition through changing the refractory fractions into more labile compounds by abrasion (Fonseca et al. 2013). During leaf decomposition process, the mass loss of the leaves is overall the greatest at the first month (Xiao et al. 2017). Moreover, the foliar mass loss is also more sensitive to environmental factors, such as UV radiation (Bosco et al. 2016), nutrients availability (Goncalves et al. 2007; Jacobson et al. 2011), forest stand characteristics and microclimate (Trogisch et al. 2016). In the hydro-fluctuation zone of the TGDR, the higher foliar mass loss rates of the three tree species at the lower elevations may be attributed to the combined actions of these factors.
Foliar decomposition rate is not only species specific but also dependent on the initial chemical composition of leaves, particularly the foliar C/N ratio (Mfilinge et al. 2002; Pettit et al. 2012). Kandelia candel leaves with lower initial C/N ratio (higher N concentration) decayed faster than Bruguiera gymnorrhiza leaves (Mfilinge et al. 2002). However, in our present study, the foliar mass loss rates of T. ascendens and T. distichum had no significant correlation with the initial contents of C and N and the ratio of C/N, while the foliar mass loss rate of S. matsudana had a negative correlation with its initial N content and a positive correlation with initial C/N ratio. Such different findings in our current study might be most likely due to different plant species with distinct leaf traits on one hand and completely different experiment conditions on the other hand. But, our findings related to the foliar mass loss rate of S. matsudana are consistent with Berg et al. (1996), who found a negative correlation between leaf decomposition and N concentration. Lee and Bukaveckas (2002) also found that the litter decomposition rate of Typha latifolia during fall in wetland showed a significantly positive correlation with foliar C/N ratio. Furthermore, in our present study, the difference of the foliar initial C/N ratio of each species across the three elevations may cause our results of the correlation studies differently. Moreover, the great variation of the hydrological condition at each elevation may also affect the leaf decomposition of the three tree species. However, the inhibition of higher C/N ratio on litter decomposition may be offset by the high availability of N in swamp water (Yang et al. 2006). Some former studies showed that the litter decomposition rate had a significant correlation with the P concentration and C/P ratio (Liu et al. 2008; Shao et al. 2014). This phenomenon also showed in our current study in leaves of both species of T. distichum and S. matsudana (Table 2). The fact that the P content restricted the foliar mass loss more than N content due to the lower P content in leaves, may be responsible for this result (Molinero et al. 1996).
In this study, all the foliar mass loss of S. matsudana in each of the three batches was significantly higher than that of T. ascendens and T. distichum, suggesting that broader leaf decomposed faster than narrow and coniferous leaves in the hydro-fluctuation zone of the TGDR. Many studies have already reported the same results (Zhang et al. 2008; Demessie et al. 2012) as what we found in our present study. Leaves of T. ascendens and T. distichum may have some chemicals such as tannin that decreased the decomposition through inhibiting the colonization of benthonic invertebrates (Guan et al. 2009). Therefore, enough attention should be paid to the effects of leaf decomposition of S. matsudana on potentially increasing eutrophication of the river water in the TGDR.
Nutrient release of tree leaves under water
In terrestrial conditions, some leaves had short or even no leaching phase, as is especially the case for some conifer leaves (Berg 2000). Thereby, the pattern of the nutrient change in the leaf depends on whether theses nutrient contents could satisfy the need of colonization and growth of microbial decomposers (Schneider et al. 2012). If foliar nutrient was not enough to microbial decomposers under terrestrial conditions, they will immobilize the nutrients from the abiotic terrestrial environment. On the contrary, the foliar nutrient will show a net release pattern if there are sufficient foliar nutrient to the decomposers in terrestrial conditions. This phenomenon was also found under aquatic conditions (Liu et al. 2008). Besides, nutrient release also correlated with the existing form and location of the nutrient (Wang et al. 2012c). Besides the leaf traits, environmental factors that affected the leaf decomposition could also affect the nutrient release by indirect influence on the abundance, assemblage, and activity of the decomposers (Teklay 2007; Zanne et al. 2015). Among these factors, oxygen condition differed greatly between terrestrial and aquatic conditions, thus affecting the decomposers differently under both conditions (Cai 2000).
