Abstract
The current work focuses on the experimental investigation to analyze the combustion and emission characteristics of a direct injection diesel engine fueled with neat biodiesel (BD100) and different proportions of cyclohexanol blends as a fuel additive in various volume fractions. Cyclohexanol is dispersed into a neat biodiesel in a volume fraction of 10, 20, and 30 vol%. The biodiesel is produced from neem oil by the conventional transesterification process. The experimental results revealed that with the increased cyclohexanol fraction, the combustion was found smooth. The addition of cyclohexanol has a positive influence on various physical and chemical properties of neat biodiesel. The in-cylinder pressure is comparatively low for diesel followed by cyclohexanol and biodiesel blends when compared with neat biodiesel. This is due to shorter ignition delay period. The heat-release rate of neat biodiesel is the highest among all fuels. The overall HC emission of BD70COH30 is 12.19% lower than BD100 and 16.34% lower than diesel. The overall CO2 emission of BD70COH30 is 13.91% higher than BD100 and 19.5% higher than diesel. The overall NOx emission of BD70COH30 is 5.31% lower than BD100 at all load engine operations. The presence of 10, 20, and 30% of cyclohexanol in biodiesel decreased smoke emissions as compared with neat biodiesel and diesel. The overall smoke emission of BD70COH30 is 19.23% lower than BD100 and 25.51% lower than diesel. The overall CO emission of cyclohexanol blended with biodiesel by 30 vol% (BD70COH30) is 17% lower than neat biodiesel and 21.8% lower than diesel. Based on the outcome of this study, neem oil biodiesel and cyclohexanol blends can be employed as a potential alternative fuel for existing unmodified diesel engines owing to its lesser emission characteristics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One of the obvious results of the modern lifestyle is increased consumption of energy. Apart from energy deficit, the other global issues like global warming and air, water and soil pollution, stringent emission norms imposed, and also the mounting of fossil fuel price are the principal motives behind expanding the exploration of unconventional energy sources (Devarajan et al. 2016; Vishnoi et al. 2017; Jiotode and Agarwal 2017). Although numerous unconventional energy sources are available, their adoption rate is very less because of its various economic constraints and lack of production (Abdullah et al. 2013; Rajesh Kumar et al. 2016a, b; Zheng et al. 2016). One of the popular alternative fuel, biodiesel continues to gain more significance as an alternative fuel owing to its renewable nature (Jatt et al. 2014; Yilmaz and Atmanli 2017; Radhakrishnan 2017). The major pollutants from the diesel engine exhaust are unburned hydrocarbons (UBHC), carbon dioxide (CO2), carbon monoxide (CO), particulate matter (PM), and oxides of nitrogen (NOx). Mitigation of these emissions is especially important due to its role in acid rain (Ibrahim 2016; Rajesh Kumar et al. 2016a, b). One way to achieve the NOx reduction is to use biofuels as an alternative source of energy in diesel engines (Nalgundwar et al. 2016; Devarajan et al. 2016). Compared with conventional, biofuels have higher oxygen content and their combustion properties result in emission reduction (Jatt et al. 2014). Biodiesel can either be used as a neat fuel or can be blended with diesel (Fangsuwannarak et al. 2016). Previous works have reported the properties of biodiesel to be closer to properties of diesel (Abu-Hamdeh and Alnefaie 2015). Also, the lubricating properties of biodiesel are much better than diesel (Abdullah et al. 2013; Devarajan et al. 2016).
Transesterification is one of the more advanced and commonly employed techniques to produce biodiesel (Yuvarajan and Venkata Ramanan 2016). Transesterification converts vegetable oils into fatty acid methyl ester (Yuvarajan and Venkata Ramanan 2016; Zheng et al. 2016). Transesterification commonly uses methanol and ethanol while the catalysts used are sodium hydroxide and potassium hydroxide (Devarajan et al. 2016; Yuvarajan and Venkata Ramanan 2016). Transesterification process involves three consecutive reversible reactions. In the first step, triglyceride reacts with alcohol to form diglyceride; di-glyceride is converted into mono-glyceride, finally, mono-glyceride is converted to glycerol (Yuvarajan and Venkata Ramanan 2016; Zheng et al. 2016). This process, when carried out by conventional heating, requires reaction time ranging from 30 min to 8 h for a reasonable conversion (Yuvarajan and Venkata Ramanan 2016; Nalgundwar et al. 2016).
The use of biodiesel from various feedstocks such as neat Jatropha oil (Jiotode and Agarwal 2017), pongamia oil (Perumal and Ilangkumaran 2017), mustard oil (Venkata Ramanan and Yuvarajan 2015), and neem oil (Ali et al. 2013; Vishnoi et al. 2017) in a compression ignition engine has been attempted by various researchers. Jiotode and Agarwal (2017) investigated research type immobile engine fueled with the biodiesel from jatropha oil to examine the feasibility of jatropha as biodiesel and to study various combustion characteristics. Jatropha-fuelled engine produced 2.2% less smoke, 6.1% less HC, and 5.9% less CO than diesel. Performance (brake thermal efficiency (BTE)) was affected by 0.9%; fuel usage (brake-specific fuel consumption (BSFC)) was also increased by 1.2%. This obtained trend in emission and performance is because of oxygen nature and lower value in heating. Perumal and Ilangkumaran (2017) employed neat pongamia biodiesel for evaluating the combustion and emission characteristics in turbocharged diesel engine under various engine-operating loads. Raw pongamia oil was converted into biodiesel by transesterification. This fuel had better properties than neat pongamia oil. Chemical and physical properties of pongamia biodiesel accomplished the American Society for Testing Materials (ASTM) standard and were found safe for use in the engine. Operating with pongamia biodiesel reduced the cylinder pressure marginally. Further, the maximum heat-release rate is decreased by 14.3% at partial engine load and 21.3% at full engine load. In addition, pongamia-fuelled engine produced 4.5% lower smoke, 4.9% lower HC, and 6.6% lower CO than diesel owing to oxygen nature. Performance (BTE) affected by 1.2%, fuel usage (BSFC) increased by 1.8% when compared with diesel fuel. This deviation in performance characteristics is attributed to its lower heating value. Venkata Ramanan and Yuvarajan (2015) analyzed the effect of mustard oil biodiesel for its performance and emission effectiveness in a diesel engine for the concentrations of 100 vol% at various engine loads at a constant speed of 1500 rpm. Neat mustard oil biodiesel has shown the best performance at full engine load. However, BTE was less than diesel at conditions owing to its lower heating value. In