Abstract
Background and aims
In wetland ecosystems, the litter of emergent macrophytes generally begins to decay while standing, but aerial decomposition has often been overlooked. The goal of this study was therefore to elucidate the processes involved in the decomposition of standing litter of emergent macrophytes in freshwater marshes in the Sanjiang Plain, Northeast China.
Methods
We used the litterbag method to quantify litter mass loss, microbial respiration rates, and nutrient dynamics of four common emergent macrophytes (Carex lasiocarpa, Deyeuxia angustifolia, Glyceria spiculosa, and Phragmites australis) during one year of aerial decomposition.
Results
Following one year of aerial decomposition, the leaf and culm mass losses were 19.3–45.1 % and 14.3–23.1 %, respectively. Litter mass loss was closely related to microbial respiration rates and initial ratios of C:N and C:P. The fact that litter N concentrations increased during aerial decomposition resulted in net N immobilization. After one year of decay, however, there was a net release of P from the standing litter in all cases, but the temporal pattern of P concentrations varied between the decomposing litter of the four different species.
Conclusions
Our results provide evidence that the decomposition of standing litter from emergent macrophytes contributes markedly to overall litter decay, and thus is a key component of C and nutrient cycles in temperate wetlands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wetlands only cover 2–6 % of the earth’s land surface, but store a large proportion of the global terrestrial carbon (C) pool in soils mainly because of their high primary productivity of plants combined with their slow decay rates under anaerobic conditions (Whiting and Chanton 2001; Kayranli et al. 2010). In most wetland ecosystems, emergent macrophytes often make substantial contributions to annual plant production (Mitsch and Gosselink 2007). Moreover, almost all emergent macrophyte biomass eventually enters the detrital food web due to its low quality forage for herbivores (Mann 1988; Kuehn et al. 2004). Therefore, emergent macrophyte litters provide the major source of energy for detritus-feeding consumers and play important roles in C and nutrient cycling in wetland ecosystems (Kuehn and Suberkropp 1998; Kuehn et al. 2011).
In wetland ecosystems, shoots (including leaves and culms) of emergent macrophytes generally do not collapse and fall onto the sediment surface immediately after senescence, but remain in the air for a long period forming so-called “standing litter” (Kuehn et al. 2004). Consequently, many fungal and bacterial taxa colonize the standing plant litters so that microbial litter decay begins in an aerial position (Newell 2001; Gessner et al. 2007; Kuehn et al. 2011). Several studies conducted over the past few decades have found substantial changes in litter mass (e.g. Newell et al. 1989; Liao et al. 2008) and nutrient concentrations (e.g. Gessner 2001; Kuehn et al. 2011) during aerial decomposition, and thus suggest that standing litter decay is a key component of C and nutrient cycling in wetland ecosystems (Liao et al. 2008; Kuehn et al. 2011). Nevertheless, most previous studies regarding emergent macrophyte decay in wetlands have focused on the decay process of plant litters at the sediment surface or in the water (e.g. Rejmankova and Sirova 2007; Longhi et al. 2008; Sun et al. 2012), and ignored the decay occurring before shoot litters enter the aquatic environment. This approach cannot reflect the natural decay sequence and may over-estimate the rate of litter decay for emergent macrophytes in wetlands partly because the standing litter does not have access to nutrients in the soil (Welsch and Yavitt 2003). Given the large amounts of standing-dead plant matter in most wetland ecosystems (Kuehn et al. 2004; Liao et al. 2008), knowledge related to the decomposition dynamics of emergent macrophyte litters in the aerial position would help completely understand wetland biogeochemical cycling.
The Sanjiang Plain, a floodplain in Northeast China, is one of the largest freshwater marshes in China (Zhao 1999). In this region, most marshes are dominated by emergent macrophytes, such as Carex lasiocarpa, Deyeuxia angustifolia, Glyceria spiculosa, and Phragmites australis. Previous studies have examined the mass loss and nutrient dynamics of macrophyte litters during decomposition at the sediment surface (Hou et al. 2012; Sun et al. 2012) or in the water (Song et al. 2011). Nevertheless, little is currently known about the litter decay processes occurring in the standing-dead phase. In these marshes, however, a large fraction of dead plant matter often remains in an aerial position. Recently, we have observed that microbially mediated C mineralization of standing litter occupies a considerable portion of annual aboveground plant productivity in a D. angustifolia-dominated freshwater marsh (Zhang et al. 2014). In the present study, we used the litterbag method to assess litter mass loss and nutrient dynamics of four common emergent macrophytes during aerial decomposition in freshwater marshes of the Sanjiang Plain. More specifically, we attempted to evaluate (1) the degree of litter mass loss during decomposition in the aerial position, and (2) whether nutrient release occurs in the standing-dead phase.