In this study, nutrient release rates of the leaves of the three tree species varied significantly among the three elevations (Fig. 4). The release rate of K in fresh leaves of the three species in the first batch was higher than the other nutrients, because potassium generally existed as metal ion, and thus being more easily released (Jacobson et al. 2011). The macroelement release rates in leaves of T. ascendens increased at lowest elevation of 165 m a.s.l. in the first batch (except C and P) and at 170 m a.s.l. in the second batch (except P, K, and Mg) (Fig. 4 A), further indicating that deeper and longer submergence may to some degree selectively stimulate the foliar macroelement release. Likewise, similar phenomena were also found in the leaves of both T. distichum and S. matsudana in terms of the foliar macroelement release rates under submergence in our present study. The reason about why such selective stimulation effect of submergence on the foliar macroelements release differed was due to the species-specific leaf traits, environmental conditions, and selective-breeding aquatic decomposers (Xie et al. 2016). Moreover, our result demonstrated that long-term deeper submergence in the hydro-fluctuation zone of the TGDR significantly increased the leaf nutrient N release of the three reforestation tree species (except foliar N of S. matsudana in the first batch). This finding from our current study was not the same as for litter decomposition at different elevation of the Xiangxi River of the TGDR (Liu et al. 2008), where variation in elevation had no significant influence on N release of leaf litter of Cotinus coggygria, Acer oliverianum, and Acer flabellatum. By contrast, Shieh et al. (2008) found that the nutrient in leaves of Machilus thunbergii, Schefflera octophylla, and Ficus erecta released rapidly in a subtropical stream of northern Taiwan of China. Former studies found that leaf nutrient release varied with the species and was associated with the leaf chemical content (Xie et al. 2016; Xiao et al. 2017). If the leaf N content is enough for the need of the decomposers, the decomposers will not need to uptake N from the outside environment (Xie et al. 2004; Xie et al. 2016). Parton et al. (2007) studied the N change in leaf litter of many plant species in a decade and found that net N release of the leaves only happened in conditions with foliar C/N ratio being lower than 40. However, Moore et al. (2006) studied the N change among six-climate region in Canada and found that such a value of C/N ratio was lower than 55. In our present study, the C/N ratios of the three species were all lower than 40; thus, the nutrient in the leaves of the three tree species presented a net release pattern.
Former plant soaking study also found that riparian plants not only released a large amount of TOC and TN but also a lot of TP (Xiao et al. 2017). Although the macroelement P is usually present in the form of bioactive substance in the leaf, and thus being more easily released (Jacobson et al. 2011), our present study found that a significant increased foliar P release only occurred in T. distichum at 170 m a.s.l. in the second batch (Fig. 4 B3), in contrast with a significantly decreased P release in T. ascendens at 165 m a.s.l. as well as in both T. distichum and S. matsudana at 170 m a.s.l. in the first batch (Fig. 4 A3, B3, and C3). Our present result was inconsistent to a previous study conducted by Liu et al. (2008), who documented that variation in elevation had no significant influence on P content of leaf litter during its decomposition in the Xiangxi River of the TGDR. However, in our current study, the result regarding foliar P release rates in T. ascendens and S. matsudana in the second batch were in support of that given by Liu et al. (2008). Such divergent results of foliar P release rates of the three tree species in this study might be caused by multiple reasons, but the differing leaf initial P content should be one of the key determinant factors to produce those diverse results. Overall, based on our observations, the leaf nutrient release will to some extent aggravate the nutrient load of the water environment of the TGDR.
In this study, the foliar nutrient release rates of the three tree species showed a significantly positive correlation with the mass loss rate except the release of K in S. matsudana (Tab. 2), suggesting that the more leaf mass loss, the higher leaf nutrient release. In our present study, the mass loss rate and nutrient release rate of broadleaf tree species (S. matsudana) were overall higher than that of the other two conifer tree species (T. ascendens and T. distichum, Figs. 3 and 4), indicating that broad leaf decomposition may be more susceptible to water submergence. This finding in our study was in keeping with that found by Hisabae et al. (2011). Therefore, adequate management of the broadleaf tree species such as sustainable harvest of its leaves and branches prior to submergence should be considered in the hydro-fluctuation zone of the TGDR.