addition, NOx emissions were found to be higher for mustard oil biodiesel when compared with diesel fuel operation. This is because of higher combustion temperature. Ali et al. (2013) analyzed the effect of neem oil biodiesel for its performance and emission characteristics in a constant speed diesel engine at various engine loads. The study revealed that neat neem oil biodiesel has shown better performance at full engine load. However, BTE was less than diesel at conditions owing to its higher calorific value. They also found that the usage of neem oil biodiesel and its blends cause problem such as poor fuel droplet formation and improper mixing of fuel with air due to its higher viscosity. In addition, NOx emissions were found to be higher for neem oil biodiesel when compared with diesel fuel operation. Increase in combustion temperature could be the main reason for increased NOx emissions. Vishnoi et al. (2017) analyzed the effect of neem oil biodiesel for its performance and emission characteristics in a constant speed diesel engine at various engine loads. They reported that the BTE of a diesel engine to be 2.3% lesser for neem oil biodiesel and diesel blends than that of diesel. Carbon deposit and chocking in fuel injector were noticed while employing neat neem oil biodiesel. Deposit and chocking were noticed and could be a result of the higher viscosity of neem oil biodiesel. However, a significant reduction of 22–28% is achieved in CO, HC, and smoke emissions while the 11.5% increase of NOx emission during neem oil biodiesel operation was also reported. Significant change in emissions was attributed to the higher oxygen availability in biodiesel. These overall results reveal that the use of biodiesel along with diesel decreases the brake thermal efficiency with an increase in brake specific fuel consumption owing to lower calorific value. Emission constituents like carbon monoxide (CO), hydrocarbon (HC) and smoke opacity was considerably reduced due to the excess availability of inbuilt oxygen with an expense of an increase in oxides of nitrogen (NOx) due to increase in combustion temperature.
Major drawbacks of biodiesel are increased kinematic viscosity, volatility, density, and NOx emission and reduced calorific value (Anderson et al. 2017; Jiotode and Agarwal 2017; Zheng et al. 2016; Ali et al. 2013). Hence, there is a necessity to address these drawbacks for wider use of biodiesel. Much research had been carried out in biodiesel operation by changing the injection parameters, engine modifications, exhaust gas recirculation, preheating, and by adding additives in the fuel formulation (Nalgundwar et al. 2016; Venkata Ramanan and Yuvarajan 2015; Perumal and Ilangkumaran 2017; Zheng et al. 2016). In order to improve the thermal efficiency and minimize the NOx emissions from biodiesel, numerous techniques have been attempted. Among various methods, fuel formulation by the use of fuel additive is one of the simplest and effective ways as they do not employ any serious significant hardware modifications. In the fuel formulation technique, two or more fuels are mixed at calculated proportions for better properties of the overall mixture. This, in turn, improves the combustion process and reduces exhaust emissions (Devarajan et al. 2017a; Ibrahim 2016). Many works have been carried out by doping alcohols with diesel, biodiesel, biodiesel and diesel blends (Devarajan et al. 2017a; Babu and Anand 2017; Joy et al. 2017; Yilmaz and Atmanli 2017; Ibrahim 2016; Radhakrishnan 2017). Devarajan et al. (2017a) reported an experimental study on palm biodiesel with the addition of cyclo-octanol as a fuel-borne oxidation catalyst in a constant-speed direct injection compression-ignition engine. The dosage level in the fuel is about 10–30 vol%. The fuel injection pressure and the compression ratio were 210 bar and 18, respectively. The result was a 2.1% reduction in brake-specific fuel consumption and 1.17% increase in brake thermal efficiency when 30 vol% of cyclo-octanol was added with palm oil biodiesel. In addition, carbon monoxide, total hydrocarbon, and smoke emissions were reduced by 11.2, 12.3, and 8.6%. Also, in-cylinder gas pressure, heat-release rate was on the higher side while the ignition delay was shorter. They reported that the cyclo-octanol promoted for the complete combustion of fuel and acts as an additional oxygen buffer which supplies surplus oxygen to smooth the progress of proper combustion of fuel. Babu and Anand (2017) investigated the experimental study on the effect of n-pentanol and n-hexanol on biodiesel-diesel blends. Waste frying oil is employed as the source for biodiesel. The fuel constituents were varied between 0 and 100 vol% in the range of 5 vol%. The dosage level of n-pentanol and n-hexanol in the fuel blends is about 5–10 vol%. The fuel injection pressure and the compression ratio were 200 bar and 16, respectively. Result found that the carbon monoxide, total hydrocarbon and smoke emissions were reduced by 22, 24.4, and 13.5% by adding n-hexanol to biodiesel-diesel blends. Additionally, in-cylinder gas pressure, heat release rate was higher while the ignition delay was shorter by adding n-hexanol to biodiesel-diesel blends. They reported that n-hexanol contains higher oxygen in their molecular structure and it requires lesser oxygen for combustion. Yilmaz and Atmanli (2017) carried out an experimental study on neat diesel with the addition of 1-pentanol as an oxygenated additive in a constant-speed direct injection compression-ignition engine. The dosage level in the fuel is about 10–35 vol%. The fuel injection pressure and compression ratio were 220 bar and 16.5, respectively. Engine test was performed at different engine loads of 0, 1.5, 2.25 and 3 kW. The constant speed engine speed of 2000 rpm has been maintained. They reported that adding 1-pentanol to diesel decreased exhaust gas temperature, CO and NOx emissions. The oxygen content of pentanol positively affects combustion efficiency and contributes to the decrease in these emissions. They also reported that the 1-pentanol holds a potential as a promising candidate for reducing the emissions associated with the diesel. In another study, Joy et al. (2017) examined an experimental study on neat diesel with the addition of n-pentanol as an oxygenated additive in a constant-speed direct injection compression-ignition engine. The dosage level in the fuel is about 10-45 vol%. Engine test was performed at different engine brake power, and at a constant speed of 1500 rpm. They reported that adding n-pentanol to diesel decreased HC, CO and NOx emissions. Further, it was reported that simultaneous reduction of NOx and smoke emissions was achieved using the combination of pentanol/diesel blends in an unmodified diesel engine. The oxygen content of pentanol positively affects combustion efficiency and contributes to the decrease in these emissions. They also suggested 1-pentanol