Materials and methods
Study site
This study was conducted in a freshwater marsh near the Sanjiang Experimental Station of Wetland Ecology, Chinese Academy of Sciences (47°35′N, 133°31’E, 56 m a. s. l.), located in the Sanjiang Plain, Northeast China. The study site has a temperate continental monsoon climate, with a mean annual temperature of 2.5 °C, precipitation of approximately 558 mm (more than 65 % falls in July and August), and a frost-free period of 125 days. The soil is mainly a typical meadow mire soil, with high organic matter content and a pH of 5–6. In this marsh, C. lasiocarpa and D. angustifolia are dominant species, and G. spiculosa and P. australis are important accompanying species. In July and October 2013, 15.5–29.2 % and 2.5–6.7 % of these macrophyte shoot litters that senesced in 2012 still remained in the aerial position, respectively. More detailed information about this study site has been provided by Song et al. (2011) and Hou et al. (2012).
Litter collection and preparation
In October 2012, we collected newly senesced shoots of three gramineous species (D. angustifolia, G. spiculosa, and P. australis) and one sedge (C. lasiocarpa) at middle height within the freshwater marsh. Shoot litters of D. angustifolia, G. spiculosa, and P. australis were separated into leaves (including leaf blade and sheath) and culms, and shoot litters of C. lasiocarpa only included leaves. Litters for a given plant tissue of a species were mixed carefully to produce one sample, and then divided into two subsamples. The first group of subsamples was used for litter decomposition experiments. The plant tissues were cut into pieces of about 5 cm long to reduce the effects of litter size on decomposition. The second group of subsamples was used to determine the initial litter moisture content and chemical properties. The subsamples were weighed, oven-dried at 65 °C for 48 h, reweighed, and then milled (<0.25 mm) for measurements of organic C, nitrogen (N), and phosphorus (P) concentrations. The organic C concentration of litter was determined using a total organic C analyzer (TOC VCPH, Shimadzu Corp., Tokyo, Japan), N concentration was analyzed using the micro-Kjeldahl method (Bremner 1996), and P concentration was measured by the ammonium molybdate method after persulfate oxidation (Kuo 1996). Appendix Table 1 shows the initial chemical properties of plant litters.
Experimental design and litter decomposition
Litter decomposition was quantified by the litterbag method (e.g. Kuehn and Suberkropp 1998; Welsch and Yavitt 2003; Liao et al. 2008). Two grams of culms or leaves were placed into 15 × 15 cm litterbags with mesh sizes of 0.8 × 0.8 cm in the upper surface and 0.3 × 0.3 cm in the lower surface. These litterbags were hung from 2 cm diameter wooden stakes with cable ties at three quarters of the middle plant height within the marsh to simulate aerial decomposition. Five litterbags of each plant tissue were randomly retrieved after 180, 270, and 360 days of incubation (i.e. April, July, and October 2013), respectively, resulting in a total of 15 litterbags per plant tissue.
After retrieval, the remaining litter was taken out of litterbags, carefully rinsed with deionized water, and then used for measurements of microbial respiration rates. Microbial respiration of standing litter was assessed using a laboratory incubation method based on the protocol of Newell et al. (1985) and Welsch and Yavitt (2003). Plant litters that remained in the litterbags were wetted until saturated with sterile deionized water, and placed into 250-ml incubation jars. The jars were sealed and incubated at 18 °C (the average air temperature from April to October at the study site) for 1 h after pre-incubated for 2 h. During the one hour incubation, four gas samples (5 mL each) were taken from the jars at 20-min intervals and analyzed for the CO2 concentration on a gas chromatograph (Agilent 4890D, Agilent Co., Santa Clara, CA, USA). Microbial respiration rates of standing litter were calculated based on the method described by Zhang et al. (2014).
Following measurement of microbial respiration, all litter samples were oven-dried at 65 °C, weighed, and then milled (<0.25 mm) for chemical analyses. Litter organic C, N, and P concentrations were determined by the methods described above. Mass loss was calculated as the difference between the initial and remaining litter mass, and expressed as the percentage of initial litter mass. The remaining N or P in the litter was calculated by multiplying the remaining litter mass by its N or P concentration, and expressed as the percentage of the initial amount of litter N or P.