Leaf initial nutrient content had a great influence on leaf nutrient release due to its close relation to leaf decomposition. High contents of foliar initial C, N, P, K, Ca, and Mg indicated that under the same conditions, the total amounts of these nutrients available for release were large, and thus facilitating the corresponding leaf nutrient release. This was proved by the correlation analysis of Table 2, in which the foliar release rates of C, N, P, and Mg of T. ascendens, C and P of T. distichum, and C, P, K, and Mg of S. matsudana were all positively correlated with their leaf initial nutrient contents. In addition, the foliar release rates of C, N, and P also showed significant relationships with the leaf initial C/P and N/P ratios across the three tree species in our study, further revealing that leaf stoichiometric characteristics of leaf carbon, nitrogen, and phosphorus would affect the nutrient release of the three tree species (Xie et al. 2017), especially the release of C, N, and P through affecting their foliar decomposition.
Conclusions
In the hydro-fluctuation zone of the TGDR, long-term deep submergence at low elevation facilitated the leaf decomposition and nutrient release of the three tree species, despite the existence of certain exception under specific conditions (e.g., the foliar C release of S. matsudana at 165 m a.s.l. in the first batch). In addition, the leaves of broadleaf tree species (S. matsudana) decomposed faster than that of the conifer species (T. ascendens and T. distichum) and released more nutrients overall. The foliar decomposition of the three tree species was subjected to leaf quality (e.g., ratios of C/P and N/P) and elevation (e.g., submergence depth and duration). Considering the extensive release of foliar nutrients, an eco-friendly method such as sustainable harvest of the leaves of the reforested stands prior to submergence should be considered in the hydro-fluctuation zone, so as to reduce the leaf biomass for decomposition and protect the water quality of the TGDR.
However, our findings can only represent the characteristics of leaf decomposition and nutrient release of deciduous tree species in the upper portion (165–175 m a.s.l.) of the hydro-fluctuation zone of the TGDR. Given that our current study was imposed by only one-cycle submergence, our findings cannot predict long-term decomposition of the leaves under conditions consisting of several water cycles of submergence. Thus, further studies on responses of leaf decomposition and nutrient release to different elevations on the entire riparian zone (between 145 and 175 m a.s.l.) and to long-lasting submergence will be needed.
References
Albariño RJ, Balseiro EG (2002) Leaf litter breakdown in Patagonian streams: native versus exotic trees and the effect of invertebrate size. Aquat Conserv Mar Freshwat Ecosyst 12:181–192. https://doi.org/10.1002/aqc.511
Alvim EACC, Medeiros ADO, Rezende RS, Goncalves Jr JF (2015) Small leaf breakdown in a savannah headwater stream. Limnologica 51:131–138
Austin AT, Vitousek PM (2000) Precipitation, decomposition and litter decomposability of Metrosideros polymorpha in native forests on Hawai’i. J Ecol 88:129–138. https://doi.org/10.1046/j.1365-2745.2000.00437.x
Berg B (2000) Litter decomposition and organic matter turn over in northern forest soils. For Ecol Manag 133:13–22
Berg B, Ekbohm G, Johansson MB, McClaugherty C, Rutigliano F, De Santo AV (1996) Maximum decomposition limits of forest litter types: a synthesis. Can J Bot 74:659–672