as a promising candidate for reducing the emissions associated with the diesel. They also reported that pentanol–diesel blends up to 45 vol% can be used in a diesel engine without any modification. In another test, Ibrahim (2016) carried out an experimental study on biodiesel and diesel blends with the addition of butanol as a fuel-borne oxidation catalyst in a constant-speed direct injection compression-ignition engine. The dosage level in the fuel is about 10–20 by volume. Results found 1.8% reduction of brake specific fuel consumption with 2.2% increase in brake thermal efficiency when 20 vol% of butanol was added with biodiesel and diesel blends. In addition, Carbon-monoxide, total hydrocarbon and smoke emissions were reduced by 6.6%, 8.9% and 9.8% by adding butanol to biodiesel and diesel blends. They reported that the butanol promotes the complete combustion of fuel and acts as an additional oxygen buffer which supplies surplus oxygen to smooth the progress of proper combustion of biodiesel and diesel blends. In a different analysis, Radhakrishnan (2017) examined an experimental study on neat palm oil biodiesel with the addition of pentanol as an additive in a constant-speed direct injection compression-ignition engine. The dosage level in the fuel is about 10–20 vol%. Engine test was performed at different engine brake power. The engine speed was maintained constant at 1500 rpm. They reported that adding n-pentanol to diesel decreased HC, CO and NOx emissions. The oxygen content of pentanol improves the combustion efficiency and contributes to the decrease in these emissions. They also reported that Pentanol–diesel blends up to 30 vol% can be used in a diesel engine without modifications. In another investigation, Rajesh Kumar et al. (2016a, b) carried out an experimental study on diesel with the addition of cyclohexanol as a fuel-borne oxidation catalyst in a constant-speed direct injection compression-ignition engine. The dosage level in the fuel is about 10–30 vol%. Result found 32.3% reduction of smoke emissions by adding 30 vol% of cyclohexanol to diesel under naturally aspirated condition. This is due to the presence of fuel-bound oxygen in cyclohexanol that increases the oxygen availability even in fuel-rich zones inhibiting the formation of soot precursors.
Aim of the study
From the above review, it is clear that the biofuels are considered as one of the potential candidates as a fuel for diesel engine applications (Tarun et al. 2014). Neem oil is chosen as the source for biodiesel. Neem oil biodiesel (BD100) has properties similar to diesel fuel. However, the operational feasibility of BD100 in a diesel engine is limited by the higher viscosity and NOx emission in the exhaust. Viscosity and NOx emissions of neat biodiesel could be reduced by adding with higher alcohols. In this work, cyclohexanol is chosen as oxygenated additive owing to its improved blend stability and higher energy density (Rajesh Kumar et al. 2016a, b). An in-depth exhaustive review of the available scientific literature has shown a gap in implementing the blending of cyclohexanol with neat neem oil biodiesel in diesel engine application. The possibility of using a higher cyclohexanol/biodiesel blends (30 vol%) was explored with an objective to reduce the viscosity of biodiesel without compromising other physical and chemical properties. By adding more %vol of cyclohexanol results in a drastic reduction in viscosity of biodiesel. However, adding beyond 30%vol results in a drastic reduction in heating value. Hence the alcohol addition is limited to 30%vol. In addition, it is thought that alcohols and biodiesel will give good results in combustion, emissions and performance while alcohols and biodiesel have low emissions and can both work without any modification in the engine. Hence, the current research effort is motivated to analyze the effect of cyclohexanol with neat neem oil biodiesel used in CI engine. In the present work, four blends of neat neem oil biodiesel and cyclohexanol and petroleum diesel is employed in CI engine for its performance and emission evaluations.
Materials and reagents
Fuel properties
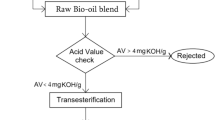
Physicochemical properties of all the fuel samples were measured according to the ASTM standards. The test and techniques carried out in many research which adapts ASTM standards are referred and the same testing procedure is performed to find out the desired properties of fuel (Abu-Hamdeh and Alnefaie 2015; Yilmaz and Atmanli 2017; Ibrahim 2016). HI904 Karl Fischer Coulometric Titrator integrated with diaphragm air pump and beaker adapter having a range of 0.0001% to 5% and resolution of 0.1 ppm to 0.0001% is used to measure the water content in the sample. DMA 4500 Density meter with the range of 0 to 3 g/cm3 and an accuracy of 5 × 10–5 g/cm3 is used to measure the density of testing fuels. A Brookfield Viscometer Model DV-I+ with UL adapter is employed to measure the viscosity of testing fuels. It consists of a set of seven spindles (RV SPINDLE SET) with accuracy ± 1%. The flash point of biodiesel is measured by flash point tester which consists of 80 ml closed copper cup, heater, and a source that gives continuous sparks. Cetane Tester SHATOX SX-200 with a range of 20–100 (CN units) and an accuracy of 0.1 °C is used to measure the cetane number. Cloud and Pour Point Analyzer (ISL CPP 5Gs) having a range of −95 to 51 °C and an accuracy of 0.1 °C is used to measure Cloud and Pour Point of the samples. The conventional transesterification process is employed for the conversion of raw oil to biodiesel. Methyl ester preparation is done following batch transesterification process in a 600 ml glass vessel reactor equipped with a magnetic stirrer, resistance heater & ‘K’ type thermocouple. Suitable arrangements were provided to control reaction temperature and stirring speed. A molar ratio of 5:1 (neem oil and methanol) and catalysts of 0.3% (wt/wt) to neem oil was used in the transesterification process adapting the standard procedure as cited in the literature (Abu-Hamdeh and Alnefaie 2015; Yilmaz and Atmanli 2017; Devarajan et al. 2017a; Yuvarajan and Venkata Ramanan 2016). A 500 g sample of neem oil in the reactor was heated to the temperature of 650 C. A measured quantity of solution containing catalysts dissolved in methanol was then added and mixed at a constant stirring speed of 340 rpm for 45 min. This ensured uniform reactivity of solution and accelerated the reaction rate. The mixture was then allowed to cool in the vessel yielding two distinct layers of ester and glycerol. Ester was then separated and washed thrice with water and dried for further analysis. Table 1 presents the fuel properties of fuels employed in this study. Table 2 presents the fatty acid composition of neem oil. A four-stroke, air-cooled, multi-cylinder, 1300 rpm constant speed AVL 5402 diesel engine is employed in this study.
