Abstract
The aim of this work was to study Cr, Ni, Zn, and P bioaccumulation in different compartments of Typha domingensis plants and sediment in a free-water surface constructed wetland for the treatment of a metallurgical effluent for 5 years. Removal efficiencies were satisfactory. To increase metal tolerance, its transport from belowground to aboveground tissues is reduced, being metal concentrations in the roots and rhizomes significantly higher than in the aerial and submerged parts of leaves. Regarding belowground tissues, metals were retained in the roots, while P was mainly accumulated in rhizomes. Bioaccumulation factors (BAFs) of Cr and Ni showed values near 1, and BAF of Zn and P were above 1 in several samplings, indicating bioaccumulation in the roots. Translocation factors (TFs) of Cr, Ni, and Zn were below 1, showing a scarce translocation from the roots to the aerial parts of the leaves, while the TF of P were above 1 in many samplings, indicating that this element is necessary for plant metabolism. The study of plant tissues where contaminants are accumulated allows gaining insight into the constructed wetland operation. The high translocation of P in T. domingensis makes this species suitable for its phytoextraction, while the low metal translocation makes T. domingensis suitable for phytostabilization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants growing in constructed wetlands (CWs) show several properties that render them an essential component (Brix 1994). There are numerous biological, chemical, and physical processes that occur among contaminants and aquatic plants. Currently, these processes and their relationship are not sufficiently known. Regarding metals, it has been proposed that metal removal mechanisms in plants are not necessarily the same for different macrophyte species and for different metals (Maine et al. 2016; Suñe et al. 2007). Among these mechanisms, root sorption (a combination of physical and chemical processes such as quelation, ionic exchange), biological processes (which include translocation to aerial parts), and precipitation induced by root exudates or microorganisms could be mentioned. Regarding plant tolerance, several species tolerate high metal concentrations in sediment because they limit the absorption and translocation to the leaves maintaining constant and relatively low concentrations in the aerial biomass, independently of the metal concentration in sediment (Bonanno 2011; Hadad et al. 2006; Vymazal 2011).
To understand contaminant dynamics, it is important to study the ecosystem compartments where they are accumulated (Bonanno et al. 2017; Eid and Shaltout 2014). Gupta et al. (2012) studied the dynamics of Cr, Ni, Cu, and Pb among the different ecosystem compartments: water, surface sediments, submerged and free-floating macrophytes, and fishes of two man-made lakes in India. These authors concluded that although a significant metal uptake by macrophytes and fishes was observed, metal concentrations in sediment were significantly higher than those of the other compartments. Regarding plant compartments, Mufarrege et al. (2015) exposed the emergent species Typha domingensis to high Cr, Ni, and Zn concentrations. These authors observed that metals were accumulated not only in the roots but also in the submerged parts of leaves in direct contact with the solution. On the other hand, macrophytes do not only remove contaminants when they are alive. It was demonstrated that plant dry biomass is able to adsorb metals (André et al. 1999; Dushenkov et al. 1995; Miretzky et al. 2006; Schneider et al. 1999, 2001; Suñé et al. 2007; Wang et al. 1998), which may be an important issue for wetland management.
In Argentina, a CW for the treatment of effluent from a metallurgical industry showed different stages as to vegetation dominance (Maine et al. 2009, 2013). The wastewater contains P, Cr, Ni, and Zn and shows high pH and conductivity. Initially, an assemblage of locally common macrophytes from the Middle Paraná River floodplain was transplanted to the wetland. Plant growth showed three different periods: the first one dominated by the floating macrophyte Eichhornia crassipes, followed by its decline and an increase of the emergent macrophyte T. domingensis cover period, and finally a T. domingensis dominance period (Maine et al. 2007, 2009). Despite the presence of metals in the effluent, the cause of disappearance of the floating macrophytes was the high values of pH and conductivity (Hadad et al. 2006). Since the emergent macrophyte T. domingensis was more tolerant to the effluent conditions than the floating species, it became dominant in the wetland over the last 12 years. A predation of T. domingensis aerial parts by capybaras (Hydrochoerus hydrochaeris) occurred during this period. However, the roots and rhizomes of T. domingensis were not damaged. After such event, T. domingensis recovered its aerial biomass and covered the entire wetland surface (Maine et al. 2013).