Bosco T, Bertiller MB, Carrera AL (2016) Combined effects of litter features, UV radiation, and soil water on litter decomposition in denuded areas of the arid Patagonian Monte. Plant Soil 406:71–82
Bruder A, Schindler MH, Moretti MS, Gessner MO (2014) Litter decomposition in a temperate and a tropical stream: the effects of species mixing, litter quality and shredders. Freshw Biol 59:438–449. https://doi.org/10.1111/fwb.12276
Cai XM (2000) Ecosystem ecology. Science Press, Beijing (in Chinese)
Chen SX, Jiang MX (2006) Leaf litter decomposition dynamics of different tree species in Xiangxi River watershed, the Three Gorges region. Acta Ecol Sin 26:2905–2912 (in Chinese)
Daniels RB, Gilliam JW (1996) Sediment and chemical load reduction by grass and riparian filters. Soil Sci Soc Am J 60:246–251. https://doi.org/10.2136/sssaj1996.03615995006000010037x
Das M, Royer TV, Leff LG (2008) Fungal communities on decaying leaves in streams: a comparison of two leaf species. Mycol Prog 7:267–275
Demessie A, Singh BR, Lal R, Strand LT (2012) Leaf litter fall and litter decomposition under Eucalyptus and coniferous plantations in Gambo District, southern Ethiopia. Acta Agric Scand Sect B 62:467–476
Dosskey MG, Vidon P, Gurwick NP, Allan CJ, Duval TP, Lowrance R (2010) The role of riparian vegetation in protecting and improving chemical water quality in streams. J Am Water Resour Assoc 46:261–277. https://doi.org/10.1111/j.1752-1688.2010.00419.x
Fan J, Zhang X, Lui X, Yan Y, Wang X (2014) Leaf litter decomposition in three subalpine forests along an elevation gradient in Tibet. Pol J Environ Stud 23:1137–1146
Fernandes I, Seena S, Pascoal C, Cássio F (2014) Elevated temperature may intensify the positive effects of nutrients on microbial decomposition in streams. Freshw Biol 59:2390–2399. https://doi.org/10.1111/fwb.12445
Ferreira V, Chauvet E (2015) Synergistic effects of water temperature and dissolved nutrients on litter decomposition and associated fungi. Glob Chang Biol 17:551–564. https://doi.org/10.1111/j.1365-2486.2010.02185.x
Fonseca ALS, Bianchini I, Pimenta CMM, Soares CBP, Mangiavacchi N (2013) The flow velocity as driving force for decomposition of leaves and twigs. Hydrobiologia 703:59–67. https://doi.org/10.1007/s10750-012-1342-3
Fonte SJ, Schowalter TD (2004) Decomposition of greenfall vs. senescent foliage in a tropical forest ecosystem in Puerto Rico. Biotropica 36:474–482. https://doi.org/10.1111/j.1744-7429.2004.tb00343.x
Fu BJ, Wu BF, Xu ZH, Cao JH, Dong N, Yang GS, Zhou YM (2010) Three Gorges Project: efforts and challenges for the environment. Prog Phys Geogr 34:741–754
Goncalves JF, Graça MAS, Callisto M (2007) Litter decomposition in a Cerrado savannah stream is retarded by leaf toughness, low dissolved nutrients and a low density of shredders. Freshw Biol 52:1440–1451. https://doi.org/10.1111/j.1365-2427.2007.01769.x
Graça MAS, Cressa C (2010) Leaf quality of some tropical and temperate tree species as food resource for stream shredders. Int Rev Hydrobiol 95:27–41. https://doi.org/10.1002/iroh.200911173
Graça MAS, Ferreira V, Canhoto C, Encalada AC, Guerrero-Bolaño F, Wantzen KM, Boyero L (2015) A conceptual model of litter breakdown in low order streams. Int Rev Hydrobiol 100:1–12. https://doi.org/10.1002/iroh.201401757
Guan Z, Zhao Y, Tong X (2009) Relationships of Syzygium jambos and Dracontomelon duperreanum leaf tannin concentration and leaf litter breakdown with the colonization of benthonic invertebrates. Chin J Appl Ecol 20:2493–2498 (in Chinese)
Hisabae M, Sone S, Inoue M (2011) Breakdown and macroinvertebrate colonization of needle and leaf litter in conifer plantation streams in Shikoku, southwestern Japan. J For Res 16:108–115. https://doi.org/10.1007/s10310-010-0210-0