Statistical analyses
All statistical analyses were carried out using the SPSS 13.0 for Windows software package (SPSS Inc. 2004), with a significance level of α = 0.05. Data were tested for normality using the Kolmogorov-Smirnov test and all data followed a normal distribution (data not shown). We used the one-way analysis of variance to test the effect of sampling date on nutrient concentrations and amounts of the remaining standing litters. Simple linear regression was used to assess the relationship between mass loss and microbial respiration rates, and Pearson product–moment correlation coefficient was used to examine the correlation between the initial chemical quality of the litter and the corresponding mass loss.
Results
Litter mass loss
For both leaves and culms, litter mass declined slowly from October 2012 to April 2013, and quickly from April to October 2013 (Fig. 1). In the initial 6 months, both leaf and culm mass losses were only about 3 % (Fig. 1). However, the culm mass loss reached 5.6–9.2 % by July 2013 and 14.3–23.1 % by October 2013 (Fig. 1a), and the leaf mass loss reached 5.3–17.3 % by July 2013 and 19.3–45.1 % by October 2013 (Fig. 1b). Among the selected macrophyte species, mass loss occurred most rapidly in G. spiculosa in all culm litters (Fig. 1a), while D. angustifolia and G. spiculosa had the greatest mass loss in all leaf litters (Fig. 1b).
During aerial decomposition, higher microbial respiration rates were observed in leaves compared with culms (Fig. 2). Moreover, mean microbial respiration rates showed a significant positive linear relationship with litter mass loss (R 2 = 0.841 and P < 0.001; Fig. 2). Litter mass loss was negatively correlated with initial C:N (r = −0.632, P < 0.001, data not shown) and C:P (r = −0.656, P < 0.001, data not shown) ratios, respectively.
Litter C, N, and P concentrations
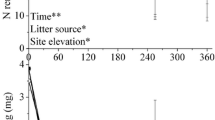
Litter C concentration generally remained constant throughout the study (428–469 mg g−1, data not shown), while N and P concentrations exhibited large variations (Fig. 3). Both leaf and culm N concentrations increased during the period of aerial decomposition (all P < 0.001, F (3, 16) = 204.2, 172.4, and 11.1 for culms of D. angustifolia, G. spiculosa, and P. australis, respectively; F (3, 16) = 57.9, 221.5, 210.4, and 125.8 for leaves of C. lasiocarpa, D. angustifolia, G. spiculosa, and P. australis, respectively; Fig. 3a and b). After 360 days of aerial decompostition, the magnitudes of increases in N concentrations ranged from 30.0 % (P. australis) to 55.8 % (D. angustifolia) for culm litters (Fig. 3a), and from 32.4 % (C. lasiocarpa) to 104.1 % (G. spiculosa) for leaf litters (Fig. 3b).
For all macrophytes, the concentrations of P in culms decreased during aerial decomposition (all P < 0.001, F (3, 16) = 219.5, 29.4, and 23.2 for D. angustifolia, G. spiculosa, and P. australis, respectively; Fig. 3c). The concentrations of P in leaves of both D. angustifolia and G. spiculosa increased in the initial 270 days (both P < 0.001, F (2, 12) = 31.7 and 200.6 for D. angustifolia and G. spiculosa, respectively), and then declined (Fig. 3d), whereas the concentration of P in leaves of C. lasiocarpa exhibited a declining trend during aerial decomposition (P < 0.001, F (3, 16) = 93.6; Fig. 3d).
Litter N and P remaining
The amount of N remaining in P. australis culms generally increased during decomposition in the aerial position (Fig. 4a), whereas the amount of N remaining of the other plant litters significantly increased during the initial 270 days (all P < 0.001, F (2, 12) = 347.7 and 152.7 for culms of D. angustifolia and G. spiculosa, respectively; F (2, 12) = 76.0, 389.9, 129.2, and 37.8 for leaves of C. lasiocarpa, D. angustifolia, G. spiculosa, and P. australis, respectively), and then declined (Fig. 4a and b). Compared with the initial amounts, the amount of N remaining by the end of the incubation period ranged from 111.4 % (P. australis) to 130.9 % (D. angustifolia) in culms (Fig. 4a), and from 106.8 % (C. lasiocarpa) to 118.0 % (G. spiculosa) in leaves (Fig. 4b).