Despite macrophytes being a key component in treatment wetlands, knowledge about their role is still insufficiently understood. The depuration efficiency in different wetland designs, the plant species to be used and their tolerance to contaminants and the characteristics of the effluent to be treated are topics that should be deepened due to their influence on the system optimization (Wu et al. 2015). The study of the compartments where contaminants are accumulated is necessary to understand the contaminant dynamics in CWs. The aim of this work was to study contaminant bioaccumulation in different compartments of T. domingensis plants and in sediment in a CW for the treatment of a metallurgical effluent for 5 years. A long-term study of the plant tissues where the contaminants are accumulated allows gaining insight into the CW operation and determining if biomass harvesting is worth performing.
Materials and methods
Description of the wetland system
A free-water surface (FWS) wetland was constructed for the final treatment of industrial wastewater and sewage from a metallurgical factory. Wastewaters from both sources receive a primary treatment (precipitation, sieving, and decantation) and are treated in the same CW. The CW has been operating since 2001. It is 50 m long, 40 m wide, and 0.4–0.5 m deep. The wetland has a central baffle to force the effluent to cover double the distance. Mean wastewater discharge is 100 m3 d−1. Water residence time ranges from 7 to 12 days. After flowing through the CW, the effluent is discharged into a 1.5-ha pond. Further design details have been explained in a previous study (Maine et al. 2009).
Sampling
This study began when the CW reached stability and maturity. The study period was between 2010 and 2015. Wastewater, sediment, and plants were sampled monthly by triplicated. The contaminant concentrations of the influent and the effluent were used to estimate the system efficiency. The sediment and plant samples were taken in the inlet zone of the CW. Surface sediment samples were collected with a PVC corer at a depth of 0–3 cm and then stored at 4 °C. T. domingensis plants were sampled by hand and separated into aerial parts of leaves, submerged parts of leaves, roots, and rhizomes. The roots and rhizomes were washed with tap water in order to separate the attached sediment.
Analytical determinations and calculations
Conductivity, pH, and dissolved oxygen (DO) were measured in situ with an YSI 33 conductivity-meter, Orion pH-meter, and Horiba OM-14 oximeter, respectively. Wastewater chemical analysis was carried out following APHA (1998). Wastewater samples were kept refrigerated during their transport to the laboratory. Soluble reactive phosphorus (SRP) was determined by the colorimetric molybdenum blue method (Murphy and Riley 1962). In the case of total phosphorus (TP), non-filtered water samples were digested with sulfuric acid-nitric acid. SRP was determined in the digested samples (Murphy and Riley, 1962). Nitrite (NO2−) was determined by coupling diazotation followed by a colorimetric technique. Nitrate (NO3−) and ammonium (NH4+) were determined by potentiometry (Orion ion selective electrodes, sensitivity 0.01 mg L−1 of N, reproducibility ± 2%). Chemical oxygen demand (COD) was determined by the open reflux method and biochemical oxygen demand (BOD) by the 5-day BOD test. For metal analysis, samples were acidified to pH < 2. Total Fe, Cr, Ni, and Zn concentrations were determined in water samples by atomic absorption spectrometry (by flame or electrothermal atomization, according to sample concentration, Perkin Elmer AAnalyst 200) and previous acid digestion with nitric acid-hydrochloric acid (APHA, 1998).
Cr, Ni, Zn, and P were measured in sediment and plant tissues. Plants and sediment samples were oven-dried at 70 °C for 48 h (APHA 1998). Dried samples were ground, sieved, and digested with a HClO4:HNO3:HCl (7:5:2) mixture, and then, metals were measured by atomic absorption spectrometry (Perkin Elmer, AAnalyst 200), and P was determined as SRP in the digested samples (Murphy and Riley, 1962).
The bioaccumulation factor (BAF) was calculated to determine the ability of plants to accumulate contaminants from the sediment:
The translocation factor (TF) was calculated to assess the plant capacity to translocate a contaminant from the roots to the aerial parts of leaves:
The mass of Cr, Ni, Zn, and P in sediment and tissues was calculated (mg m−2) using the dry plant biomass and the dry sediment mass. To estimate dry biomass, T. domingensis stands were sampled with a 0.50 × 0.50 m−2. Four replicates were collected. Plants were harvested, separated into aerial and submerged parts of leaves, roots, and rhizomes, and dried at 105 °C until a constant weight was reached (APHA 1998). For sediment sampling, it was considered an active sediment layer of 3 cm, according to our previous work (Di Luca et al. 2011). Sediment samples were dried at 105 °C until constant weight. The mean contaminant retention percentage by macrophyte tissues and sediment was calculated.