Huang Y, Fan D, Liu D, Song L, Ji D, Hui E (2016) Nutrient estimation by HJ-1 satellite imagery of Xiangxi bay, Three Gorges Reservoir, China. Environ Earth Sci 75:633. https://doi.org/10.1007/s12665-016-5421-z
Jacobson TK, Bustamante MM, Kozovits AR (2011) Diversity of shrub tree layer, leaf litter decomposition and N release in a Brazilian Cerrado under N, P and N plus P additions. Environ Pollut 159:2236–2242. https://doi.org/10.1016/j.envpol.2010.10.019
Jie S, Fan D, Xie Z, Zhang X, Xiong G (2012) Features of leaf photosynthesis and leaf nutrient traits in reservoir riparian region of Three Gorges Reservoir, China. Acta Ecol Sin 32:1723–1733 (in Chinese)
Jordan TE, Correll DL, Weller DE (1993) Nutrient interception by a riparian forest receiving inputs from adjacent cropland. J Environ Qual 22:467–473. https://doi.org/10.2134/jeq1993.00472425002200030010x
Kalburtji KL, Mosjidis JA, Mamolos AP (1999) Litter dynamics of low and high tannin sericea lespedeza plants under field conditions. Plant Soil 208:271–281
Kelley RH, Jack JD (2002) Leaf litter decomposition in an ephemeral karst lake (Chaney Lake, Kentucky, U.S.A.). Hydrobiologia 482:41–47
Kröger R, Holland MM, Moore MT, Cooper CM (2007) Plant senescence: a mechanism for nutrient release in temperate agricultural wetlands. Environ Pollut 146:114–119. https://doi.org/10.1016/j.envpol.2006.06.005
Langer ER, Steward GA, Kimberley MO (2008) Vegetation structure, composition and effect of pine plantation harvesting on riparian buffers in New Zealand. For Ecol Manag 256:949–957. https://doi.org/10.1016/j.foreco.2008.05.052
Lee A, Bukaveckas PA (2002) Surface water nutrient concentrations and litter decomposition rates in wetlands impacted by agriculture and mining activities. Aquat Bot 74:273–285
Li AOY, Lily CY, Dudgeon D (2009) Effects of leaf toughness and nitrogen content on litter breakdown and macroinvertebrates in a tropical stream. Aquat Sci 71:80–93. https://doi.org/10.1007/s00027-008-8117-y
Li C, Zhong Z, Geng Y, Schneider R (2010) Comparative studies on physiological and biochemical adaptation of Taxodium distichum and Taxodium ascendens seedlings to different soil water regimes. Plant Soil 329:481–494. https://doi.org/10.1007/s11104-009-0174-z
Liu X, Jiang M, Deng H (2008) Dynamics of nitrogen and phosphorus content during leaf litter decomposition in Xiangxi River watershed, the Three Gorges region. Journal of Wuhan Botanical Research 26:613–619 (in Chinese)
Liu L, Liu D, Johnson DM, Yi Z, Huang Y (2012) Effects of vertical mixing on phytoplankton blooms in Xiangxi bay of Three Gorges Reservoir: implications for management. Water Res 46:2121–2130. https://doi.org/10.1016/j.watres.2012.01.029
Liu G, Sun J, Tian K, Xiao D, Yuan X (2017) Long-term responses of leaf litter decomposition to temperature, litter quality and litter mixing in plateau wetlands. Freshw Biol 62:178–190. https://doi.org/10.1111/fwb.12860
Ma L, Yuan J, Sun X, Zhang M (2012) Spatial distribution and release of nitrogen in soils in the water fluctuation zone of the Three Gorges Reservoir. J Food Agric Environ 10:787–791
Ma W, Liu Y, Zhou C, Wang T, Wei H (2017) Effect of water-level change on nutritional characteristics of Taxodium distichum in the hydro-fluctuation belt of the Three Gorges Reservoir. Acta Ecol Sin 37:1128–1136 (in Chinese)
Martínez A, Larrañaga A, Pérez J, Descals E, Pozo J (2014) Temperature affects leaf litter decomposition in low-order forest streams: field and microcosm approaches. FEMS Microbiol Ecol 87:257–267. https://doi.org/10.1111/1574-6941.12221