Engine setup
In the present work, the fuel tests were performed on a four-stroke single cylinder, air cooled type, naturally aspirated, Direct Injection (DI) diesel engine (AVL 5402). The maximum torque is 28.2 Nm at 1500 rpm while the optimum engine power is 3.7 kW. The engine technical specifications are tabulated in Table 3. The experimental setup consists of an engine, a dynamometer, fuel supply system, data acquisition unit, emission analyzer and a smoke opacimeter. The engine under testing is coupled to a dynamometer of eddy-current type (Dynalec make) along with an electronic exciter for measuring and adjusting the engine load. For the fuel consumption measurement, the time required for 100 cm3 of fuel consumption is recorded with the aid of burette setup and a stopwatch. The exhaust gas and engine oil temperature were obtained by a K-Type Thermocouple which is connected to the digital display unit. For collecting the exhaust gas samples a measuring probe is connected to the exhaust tailpipe of the engine. The collected emissions of HC, NOx, CO2 and CO were obtained by using the AVL make (AVL digas 444 model) emission analyzer and similarly smoke opacity by using AVL 437C model opacimeter. Before conducting the experiments, the gas analyzers were calibrated using standard gases. The combustion chamber pressure is obtained using a Kistler make miniature pressure transducer which is connected to a computer-based Data Acquisition System (DAQ). The degree of crank angle as well the position of Top Dead Centre (TDC) is obtained using an encoder. The acquired output data is fed into the combustion analysis software (AVL INDMICRA) with the aid of DAQ system. The combustion analyzer provides the results of the characteristics of combustion such as in-cylinder gas pressure variation, heat-release rate (HRR), peak pressure, and ignition delay period of testing fuel samples. Repeating the same for 100 cycles to obtain all the parameters and considering the average of all these values is taken to reduce the influence of cycle-by-cycle variation. All these investigations were performed at steady-state conditions in order to ensure the reliability of recordings. Before the readings are acquired, the engine is run for about 15 min to allow it to attain steady-state conditions. Before starting the test, diesel fuel is used to run the engine for a few minutes to warm up and after each test, the engine is made to run by fuelling with diesel in order to flush out the biodiesel and cyclohexanol blends from the fuel injection system. Table 4 shows the details of gas analyzer and smoke meter range, accuracy, and uncertainty details of quantities measured. Uncertainty analysis is performed to evaluate the accurateness of engine performance measurements. Estimation of uncertainty of some important parameters from known measured values are evaluated with respect to square root method, and the same has been shown in Table 4. To ensure the correctness in the engine testing, each test should be repeated three times and the averaged results are mentioned in the subsequent section.
Results and discussion
Brake-specific hydrocarbon emission
Figure 1 shows the variation in HC with brake power for diesel, BD100, and BD100 blended with cyclohexanol in different proportions. HC increases with brake power due to the presence of more fuel inside the combustion chamber. Biofuels have lower HC emission than diesel. It contains extra oxygen molecules which are taking part in the combustion and leads to the lower HC emission (Yuvarajan et al. 2016, Devarajan et al. 2017a). It is found that addition of cyclohexanol to BD100 further decreases the HC. Cyclohexanol promotes the complete combustion and acts as an additional oxygen buffer which supplies surplus oxygen to smooth the progress of proper combustion of fuel (Babu and Anand 2017). The maximum reduction in HC emission is observed for BD70COH30 and this is attributed to complete combustion aided by surplus oxygen from cyclohexanol (Babu and Anand 2017; Devarajan et al. 2017a). Further, the addition of cyclohexanol reduces the viscosity of BD100, which in turn enhance the evaporation rate and result in better mixing with available air. In the earlier study, Yuvarajan and Venkata Ramanan (2016) stated that the low viscosity of fuel could be a dominant factor for higher evaporation rate and better mixing process. From the results, the overall HC emission of BD70COH30 is 12.19% lower than BD100 and 16.34% lower than diesel. Babu and Anand (2017) confirmed that the higher oxygen content in alcohol and biodiesel is a dominant factor for lowering HC emissions. In another investigation, Rajesh Kumar et al. (2016a, b) found a significant reduction in HC emission by adding n-octanol in diesel, stating improvement in combustion efficiency as a cause. In addition, this result is in accordance with other research carried out in different higher alcohols and biodiesel blends (Cai et al. 2015; Devarajan et al. 2017a).
Brake-specific carbon dioxide emissions
Figure 2 shows the variation in brake-specific carbon di-oxide (BSCO2) with brake power for Diesel, BD100, and BD100 blended with cyclohexanol in different proportions. CO2 increases with brake power for all fuels tested due to the presence of more fuel inside the combustion chamber causing complete combustion and to convert the CO into CO2 emissions (Venkata Ramanan and Yuvarajan 2015; Devarajan et al. 2017a). The result shows that carbon dioxide emission for BD100 and cyclohexanol blends is more than that of diesel. Since diesel constitutes the pure hydrocarbon chain, lower CO2 is obvious. It is found that addition of cyclohexanol to BD100 increases the BSCO2 emissions. Cyclohexanol enhances the combustion rate and also supplies more oxygen, which increases CO2 emissions (Babu and Anand 2017). The maximum increase in CO2 emission for cyclohexanol blends is observed for BD70COH30, and this is owing to the complete combustion with aid surplus oxygen molecules present in cyclohexanol (Babu and Anand 2017; Devarajan et al. 2017a). Further, the reduction in viscosity of mixtures also played a key role in an increase in BSCO2 emission (Yuvarajan et al. 2016). In addition, owing to the lesser viscous nature of cyclohexanol and biodiesel blends, the breakup and atomization of the spray are enhanced for and hence more oxygen is entrained by the spray plume causing complete combustion and higher CO2 emissions. Babu and Anand (2017) stated that the lower kinematic viscosity and density is a dominant factor which improves the vaporization of fuel and enhances CO2 emissions. Vedaraman et al. (2012) stated that the fuel with lower viscosity improves the penetration rate and lower the droplet size of the fuel which in turn improves the atomization and mixing of fuel with air and improve CO2 emissions. From the results, the cumulative CO2 emission of BD70COH30 is 13.91% higher than BD100 and 19.5% higher than diesel. Blending 30 vol% of cyclohexanol increases significant CO2 emissions of BD100. However, BSCO2 emissions of diesel fuel are lower than for other fuels. This result is in accordance with other research carried out in other higher alcohols and biodiesel blends (Cai et al. 2015; Babu and Anand 2017; Devarajan et al. 2017b).