Statistical analysis
In order to ascertain statistical differences in contaminant concentrations in water, sediment, and plant tissues between the inlet and outlet, paired tests were used. ANOVA was carried out to determine if there were significant differences in contaminant concentrations among the sediment and the different plant tissues, in the BAF and TF among the different contaminants, and among the mass of the different contaminants. To differentiate means where appropriate, Duncan’s test was used. Normality of residuals was tested graphically. Bartlett’s test was used to verify that variances were homogeneous. In all comparisons, a level of p < 0.05 was used.
QA/QC
To wash all glassware, 2 M HNO3 was used. To prepare all solutions, analytical grade reagents and Milli-Q water were used. A precision lower than 5% (coefficient of variation) was determined in all replicate analyses. Detection limits for water were Cr = 2 μg L−1, Ni = 3 μg L−1, Zn = 3 μg L−1, and P = 5 μg L−1, and for macrophyte tissues and sediment were Cr = 2 μg g−1, Ni = 3 μg g−1, Zn = 3 μg g−1, and P = 0.5 μg g−1.
Results and discussion
Table 1 shows mean values, concentration ranges, and removal efficiencies of the parameters measured in water. High pH and conductivity were determined in the influent. The chemical composition of the wastewater showed significantly high variability, which is a common characteristic of industrial effluents. However, after flowing through the CW, the effluent presented not only significantly lower concentrations (paired test, p < 0.05) but also significantly lower variability of the parameters measured compared with that of the influent, which proves the buffer capacity of the system.
Metals were efficiently removed from the wastewater (paired test, p < 0.05). It is important to highlight that this CW was meant for a final treatment, and metal concentrations in the inlet were low. Contaminant removal efficiencies were satisfactory, except for SRP and NH4+, probably due to low DO concentration. CW performance was steady over the operation period, allowing the effluent to meet law regulatory limits. A more detailed analysis of the removal efficiency was reported by Maine et al. (2017).
The sediment in the inlet zone of the CW showed significantly higher Cr, Ni, Zn, and P concentrations than those registered in the outlet zone (paired test, p < 0.05) (Fig. 1), and the concentrations in the latter remained showing no significant differences along the study (paired test, p < 0.05). Comparing studied contaminant concentrations in sediment, Zn showed the lowest ones reflecting the low Zn concentration in the influent. Sediment sorption is considered the main long-term contaminant accumulation mechanism (Machemer et al. 1993; Maine et al. 2009; Wood and Shelley, 1999). Di Luca et al. (2011) evaluated metal retention and distribution in the sediment of the studied CW. These authors concluded that sediment may be expected to continue retaining Ni and Zn mainly stored in carbonate fraction, Cr was mainly associated with the Fe–Mn oxide fraction, while Fe was mainly associated with the residual fraction. These metals will not be released into water while the chemical and environmental conditions remain unchanged. Aquatic plants can modify the biogeochemistry of the sediment by altering redox conditions, pH, and organic matter content, influencing contaminant accumulation in this compartment (Di Luca et al. 2016).
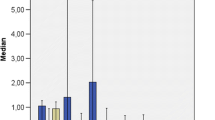
Cr and Ni concentrations were significantly higher in sediment than in plant tissues (ANOVA, p < 0.05), while in some samplings, the concentrations of Zn and P were significantly higher in roots than in sediment. Cr, Ni, and Zn concentrations in roots and rhizomes were significantly higher than in the aerial and submerged parts of leaves (ANOVA, p < 0.05) (Fig. 2). This indicates a scarce metal translocation from belowground to aboveground parts. To increase metal tolerance, transport from belowground to aboveground tissues is reduced. The exclusion of metals in roots is a macrophyte tolerance strategy to avoid the damage of vital tissues (Chaney 1993; Göthberg et al. 2004; Hechmi et al. 2014; Kabata-Pendias and Pendias 2011; Loneragan and Webb 1993; Marchand et al. 2010; Shanker et al. 2005). The tolerance mechanisms consist of metal deposition on the root surface and inside the vacuoles and the root cell walls (Taylor and Crowder 1983).
BAF of Cr and Ni showed values near 1. BAFs of Zn and P were above 1 indicating that roots bioaccumulate them (Fig. 3). In all samplings, metal TFs were lower than 1 showing a scarce translocation of Cr, Ni, and Zn from the roots to the aerial parts of leaves.