Mfilinge P, Atta N, Tsuchiya M (2002) Nutrient dynamics and leaf litter decomposition in a subtropical mangrove forest at Oura Bay, Okinawa, Japan. Trees 16:172–180. https://doi.org/10.1007/s00468-001-0156-0
Molinero J, Pozo J, Gonzalez E (1996) Litter breakdown in streams of the Agüera catchment: influence of dissolved nutrients and land use. Freshw Biol 36:745–756
Moore TR, Trofymow JA, Prescott CE, Fyles J, Titus BD, CIDET Working Group (2006) Patterns of carbon, nitrogen and phosphorus dynamics in decomposing foliar litter in Canadian forests. Ecosystems 9:46–62. https://doi.org/10.1007/s10021-004-0026-x
Neckles HA, Neill C (1994) Hydrologic control of litter decomposition in seasonally flooded prairie marshes. Hydrobiologia 286:155–165
Parton W, Silver WL, Burke IC, Grassens L, Harmon ME, Currie WS, King JY, Adair EC, Bmndt LA, Hart SC, Fasth B (2007) Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315:361–364. https://doi.org/10.1126/science.1134853
Peng C, Zhang L, Qin H, Li D (2014) Revegetation in the water level fluctuation zone of a reservoir: an ideal measure to reduce the input of nutrients and sediment. Ecol Eng 71:574–577
Pettit E, Tegan D, Fellman JB, Grierson PF, Warfe D, Davies P (2012) Leaf litter chemistry, decomposition and assimilation by macroinvertebrates in two tropical streams. Hydrobiologia 680:63–77. https://doi.org/10.1007/s10750-011-0903-1
Ren C, Wang L, Zheng B, Qian J, Ton H (2016) Ten-year change of total phosphorous pollution in the Min River, an upstream tributary of the Three Gorges Reservoir. Environ Earth Sci 75:1–11. https://doi.org/10.1007/s12665-016-5789-9
Rincón J, Covich A (2014) Effects of insect and decapod exclusion and leaf litter species identity on breakdown rates in a tropical headwater stream. Rev Biol Trop 62:143–154
Schmidt A, John K, Auge H, Brandl R, Horgan FG, Settele J, Zaitsevb AS, Woltersb V, Schädler M (2016) Compensatory mechanisms of litter decomposition under alternating moisture regimes in tropical rice fields. Appl Soil Ecol 107:79–90
Schneider T, Keiblinger KM, Schmid E, Sterflingergleixner K, Ellersdorfer G, Roschitzki B, Richter A, Eberl L, Zechmeister-Boltenstern S, Riedel K (2012) Who is who in litter decomposition? Metaproteomics reveals major microbial players and their biogeochemical functions. ISME J 6:1749–1762. https://doi.org/10.1038/ismej.2012.11
Shao X, Liang X, Wu M, Ye X, Jiang K (2014) Decomposition and phosphorus dynamics of the litter in standing and litterbag of the Hangzhou Bay coastal wetland. Environ Sci 35:3381–3388 (in Chinese)
Shieh SH, Wang CP, Hsu CB, Yang PS (2008) Leaf breakdown in a subtropical stream: nutrient release patterns. Fundam Appl Limnol 171:273–284. https://doi.org/10.1127/1863-9135/2008/0171-0273
Sun Z, Mou X, Liu JS (2012) Effects of flooding regimes on the decomposition and nutrient dynamics of Calamagrostis angustifolia, litter in the Sanjiang plain of China. Environ Earth Sci 66:2235–2246. https://doi.org/10.1007/s12665-011-1444-7
Taylor AR, Schroter D, Pflug A, Wolters V (2004) Response of different decomposer communities to the manipulation of moisture availability: potential effects of changing precipitation patterns. Glob Chang Biol 10:1313–1324. https://doi.org/10.1111/j.1365-2486.2004.00801.x
Teklay T (2007) Decomposition and nutrient release from pruning residues of two indigenous agroforestry species during the wet and dry seasons. Nutr Cycl Agroecosyst 77:115–126
Tonin AM, Restello RM, Hepp LU (2014) Chemical change of leaves during breakdown affects associated invertebrates in a subtropical stream. Acta Limnol Bras 26:235–244. https://doi.org/10.1590/S2179-975X2014000300003