Brake-specific oxides of nitrogen emission
Variation in NOx emission with brake power for diesel, BD100, and BD100 blended with cyclohexanol in different proportions is shown in Fig. 3. NOx emission for the BD100 is found to be more comparable with the diesel fuel as a result of the higher oxygen content of the fuel and high temperature inside the chamber during combustion (Devarajan et al. 2016; Devarajan et al. 2017a). NOx emission is highest for pure BD100 and lowest for BD70COH30. The possible reason for lower NOx emission could be due to the high latent heat of vaporization and comparatively lower calorific value of alcohol-biodiesel blends cause lower combustion temperature and lower NOx emission (Yuvarajan et al. 2016; Babu and Anand 2017). The high latent heat of evaporation of BD70CH30 may allow for absorption of heat from the combustion chamber and lead to a reduction of NOx formation (Yilmaz and Atmanli 2017). In addition, fuel with lower viscosity increases the atomization process and reduces the NOx emission (Yuvarajan et al. 2018; Yilmaz and Atmanli 2017). Babu and Anand (2017) stated that the high latent heat of vaporization and lower calorific value of hexanol in biodiesel results in lower combustion temperature and lower NOx emissions. In another investigation, Rajesh et al. (2016) found a significant reduction in NOx emission by adding n-octanol in diesel stating leaner mixture of alcohol and diesel as a cause. From the results, the overall NOx emission of BD70COH30 is 5.31% lower than BD100 at all load engine operations. NOx emission reduces with the inclusion of cyclohexanol to BD100. The maximum reduction in NOx emission is observed by doping 30 vol% of cyclohexanol to BD100. This result is in accordance with other research carried out in different higher alcohols and biodiesel blends (Babu and Anand 2017; Yilmaz and Atmanli 2017; Devarajan et al. 2017a; Devarajan et al. 2017b).
Smoke opacity
Figure 4 shows the variation in smoke emission with brake power for diesel, BD100, and BD100 blended with cyclohexanol in different proportions. The smoke opacity value is increased with the increase in the engine loading condition and this is because of injecting the higher amount of fuel in order to attain the engine power (Devarajan et al. 2017a). Smoke is formed mainly in the region of rich fuel mixture zone and hence it will be higher at 100% engine load condition for tested fuels (Devarajan et al. 2016). Smoke value for all biofuels is lower when compared with diesel fuel and this is because of higher content oxygen in the fuel tends to increase the oxidation reaction (Devarajan et al. 2016; Yilmaz and Atmanli 2017). Further, the excess oxygen concentration in the biofuels causes to undergo a complete combustion and thereby reduces the smoke emission. Biodiesel with cyclohexanol blends shows a lesser smoke emission as compared with the neat biodiesel. This is due to enhancement in soot oxidation (Babu and Anand 2017). The enhance oxidation is due to the local lambdas and temperature attained during the combustion. Besides, owing to the lesser viscous nature of cyclohexanol and biodiesel blends, the breakup and atomization of the spray are enhanced for and hence more oxygen is entrained by the spray plume causing complete combustion and lower smoke emissions (Yuvarajan and Venkata Ramanan 2016). The other possible reason may be attributed to the higher oxygen concentration of cyclohexanol and biodiesel blends and therefore proper fuel burning during the combustion process resulting in less smoke formation. Babu and Anand (2017) stated that the lower kinematic viscosity and density is a dominant factor which improves the vaporization of fuel and reduces smoke emissions. Further, they also confirmed that higher oxygen availability in the biodiesel-alcohol mixture reduces the smoke emissions. In another investigation, Rajesh et al. (2016) found a significant reduction in smoke emission by adding n-octanol in diesel. They concluded that the oxygen atoms bonded to the hydroxyl group of n-octanol reduce soot formation by inhibiting soot precursors and increases the availability of oxygen even in fuel-rich zones. From the results, the overall smoke emission of BD70COH30 is 19.23% lower than BD100 and 25.51% lower than diesel. Blending 30 vol% of cyclohexanol reduces significant smoke emissions. This result is in accordance with other research carried out employing different higher alcohols and biodiesel blends (Babu and Anand 2017; Yilmaz and Atmanli 2017; Devarajan et al. 2017b).
Brake-specific carbon monoxide emissions
Figure 5 shows the variation in brake-specific carbon monoxide (BSCO) with brake power for diesel, BD100, and BD100 blended with cyclohexanol in different proportions. CO increases with brake power for all fuels tested due to the presence of more fuel inside the combustion chamber causing complete combustion and to convert the CO into CO2 emissions (Yuvarajan et al. 2016; Devarajan et al. 2017a). It is observed that the carbon monoxide emission for BD100 and BD100 blended with cyclohexanol is less than diesel owing to the presence of inbuilt surplus oxygen molecules in the biodiesel. CO emission reduces with increase in cyclohexanol content. The lowest value of CO emissions is observed for BD70COH30. Complete combustion of BD100 and cyclohexanol blends results in lower CO emissions owing to surplus oxygen molecules present in cyclohexanol (Devarajan et al. 2017a). In addition, BD100 and cyclohexanol blends have a lower kinematic viscosity than from BD100. Fuel with lower viscosity improves the atomization and vaporization of fuel with air and reduces CO emissions (Venkata Ramanan and Yuvarajan 2015). Babu and Anand (2017) stated that the lower kinematic viscosity, density, and oxygen content of n-hexanol in the biodiesel-diesel blends are the dominant factor to improve the vaporization of fuel and reduce CO emissions. From the results, the overall CO emission of cyclohexanol blended with biodiesel by 30 vol% (BD70COH30) is 17% lower than neat biodiesel and 21.8% lower than diesel. Blending 30 vol% of cyclohexanol reduces significant CO2 emissions of BD100 (Babu and Anand, 2017). This result is in accordance with other research carried out in different higher alcohols and biodiesel blends (Yilmaz and Atmanli 2017; Devarajan et al. 2017b).