In most samplings, P showed BAF and TF values higher than 1, demonstrating that it is taken from the sediment by roots and then it is translocated to the aerial parts of leaves. The BAF values for Zn higher than 1 indicate that this metal is accumulated in root tissues to a higher extent than Cr and Ni. Bragato et al. (2009) reported that Zn was the most accumulated metal in Phragmites australis in a CW in North Italy. Zn is a micronutrient for plants and it is both a structural and a catalytic component in many proteins and enzymes (Marschner 1995; Van Assche and Clijsters 1986). Nyquist and Greger (2007) studied the stress effects in response to Zn, Cu, and Cd exposure on Elodea canadensis. They reported that most of the accumulated Zn and Cu are transported inside the cell through the cell membrane, whereas Cd remains in the cell membrane. Probably, this could be explained by the essentiality of these elements that would increase uptake rates and translocation within the plant. While in the cell, Zn is quickly subjected to enzyme synthesis, needed for other physiologically active molecules, or incorporated in membranes or other cellular components (Rengel 1999).
Regarding belowground tissues, metal concentrations in the roots were significantly higher than those of rhizomes (ANOVA, p < 0.05) (Fig. 2). Metals are retained by roots, while P is mainly accumulated in rhizomes. Since P is a plant nutrient, it is stored to carry out photosynthesis and develop biomass. The same result was reported for T. domingensis plants growing in natural wetlands from the Middle Paraná River floodplain (Argentina) as a strategy for biomass increase (Hadad and Maine 2007). The high translocation of P to aboveground organs in T. domingensis makes this species suitable for its phytoextraction from the system, while the low metal translocation makes T. domingensis suitable for phytostabilization. In the case of metals, Eid and Shaltout (2014) concluded that higher translocation of Cd in the aboveground organs of P. australis allows its phytoextraction, while the lower translocation for Cu, Fe, and Zn enhances their phytostabilization.
It was widely reported that metal concentrations in macrophytes growing in constructed and natural wetlands are usually higher in belowground than in aboveground parts (Bonanno 2011; Hadad et al. 2006, 2007, 2011; Larsen and Schierup 1981; Maddison et al. 2009; Peverly et al. 1995; Nuñez et al., 2011; Vymazal, 2011, 2013; Windham et al. 2003; Zhang et al. 2014). Most studies only consider above and belowground parts. In our work, we consider the submerged parts of leaves which are a rarely studied plant compartment. It is remarkable that such plant compartment showed significantly higher metal concentrations than the aerial parts of leaves. The tissues of the submerged parts of leaves were in direct contact with the wastewater. For this reason, not only translocation but also adsorption from water were probably the mechanisms responsible for metal uptake in these tissues. Mufarrege et al. (2015) reported a similar result studying T. domingensis responses when exposed to Cr, Ni, and Zn during greenhouse experiments. This result acquires importance because the submerged parts of leaves are not considered in studies on contaminant bioaccumulation by macrophytes. In comparison with the other plant compartments, metal bioaccumulation in the aerial parts of leaves was negligible (ANOVA, p < 0.05). However, leaves transport oxygen to the belowground tissues and sediment, enhancing the contaminant removal processes.
In comparison with submerged macrophytes (Harguinteguy et al. 2014; Nyquist and Greger 2007; Yabanli et al. 2014), T. domingensis has a significantly higher biomass, which enhances efficiency in contaminant removal and extraction from the system. In CW, contaminant accumulation in the biomass of emergent macrophytes could allow phytoextraction from the system by harvesting, and phytostabilization by accumulation in the belowground tissues (Brezinová and Vymazal 2015). Another advantage of T. domingensis is that it is found worldwide in remarkable abundance, being available in natural wetlands close to the sites where treatment wetlands may be constructed. Guittonny-Philippe et al. (2015) highlight the use of native macrophytes growing at short distances from industrial discharges to be treated.
Figure 4 shows the estimated mean contaminant retention by macrophyte tissues and sediment in the inlet area of CW (%). Metals and P were retained by plants and sediment; however, sediment was the main accumulation compartment (ANOVA, p < 0.05). As it can be seen, macrophytes are not important sinks for metal removal, which is in agreement with the findings of Mays and Edward (2001) and Lee and Scholz (2007). This is an advantage since metals were phytostabilized in wetland sediment. Plants certainly contribute to metal trapping into the substrate via rhizodeposition (Kidd et al. 2009).