The amount of P remaining in leaves of D. angustifolia increased by 9.0 % after 270 days of aerial decomposition, and then declined (Fig. 4d), while the amount of P remaining of the other plant litters generally decreased during aerial decomposition (all P < 0.001, F (3, 16) = 340.2, 71.3, and 39.7 for culms of D. angustifolia, G. spiculosa, and P. australis, respectively; F (3, 16) = 222.0, 355.4, and 43.8 for leaves of C. lasiocarpa, G. spiculosa, and P. australis, respectively; Fig. 4c and d). At the end of the incubation period, the amounts of P remaining in culms were 58.9 %, 53.0 %, and 62.1 % for D. angustifolia, G. spiculosa, and P. australis, respectively (Fig. 4c). The amounts of P remaining in leaves ranged from 58.4 % (G. spiculosa) to 69.3 % (P. australis) after one year of decomposition (Fig. 4d).
Discussion
Notably, the litter mass loss following one year of aerial decomposition was 19.3–45.1 % for leaves, and 14.3–23.1 % for culms in this temperate freshwater marsh. These observations were comparable to those reported by most previous studies regarding standing litter decomposition in both freshwater and salt marshes (e.g. Christian et al. 1990; Kuehn and Suberkropp 1998; Welsch and Yavitt 2003), although the annual average air temperature of the Sanjiang Plain was much lower than that in the areas of those previous studies (Zhang et al. 2014). More rapid litter mass losses during aerial decay have been found in subtropical wetlands (e.g. Newell et al. 1989; Liao et al. 2008). Evidently, a considerable amount of macrophyte litters is decomposed during the standing-dead phase, suggesting that decomposition of standing litters is an important component of litter decay in wetland ecosystems. The majority of past litter decomposition studies, however, have ignored the aerial decay phase. In order to gain a comprehensive understanding of litter decomposition in wetland ecosystems, additional studies are essential to quantify decomposition processes of aerial litters.
Microorganisms, especially fungi, play a key role in decay processes of standing litters in wetland ecosystems (Kuehn et al. 2011). Gessner (2001) estimated that fungal assimilation (biomass production and respiration) accounted for about 50 % of leaf mass loss of P. australis during the aerial decay phase in the littoral zone of a hardwater lake. In this study, we observed a strong positive linear relationship between mass loss and litter-associated microbial CO2 evolution rates, which further confirmed the leading role of microorganisms in standing litter decomposition in wetlands. Microbial colonization and activity are substantially influenced by temperature (Kuehn et al. 2004; Zhang et al. 2014); as a result, standing litters decomposed slowly during the initial 6 months with an average air temperature of about −12 °C, but rapidly in the following 6 months with an average air temperature of about 18 °C.
Besides decomposer activities, litter decomposition is generally controlled by the initial chemical quality of litter, such as C:N and C:P ratios (Aerts and Chapin 1999). Previous studies observed that the standing litter with greater C:N and/or C:P ratios often had slower decay rates in wetlands (Kuehn et al. 2004; Liao et al. 2008). In this study, we also found a negative correlation between litter mass loss and initial C:N or C:P ratio. These results indicate that litter chemical quality is an important factor controlling decay rates of standing litter in wetland ecosystems.
In this study, we observed a net N immobilization in both leaves and culms during the study period, and thus an increase in the total quantity of N in standing litters. Generally, net accumulation or release of N is related to a “critical” litter C:N ratio or N concentration, and N release can begin at a C:N ratio of between 25 and 30 or at a N concentration above 20 mg g−1 (Moore et al. 2006; Jacob et al. 2009). In this study, the initial N concentration and C:N ratio of the standing litters were 1.97–10.09 mg g−1 and 46–238, respectively (Appendix Table 1), resulting in a net accumulation of N in standing litters. The increased amounts of N found in standing litters may have been attributed to microbial immobilization of N from atmospheric dry and wet deposition and the conservation of nutrients within the litter (Gessner 2001; van Ryckegem et al. 2006). Since N concentration and C:N ratio were the main litter quality variables controlling decay rates (Moore et al. 2006; Jacob et al. 2009), increased N concentration and decreased C:N ratio during aerial decomposition would exert a profound impact on subsequent litter decomposition in the water or at the sediment surface in wetland ecosystems.
Interestingly, the total amount of P in decomposing standing litters exhibited a net release after one year of aerial decomposition, although the litter P concentration either increased or declined. However, Kuehn and Suberkropp (1998) found that both the concentration and total amount of P in Juncus effusus standing litters increased during decomposition. These inconsistent results may be explained by the differences in litter P concentrations and C:P ratios. In our study, the C:P ratio (798–2934; Appendix Table 1) of standing litters was much lower than that of J. effusus litters (4300; Kuehn and Suberkropp 1998). Moreover, decomposition of standing litter was not P-limited in this study (Güsewell and Verhoeven 2006). Thus, P in decomposing litters was sufficient to meet the demands of microbial assemblages inhabiting standing litters, allowing excess P to be released (Aerts and Chapin 1999).