In-cylinder pressure variation
Combustion characteristics were analyzed based on in-cylinder pressure measurements. Figure 6a shows the in-cylinder pressure with brake power for diesel, BD100, and BD100 blended with cyclohexanol in different proportions for different loads on the engine. The in-cylinder pressure development in diesel engine depends on the quantity of fuel burned during the uncontrolled combustion (premixed combustion) phase. The variation in in-cylinder gas pressure clearly indicates the mixing and burning ability of fuel with air (Mahalingam et al. 2017). During the experiment, the center of combustion is not kept constant. Further, the injection duration is varied to adjust fuel quantity and to produce the required power output.
The Peak pressure of diesel is lesser than other test fuels. This is due to better atomization, better combustion and shorter ignition delay characteristics of diesel. In addition, the lower kinematic viscosity of diesel is beneficial to improve the fuel spray characteristics, fuel-air mixing and evaporation process, thereby resulting in shorter ignition delay and complete combustion. Peak in-cylinder gas pressure is slightly lower for biodiesel and alcohol blends (BD90COH10, BD80COH20, and BD70COH30) when compared with neat biodiesel (BD100). This is because of low viscous nature of blends, which could enhance the evaporation rate and thereby better mix with available air, lower ignition delay and lesser accumulation of fuel, and lower peak pressure. In an earlier study, Yuvarajan and Venkata Ramanan (2016) stated that the low viscosity of a fuel could be a dominant factor for high evaporation rate and better mixing process. This process pointed to complete combustion process and produces lower in-cylinder gas pressure. Similar results were cited in the experimental work by Babu and Anand (2017). This result supplements the observation of higher NOx emissions for BD100 when compared and other test fuels (BD90COH10, BD80COH20, and BD70COH30).
In addition, since BD100 has a lesser compressibility than BD90COH10, BD80COH20, and BD70COH30 due to higher density and kinematic viscosity, the time is taken for the fuel to reach combustion chamber is shorter, causing earlier fuel injection and longer combustion duration (Yuvarajan and Venkata Ramanan 2016). Further, the higher kinematic viscosity of BD100 reduces leakages in the fuel pump clearances, leading to an increase in the injection line pressure. Therefore, a quicker and earlier needle opening happens to BD100 causing advanced fuel injection (Yuvarajan and Venkata Ramanan 2016). Since the density and kinematic viscosity of BD100 are higher, the time taken for the fuel to mix with the air in the cylinder is more and results in the relatively longer combustion period. The advancement of fuel injection timing will increase NOx emissions for BD100. This is in agreement with the results obtained from NOx emissions. This result is in accordance with other research carried out in different higher alcohols and biodiesel blends (Babu and Anand 2017; Yilmaz and Atmanli 2017; Devarajan et al. 2017b).
Combustion noise
Combustion noise is produced by spontaneous ignition of fuel inside the combustion chamber, and it originates due to rapid fluctuations of in-cylinder pressure. Combustion noise gradually increased with increasing engine load. This is attributed to combustion of higher fuel quantity at higher engine loads. In general, baseline diesel produces the least amount of noise (gradients of the in-cylinder pressure trace). This is particularly visible at higher engine loads. Compared with baseline diesel, biodiesel and cyclohexanol blends were noisier. Among these, BD100 was the noisiest, while BD70COH30 was less noisy. As mentioned earlier, combustion and noise characteristics of biodiesel and cyclohexanol blends are primarily influenced by its lower fuel viscosity in comparison with neat biodiesel (BD100). Lower viscosity of BD100 blended with cyclohexanol in different proportions tends to reduce the ignition delay and produce lower noise levels (Devarajan et al. 2017b). At lower engine loads, fuel-air mixture is leaner hence the effect of viscosity is not significant. However, with increasing engine loads, fuel-air mixture becomes richer and the effect of lower fuel viscosity of biodiesel and cyclohexanol blends becomes significant (Yilmaz and Atmanli 2017). Since BD100 is more viscous, it is the noisiest.
Cylinder temperature
The average value of the cylinder temperature of burned and unburned gas existing in the combustion chamber during a cycle is called mean gas temperature. The gas in the cylinder is the mixture of burned and unburned fuel-air mixture. Mean gas temperature determines the rate of reaction through the combustion of fuel. The variation of mean cylinder temperature with a crank angle of the engine operated with tested fuels is represented in Fig. 7. The use of the BD100 led to a higher value of peak temperature when compared with pure diesel. The reason can be attributed to the adverse effect of higher viscosity which leads to inefficient utilization of fuel energy contents (Devarajan et al. 2017b; Babu and Anand 2017). However, the hindrance to receiving this peak value can be attributed to the increase in the ignition delay period necessary to balance the effect of the high viscosity of fuel burned that worsens the processes of fuel atomization and evaporation (Yuvarajan and Venkata Ramanan 2016). The addition of cyclohexanol to BD100 mixture led to accelerating the combustion process. The cyclohexanol and biodiesel blends (BD70COH30, BD80COH20, and BD90COH10) have a lower viscosity than that of neat biodiesel. Therefore, the evaporation rate of fuel droplet is increased, which resulted in a shortened ignition delay (Mahalingam et al. 2017). This effect, in turn, improved the fuel droplet atomization and the combustion process and lowers the peak combustion temperature (Babu and Anand 2017).
Heat-release rate
The rate of release of the heat content during the burning of fuel inside the cylinder indirect injection diesel engine is called as heat-release rate. Heat-release rates (HRR) were deduced from the pressure data that is averaged over 100 cycles to minimize the effects of cycle-to-cycle variations. Figure 8 shows the HRR with brake power for diesel, BD100, and BD100 blended with cyclohexanol in different proportions for 100% load on the engine. The start of the combustion process is read from heat-release rate, where its value changes from negative to positive value. The peak value of heat-release rate normally occurs for all fuels only at full-engine load conditions. This is due to the facts that in full-engine load, the quantity of fuel admitted is more. As noted in the figure, the heat-release rate follows comparable trends for all tested fuels. This can be viewed as a heat-release rate is comparatively higher when the diesel engine fuelled with neat biodiesel when compared with a diesel engine at the same load on the engine.