Among plant compartments, the submerged parts of leaves were the main contaminant compartment due to a higher biomass and contaminant concentration in comparison with the other plant compartments (ANOVA, p < 0.05). The bioaccumulation of Zn and P in the aerial parts of leaves was significantly higher than that of Cr and Ni due to higher translocation. The results obtained could be used to know in more detail about the CW functioning and the assessment of harvesting in order to remove contaminants from the system, definitely.
Conclusions
-
Contaminant removal efficiencies were satisfactory in the studied CW and T. domingensis showed tolerance to the wastewater.
-
The concentrations of Zn and P were significantly higher in the roots than in sediment, indicating that root plants are a compartment to be considered during the effluent treatment using CWs.
-
Cr, Ni, and Zn were scarcely translocated from the roots to the aerial parts of leaves, while P showed a high translocation. This implies that Cr, Ni, and Zn were phytostabilized in the belowground tissues and sediment, while P could be phytoextracted from the system by harvesting the aboveground parts due to the higher accumulation of this element in the aboveground tissues.
-
The results obtained could be used to predict the accumulation pattern of contaminants in different compartments to carry out a suitable management in a CW.
References
André I, Schneider H, Rubio J (1999) Sorption of heavy metal ions by the nonliving biomass of freshwater macrophytes. Environ Sci Technol 33:2213–2217
APHA, AWWA, WEF (1998) Standard methods for the examination of water and wastewater. American Public Health Association, Washington D.C
Bonanno G (2011) Trace element accumulation and distribution in the organs of Phragmites australis (common reed) and biomonitoring applications. Ecotoxicol Environ Saf 74:1057–1064
Bonanno G, Borg JA, Di Martino V (2017) Levels of heavy metals in wetland and marine vascular plants and their biomonitoring potential: a comparative assessment. Sci Total Environ 576:796–806
Bragato C, Schiavon M, Polese R, Ertani A, Pittarello M, Malagoli M (2009) Seasonal variations of Cu, Zn, Ni and Cr concentration in Phragmites australis (Cav.) Trin ex steudel in a constructed wetland of North Italy. Desalination 246:35–44
Brezinová TB, Vymazal J (2015) Evaluation of heavy metals seasonal accumulation in Phalaris arundinacea in a constructed treatment wetland. Ecol Eng 79:94–99
Brix H (1994) Functions of macrophytes in constructed wetlands. Water Sci Technol 29(4):71–78
Chaney R (1993) Zinc phytotoxicity. In: Robson A (ed) Zinc in soils and plants. Kluwer, Dordercht, pp 135–150
Di Luca GA, Maine MA, Mufarrege MM, Hadad HR, Sánchez GC, Bonetto CA (2011) Metal retention and distribution in the sediment of a constructed wetland for industrial wastewater treatment. Ecol Eng 37:1267–1275
Di Luca GA, Mufarrege MM, Hadad HR, Maine MA (2016) Distribution of high Zn concentrations in unvegetated and Typha domingensis Pers. vegetated sediments. Environ Earth Sci 75:773. https://doi.org/10.1007/s12665-016-5575-8
Dushenkov VP, Kumar NBA, Motto H, Raskin Y (1995) Rhizofiltration: the use of plants to remove heavy metals from aqueous streams. Environ Sci Technol 29:1239–1245
Eid EM, Shaltout KH (2014) Monthly variations of trace elements accumulation and distribution in above- and below-ground biomass of Phragmites australis (Cav.) Trin. ex Steudel in Lake Burullus (Egypt): a biomonitoring application. Ecol Eng 73:17–25
Göthberg A, Greger M, Holm K, Bengtsson BE (2004) Influence of nutrient levels on uptake and effects of mercury, cadmium and lead in water spinach. J Environ Qual 33:1247–1255
Guittonny-Philippe A, Petit ME, Masotti V, Monnier Y, Malleret L, Coulomb B, Combroux I, Baumberger T, Viglione J, Laffont-Schwob I (2015) Selection of wild macrophytes for use in constructed wetlands for phytoremediation of contaminant mixtures. J Environ Manag 147:108–123