Litter N and P concentrations, and the N:P ratio can be indicative of whether N or P limits litter decomposition (Hobbie and Vitousek 2000; Güsewell and Verhoeven 2006). Güsewell and Verhoeven (2006) suggested that the critical N concentration and N:P ratio (threshold between N and P limitation) were 11.3 mg g−1 and 25, respectively, for graminoid litters. In this study, the N concentration and N:P ratio in decomposing standing litters were generally lower than the proposed threshold values (Appendix Table 1). Therefore, decomposition of standing litters is more likely to be N-limited in this freshwater marsh.
In recent decades, increased nutrient inputs to freshwater marshes induced by N deposition and fertilizer application during the agricultural activities can substantially alter the C:N:P stoichiometric ratios in plant litter from the Sanjiang Plain (Mao et al. 2013). This not only alters initial litter chemical quality, but also causes a shift in limiting nutrients during standing litter decomposition. Therefore, further studies are needed to reveal the effects of increased nutrient inputs on litter decomposition and nutrient cycling during the stand-dead phase of emergent macrophytes.
Conclusions
In this study, we used the litterbag method to investigate litter decomposition of four emergent macrophytes in the standing-dead position in a freshwater marsh in the Sanjiang Plain, Northeast China. Following one year of aerial decomposition, the leaf and culm mass loss reached 19.3–45.1 % and 14.3–23.1 %, respectively, in this temperate freshwater marsh. During the study period, standing litters exhibited a net N immobilization, but showed a net P release. Our results suggest that, in temperate wetland ecosystems, plant litter decomposition in the standing-dead phase not only accounts for a considerable portion of overall mass loss during the processes of litter decay, but also causes substantial variations of nutrient concentrations and stoichiometric ratios in decomposing litters. Given the predominance of standing litter after emergent macrophytes senescence in most wetlands (Christian et al. 1990; Kuehn and Suberkropp 1998; Liao et al. 2008), this study could provide insights into litter decomposition and thus C and nutrient cycles in wetland ecosystems.
References
Aerts R, Chapin FS (1999) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–67
Bremner JM (1996) Nitrogen: total. In: Sparks DL (eds) Methods of soil analysis. Part 3. Chemical methods. SSSA Inc., Madison, pp 1085–1121
Christian RR, Bryant WL Jr, Brinson MM (1990) Juncus roemerianus production and decomposition along gradients of salinity and hydroperiod. Mar Ecol Prog Ser 68:137–145
Gessner MO (2001) Mass loss, fungal colonisation and nutrient dynamics of Phragmites australis leaves during senescence and early aerial decay. Aquat Bot 69:325–339
Gessner MO, Gulis V, Kuehn KA, Chauvet E, Suberkropp K (2007) Fungal decomposers of plant litter in aquatic habitats. In: Kubieck CP, Druzhinina IS (eds) The mycota, vol IV, Environmental and microbial relationships, 2nd ed. Spinger, Berlin, pp 301–324
Güsewell S, Verhoeven JT (2006) Litter N:P ratios indicate whether N or P limits the decomposability of graminoid leaf litter. Plant Soil 287:131–143
Hobbie SE, Vitousek PM (2000) Nutrient limitation of decomposition in Hawaiian forests. Ecology 81:1867–1877
Hou C, Song C, Li Y, Guo Y, Yang G (2012) Litter decomposition and nutrient dynamics of Carex lasiocarpa under different water conditions. Acta Ecol Sin 32:650–658
Jacob M, Weland N, Platner C, Schaefer M, Leuschner C, Thomas FM (2009) Nutrient release from decomposing leaf litter of temperate deciduous forest trees along a gradient of increasing tree species diversity. Soil Biol Biochem 41:2122–2130
Kayranli B, Scholz M, Mustafa A, Hemark A (2010) Carbon storage and fluxes within freshwater wetlands: a critical review. Wetlands 30:111–124
Kuehn KA, Suberkropp K (1998) Decompisition of standing litter of the freshwater emergent macrophyte Juncus effusus. Freshw Biol 40:717–727
Kuehn KA, Steiner D, Gessner MO (2004) Diel mineralization patterns of standing-dead plant litter: implications for CO2 flux from wetlands. Ecology 85:2504–2518