The maximum heat-release rate of diesel is lower than other test fuels (BD100, BD90COH10, BD80COH20, and BD70COH30). Since the calorific value of diesel is higher than biofuels, the quantity of diesel combusted are less and hence resulting in lower heat-release rate (Devarajan et al. 2017b). The HRR of biodiesel and alcohol blends (BD90COH10, BD80COH20, and BD70COH30) is lower than neat biodiesel (BD100) but higher than diesel. Addition of alcohol resulted in the enhancement of its combustion characteristics. Since the density and kinematic viscosity of BD100 are higher, the fuel droplet size increased which in turn reduced the mass fraction burnt in the premixed combustion phase, thereby resulting in a higher heat-release rate (Babu and Anand 2017). Further, the density and kinematic viscosity of BD90CH10, BD80CH20, and BD70CH30 is considerably lower due to the addition of alcohol, the fuel droplet size reduced, which in turn reduced the mass of fuel burnt in premixed combustion phase and results in lower heat-release rate (Yuvarajan and Venkata Ramanan 2016). This result is in accordance with other research carried out in different higher alcohols and biodiesel blends (Babu and Anand 2017; Yuvarajan and Venkata Ramanan 2016; Devarajan et al. 2017b).
Total heat-release rate
Figure 9 shows the total heat-release rate of the tested fuels with crank angle. It can be seen that the total heat-release rate, which is directly proportional to combustion efficiency in constant-volume adiabatic combustion, is highest for diesel followed by BD70COH30, BD80COH20, BD90COH10, and BD100. This could be attributed the higher calorific value of diesel (Babu and Anand 2017). In addition, the total heat-release rate for BD100 is least among all fuels. BD100 fuel had a high viscosity, resulting in poor atomization, reduced spray penetration, decreased cone angle, and greater fuel droplet size than other test fuels, which results in the lower amount of air entertainment and poor combustion, leading to lower total heat-release rate (Yuvarajan and Venkata Ramanan 2016). Further, Fuel with a lesser viscosity (BD90COH10, BD80COH20, BD70COH30, and diesel) when compared with BD100 reduces the combustion duration and, correspondingly, reduces the time of heat transfer compared with diffused dominant combustion, resulting in a further increase of the total heat-release rate. This result is in accordance with other research carried out in different higher alcohols and biodiesel blends (Babu and Anand 2017; Yuvarajan and Venkata Ramanan 2016; Devarajan et al. 2017b).
Conclusion
The experimental studies were conducted on a four-stroke single-cylinder, naturally aspirated, water-cooled, direct-injection diesel engine using neat biodiesel with the addition of cyclohexanol. Based on the result, the following conclusions were drawn
-
The in-cylinder pressure and temperature are comparatively low for diesel followed by cyclohexanol and biodiesel blends when compared with neat biodiesel.
-
The heat-release rate of BD100 is the highest among all fuels. The higher kinematic viscosity of BD100 caused more ignition lag, leading to higher HRR than diesel.
-
The addition of cyclohexanol has a direct positive influence on physical and chemical properties of biodiesel. The improvement in viscosity and volatility is better by appending 30%vol of cyclohexanol to biodiesel. However, the addition of cyclohexanol decreased the calorific value of neat biodiesel. Nevertheless, properties of diesel are found supreme than other tested fuels
-
The presence of 10, 20, and 30% of cyclohexanol in biodiesel decreased HC emissions as compared with neat biodiesel and diesel. The overall HC emission of BD70COH30 is 12.19% lower than BD100 and 16.34% lower than diesel. Further, diesel emitted more HC emissions among all tested fuels. In addition, appending different blends of cyclohexanol into biodiesel decreased NOx emissions as compared with neat biodiesel. The overall NOx emission of BD70COH30 is 5.31% lower than BD100 at all load engine operations. However, NOx emissions remained low for diesel at all engine loads when compared with other tested fuels.
-
Smoke emission in biodiesel-cyclohexanol and neat biodiesel is lower when compared with diesel due to the oxygen available in biofuel. The presence of 10, 20, and 30% of cyclohexanol in biodiesel decreased smoke emissions as compared with neat biodiesel and diesel. The overall smoke emission of BD70COH30 is 19.23% lower than BD100 and 25.51% lower than diesel.
-
The overall CO emission of cyclohexanol blended with biodiesel by 30 vol% (BD70COH30) is 17% lower than neat biodiesel and 21.8% lower than diesel. The overall CO2 emission of BD70COH30 is 13.91% higher than BD100 and 19.5% higher than diesel.
Abbreviations
- ASTM:
-
American Society for Testing Materials
- BD100:
-
Neat biodiesel
- BD70COH30:
-
70% of biodiesel + 30% of cyclohexanol (by volume)
- BD80COH20:
-
80% of biodiesel + 20% of cyclohexanol (by volume)
- BD90COH10:
-
90% of biodiesel + 10% of cyclohexanol (by volume)
- BSCO:
-
Brake-specific carbon monoxide
- BSEC:
-
Brake-specific energy consumption
- BSFC:
-
Brake-specific fuel consumption
- BTE:
-
Brake thermal efficiency
- CA:
-
Crank angle
- CO2 :
-
Carbon dioxide
- CO:
-
Carbon monoxide
- COH:
-
Cyclohexanol
- DAQ:
-
Data acquisition
- DI:
-
Direct injection
- EGR:
-
Exhaust gas recirculation
- HRR:
-
Heat-release rate
- IMEP:
-
Indicated mean effective pressure
- IT:
-
Injection timing
- NOx :
-
Oxides of nitrogen
- TDC:
-
Top dead center
- THRR:
-
Total heat-release rate
- UBHC:
-
Unburned hydrocarbons
References
Abdullah MA, Ani FN, Hassan M (2013) The effects of injection parameters on the performance of common rail light duty engine fueled with neem oil biodiesel. Appl Mech Mater 465-466:322–326
Abu-Hamdeh NH, Alnefaie KA (2015) A comparative study of almond and neem oils as two bio-diesel fuels for diesel engine in terms of emissions and performance. Fuel 150:318–324
Ali MH, Mashud M, Rubel MR, Ahmad RH (2013) Biodiesel from neem oil as an alternative fuel for diesel engine. Procedia Eng 56:625–630. https://doi.org/10.1016/j.proeng.2013.03.169