Gupta B, Kumar R, Rani M, Agarwal T (2012) Dynamics of toxic heavy metals in different compartments of a highly urbanized closed aquatic system. J Environ Monit 14:916–924
Hadad HR, Maine AM (2007) Phosphorous amount in floating and rooted macrophytes growing in wetlands from the Middle Paraná River floodplain (Argentina). Ecol Eng 31(4):251–258
Hadad HR, Maine MA, Bonetto CA (2006) Macrophyte growth in a pilot-scale constructed wetland for industrial wastewater treatment. Chemosphere 63:1744–1753
Hadad HR, Maine MA, Natale GS, Bonetto C (2007) The effect of nutrient addition on metal tolerance in Salvinia herzogii. Ecol Eng 31:122–131
Hadad HR, Maine MA, Mufarrege MM, Del Sastre MV, Di Luca GA (2011) Bioaccumulation kinetics and toxic effects of Cr, Ni and Zn on Eichhornia crassipes. J Hazard Mater 190:1016–1022
Harguinteguy CA, Cirelli AF, Pignata ML (2014) Heavy metal accumulation in leaves of aquatic plant Stuckenia filiformis and its relationship with sediment and water in the Suquía river (Argentina). Microchem J 114:111–118
Hechmi N, Aissa NB, Abdenaceur H, Jedidi N (2014) Evaluating the phytoremediation potential of Phragmites australis grown in pentachlorophenol and cadmium co-contaminated soils. Environ Sci Pollut Res 21(2):1304–1313
Kabata-Pendias A, Pendias H (2011) Trace elements in soils and plants. CRC Press, Florida
Kidd P, Barcelo J, Pilar Bernal M, Navari-Izzo F, Poschenrieder C, Shilev S, Clemente R, Monterroso C (2009) Trace element behaviour at the root-soil interface: implications in phytoremediation. Environ Exp Bot 67:243–259
Larsen VJ, Schierup HH (1981) Macrophyte cycling of zinc, copper, lead and cadmium in the littoral zone of a polluted and a non- polluted lake. II. Seasonal changes in heavy metal content of aboveground biomass and decomposing leaves of Phragmites australis (Cav.) Trin. Aquat Bot 11:211–230
Lee BH, Scholz M (2007) What is the role of Phragmites australis in experimental constructed wetland filters treating urban runoff? Ecol Eng 29:87–95
Loneragan J, Webb M (1993) Interactions between zinc and other nutrients affecting the growth of plants. In: Robson A (ed) Zinc in soils and plants. Kluwer, Dordercht, pp 119–131
Machemer S, Reynolds J, Laudon L, Wildeman T (1993) Balance of S in a constructed wetland built to treat acid mine drainage, Idaho Springs, Colorado, USA. Appl Geochem 8:587–603
Maddison M, Soosaar K, Mauring T, Mander Ü (2009) The biomass and nutrient and heavy metal content of cattails and reeds in wastewater treatment wetlands for the production of construction material in Estonia. Desalination 246:120–128
Maine MA, Suñé N, Hadad H, Sánchez G, Bonetto C (2007) Removal efficiency of a constructed wetland for wastewater treatment according to vegetation dominance. Chemosphere 68:1105–1113
Maine MA, Suñé N, Hadad H, Sánchez G, Bonetto C (2009) Influence of vegetation on the removal of heavy metals and nutrients in a constructed wetland. J Environ Manag 90:355–363
Maine MA, Hadad HR, Sánchez GC, Mufarrege MM, Di Luca GA, Caffaratti SE, Pedro MC (2013) Sustainability of a constructed wetland faced with a depredation event. J Environ Manag 128:1–6
Maine MA, Hadad HR, Sánchez G, Caffaratti S, Pedro MC (2016) Kinetics of Cr(III) and Cr(VI) removal from water by two floating macrophytes. Int J Phytorem 18(3):261–268
Maine MA, Hadad HR, Sánchez GC, Di Luca GA, Mufarrege MM, Caffaratti SE, Pedro MC (2017) Long-term performance of two free-water surface wetlands for metallurgical effluent treatment. Ecol Eng 98:372–377
Marchand L, Mench M, Jacob DL, Otte ML (2010) Metal and metalloid removal in constructed wetlands, with emphasis on the importance of plants and standardized measurements: a review. Environ Pollut 158:3447–3461
Marschner H (1995) Mineral nutrition of higher plants. Academic Press, New York, Boston, London
Mays PA, Edwards GS (2001) Comparison of heavy metal accumulation in a natural wetland and constructed wetlands receiving acid mine drainage. Ecol Eng 18:251–252