Trogisch S, He J, Hector A, Scherer-Lorenzen M (2016) Impact of species diversity, stand age and environmental factors on leaf litter decomposition in subtropical forests in China. Plant Soil 400:337–350. https://doi.org/10.1007/s11104-015-2737-5
van Diepen LTA, Frey SD, Sthultz CM, Morrison EW, Minocha R, Pringle A (2016) Changes in litter quality caused by simulated nitrogen deposition reinforce the N-induced suppression of litter decay. Ecosphere 6:1–16. https://doi.org/10.1890/ES15-00262.1
Vitousek PM, Turner DR, Parton WJ, Sanford RL (1994) Litter decomposition on the Mauna Loa environmental matrix, Hawai’i: patterns, mechanisms, and models. Ecology 75:418–429
Wallis E, Raulings E (2011) Relationship between water regime and hummock-building by Melaleuca ericifolia and Phragmites australis in a brackish wetland. Aquat Bot 95:182–188. https://doi.org/10.1016/j.aquabot.2011.05.006
Wang J, Zhu B, Wang T, Yi L (2012a) Nitrogen and phosphorus release from herbaceous vegetation under simulated inundation experiment of water-level fluctuation zone in the Three Gorges Reservoir area. Environ Sci 33:1144–1151 (in Chinese)
Wang Q, Yuan X, Liu H, Zhang Y, Cheng Z, Li B (2012b) Effect of long-term winter flooding on the vascular flora in the drawdown area of the Three Gorges Reservoir, China. Pol J Ecol 60:95–106
Wang X, Gao M, Yang F, Guo Y, Ma C (2012c) Litter decomposition and nutrient dynamics of Larix principis-rupprechtii plantations of different ages. Journal of Northeast Forestry University 40(56–60):66 (in Chinese)
Wang C, Li C, Wei H, Xie Y, Han W (2016a) Effects of long-term periodic submergence on photosynthesis and growth of Taxodium distichum and Taxodium ascendens saplings in the hydro-fluctuation zone of the Three Gorges Reservoir of China. PLoS One 11:e0162867. https://doi.org/10.1371/journal.pone.0162867
Wang J, You Y, Tang Z, Sun X, Sun OJ (2016b) A comparison of decomposition dynamics among green tree leaves, partially decomposed tree leaf litter and their mixture in a warm temperate forest ecosystem. J For Res 27:1037–1045. https://doi.org/10.1007/s11676-016-0248-8
Wieder WR, Cleveland CC, Townsend AR (2009) Controls over leaf litter decomposition in wet tropical forests. Ecology 90:3333–3341
Willison JHM, Li R, Yuan X (2013) Conservation and ecofriendly utilization of wetlands associated with the Three Gorges Reservoir. Environ Sci Pollut Res 20:6907–6916. https://doi.org/10.1007/s11356-012-1438-3
Xiao L, Zhu B, Kumwimba MN, Jiang S (2017) Plant soaking decomposition as well as nitrogen and phosphorous release in the water-level fluctuation zone of the Three Gorges Reservoir. Sci Total Environ 592:527–534
Xie Y, Yu D, Ren B (2004) Effects of nitrogen and phosphorus availability on the decomposition of aquatic plants. Aquat Bot 80:29–37. https://doi.org/10.1016/j.aquabot.2004.07.002
Xie Y, Xie Y, Hu C, Chen X, Li F (2016) Interaction between litter quality and simulated water depth on decomposition of two emergent macrophytes. J Limnol 75:36–43. https://doi.org/10.4081/jlimnol.2015.1119
Xie Y, Xie Y, Xiao H, Chen X, Li F (2017) Controls on litter decomposition of emergent macrophyte in Dongting Lake wetlands. Ecosystems 20:1–7. https://doi.org/10.1007/s10021-017-0119-y
Xu X, Tan Y, Yang G (2013) Environmental impact assessments of the Three Gorges Project in China: issues and interventions. Earth-Sci Rev 124:115–125
Yang J, Liu J, Yu J, Wang J, Li X, Sun Z (2006) Decomposition and nutrient dynamics of marsh litter in the Sanjiang Plain, China. Acta Ecol Sin 26:1297–1302 (in Chinese)