Anderson A, Devarajan Y, & Nagappan B (2017) Effect of injection parameters on the reduction of NOx emission in neat bio-diesel fuelled diesel engine. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 40(2):186–192. https://doi.org/10.1080/15567036.2017.1407844
Babu D, Anand R (2017) Effect of biodiesel-diesel-n-pentanol and biodiesel-diesel- n -hexanol blends on diesel engine emission and combustion characteristics. Energy 133:761–776. https://doi.org/10.1016/j.energy.2017.05.103
Cai L, Uygun Y, Togbé C, Pitsch H, Olivier H, Dagaut P, Sarathy SM (2015) An experimental and modeling study of n-octanol combustion. Proceedings of the Combustion Institute 351:419–427
Devarajan Y, Jayabal RK, Ragupathy D, Venu H (2016) Emissions analysis on second generation biodiesel. Front Environ Sci Eng 111. https://doi.org/10.1007/s11783-017-0891-0
Devarajan Y, Munuswamy DB, Mahalingam A, Nagappan B (2017a) Performance, combustion, and emission analysis of neat palm oil biodiesel and higher alcohol blends in a diesel engine. Energy Fuel 31(12):13796–13801. https://doi.org/10.1021/acs.energyfuels.7b02939
Devarajan Y, Nagappan BK, Munuswamy DB (2017b) Performance and emissions analysis on diesel engine fuelled with cashew nut shell biodiesel and pentanol blends. Korean J Chem Eng 344:1021–1026
Fangsuwannarak K, Wanriko P, Fangsuwannarak T (2016) Effect of bio-polymer additive on the fuel properties of neem biodiesel and on engine performance analysis and exhaust emission. Energy Procedia 100:227–236
Ibrahim A (2016) Performance and combustion characteristics of a diesel engine fuelled by butanol–biodiesel–diesel blends. Appl Therm Eng 103:651–659
Jaat M, Khalid A, Manshoor B, Ramsy H, Mustaffa N (2014) An experimental study on the performance and emissions of diesel engine fuelled with biodiesel derived from neem oil. Appl Mech Mater 699:654–659
Jiotode Y, Agarwal AK (2017) Endoscopic combustion characterization of Jatropha biodiesel in a compression ignition engine. Energy 119:845–851. https://doi.org/10.1016/j.energy.2016.11.056
Joy N, Devarajan Y, Nagappan B, & Anderson A (2017) Exhaust emission study on neat biodiesel and alcohol blends fueled diesel engine. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 40(1):115–119. https://doi.org/10.1080/15567036.2017.1405119-1
Mahalingam A, Devarajan Y, Radhakrishnan S, Vellaiyan S, & Nagappan B (2017) Emissions analysis on mahua oil biodiesel and higher alcohol blends in diesel engine. Alex Eng J. https://doi.org/10.1016/j.aej.2017.07.009
Nalgundwar A, Paul B, Sharma SK (2016) Comparison of performance and emissions characteristics of DI CI engine fueled with dual biodiesel blends of neem and jatropha. Fuel 173:172–179
Perumal V, Ilangkumaran M (2017) Experimental analysis of engine performance, combustion and emission using pongamia biodiesel as fuel in CI engine. Energy 129:228–236. https://doi.org/10.1016/j.energy.2017.04.120
Radhakrishnan S (2017) Emissions analysis on diesel engine fueled with palm oil biodiesel and pentanol blends. Journal of Oil Palm Research 29:380–386. https://doi.org/10.21894/jopr.2017.2903.11
Rajesh Kumar B, Saravanan S, Niranjan Kumar R, Nishanth B, Rana D, Nagendran A (2016a) Effect of lignin-derived cyclohexanol on combustion, performance and emissions of a direct-injection agricultural diesel engine under naturally aspirated and exhaust gas recirculation (EGR) modes. Fuel 181:630–642. https://doi.org/10.1016/j.fuel.2016.05.052
Rajesh Kumar B, Saravanan S, Rana D, Anish V, Nagendran A (2016b) Effect of a sustainable biofuel-n-octanol-on the combustion, performance and emissions of a DI diesel engine under naturally aspirated and exhaust gas recirculation (EGR) modes. Energy Convers Manag 118:275–286. https://doi.org/10.1016/j.enconman.2016.04.001
Tarun Y, Thamotharan C, Mukherjee K (2014) Evaluation of engine performance, emissions, of a twin cylinder diesel engine fuelled with waste plastic oil and diesel blends with a fraction of methanol. Int J Eng Technol 32
Vedaraman N, Puhan S, Nagarajan G, Ramabrahmam BV, Velappan KC (2012) Methyl ester of Sal oil (Shorea robusta) as a substitute to diesel fuel—a study on its preparation, performance and emissions in direct injection diesel engine. Ind Crop Prod 36(1):282–288. https://doi.org/10.1016/j.indcrop.2011.09.003
Venkata RM, & Yuvarajan D (2015) Emissions Analysis of Preheated Methyl Ester on CI Engine. Appl Mech Mater 812:21–25. https://doi.org/10.4028/www.scientific.net/amm.812.21
Vishnoi PK, Gupta VK, Bisht VS, Patil AK (2017) Performance evaluation of CI engine on fuel blends of neem oil biodiesel and diesel. J Biofuels 8(2):98. https://doi.org/10.5958/0976-4763.2017.00013.7
Yilmaz N, Atmanli A (2017) Experimental assessment of a diesel engine fueled with diesel-biodiesel-1-pentanol blends. Fuel 191:190–197
Yuvarajan D, Venkata Ramanan M (2016) Experimental analysis on neat mustard oil methyl ester subjected to ultrasonication and microwave irradiation in four stroke single cylinder diesel engine. J Mech Sci Technol 301:437–446
Yuvarajan D, Ravikumar J, Babu MD (2016) Simultaneous optimization of smoke and NOx emissions in a stationary diesel engine fuelled with diesel–oxygenate blends using the grey relational analysis in the Taguchi method. Anal Methods 832:6222–6230
Yuvarajan D, Dinesh BM, BeemKumar N & Amith KP (2018) Experimental investigation on the influence of titanium dioxide nanofluid on emission pattern of biodiesel in a diesel engine. Atmos Pollut Res 9(1):47–52. https://doi.org/10.1016/j.apr.2017.06.003
Zheng Z, Wang X, Yue L, Liu H, Yao M (2016) Effects of six-carbon alcohols, ethers and ketones with chain or ring molecular structures on diesel low temperature combustion. Energy Convers Manag 124:480–491. https://doi.org/10.1016/j.enconman.2016.07.057
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Devarajan, Y., Mahalingam, A., Munuswamy, D.B. et al. Emission and combustion profile study of unmodified research engine propelled with neat biofuels. Environ Sci Pollut Res 25, 19643–19656 (2018). https://doi.org/10.1007/s11356-018-2137-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2137-5