Miretzky P, Saralegui A, Fernandez Cirelli A (2006) Simultaneous heavy metal removal mechanism by dead macrophytes. Chemosphere 66(2):247–254
Mufarrege MM, Hadad HR, Di Luca GA, Maine MA (2015) The ability of Typha domingensis to accumulate and tolerate high concentrations of Cr, Ni, and Zn. Environ Sci Pollut Res 22:286–292
Murphy J, Riley J (1962) A modified single solution method for determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nuñez SER, Negrete JLM, Rios JEA, Hadad HR, Maine MA (2011) Hg, Cu, Pb, Cd, and Zn accumulation in macrophytes growing in tropical wetlands. Water Air Soil Pollut 216:361–373
Nyquist J, Greger M (2007) Uptake of Zn, Cu, and Cd in metal loaded Elodea canadensis. Environ Exp Bot 60:219–226
Peverly JH, Surface JM, Wang T (1995) Growth and trace metal absorption by Phragmites australis in wetlands constructed for landfill leachate treatment. Ecol Eng 5:21–35
Rengel Z (1999) Heavy metal as essential nutrients. In: Prasad MNV, Hagemeyer J (eds) Heavy metal stress in plants: from molecules to ecosystems. Springer-Verlag, Berlin, Heidelberg, New York, pp 231–251
Schneider IAH, Smith RW, Rubio J (1999) Effect of mining chemicals on biosorption of Cu(II) by the non-living biomass of the macrophyte Potamogeton lucens. Miner Eng 12(3):255–260
Schneider IAH, Rubio J, Smith RW (2001) Biosorption of metals onto plant biomass: exchange adsorption or surface precipitation? Int J Min Process 62(1–4):111–120
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31:739–753
Suné N, Sanchez G, Caffaratti S, Maine MA (2007) Cadmium and chromium removal kinetics by two aquatic macrophytes. Environ Pollut 145:467–473
Taylor GJ, Crowder AA (1983) Uptake and accumulation of copper, nickel, and iron by Typha latifolia grown in solution culture. Can J Bot 61:1825–1830
Van Assche F, Clijsters H (1986) Inhibition of photosynthesis in Phaseolus vulgaris by treatment with toxic concentrations of zinc: effects on electron transport and photophoshorylation. Physiol Plantarum 66:717–721
Vymazal J (2011) Constructed wetlands for wastewater treatment: five decades of experience. Environ Sci Technol 45:61–69
Vymazal J (2013) Emergent plants used in free water surface constructed wetlands: a review. Ecol Eng 61:582–592
Wang G, Fuerstenau MC, Smith RW (1998) Sorption of heavy metals onto nonliving water hyacinth roots. Min Process Ext Met Rev Int J 19(1):309–322
Windham L, Weis JS, Weis P (2003) Uptake and distribution of metals in two dominant salt marsh macrophytes: Spartina alterniflora (cordgrass) and Phragmites australis (common reed). Est Coast Shelf Sci 56:63–72
Wood T, Shelley MA (1999) Dynamic model of bioavailability of metals in constructed wetland sediments. Ecol Eng 12:231–252
Wu S, Wallace S, Brix H, Kuschk P, Kirui WK, Masi F, Dong R (2015) Treatment of industrial effluents in constructed wetlands: challenges, operational strategies and overall performance. Environ Pollut 201:107–120
Yabanli M, Yozukmaz A, Sel F (2014) Heavy metal accumulation in the leaves, stem and root of the invasive submerged macrophyte Myriophyllum spicatum L. (Haloragaceae): an example of Kadin Creek (Mugla, Turkey). Braz Arch Biol Technol 57(3):434–440
Zhang DQ, Jinadasa KB, Richard MG, Liu Y, Ng WJ, Tan SK (2014) Application of constructed wetlands for wastewater treatment in developing countries: a review of recent developments (2000–2013). J Environ Manag 141:116–131
Funding
This study is funded by the following Argentine institutions: Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Universidad Nacional del Litoral (UNL)-CAI+D Project, and Agencia de Promoción Científica y Tecnológica.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Hadad, H.R., Mufarrege, M.d., Di Luca, G.A. et al. Long-term study of Cr, Ni, Zn, and P distribution in Typha domingensis growing in a constructed wetland. Environ Sci Pollut Res 25, 18130–18137 (2018). https://doi.org/10.1007/s11356-018-2039-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2039-6