Yang F, Wang Y, Chan Z (2014) Perspectives on screening winter-flood-tolerant woody species in the riparian protection forests of the Three Gorges Reservoir. PLoS One 9:e108725. https://doi.org/10.1371/journal.pone.0108725
Ye C, Li S, Zhang Y, Zhang Q (2011) Assessing soil heavy metal pollution in the water-level-fluctuation zone of the Three Gorges Reservoir, China. J Hazard Mater 191:366–372. https://doi.org/10.1016/j.jhazmat.2011.04.090
Ye C, Cheng X, Zhang Y, Wang Z, Zhang Q (2012) Soil nitrogen dynamics following short-term revegetation in the water level fluctuation zone of the Three Gorges Reservoir, China. Ecol Eng 38:37–44. https://doi.org/10.1016/j.ecoleng.2011.10.005
Yuan X, Zhang Y, Liu H, Xiong S, Li B, Deng W (2013) The littoral zone in the Three Gorges Reservoir, China: challenges and opportunities. Environ Sci Pollut Res 20:7092–7102. https://doi.org/10.1007/s11356-012-1404-0
Yue K, Yang W, Peng C, Peng Y, Zhang C, Huang C, Tan Y, Wu F (2016) Foliar litter decomposition in an alpine forest meta-ecosystem on the eastern Tibetan Plateau. Sci Total Environ 566-567:279–287
Zanne AE, Oberle B, Dunham KM, Milo AM, Walton ML, Young DF (2015) A deteriorating state of affairs: how endogenous and exogenous factors determine plant decay rates. J Ecol 103:1421–1431. https://doi.org/10.1111/1365-2745.12474
Zhang Q, Lou Z (2011) The environmental changes and mitigation actions in the Three Gorges Reservoir region, China. Environ Sci Pol 14:1132–1138. https://doi.org/10.1016/j.envsci.2011.07.008
Zhang P, Tian X, He X, Song F, Ren L, Jiang P (2008) Effect of litter quality on its decomposition in broadleaf and coniferous forest. Eur J Soil Biol 44:392–399. https://doi.org/10.1016/j.ejsobi.2008.04.005
Zhang Z, Peng J, Wan C, Zheng Z, Qiu D (2010) Distribution and decomposition dynamics of herb plants in Pengxihe water-fluctuation-zone of the Three Gorges Reservoir. Acta Prataculturae Sinica 19:146–152 (in Chinese)
Zhang X, Song C, Mao R, Yang G, Tao B, Shi F, Zhu X, Hou A (2014) Litter mass loss and nutrient dynamics of four emergent macrophytes during aerial decomposition in freshwater marshes of the Sanjiang Plain, Northeast China. Plant Soil 385:139–147. https://doi.org/10.1007/s11104-014-2217-3
Zhang S, Li R, Wang W, Lu W, Ren Y, Fu S (2015) Tree species selection in the riparian zone of Meishan reservoir. Journal of Anhui Agricultural University 42:357–362 (in Chinese)
Zhu W, Wang J, Zhang Z, Ren F, Chen L, He J (2016) Changes in litter quality induced by nutrient addition alter litter decomposition in an alpine meadow on the Qinghai-Tibet Plateau. Sci Rep 6:34290. https://doi.org/10.1038/srep34290
Acknowledgments
The authors acknowledge financial support from the Chongqing Municipality Key Forestry Research Project (No. Yulinkeyan 2016-8), the International Cooperation Program of Ministry of Science and Technology (No. 2015DFA90900), China Central Finance Forestry Extension Program (Yulinketui 2014-10), and Chongqing Postgraduate Research and Innovation Project (No. CYB16066). We specially thank the anonymous reviewers and editor Dr. Philippe Garrigues for their valuable comments on our earlier draft of this manuscript. The authors would also like to thank Joanne Cabato and Florence Delavaud for their kind assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Wang, C., Xie, Y., Ren, Q. et al. Leaf decomposition and nutrient release of three tree species in the hydro-fluctuation zone of the Three Gorges Dam Reservoir, China. Environ Sci Pollut Res 25, 23261–23275 (2018). https://doi.org/10.1007/s11356-018-2357-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2357-8