Kuehn KA, Ohsowski BM, Francoeur SN, Neely RK (2011) Contributions of fungi to carbon flow and nutrient cycling from standing dead Typha angustifolia leaf litter in a temperate freshwater marsh. Limnol Oceanogr 56:529–539
Kuo S (1996) Phosphorus. In: Sparks DL (eds) Methods of soil analysis. Part 3. Chemical methods. SSSA Inc., Madison, pp 908–910
Liao C, Luo Y, Fang C, Chen J, Li B (2008) Litter pool sizes, decomposition, and nitrogen dynamics in Spartina alterniflora-invaded and native coastal marshlands of the Yangtze Estuary. Oecologia 156:589–600
Longhi D, Bartoli M, Viaroli P (2008) Decomposition of four macrophytes in wetland sediments: organic matter and nutrient decay and associated benthic processes. Aquat Bot 89:303–310
Mann KH (1988) Production and use of detritus in various freshwater, estuarine and coastal marine ecosystems. Limnol Oceanogr 33:910–930
Mao R, Song C, Zhang X, Wang X, Zhang Z (2013) Response of leaf, sheath and stem nutrient resorption to 7 years of N addition in freshwater wetland of Northeast China. Plant Soil 364:385–394
Mitsch WJ, Gosselink JG (2007) Wetlands (4th ed.). John Wiley and Sons, New York
Moore TR, Trofymow JA, Prescott CE, Fyles J, Titus BD, CIDET Working Group (2006) Patterns of carbon, nitrogen and phosphorus dynamics in decomposing foliar litter in Canadian forests. Ecosystems 9:46–62
Newell SY (2001) Fungal biomass and productivity in standing-decaying leaves of black needlerush (Juncus roemerianus). Mar Freshw Res 52:249–255
Newell SY, Fallon RD, Rodriguez RMC, Groene LC (1985) Influence of rain, tidal wetting and relative humidity on release of carbon dioxide by standing-dead salt-marsh plants. Oecologia 68:73–79
Newell SY, Fallon RD, Miller JD (1989) Decomposition and microbial dynamics for standing, naturally positioned leaves of the salt-marsh grass Spartina alterniflora. Mar Biol 101:471–481
Rejmankova E, Sirova D (2007) Wetland macrophyte decomposition under different nutrient conditions: relationships between decomposition rate, enzyme activities and microbial biomass. Soil Biol Biochem 39:526–538
Song C, Liu D, Yang G, Song Y, Mao R (2011) Effect of nitrogen addition on decomposition of Calamagrostis angustifolia litters from freshwater marshes of Northeast China. Ecol Eng 37:1578–1582
SPSS Inc. (2004) SPSS 13.0 base users guide. SPSS Inc., Chicago
Sun Z, Mou X, Liu J (2012) Effects of flooding regimes on the decomposition and nutrient dynamics of Calamagrostis angustifolia litter in the Sanjiang plain of China. Environ Earth Sci 66:2235–2246
van Ryckegem G, van Driessche G, van Beeumen J, Verbeken A (2006) The estimated impact of fungi on nutrient dynamics during decomposition of Phragmites australis leaf sheaths and stems. Microb Ecol 52:564–574
Welsch M, Yavitt JB (2003) Early stages of decay of Lythrum salicaria L. and Typha latifolia L. in a standing-dead position. Aquat Bot 75:45–57
Whiting GJ, Chanton JP (2001) Greenhouse carbon balance of wetlands: methane emission versus carbon sequestration. Tellus B 53:521–528
Zhang X, Mao R, Gong C, Qiao T, Song C (2014) CO2 evolution from standing litter of the emergent macrophyte Deyeuxia angustifolia in the Sanjiang plain, Northeast China. Ecol Eng 63:45–49
Zhao K (1999) Chinese mires. Science Press, Beijing
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Nos. 31100357, 41103037, 41125001 and 41171169) and “Strategic Priority Research Program – Climate Change: Carbon Budget and Related Issue” of the Chinese Academy of Sciences (No. XDA05050508). We thank the editor (Dr. Alfonso Escudero) and two anonymous reviewers for their constructive comments on the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Alfonso Escudero..
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 41 kb)
Rights and permissions
About this article
Cite this article
Zhang, X., Song, C., Mao, R. et al. Litter mass loss and nutrient dynamics of four emergent macrophytes during aerial decomposition in freshwater marshes of the Sanjiang plain, Northeast China. Plant Soil 385, 139–147 (2014). https://doi.org/10.1007/s11104-014-2217-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2217-3